Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Process Parameters for NP Preparation
2.2. Loading Content
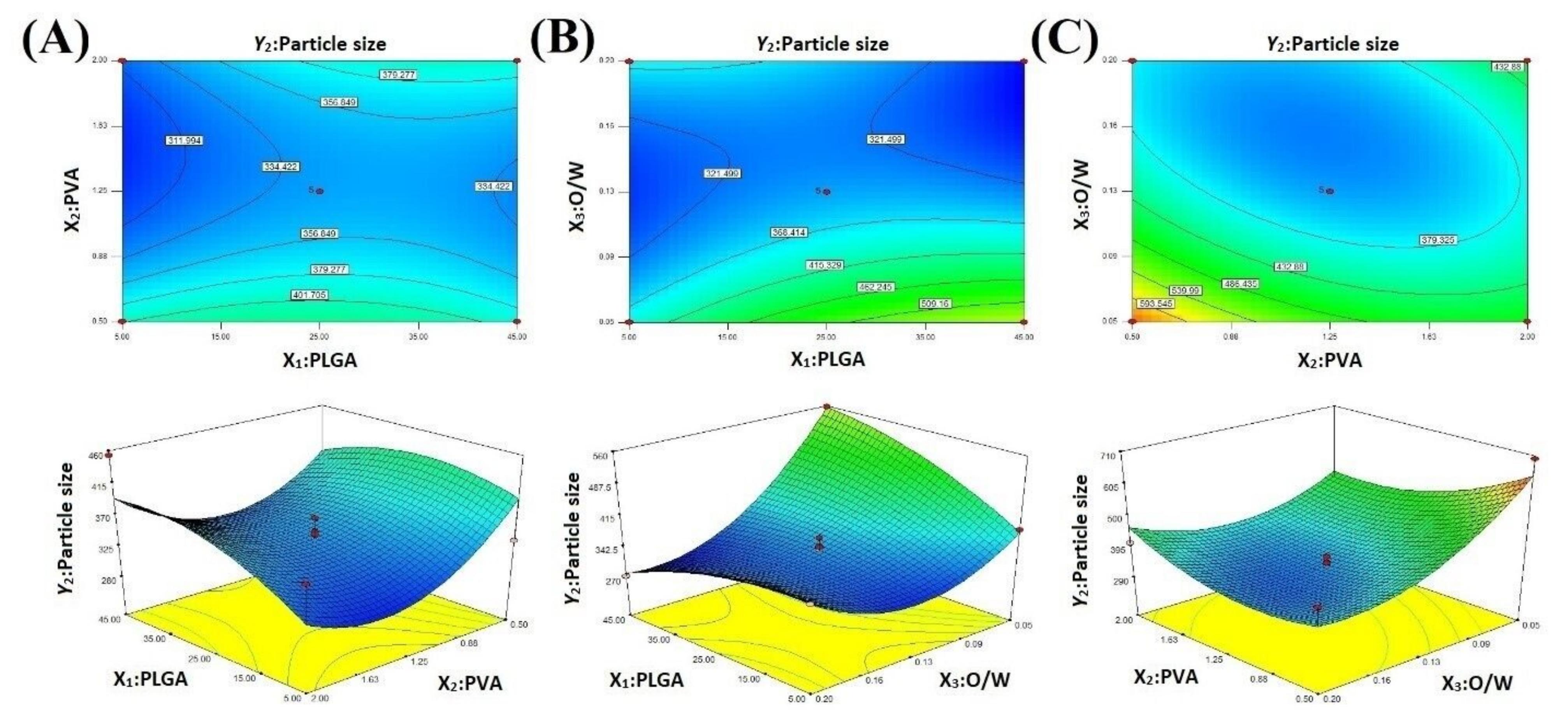
2.3. Particle Size
2.4. Optimization
2.5. Analysis of the MeP-NPs
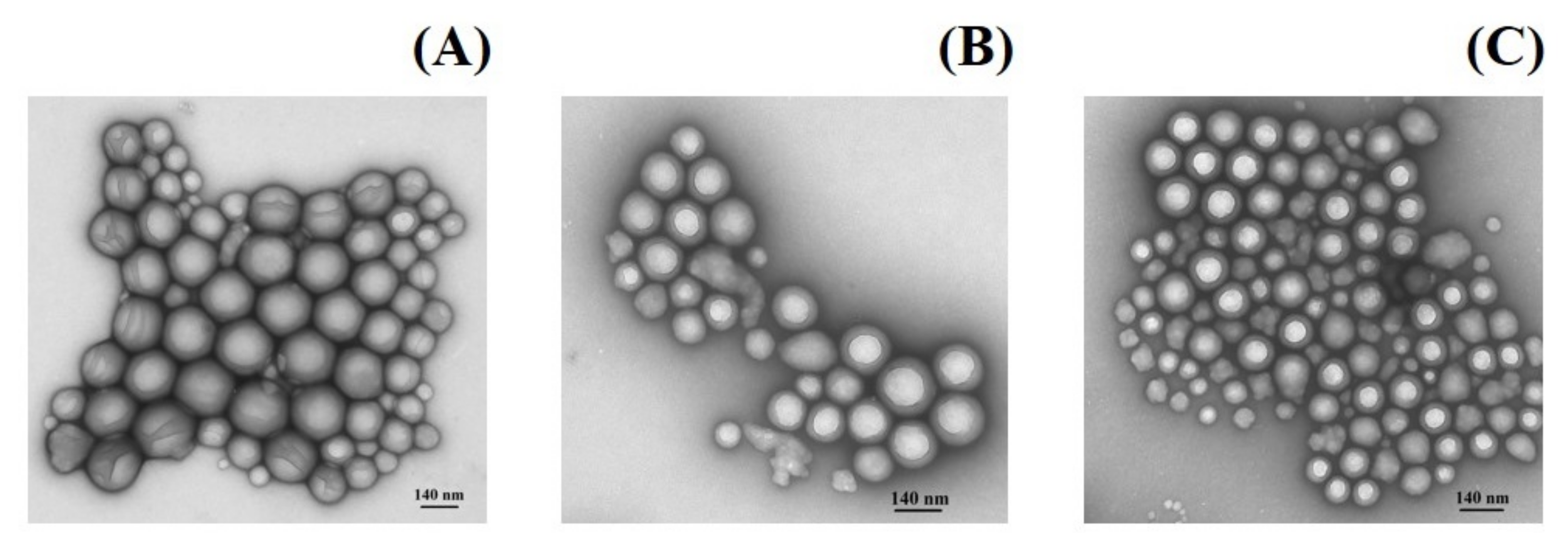
2.6. Morphology
2.7. X-ray Diffraction (XRD) Study
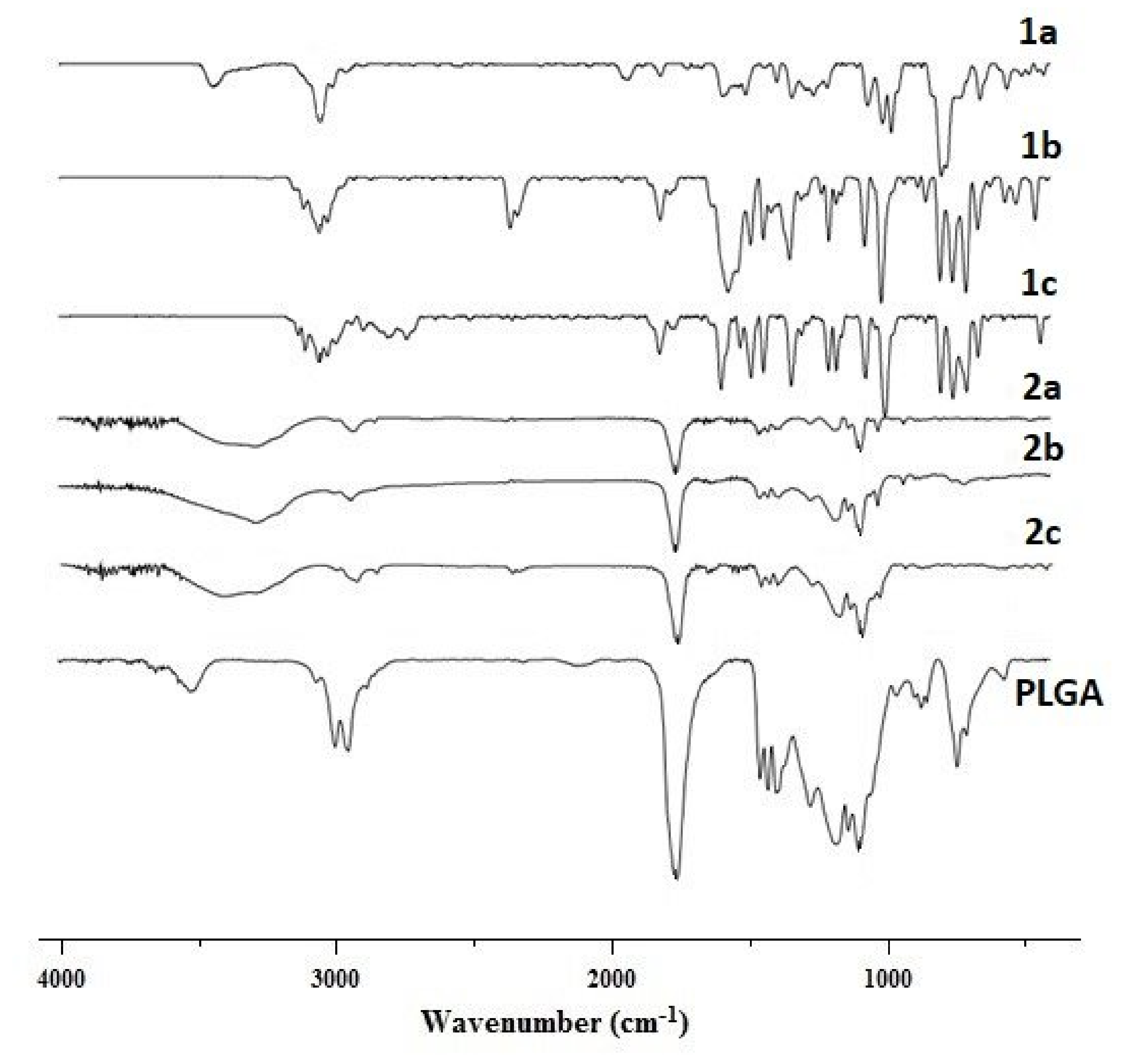
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
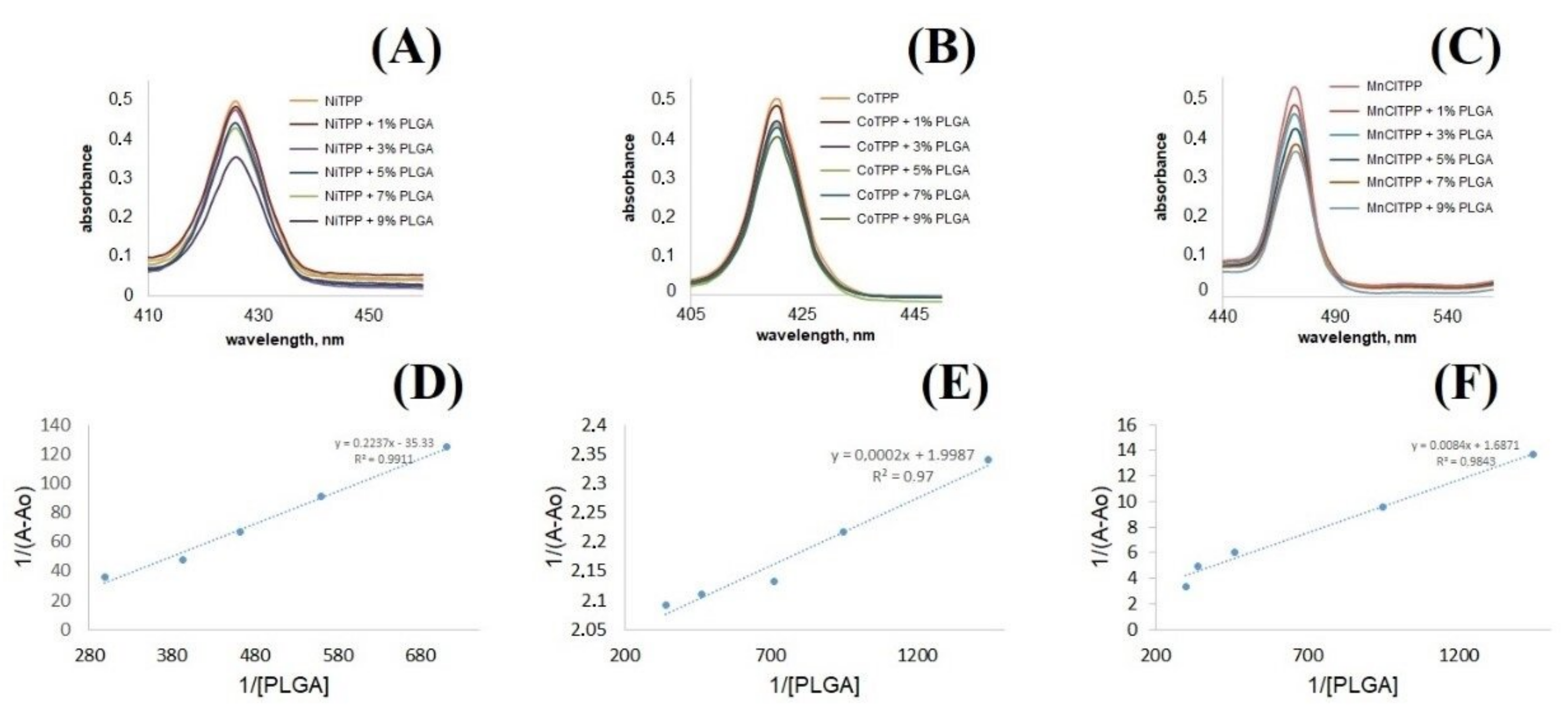
2.9. Interactions between PLGA and MeP
2.10. Hemolytic Activity Study
2.11. In Vitro Drug Release
2.12. In Vitro Cytotoxicity of MeP and MeP-NPs
2.13. ROS Formation Analysis
2.14. Acute Toxicity Analysis
2.15. In Vivo Pharmacokinetic Study
3. Materials and Methods
3.1. Materials
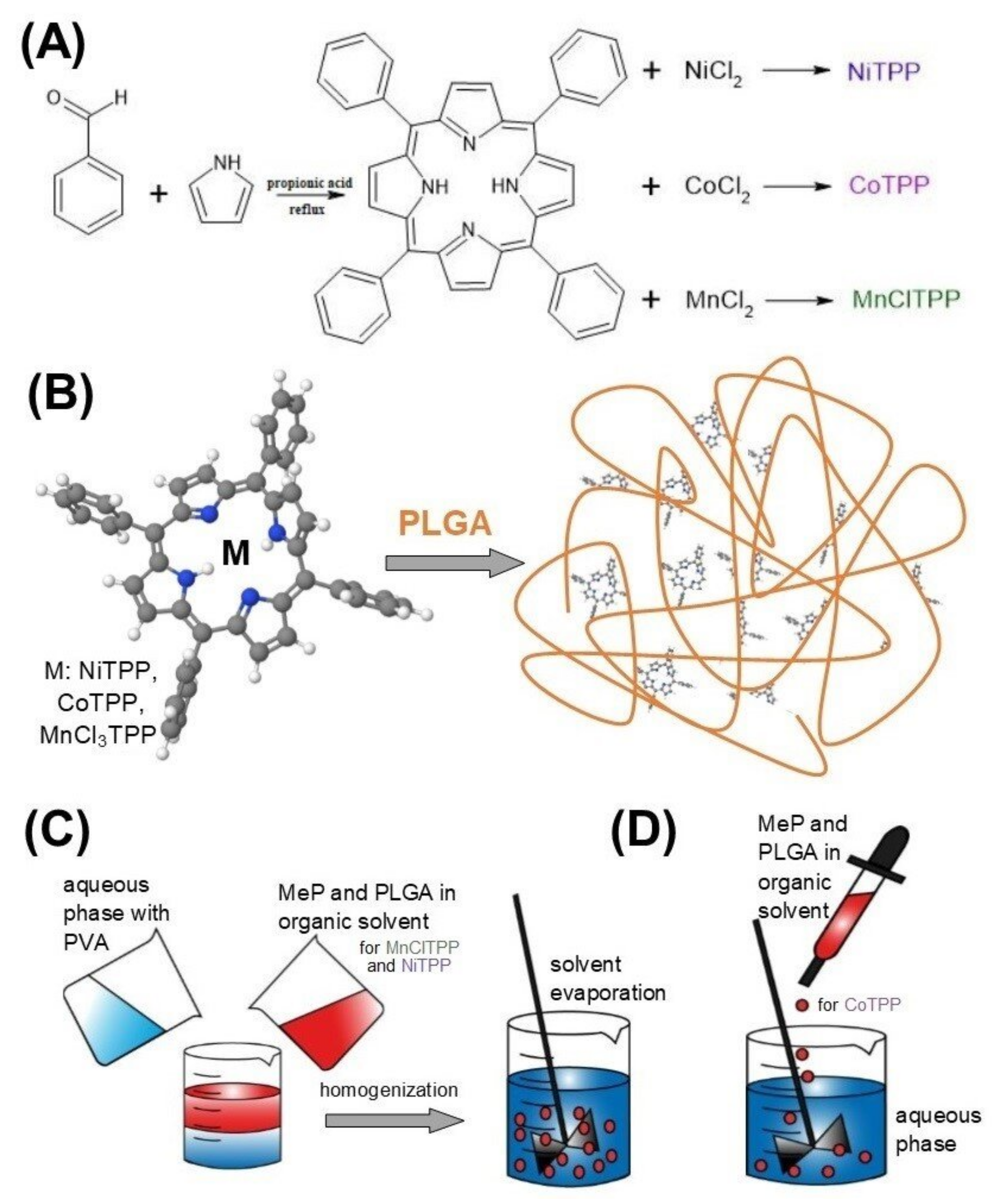
3.2. Synthesis of MeP
3.3. Preparation of Nanoparticles
3.4. Box–Behnken Experimental Design
3.5. Characterization of NPs
3.5.1. Encapsulation Efficiency (EE) and MeP Loading Content (LC)
3.5.2. Size (PS), Zeta Potential, and Polydispersity Index (PDI) Measurement
3.5.3. Nanoparticle Morphology
3.5.4. X-ray Diffraction Study (XRD)
3.5.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.6. In Vitro Release Study
3.7. Interactions between PLGA and MeP
3.8. Hemolytic Activity Study
3.9. Cell Culture
3.10. Cytotoxicity Assay on MCF-7, SK-OV-3, and HeLa Cells
3.11. Intracellular ROS Measurement
3.12. Acute Toxicity Analysis
3.13. In Vivo Pharmacokinetic Study
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Takano, Y.; Kondo, H.X.; Kanematsu, Y.; Imada, Y. Computational study of distortion effect of Fe-porphyrin found as a biological active site. Jpn. J. Appl. Phys. 2020, 59, 010502. [Google Scholar] [CrossRef]
- Lu, C.; Xie, Z.; Guo, J.; Song, Y.; Xing, Y.; Han, Y.; Li, H.; Hou, Y. Chlorophyll as natural redox mediators for the denitrification process. Int. Biodeterior. Biodegrad. 2020, 148, 104895. [Google Scholar] [CrossRef]
- Chaves, J.C.A.; Santos, C.G.; Miranda, É.G.A.; Junior, J.T.A.; Nantes, I.L. Free-Base and Metal Complexes of 5,10,15,20-Tetrakis(N-Methyl Pyridinium L)Porphyrin: Catalytic and Therapeutic Properties. In Phtalocyanines and Some Current Applications; Yilmaz, Y., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 1–28. [Google Scholar]
- Choby, J.E.; Skaar, E.P. Heme Synthesis and Acquisition in Bacterial Pathogens. J. Mol. Biol. 2016, 428, 3408–3428. [Google Scholar] [CrossRef] [Green Version]
- Buytendyk, A.M.; Graham, J.D.; Gould, J.; Bowen, H. Parent Anions of Iron, Manganese, and Nickel Tetraphenyl Porphyrins: Photoelectron Spectroscopy and Computations. J. Phys. Chem. A 2015, 119, 8643–8646. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Liu, H.; Yang, Y.; Liu, H.; Li, S.; Zhang, P.; Han, B. Base-Free Aerobic Oxidation of Alcohols over Copper-Based Complex under Ambient Condition. ACS Sustain. Chem. Eng. 2018, 6, 2362–2369. [Google Scholar] [CrossRef]
- Barona-Castaño, J.C.; Carmona-Vargas, C.C.; Brocksom, T.J.; de Oliveira, K.T. Porphyrins as Catalysts in Scalable Organic Reactions. Molecules 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta, V.; Singhal, R.; de la Cruz, P.; Sharma, G.D.; Langa, F. Reducing energy loss in organic solar cells by changing the central metal in metalloporphyrins. ChemSusChem 2021, 14, 3494–3501. [Google Scholar] [CrossRef]
- Zang, L.; Huimin, Z. Stabilization of a gadolinium (III)-porphyrin in aqueous solution for oxygen sensing. Dyes Pigments 2020, 183, 108731. [Google Scholar] [CrossRef]
- Tahara, K.; Pan, L.; Ono, T.; Hisaeda, Y. Learning from B12 enzymes: Biomimetic and bioinspired catalysts for eco-friendly organic synthesis. Beilstein J. Org. Chem. 2018, 14, 2553–2567. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.K.; Herges, R. Insertion of Ni(I) into Porphyrins at Room Temperature: Preparation of Ni(II)porphyrins, and Ni(II)chlorins and Observation of Hydroporphyrin Intermediates. Inorg. Chem. 2018, 57, 3177–3318. [Google Scholar] [CrossRef]
