Enhanced Muscle Strength in Dyslipidemic Mice and Its Relation to Increased Capacity for Fatty Acid Oxidation
Abstract
:1. Introduction
2. Results
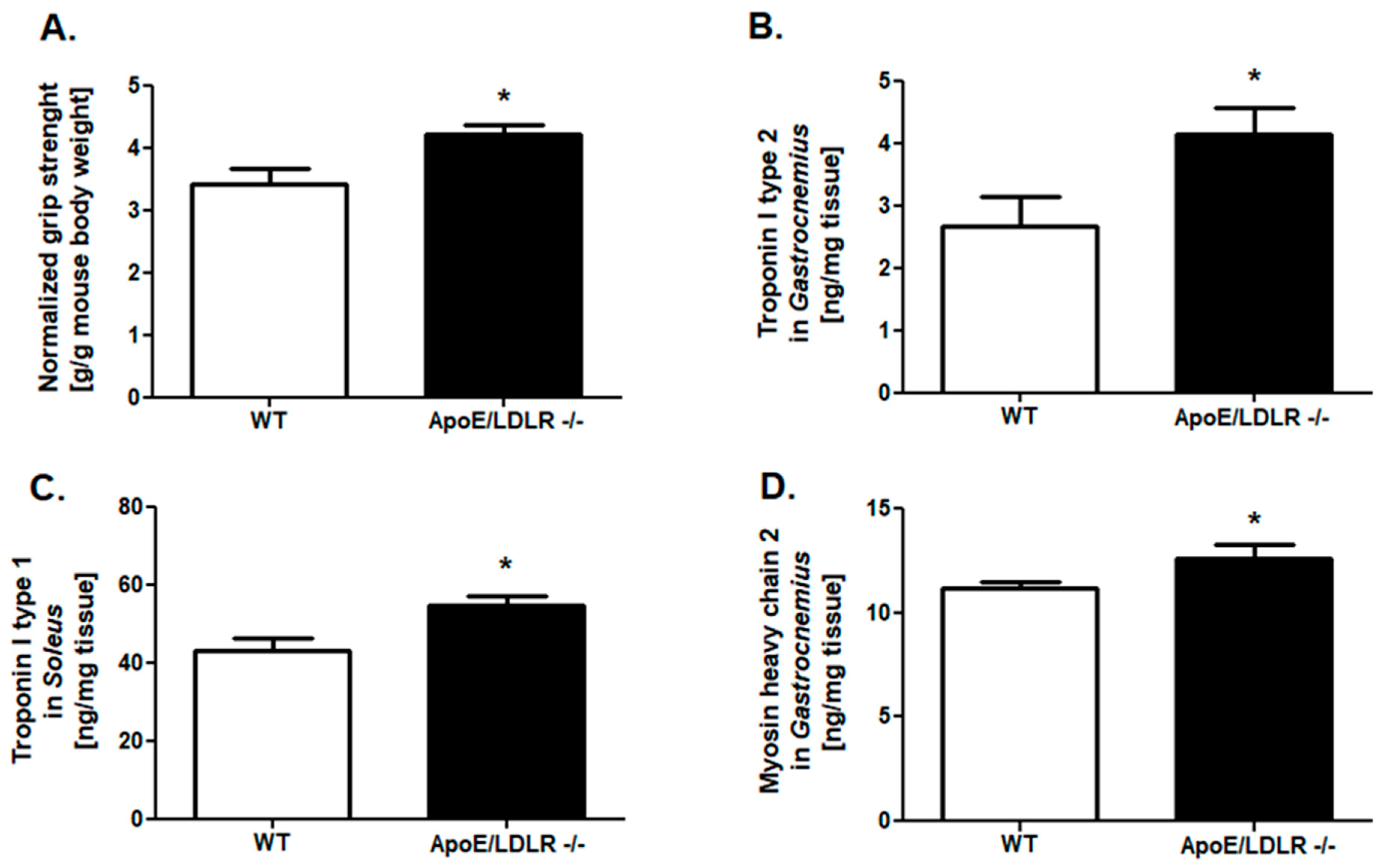
2.1. Improved Grip Strength and Skeletal Muscle Troponins Content in ApoE/LDLR -/- Mice
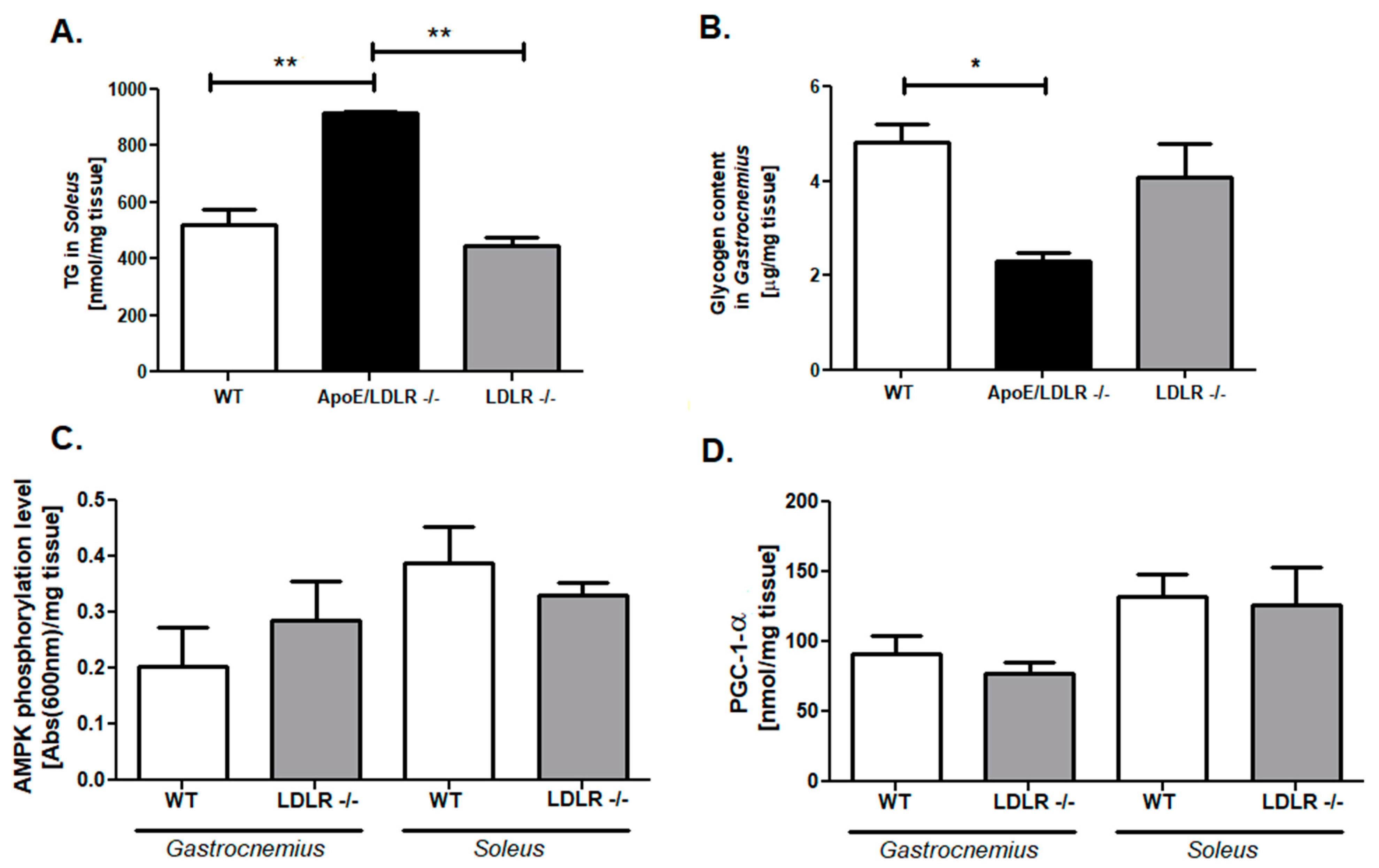
2.2. Compensatory Changes in Mitochondria in Skeletal Muscle in ApoE/LDLR -/- Mice
2.3. Enhanced Adenine Nucleotides Pool Is Accompanied by an Elevation of Triglycerides and Depletion of Glycogen Stores in ApoE/LDLR -/- Mice Skeletal Muscle
2.4. Free Fatty Acids Elevation as a Key Factor for Skeletal Muscle Function Improvement in ApoE/LDLR -/- Mice Model
2.5. Characterization of Fatty Acids Composition in ApoE/LDLR -/- Mice Serum
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Animals
5.1.1. Forelimb Grip Strength Measurement
5.1.2. Ranolazine Treatment and Evaluation of Exercise Capacity
5.1.3. Skeletal Muscle Isolation and Serum Collection
5.2. Evaluation of Skeletal Muscle Protein Levels
5.3. Investigation of Skeletal Muscle Mitochondrial Chain Complexes Activities
5.4. Measurement of Total Phosphocreatine and Creatine, Nicotinamide Dinucleotides, and Adenine Nucleotides Pool
5.5. Evaluation of Skeletal Muscle Triglycerides, Glycogen Stores, and Citric Synthase Activity
5.6. Investigation of Serum-Free Fatty Acids, Glucose, and Lipid Profiles
5.7. Fatty Acids Serum Composition
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2014, 96, 183–195. [Google Scholar] [CrossRef]
- Ahmad, K.; Shaikh, S.; Ahmad, S.S.; Lee, E.J.; Choi, I. Cross-Talk Between Extracellular Matrix and Skeletal Muscle: Implications for Myopathies. Front. Pharmacol. 2020, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.-G.; Lee, E.J.; Choi, I. Dermatopontin in Skeletal Muscle Extracellular Matrix Regulates Myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [Green Version]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C.; Kostrominova, T.Y.; Mann, M.; Murgia, M. Mitochondrial specialization revealed by single muscle fiber proteomics: Focus on the Krebs cycle. Scand. J. Med. Sci. Sports 2015, 25, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Metab. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojewoda, M.; Tyrankiewicz, U.; Gwozdz, P.; Skorka, T.; Jabłońska, M.; Orzylowska, A.; Jasinski, K.; Jasztal, A.; Przyborowski, K.; Kostogrys, R.B.; et al. Exercise capacity and cardiac hemodynamic response in female ApoE/LDLR−/− mice: A paradox of preserved V’O2max and exercise capacity despite coronary atherosclerosis. Sci. Rep. 2016, 6, 24714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olkowicz, M.; Tomczyk, M.; Debski, J.; Tyrankiewicz, U.; Przyborowski, K.; Borkowski, T.; Zabielska-Kaczorowska, M.; Szupryczynska, N.; Kochan, Z.; Smeda, M.; et al. Enhanced cardiac hypoxic injury in atherogenic dyslipidaemia results from alterations in the energy metabolism pattern. Metabolism 2020, 114, 154400. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Targosz-Korecka, M.; Suraj-Prażmowska, J.; Proniewski, B.; Jasztal, A.; Marczyk, B.; Sternak, M.; Przybyło, M.; Kurpinska, A.; Walczak, M.; et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J. Am. Hear. Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef] [Green Version]
- Przyborowski, K.; Kassassir, H.; Wojewoda, M.; Kmiecik, K.; Sitek, B.; Siewiera, K.; Zakrzewska, A.; Rudolf, A.M.; Kostogrys, R.B.; Watala, C.; et al. Effects of a single bout of strenuous exercise on platelet activation in female ApoE/LDLR−/− mice. Platelets 2017, 28, 657–667. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Stump, C.S.; Henriksen, E.J.; Wei, Y.; Sowers, J.R. The metabolic syndrome: Role of skeletal muscle metabolism. Ann. Med. 2006, 38, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Steinberg, G.; Chen, Z.-P.; Kemp, B.E.; Febbraio, M.A. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J. Physiol. 2006, 574, 139–147. [Google Scholar] [CrossRef]
