Bioconversion of Lignocellulosic Biomass into Value Added Products under Anaerobic Conditions: Insight into Proteomic Studies
Abstract
1. Introduction
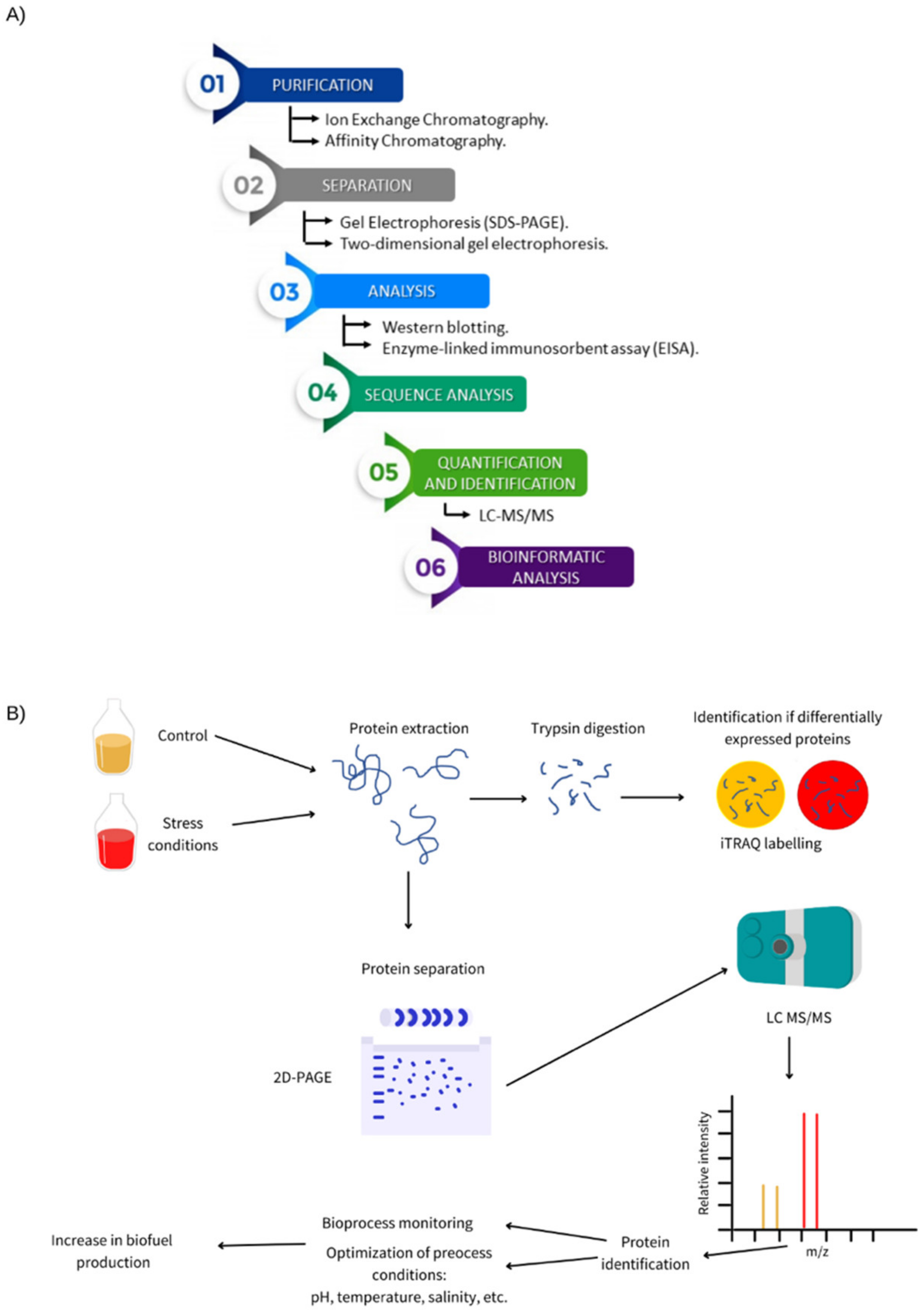
2. Importance of Proteomic Technologies in Bioprocesses
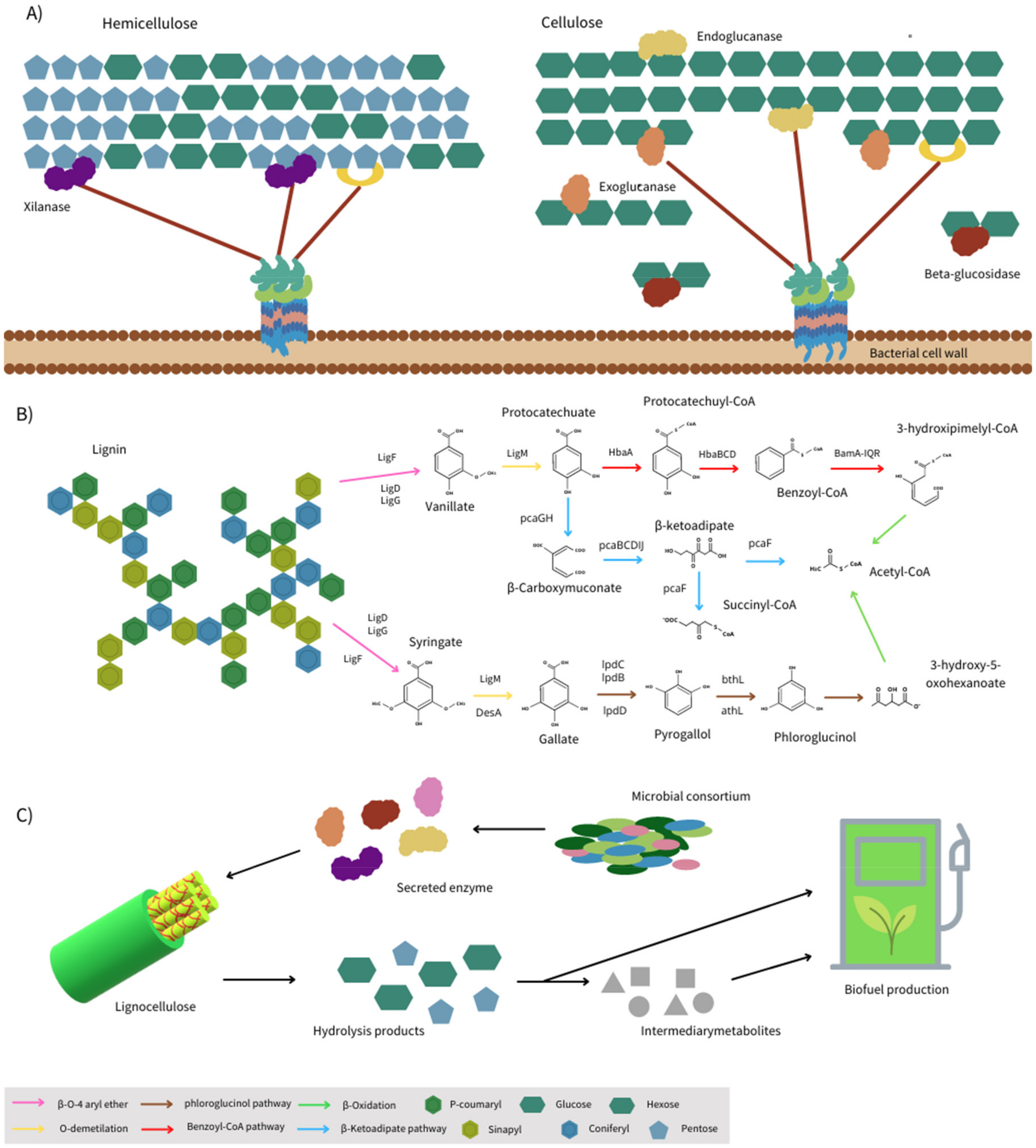
3. Proteins Involved in Lignocellulose Utilization
3.1. Enzymes Targeting Lignocellulosic Polysaccharides
3.2. Enzymes Involved in Lignin Degradation
4. Biofuel Production from Lignocellulosic Biomass
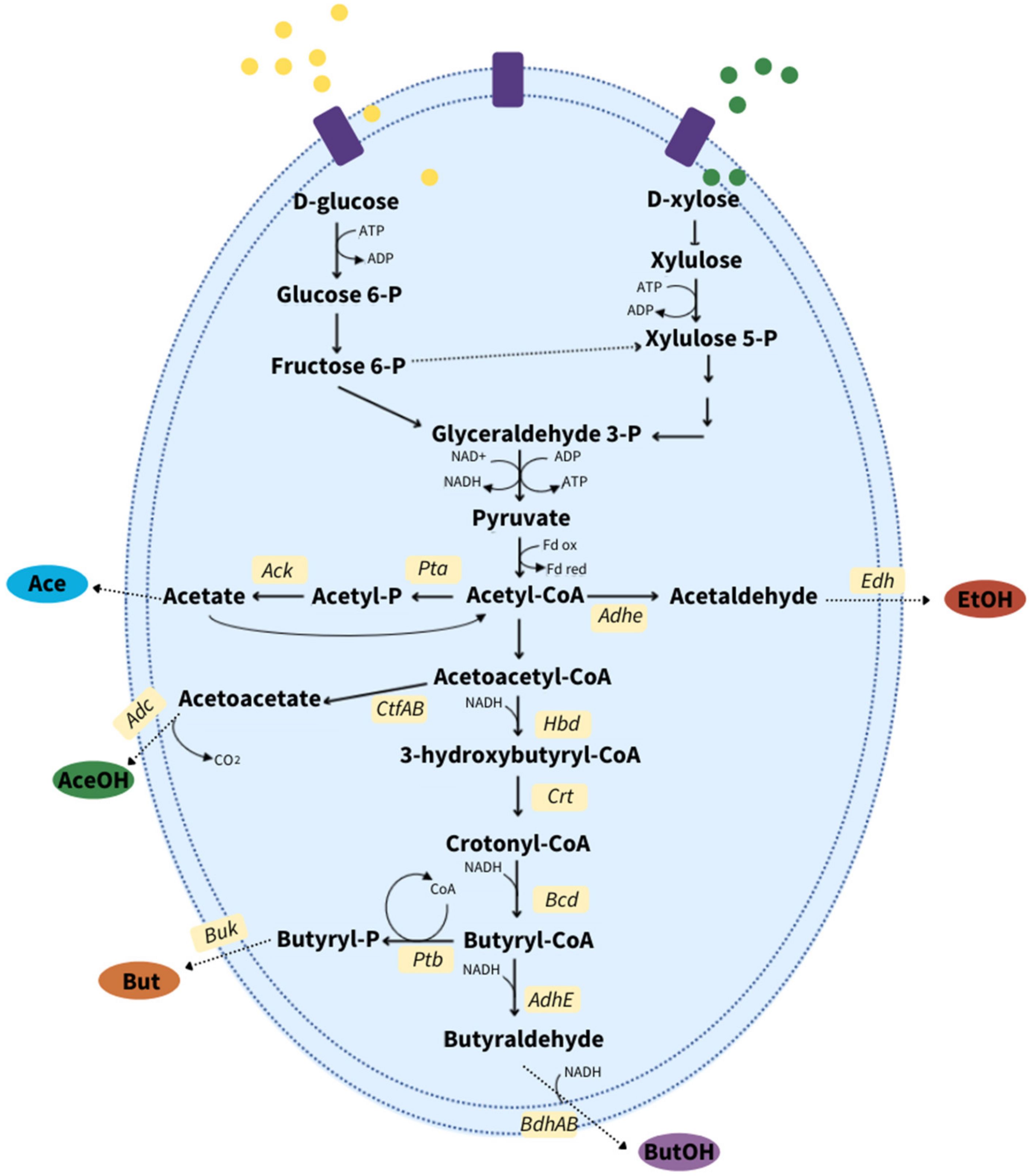
4.1. Proteomics of Ethanol Production
4.2. Proteomics in the Production of Acids and Solvents