- Wu, K.; Guo, J.; Wang, C. An Elastic Monolithic Catalyst: A Microporous Metalloporphyrin-Containing Framework-Wrapped Melamine Foam for Process-Intensified Acyl Transfer. Angew. Chem. Int. Ed. 2016, 55, 6013. [Google Scholar] [CrossRef] [PubMed]
- Mahyari, M.; Bide, Y.; Gavgani, J.N. Iron(III) porphyrin supported on S and N co-doped graphene quantum dot as an efficient photocatalyst for aerobic oxidation of alcohols under visible light irradiation. Appl. Catal. A Gen. 2016, 517, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Fodor, M.A.; Horváth, O.; Fodor, L.; Vazdar, K.; Grampp, G.; Wankmüller, A. Photophysical and photochemical properties of manganese complexes with cationic porphyrin ligands: Effects of alkyl substituents and micellar environment. J. Photochem. Photobiol. A Chem. 2016, 328, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Föhlinger, J.; Petersson, J.; Hammarström, L.; Imahori, H. A Ruthenium Complex–Porphyrin–Fullerene-Linked Molecular Pentad as an Integrative Photosynthetic Model. Angew. Chem. Int. Ed. 2017, 56, 3329. [Google Scholar] [CrossRef] [PubMed]
- Horváth, O.; Valicsek, Z.; Fodor, M.A.; Major, M.M.; Imran, M.; Grampp, G.; Wankmüller, A. Visible light-driven photophysics and photochemistry of water-soluble metalloporphyrins. Coord. Chem. Rev. 2016, 325, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tian, X.-T.; Shang, Y.; Li, Y.-H.; Yin, X.-B. Theranostic Mn-Porphyrin Metal–Organic Frameworks for Magnetic Resonance Imaging-Guided Nitric Oxide and Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 28390–28398. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [Green Version]
- Tovmasyan, A.; Batinic-Haberle, I.; Benov, L. Antibacterial Activity of Synthetic Cationic Iron Porphyrins. Antioxidants 2020, 9, 972. [Google Scholar] [CrossRef]
- Li, L.; Tovmasyan, A.; Sheng, H.; Xu, B.; Sampaio, R.S.; Reboucas, J.S.; Warner, D.S.; Batinic-Haberle, I.; Spasojevic, I. Fe porphyrin-based SOD mimic and redox-active compound, (OH)FeTnHex-2-PyP4+, in a rodent ischemic stroke (MCAO) model: Efficacy and pharmacokinetics as compared to its Mn analogue, (H2O)MnTnHeX-2-PyP5+. Antioxidants 2020, 9, 467. [Google Scholar] [CrossRef]
- Chatterjee, A.; Zhu, Y.; Tong, Q.; Kosmacek, E.A.; Lichter, E.Z.; Oberley-Deegan, R.E. The addition of manganese porphyrins during radiation inhibits prostate cancer growth and simultaneously protects normal prostate tissue from radiation damage. Antioxidants 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic acid in cancer treatment: Let the phoenix fly. Cancer Cell. 2018, 34, 700–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendiran, V.; Ghosh, S.; Lovell, J.F. Porphyrin and Phthalocyanine Radiolabeling. In Radionanomedicine. Biological and Medical Physics, Biomedical Engineering; Lee, D., Ed.; Springer: Cham, Switzerland, 2018; pp. 49–78. [Google Scholar]
- Tovmasyan, A.; Sampaio, R.S.; Boss, M.K.; Bueno-Janice, J.C.; Bader, B.H.; Thomas, M.; Reboucas, J.S.; Orr, M.; Chandler, J.D.; Go, Y.M.; et al. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Biol. Med. 2015, 89, 1231–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batinić-Haberle, I.; Rajić, Z.; Benov, L. A Combination of Two Antioxidants (An SOD Mimic and Ascorbate) Produces a Pro-Oxidative Effect Forcing Escherichia coli to Adapt Via Induction of oxyR Regulon. Anti-Cancer Agents Med. Chem. 2011, 11, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Clair, D.S.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 2011, 1822, 794–814. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.K.; Tovmasyan, A.; Batinic-Haberle, I.; Devi, G.R. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radic. Biol. Med. 2013, 68, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girdhar, V.; Patil, S.; Banerjee, S.; Singhvi, G. Nanocarriers For Drug Delivery: Mini Review. Curr. Nanomed. 