- Clark, H.; Saggerson, D.; Carling, D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. JBIC J. Biol. Inorg. Chem. 2004, 271, 2215–2224. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Scaini, G.; Rochi, N.; Benedet, J.; Ferreira, G.K.; Teodorak, B.P.; Comim, C.M.; Constantino, L.; Vuolo, F.; Constantino, L.C.; Quevedo, J.; et al. Inhibition of brain citrate synthase activity in an animal model of sepsis. Rev. Bras. Ter. Intensiv. 2011, 23, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauerfeld, C.; Talwar, H.; Zhang, K.; Liu, Y.; Samavati, L. MKP-1 Modulates Mitochondrial Transcription Factors, Oxidative Phosphorylation, and Glycolysis. ImmunoHorizons 2020, 4, 245–258. [Google Scholar] [CrossRef]
- Huo, L.; Scarpulla, R.C. Mitochondrial DNA Instability and Peri-Implantation Lethality Associated with Targeted Disruption of Nuclear Respiratory Factor 1 in Mice. Mol. Cell. Biol. 2001, 21, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Baar, K.; Song, Z.; Semenkovich, C.F.; Jones, T.E.; Han, D.-H.; Nolte, L.A.; Ojuka, E.O.; Chen, M.; Holloszy, J.O. Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J. 2003, 17, 1666–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röckl, K.S.; Hirshman, M.F.; Brandauer, J.; Fujii, N.; Witters, L.A.; Goodyear, L.J. Skeletal Muscle Adaptation to Exercise Training. Diabetes 2007, 56, 2062–2069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-Y.; Lin, J.; Wu, Z.; Wu, H.; Tarr, P.T.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional Co-Activator PGC-1 Alpha Drives the Formation of Slow-Twitch Muscle Fibres. Nature 2002, 418, 797–801. [Google Scholar]
- Van de Locht, M.; Borsboom, T.C.; Winter, J.M.; Ottenheijm, C.A.C. Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics. Int. J. Mol. Sci. 2021, 22, 9187. [Google Scholar] [CrossRef]
- Tomczyk, M.; Olkowicz, M.; Slominska, E.M.; Smolenski, R.T. High Throughput Procedure for Comparative Analysis of In Vivo Cardiac Glucose or Amino Acids Use in Cardiovascular Pathologies and Pharmacological Treatments. Metabolites 2021, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Mateuszuk, L.; Jasztal, A.; Maslak, E.; Gasior-Glogowska, M.; Baranska, M.; Sitek, B.; Kostogrys, R.; Zakrzewska, A.; Kij, A.; Walczak, M.; et al. Antiatherosclerotic Effects of 1-Methylnicotinamide in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice: A Comparison with Nicotinic Acid. J. Pharmacol. Exp. Ther. 2015, 356, 514–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caligiuri, G.; Levy, B.; Pernow, J.; Thorén, P.; Hansson, G.K. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc. Natl. Acad. Sci. USA 1999, 96, 6920–6924. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef]