2018, 8, 88–99. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; Jain, D.K. Nanotechnology Based Approaches for Enhancing Oral Bioavailability of Poorly Water Soluble Antihypertensive Drugs. Scientifica 2016, 2016, 8525679. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Frère, A.; Evrard, B.; Mottet, D.; Piel, G. Polymeric Nanoparticles as siRNA Drug Delivery System for Cancer Therapy: The Long Road to Therapeutic Efficiency. Nanoarchitecton. Smart Deliv. Drug Target. 2016, 503–540. [Google Scholar]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivarajakumar, R.; Mallukaraj, D.; Kadavakollu, M.; Neelakandan, N.; Chandran, S.; Bhojaraj, S.; Karri, V.V.S.R. Nanoparticles for the Treatment of Lung Cancers. J. Young Pharm. 2018, 10, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Sokol, M.B.; Nikolskaya, E.D.; Yabbarov, N.; Zenin, V.; Faustova, M.; Belov, A.; Zhunina, O.; Mollaev, M.; Zabolotscky, A.; Tereshenko, O.; et al. Development of novel PLGA nanoparticles with co-encapsulation of docetaxel and abiraterone acetate for a highly efficient delivery into tumor cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1150–1158. [Google Scholar] [CrossRef]
- Sokol, M.B.; Faustova, M.R.; Nikolskaya, E.D.; Zhunina, O.A.; Fomicheva, M.V.; Petrov, R.V.; Yabbarov, N.G. Cellular internalization of targeted and non-targeted delivery systems for contrast agents based on polyamidoamine dendrimers. Russ. Chem. Bull. 2020, 69, 793–803. [Google Scholar] [CrossRef]
- Yabbarov, N.G.; Posypanova, G.A.; Vorontsov, E.A.; Obydenny, S.I.; Severin, E.S. A new system for targeted delivery of doxorubicin into tumor cells. J. Control. Release 2013, 168, 135–141. [Google Scholar] [CrossRef]
- Khan, I.; Gothwal, A.; Sharma, A.K.; Kesharwani, P.; Gupta, L.; Iyer, A.K.; Gupta, U. PLGA Nanoparticles and Their Versatile Role in Anticancer Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 159–193. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Lozano, O.; Rodríguez-Varela, A.; García-Rivas, G. Optimization of PLGA-Resveratrol nanoparticle synthesis through combined response surface methodologies. Mater. Today Proc. 2019, 13, 384–389. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.-H.; Adibkia, K. Application of Box–Behnken design to prepare gentamicin-loaded calcium carbonate nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, S.; Cózar-Bernal, M.J.; Rezgui, F.; Álvarez, A.M.R.; González-Rodríguez, M.L. Optimization of preparation method by W/O/W emulsion for entrapping metformin hydrochloride into poly (lactic acid) microparticles using Box-Behnken design. J. Drug Deliv. Sci. Technol. 2019, 51, 419–429. [Google Scholar] [CrossRef]
- Faustova, M.R.; Nikolskaya, E.D.; Mollaev, M.D.; Sokol, M.B.; Zabolotskiy, A.I.; Zhunina, O.A.; Fomicheva, M.V.; Shvets, V.I.; Lobanov, A.V.; Yabbarov, N.G. Polymer particles containing Fe-based metalloporphyrin as a highly efficient stimulator of reactive oxygen species formation in vitro and in vivo. Russ. Chem. Bull. 2019, 68, 2216–2224. [Google Scholar] [CrossRef]
- Faustova, M.R.; Nikolskaya, E.D.; Zhunina, O.A.; Mollaev, M.D.; Yabbarov, N.G.; Lobanov, A.V.; Melnikov, M.Y.; Severin, E.S. Polymer nanoparticles loaded with FeCl-tetraphenylporphyrin for binary catalytic therapy of neoplasms. Russ. Chem. Bull. 2018, 67, 359–365. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Factorial Design as a Tool for the Optimization of PLGA Nanoparticles for the Co-Delivery of Temozolomide and O6-Benzylguanine. Pharmaceutics 2019, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Pimple, S.; Manjappa, A.S.; Ukawala, M.; Murthy, R.S.R. PLGA nanoparticles loaded with etoposide and quercetin dihydrate individually: In vitro cell line study to ensure advantage of combination therapy. Cancer Nanotechnol. 2012, 3, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2015, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Mwiiri, F.K.; Rolf, D. Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing. Molecules 2020, 25, 4799. [Google Scholar] [CrossRef]
- Jonderian, A.; Maalouf, R. Formulation and In vitro Interaction of Rhodamine-B Loaded PLGA Nanoparticles with Cardiac Myocytes. Front. Pharmacol. 2016, 7, 458. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, P.; Haddadi, A. A robust systematic design: Optimization and preparation of polymeric nanoparticles of PLGA for docetaxel intravenous delivery. Mater. Sci. Eng. C 2019, 104, 109950. [Google Scholar] [CrossRef]
- Rouhani, H.; Sepehri, N.; Montazeri, H.; Khoshayand, M.R.; Ghahremani, M.H.; Ostad, S.N.; Atyabi, F.; Dinarvand, R. Zinc Protoporphyrin Polymeric Nanoparticles: Potent Heme Oxygenase Inhibitor for Cancer Therapy. Pharm. Res. 2014, 31, 2124–2139. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, D.; Souto, C.A.Z.; Araujo, M.B.; de Souza Berger, C.; da Silva, L.C.D.; Baratti, M.O.; Ribeiro, J.N.; Endringer, D.C.; Guimaraes, M.C.C.; da Silva, A.R. PLGA-PEG nanoparticles containing gallium phthalocyanine: Preparation, optimization and analysis of its photodynamic efficiency on red blood cell and Hepa-1C1C7. J. Photochem. Photobiol. B Biol. 2019, 198, 111582. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Pramual, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiri, N. Polymer-lipid-PEG hybrid nanoparticles as photosensitizer carrier for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2017, 173, 12–22. [Google Scholar] [CrossRef]
- Langlois, C.T.; Oikawa, T.; Bayle-Guillemaud, P.; Ricolleau, C. Energy-filtered electron microscopy for imaging core–shell nanostructures. J. Nanopart. Res. 2008, 10, 997–1007. [Google Scholar] [CrossRef]
- Maclean, A.L.; Foran, G.J.; Kennedy, B.J.; Turner, P.; Hambley, T.W. Structural Characterization of Nickel(II) Tetraphenylporphyrin. Aust. J. Chem. 1996, 49, 1273–1278. [Google Scholar] [CrossRef]
- Mahajan, N.M.; Sakarkar, D.M.; Manmode, A.S. Preparation and Characterization of Meselamine Loaded PLGA Nanoparticles. Int. J. Pharm. Pharm. Sci. 2011, 3, 208–214. [Google Scholar]
- Singh, G.; Tanurajvir, K.; Ravinder, K.; Kaur, A. Recent biomedical applications and patents on biodegradable polymer-PLGA. Int. J. Pharmacol. Pharm. Sci. 2014, 1, 30–42. [Google Scholar]
- Sun, Z.C.; She, Y.B.; Zhou, Y.; Song, X.F.; Li, K. Synthesis, characterization and spectral properties of substituted tetraphenylporphyrin iron chloride complexes. Molecules 2011, 16, 2960–2970. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.W.; Martell, A.E. Metal Chelates of Tetraphenylporphine and of Some p-Substituted Derivatives. J. Am. Chem. Soc. 1959, 81, 5111–5119. [Google Scholar] [CrossRef]
- Nikolskaya, E.; Sokol, M.; Faustova, M.; Zhunina, O.; Mollaev, M.; Yabbarov, N.; Tereshchenko, O.; Popov, R.; Severin, E. The comparative study of influence of lactic and glycolic acids copolymers type on properties of daunorubicin loaded nanoparticles and drug release. Acta Bioeng. Biomech. 2018, 20, 65–77. [Google Scholar] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Jhonsi, M.A.; Ananth, D.A.; Nambirajan, G.; Sivasudha, T.; Yamini, R.; Bera, S.; Kathiravan, A. Antimicrobial activity, cytotoxicity and DNA binding studies of carbon dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, H.; Moosavi, A. Investigation of the interactions between Melittin and the PLGA and PLA polymers: Molecular dynamic simulation and binding free energy calculation. Mater. Res. Express 2019, 6, 055318. [Google Scholar] [CrossRef]
- Rana, A.; Westein, E.; Niego, B.E.; Hagemeyer, C.E. Shear-dependent platelet aggregation: Mechanisms and therapeutic opportunities. Front. Cardiovasc. Med. 2019, 6, 141. [Google Scholar] [CrossRef]