- He, K.; Wang, J.; Shi, H.; Yu, Q.; Zhang, X.; Guo, M.; Sun, H.; Lin, X.; Wu, Y.; Wang, L.; et al. An interspecies study of lipid profiles and atherosclerosis in familial hypercholesterolemia animal models with low-density lipoprotein receptor deficiency. Am. J. Transl. Res. 2019, 11, 3116–3127. [Google Scholar]
- Vance, J.E.; Hayashi, H.; Karten, B. Cholesterol homeostasis in neurons and glial cells. Semin. Cell Dev. Biol. 2005, 16, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, M.J.; Shooter, E.M.; Pitas, R.E.; Mahley, R.W. Lipoprotein Uptake by Neuronal Growth Cones in Vitro. Science 1987, 236, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Deng, A.; Irizarry, M.C.; Fitzgerald, M.L.; Rebeck, G.W. Induction of the Cholesterol Transporter ABCA1 in Central Nervous System Cells by Liver X Receptor Agonists Increases Secreted Aβ Levels. J. Biol. Chem. 2002, 277, 48508–48513. [Google Scholar] [CrossRef] [Green Version]
- Galgani, J.E.; Uauy, R.D.; Aguirre, C.; Díaz, E.O. Effect of the dietary fat quality on insulin sensitivity. Br. J. Nutr. 2008, 100, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.; Martinez, K.; Chuang, C.-C.; LaPoint, K.; McIntosh, M. Saturated Fatty Acid-Mediated Inflammation and Insulin Resistance in Adipose Tissue: Mechanisms of Action and Implications. J. Nutr. 2008, 139, 1–4. [Google Scholar] [CrossRef]
- Decsi, T.; Molnár, D.; Koletzko, B. Long-chain polyunsaturated fatty acids in plasma lipids of obese children. Lipids 1996, 31, 305–311. [Google Scholar] [CrossRef]
- Mika, A.; Kaska, L.; Korczynska, J.; Mirowska, A.; Stepnowski, P.; Proczko, M.; Ratnicki-Sklucki, K.; Goyke, E.; Sledzinski, T. Visceral and subcutaneous adipose tissue stearoyl-CoA desaturase-1 mRNA levels and fatty acid desaturation index positively correlate with BMI in morbidly obese women. Eur. J. Lipid Sci. Technol. 2015, 117, 926–932. [Google Scholar] [CrossRef]
- Ragino, Y.I.; Shramko, V.; Stakhneva, E.M.; Chernyak, E.; Morozov, S.V.; Shakhtshneider, E.V.; Polonskaya, Y.V.; Shcherbakova, L.V.; Chernyavskiy, A.M. Changes in the blood fatty-acid profile associated with oxidative-antioxidant disturbances in coronary atherosclerosis. J. Med Biochem. 2019, 39, 46–53. [Google Scholar] [CrossRef]
- Zwara, A.; Wertheim-Tysarowska, K.; Mika, A. Alterations of Ultra Long-Chain Fatty Acids in Hereditary Skin Diseases—Review Article. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, B.; Heeren, J.; Blaeser, M.; Radner, H.; Kayser, D.; Aydin, B.; Merkel, M. Lipoprotein lipase-facilitated uptake of LDL is mediated by the LDL receptor. J. Lipid Res. 2007, 48, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niebauer, J.; Maxwell, A.J.; Lin, P.S.; Tsao, P.S.; Kosek, J.; Bernstein, D.; Cooke, J. Impaired aerobic capacity in hypercholesterolemic mice: Partial reversal by exercise training. Am. J. Physiol. Circ. Physiol. 1999, 276, H1346–H1354. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.S.; Albadawi, H.; Robaldo, A.; Peck, M.A.; Abularrage, C.J.; Yoo, H.-J.; Lamuraglia, G.M.; Watkins, M.T. Divergent systemic and local inflammatory response to hind limb demand ischemia in wild-type and ApoE–/– mice. J. Surg. Res. 2013, 183, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Robertson, T.A.; Dutton, N.S.; Martins, R.N.; Roses, A.D.; Kakulas, B.A.; Papadimitriou, J.M. Beta-amyloid protein-containing inclusions in skeletal muscle of apolipoprotein-E-deficient mice. Am. J. Pathol. 1997, 150, 417–427. [Google Scholar] [PubMed]
- Sellers, S.L.; Milad, N.; White, Z.; Pascoe, C.; Chan, R.; Payne, G.W.; Seow, C.Y.; Rossi, F.; Seidman, M.A.; Bernatchez, P. Increased nonHDL cholesterol levels cause muscle wasting and ambulatory dysfunction in the mouse model of LGMD2B. J. Lipid Res. 2018, 59, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, G.W.; Brand, M.; Petrosyan, S.; Ashok, D.; Elorza, A.A.; Ferrick, D.A.; Murphy, A.N. High Throughput Microplate Respiratory Measurements Using Minimal Quantities of Isolated Mitochondria. PLoS ONE 2011, 6, e21746. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Toczek, M.; Smeets, C.J.L.M.; Franklin, S.A.; Bondulich, M.K.; Jolinon, N.; Muller, T.; Ahmed, M.; Dick, J.R.T.; Piotrowska, I.; et al. HDAC4-Myogenin Axis as an Important Marker of HD-Related Skeletal Muscle Atrophy. PLoS Genet. 2015, 11, e1005021. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, A.; Jakubiak, A.; Mierzejewska, P.; Zwara, A.; Liakh, I.; Sledzinski, T.; Mika, A. The Effect of a High-Fat Diet on the Fatty Acid Composition in the Hearts of Mice. Nutrients 2020, 12, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]








| Parameter [%] | WT | ApoE/LDLR -/- | p |
|---|---|---|---|
| C10:0 | 0.10 ± 0.003 | traces | - |
| C12:0 | 0.30 ± 0.04 | 0.04 ± 0.004 | *** |
| C13:0 | 0.03 ± 0.003 | 0.01 ± 0.002 | *** |
| C14:0 | 1.03 ± 0.10 | 0.22 ± 0.04 | *** |
| C15:0 | 0.51 ± 0.05 | 0.24 ± 0.01 | ** |
| C16:0 | 24.12 ± 1.53 | 18.05 ± 0.62 | * |
| C17:0 | 0.47 ± 0.03 | 0.56 ± 0.05 | n/s (0.13) |
| C18:0 | 9.78 ± 1.08 | 8.37 ± 0.24 | n/s (0.3) |
| C19:0 | 0.16 ± 0.008 | 0.22 ± 0.011 | ** |
| C20:0 | 0.27 ± 0.04 | 0.29 ± 0.03 | n/s (0.7) |
| C21:0 | 0.05 ± 0.007 | 0.04 ± 0.003 | n/s (0.1) |
| C22:0 | 0.30 ± 0.04 | 0.21 ± 0.02 | n/s (0.07) |
| C24:0 | 0.30 ± 0.03 | 0.20 ± 0.01 | n/s (0.051) |
| C25:0 | 0.06 ± 0.009 | 0.06 ± 0.009 | n/s (0.9) |
| C26:0 | 0.04 ± 0.008 | 0.01 ± 0.001 | * |
| Total SFA | 37.51 ± 2.69 | 28.57 ± 0.52 | * |
| C14:1 | 0.02 ± 0.005 | 0.02 ± 0.003 | n/s (0.6) |
| C16:1 | 4.26 ± 0.33 | 3.10 ± 0.10 | ** |
| C18:1 | 18.93 ± 1.45 | 24.78 ± 0.79 | ** |
| C19:1 | 0.01 ± 0.003 | 0.01 ± 0.002 | n/s (0.4) |
| C20:1 | 0.34 ± 0.03 | 1.04 ± 0.10 | *** |
| C22:1 | 0.51 ± 0.08 | 0.10 ± 0.02 | ** |
| C24:1 | 0.60 ± 0.09 | 0.34 ± 0.03 | * |
| Total MUFA | 24.67 ± 1.76 | 29.38 ± 0.74 | n/s (0.07) |
| C16:2n-6 | 0.04 ± 0.008 | 0.05 ± 0.01 | n/s (0.6) |
| C18:2n-6 | 25.72 ± 1.57 | 29.90 ± 0.95 | n/s (0.07) |
| C20:2n-6 | 0.09 ± 0.01 | 0.1 ± 0.008 | n/s (0.6) |
| C20:3n-6 | 0.56 ± 0.075 | 0.53 ± 0.053 | n/s (0.7) |
| C20:4n-6 | 6.12 ± 1.39 | 5.54 ± 0.42 | n/s (0.7) |
| C22:4n-6 | 0.04 ± 0.005 | 0.05 ± 0.004 | n/s (0.3) |