- Agnoletti, M.; Rodríguez-Rodríguez, C.; Kłodzińska, S.N.; Esposito, T.V.; Saatchi, K.; Mørck Nielsen, H.; Hafeli, U.O. Monosized Polymeric Microspheres Designed for Passive Lung Targeting: Biodistribution and Pharmacokinetics after Intravenous Administration. ACS Nano 2020, 14, 6693–6706. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Mooberry, L.; Sabnis, N.; Garud, A.; Dossou, A.S.; Lacko, A. Reconstituted HDL: Drug delivery platform for overcoming biological barriers to cancer therapy. Front. Pharmacol. 2018, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Libi, S.; Calenic, B.; Astete, C.E.; Kumar, C.; Sabliov, C.M. Investigation on hemolytic effect of poly (lactic co-glycolic) acid nanoparticles synthesized using continuous flow and batch processes. Nanotechnol. Rev. 2017, 6, 209–220. [Google Scholar] [CrossRef]
- de la Harpe, K.M.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The hemocompatibility of nanoparticles: A review of cell–nanoparticle interactions and hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [Green Version]
- Libi, S. Poly (Lactic Co-Glycolic) Acid Nanoparticles: Synthesis Using Millifluidic Chip and Interaction with Red Blood Cells. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2015. [Google Scholar]
- Derman, S.; Uzunoglu, D.; Acar, T.; Arasoglu, T.; Ucak, S.; Ozalp, V.C.; Mansuroglu, B. Antioxidant Activity and Hemocompatibility Study of Quercetin Loaded PLGA Nanoparticles. Iran. J. Pharm. Res. IJPR 2020, 19, 424–435. [Google Scholar] [PubMed]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Liang, Q.; Xiang, H.; Li, X.; Luo, C.; Ma, X.; Zhao, W.; Chen, J.; Tian, Z.; Li, X.; Song, X. Development of Rifapentine-Loaded PLGA-Based Nanoparticles: In vitro Characterisation and in vivo Study in Mice. Int. J. Nanomed. 2020, 15, 7491. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Luo, L.; Wang, Y.; Zhong, Y.; Dai, H.B.; Sun, D.; Luo, M.-L.; Wu, W.; Wang, G.X. Dual tumor-targeted poly (lactic-co-glycolic acid)–polyethylene glycol–folic acid nanoparticles: A novel biodegradable nanocarrier for secure and efficient antitumor drug delivery. Int. J. Nanomed. 2017, 12, 5745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtovaara, B.C.; Verma, M.S.; Gu, F.X. Synthesis of curdlan-graft-poly(ethylene glycol) and formulation of doxorubicin-loaded core–shell nanoparticles. J. Bioact. Polym. 2012, 27, 3–17. [Google Scholar] [CrossRef]
- Scheeren, L.E.; Nogueira-Librelotto, D.R.; Macedo, L.B.; de Vargas, J.M.; Mitjans, M.; Vinardell, M.P.; Rolim, C.M. Transferrin-conjugated doxorubicin-loaded PLGA nanoparticles with pH-responsive behavior: A synergistic approach for cancer therapy. J. Nanopart. Res. 2020, 22, 72. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Zheng, M.; Guo, Q.; Wang, Y.; Wang, H.; Xie, X.; Huang, F.; Gong, R. Folate mediated self-assembled phytosterol-alginate nanoparticles for targeted intracellular anticancer drug delivery. Colloids Surf. B Biointerfaces 2015, 129, 63–70. [Google Scholar] [CrossRef]
- Abouelmag, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Gowthamarajan, K.; Singh, S.K. Dissolution testing for poorly soluble drugs: A continuing perspective. Dissolut. Technol. 2010, 17, 24–32. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B 2016, 105, 1692–1716. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, D.R.; Domínguez-Ríos, R.; Juárez, J.; Valdés, M.; Hassan, N.; Quintero-Ramos, A.; del Toro-Arreola, A.; Barbosa, S.; Taboada, P.; Topete, A.; et al. Biodegradable photoresponsive nanoparticles for chemo-, photothermal and photodynamic therapy of ovarian cancer. Mater. Sci. Eng. C 2020, 116, 111196. [Google Scholar] [CrossRef]
- Lu, B.; Xikun, L.; Yuan, L. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Kang, G.; Phan, D.T.; Hsu, M.N.; Por, Y.C.; Chen, C.H. Polymerization-induced phase separation formation of structured hydrogel particles via microfluidics for scar therapeutics. Sci. Rep. 2018, 8, 2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhuraj, R.S.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Dual drug delivery of curcumin and niclosamide using PLGA nanoparticles for improved therapeutic effect on breast cancer cells. J. Polym. Res. 2020, 27, 133. [Google Scholar]
- Upson, S.J.; Partridge, S.W.; Tcacencu, I.; Fulton, D.A.; Corbett, I.; German, M.J.; Dalgarno, K.W. Development of a methacrylate-terminated PLGA copolymer for potential use in craniomaxillofacial fracture plates. Mater. Sci. Eng. C 2016, 69, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.; Cristopher, D.; Sharma, S.; Soniwala, M.; Chavda, J. Inhalable linezolid loaded PLGA nanoparticles for treatment of tuberculosis: Design, development and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 102013. [Google Scholar] [CrossRef]
- Almeida, K.B.; Ramos, A.S.; Nunes, J.B.; Silva, B.O.; Ferraz, E.R.; Fernandes, A.S.; Falcão, D.Q. PLGA nanoparticles optimized by Box-Behnken for efficient encapsulation of therapeutic Cymbopogon citratus essential oil. Colloids Surf. B Biointerfaces 2019, 181, 935–942. [Google Scholar] [CrossRef]
- Chime, S.A.; Onunkwo, G.C.; Onyishi, I.I. Kinetics and mechanisms of drug release from swellable and non swellable matrices: A review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 97–103. [Google Scholar]
- Bajpai, A.; Raj, V. Hydrophobically modified guar gum films for wound dressing. Polym. Bull. 2021, 78, 4109–4128. [Google Scholar] [CrossRef]
- Heidarian, M.; Khazaei, A.; Saien, J. Grafting drugs to functionalized single-wall carbon nanotubes as a potential method for drug delivery. Phys. Chem. Res. 2021, 9, 57–68. [Google Scholar]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Folate-targeted nanomicelles containing silibinin as an active drug delivery system for liver cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102157. [Google Scholar] [CrossRef]
- Lim, E.B.; Vy, T.A.; Lee, S.W. Comparative release kinetics of small drugs (ibuprofen and acetaminophen) from multifunctional mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Zabolotsky, A.; Mollaev, M.; Zhunina, O.; Fomicheva, M.; Lobanov, A.; Severin, E.; Yabbarov, N. High-effective reactive oxygen species inducer based on Mn-tetraphenylporphyrin loaded PLGA nanoparticles in binary catalyst therapy. Free Radic. Biol. Med. 2019, 143, 522–533. [Google Scholar] [CrossRef]
- Riva, B.; Bellini, M.; Corvi, E.; Verderio, P.; Rozek, E.; Colzani, B.; Avvakumova, S.; Radeghieri, A.; Rizzuto, M.A.; Morasso, C.; et al. Impact of the strategy adopted for drug loading in nonporous silica nanoparticles on the drug release and cytotoxic activity. J. Colloid Interface Sci. 2018, 519, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.I.; Leshchenko, V.V. Large-scale DNA fragmentation and tumor cell death using binary system: Ascorbic acid—cobalt metal complexes in vitro. Cytology 2012, 54, 417–420. [Google Scholar]
- Scheuhammer, A.M.; Cherian, M.G. Binding of manganese in human and rat plasma. Biochim. Biophys. Acta Gen. Subj. 1985, 840, 163–169. [Google Scholar] [CrossRef]
- Mollaeva, M.; Nikolskaya, E.; Sokol, M.; Fomicheva, M.; Yabbarov, N. Influence of MnClTPP/AA Catalyst System on Antioxidant Defense of Tumor Cells. Free Radic. Biol. Med. 2020, 159, S57. [Google Scholar] [CrossRef]
- Rhee, J.K.; Baksh, M.; Nycholat, C.; Paulson, J.C.; Kitagishi, H.; Finn, M.G. Glycan-targeted virus-like nanoparticles for photodynamic therapy. Biomacromolecules 2012, 13, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Nikhileshwar Reddy, Y.; Singh Thakur, N.; Bhaumik, J. Harnessing the Photocatalytic Potential of Polypyrroles in Water through Nanointervension: Synthesis and Photophysical Evaluation of Biodegradable Polypyrrolic Nanoencapsulates. ChemNanoMat 2020, 6, 239–247. [Google Scholar] [CrossRef]