| Total PUFA n-6 | 32.57 ± 2.91 | 36.16 ± 1.01 | n/s (0.3) |
| C18:3n-3 | 0.23 ± 0.71 | 0.21 ± 0.04 | n/s (0.8) |
| C20:4n-3 | 0.05 ± 0.009 | 0.03 ± 0.004 | n/s (0.2) |
| C20:5n-3 | 1.10 ± 0.33 | 1.85 ± 0.24 | n/s (0.1) |
| C22:5n-3 | 0.34 ± 0.07 | 0.48 ± 0.06 | n/s (0.2) |
| C22:6n-3 | 3.54 ± 0.78 | 3.27 ± 0.23 | n/s (0.2) |
| Total PUFA n-3 | 5.26 ± 1.19 | 5.85 ± 0.44 | n/s (0.6) |
| Parameter | WT | ApoE/LDLR -/- | p |
|---|---|---|---|
| SFA/MUFA ratio | 1.52 ± 0.09 | 0.97 ± 0.02 | *** |
| SFA/PUFA ratio | 0.99 ± 0.20 | 0.68 ± 0.02 | n/s (0.1) |
| PUFA n-6/PUFA n-3 ratio | 6.19 ± 2.50 | 6.18 ± 0.63 | n/s (0.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.; Braczko, A.; Jablonska, P.; Mika, A.; Przyborowski, K.; Jedrzejewska, A.; Krol, O.; Kus, F.; Sledzinski, T.; Chlopicki, S.; et al. Enhanced Muscle Strength in Dyslipidemic Mice and Its Relation to Increased Capacity for Fatty Acid Oxidation. Int. J. Mol. Sci. 2021, 22, 12251. https://doi.org/10.3390/ijms222212251
Tomczyk M, Braczko A, Jablonska P, Mika A, Przyborowski K, Jedrzejewska A, Krol O, Kus F, Sledzinski T, Chlopicki S, et al. Enhanced Muscle Strength in Dyslipidemic Mice and Its Relation to Increased Capacity for Fatty Acid Oxidation. International Journal of Molecular Sciences. 2021; 22(22):12251. https://doi.org/10.3390/ijms222212251
Chicago/Turabian StyleTomczyk, Marta, Alicja Braczko, Patrycja Jablonska, Adriana Mika, Kamil Przyborowski, Agata Jedrzejewska, Oliwia Krol, Filip Kus, Tomasz Sledzinski, Stefan Chlopicki, and et al. 2021. "Enhanced Muscle Strength in Dyslipidemic Mice and Its Relation to Increased Capacity for Fatty Acid Oxidation" International Journal of Molecular Sciences 22, no. 22: 12251. https://doi.org/10.3390/ijms222212251
APA StyleTomczyk, M., Braczko, A., Jablonska, P., Mika, A., Przyborowski, K., Jedrzejewska, A., Krol, O., Kus, F., Sledzinski, T., Chlopicki, S., Slominska, E. M., & Smolenski, R. T. (2021). Enhanced Muscle Strength in Dyslipidemic Mice and Its Relation to Increased Capacity for Fatty Acid Oxidation. International Journal of Molecular Sciences, 22(22), 12251. https://doi.org/10.3390/ijms222212251