- Vepris, O.; Eich, C.; Feng, Y.; Zhang, H.; Kaijzel, E.L.; Cruz, L.J. Triplet-Triplet Annihilation PLGA-Nanoparticles for Cancer Bioimaging. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.A.; El-Sabbagh, H.M. In vitro–in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomed. 2019, 14, 7191. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Barnett, G.H.; Hudson, M.F.; Smith, K.M. Concerning meso-tetraphenylporphyrin purification. J. Chem. Soc. Perkin Trans. 1975, 1, 1401–1403. [Google Scholar] [CrossRef]
- Singh, C.; Chaubey, S.; Singh, P.; Sharma, K.; Kumar, A.; Yadav, R.K.; Dwivedi, D.K.; Baeg, J.-O.; Kumar, U.; Yadav, B.C.; et al. Self-assembled carbon nitride/cobalt (III) porphyrin photocatalyst for mimicking natural photosynthesis. Diam. Relat. Mater. 2020, 101, 107648. [Google Scholar] [CrossRef]
- Polat, S.; Sayan, P. Application of response surface methodology with a Box–Behnken design for struvite precipitation. Adv. Powder Technol. 2019, 30, 2396–2407. [Google Scholar] [CrossRef]
- Ji, C.; Shen, C.; Zhou, Y.; Zhu, K.; Sun, Z.; Zuo, Z.; Zhao, M. AhR agonist activity confirmation of polyhalogenated carbazoles (PHCZs) using an integration of in vitro, in vivo, and in silico models. Environ. Sci. Technol. 2019, 53, 14716–14723. [Google Scholar] [CrossRef] [PubMed]
- Menilli, L.; Monteiro, A.R.; Lazzarotto, S.; Morais, F.M.P.; Gomes, A.T.P.C.; Moura, N.M.M.; Fateixa, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Trindade, T.; et al. Graphene Oxide and Graphene Quantum Dots as Delivery Systems of Cationic Porphyrins: Photo-Antiproliferative Activity Evaluation towards T24 Human Bladder Cancer Cells. Pharmaceutics 2021, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, Y.; Wang, Y.; Xu, C.; Dong, C.; Li, C.; Ren, S.; Zhang, W.; Lu, Y.; Dai, Y.; et al. Pharmacokinetics study of hemin in rats by applying 58Fe-extrinsically labeling techniques in combination with ICP-MS method. J. Pharm. Biomed. Anal. 2014, 88, 331–336. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Khashaba, P.Y.; Shahin, R.Y.; El-Wekil, M.M. Tunable ternary nanocomposite prepared by electrodeposition for biosensing of centrally acting reversible acetyl cholinesterase inhibitor donepezil hydrochloride in real samples. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 76–85. [Google Scholar] [CrossRef]
- Serrano-Román, J.; Nicasio-Torres, P.; Hernández-Pérez, E.; Jiménez-Ferrer, E. Pharmacokinetic Study of Anti-osteoarthritic Compounds of a Standardized Fraction from Sphaeralcea angustifolia. Pharmaceuticals 2021, 14, 610. [Google Scholar] [CrossRef] [PubMed]





| No. | Independent Variable | Dependent Variable | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| 1 | −1 | 0 | −1 | 4.6 ± 0.2 | 395.0 ± 4.2 |
| 2 | −1 | 0 | 1 | 13.5 ± 0.5 | 376.3 ± 9.7 |
| 3 | 0 | 0 | 0 | 3.1 ± 0.9 | 350.3 ± 6.8 |
| 4 | 1 | 0 | 1 | 2.9 ± 0.2 | 272.0 ± 4.1 |
| 5 | −1 | 1 | 0 | 6.7 ± 0.4 | 372.1 ± 6.6 |
| 6 | 0 | 0 | 0 | 3.2 ± 0.3 | 370.2 ± 3.7 |
| 7 | 1 | 1 | 0 | 2.6 ± 0.4 | 453.5 ± 9.6 |
| 8 | 0 | −1 | 1 | 5.3 ± 0.7 | 434.8 ± 11.3 |
| 9 | 0 | 0 | 0 | 3.0 ± 0.5 | 338.2 ± 5.8 |
| 10 | 1 | 0 | −1 | 1.9 ± 0.1 | 557.1 ± 7.3 |
| 11 | 1 | −1 | 0 | 4.8 ± 0.8 | 337.6 ± 4.9 |
| 12 | 0 | 0 | 0 | 2.5 ± 0.9 | 346.7 ± 8.3 |
| 13 | 0 | 0 | 0 | 3.3 ± 0.7 | 299.9 ± 2.2 |
| 14 | −1 | −1 | 0 | 19.9 ± 0.4 | 341.2 ± 5.5 |
| 15 | 0 | 1 | −1 | 2.6 ± 0.5 | 418.7 ± 4.2 |
| 16 | 0 | 1 | 1 | 4.7 ± 0.8 | 408.7 ± 2.2 |
| 17 | 0 | −1 | −1 | 4.3 ± 0.7 | 700.4 ± 19.8 |
| Models | R2 | Adjusted R2 | F-Value | p-Value Prob > F |
|---|---|---|---|---|
| Response Y1 (NiTPP loading content, %) | ||||
| Linear | 0.5590 | 0.4572 | 215.08 | <0.0001 |
| 2FI | 0.6943 | 0.5109 | 223.43 | <0.0001 |
| Quadratic | 0.9049 | 0.7827 | 138.07 | 0.0002 |
| Response Y2 (particle size, nm) | ||||
| Linear | 0.2887 | 0.1246 | 19.04 | 0.0061 |
| 2FI | 0.5068 | 0.2110 | 19.60 | 0.0062 |
| Quadratic | 0.8258 | 0.6019 | 12.98 | 0.0158 |
| MeP | Conditions | Responses | Observed Data | Predicted Data | Relative Error, % |
|---|---|---|---|---|---|
| NiTPP-NPs | X1: 5 mg X2: 1.47% X3: 1:7 Desirability 0.835 | Y1, % | 11.1 | 10.1 | −9.9 |
| Y2, nm | 322.9 | 296.9 | −8.8 | ||
| CoTPP-NPs | X1: 7.2 mg X2: 2% X3: 1:5 Desirability 0.750 | Y1, % | 8.7 | 10.3 | 15.2 |
| Y2, nm | 344.5 | 410.5 | 16.1 | ||
| MnClTPP-NPs | X1: 5 mg X2: 1.42% X3: 1:5.6 Desirability 0.837 | Y1, % | 28.9 | 22.6 | −27.8 |
| Y2, nm | 205.2 | 247.4 | 17.1 |
| MeP | LC, % | EE, % | PS, nm | Zeta Potential, mV | PDI |
|---|---|---|---|---|---|
| NiTPP-NPs | 11.1 ± 0.6 | 24.1 ± 0.9 | 322.9 ± 9.7 | −14.7 ± 1.7 | 0.172 |
| CoTPP-NPs | 8.7 ± 0.4 | 79.7 ± 2.2 | 344.5 ± 15.6 | −10.7 ± 2.3 | 0.191 |
| MnClTPP-NPs | 28.9 ± 1.6 | 79.9 ± 1.8 | 205.2 ± 10.2 | +18.1 ± 1.6 | 0.140 |
| MeP | NiTPP | CoTPP | MnClTPP |
|---|---|---|---|
| Kd | 0.2 × 10−3 | 0.2 × 10−3 | 10 × 10−3 |
| EE, % | 24.2 | 79.7 | 79.9 |
| LC, % | 11.1 | 8.7 | 28.9 |
| Scheme | Concentration, mg/mL | Hemolysis, % |
|---|---|---|
| blank NPs | 25 | 3.8 |
| 2.5 | 1.4 | |
| 0.25 | 0.8 | |
| NiTPP-NPs | 25 | 6.5 |
| 2.5 | 2.2 | |
| 0.25 | 0.3 | |
| CoTPP-NPs | 25 | 3.3 |
| 2.5 | 1.3 | |
| 0.25 | 0.3 | |
| MnClTPP-NPs | 25 | 107.5 |
| 2.5 | 102.5 | |
| 0.25 | 88.1 | |
| 0.09 | 23.4 | |
| 0.009 | 1.8 |
| MeP | Mathematical Model | ||||
|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Hixson-Crowell | Korsmeyer–Peppas | |
| R2 | R2 | R2 | R2 | R2 | |
| NiTPP-NPs | −10.1967 | 0.7965 | −7.4722 | −8.4886 | 0.9948 |
| CoTPP-NPs | −5.7916 | 0.6060 | −3.5087 | −4.2651 | 0.9876 |
| MnClTPP-NPs | −2.8711 | 0.0180 | −1.3010 | −1.8479 | 0.9763 |
| Dose, mg/kg | Formulation (Mice Survived) | |||||
|---|---|---|---|---|---|---|
| NiTPP | CoTPP | MnClTPP | NiTPP-NPs | CoTPP-NPs | MnClTPP-NPs | |
| 6.25 | 3/3 | 3/3 | 3/3 | - | - | - |
| 12.5 | 3/3 | 3/3 | 3/3 | - | - | - |
| 25 | 1/3 | 2/3 | 3/3 | - | - | - |
| 50 | 0/3 | 0/3 | 2/3 | - | - | - |
| 100 | - | - | - | 3/3 | 3/3 | 3/3 |
| 200 | - | - | - | 3/3 | 3/3 | 3/3 |
| Parameters | Formulations | Units | |||||
|---|---|---|---|---|---|---|---|
| MnClTPP | MnClTPP-NPs | CoTPP | CoTPP-NPs | NiTPP | NiTPP-NPs | ||
| AUCinf | 15,362 ± 5377 | 9362 ± 3745 * | 60,370 ± 18,111 | 1406 ± 211 * | 612 ± 184 | 1133 ± 453 | (µg/mL) * (h) |
| Vd | 0.013 ± 0.004 | 0.188 ± 0.066 * | 0.003 ± 0.001 | 3.083 ± 0.462 * | 2.683 ± 0.805 | 2.614 ± 0.915 | L/kg |
| t1/2 | 44 ± 14 | 37 ± 14 * | 17 ± 4 | 33 ± 5 * | 19 ± 6 | 24 ± 8 | h |
| MRT | 62 ± 21 | 53 ± 21 * | 27 ± 7 | 66 ± 8 * | 25 ± 6 | 45 ± 16 | h |
| Variation Factors | Levels | ||
|---|---|---|---|
| Low | High | ||
| X1 | PLGA, mg | 5 | 45 |
| X2 | PVA, % | 0.5 | 2 |
| X3 | O/W (v/v) | 0.05 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. https://doi.org/10.3390/ijms222212261
Mollaeva MR, Yabbarov N, Sokol M, Chirkina M, Mollaev MD, Zabolotskii A, Seregina I, Bolshov M, Kaplun A, Nikolskaya E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. International Journal of Molecular Sciences. 2021; 22(22):12261. https://doi.org/10.3390/ijms222212261
Chicago/Turabian StyleMollaeva, Mariia R., Nikita Yabbarov, Maria Sokol, Margarita Chirkina, Murad D. Mollaev, Artur Zabolotskii, Irina Seregina, Mikhail Bolshov, Alexander Kaplun, and Elena Nikolskaya. 2021. "Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles" International Journal of Molecular Sciences 22, no. 22: 12261. https://doi.org/10.3390/ijms222212261
APA StyleMollaeva, M. R., Yabbarov, N., Sokol, M., Chirkina, M., Mollaev, M. D., Zabolotskii, A., Seregina, I., Bolshov, M., Kaplun, A., & Nikolskaya, E. (2021). Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. International Journal of Molecular Sciences, 22(22), 12261. https://doi.org/10.3390/ijms222212261