4.3. Proteomics of Methane and Hydrogen Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Xin, F.; Lu, J.; Dong, W.; Zhang, W.; Zhang, M.; Wu, H.; Ma, J.; Jiang, M. State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour. Technol. 2017, 245, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Hwang, K.R.; Kim, C.; Kim, J.R.; Lee, J.S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Rosnow, J.J.; Anderson, L.N.; Nair, R.N.; Baker, E.S.; Wright, A.T. Profiling microbial lignocellulose degradation and utilization by emergent omics technologies. Crit. Rev. Biotechnol. 2016, 37, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Cho, E.J.; Lee, D.-S.; Lee, S.J.; Lee, Y.J.; Bae, H.-J. Lignocellulose conversion for biofuel: A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels 2015, 8, 1–11. [Google Scholar] [CrossRef]
- Shen, L.; Su, Y.; Sun, Y.; Wang, G.; Chen, H.; Yu, X.; Zhang, S.; Chen, G. Establishment of a highly efficient and low cost mixed cellulase system for bioconversion of corn stover by Trichoderma reesei and Aspergillus niger. Biocatal. Agric. Biotechnol. 2021, 32, 101849. [Google Scholar] [CrossRef]
- Lee, J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 1997, 56, 1–24. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef] [PubMed]
- Akinosho, H.; Yee, K.; Close, D.; Ragauskas, A. The emergence of Clostridium thermocellum as a high utility candidate for consolidated bioprocessing applications. Front. Chem. 2014, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, S.; Kurosaki, M.; Kokuzawa, T.; Hirano, K.; Takano, H.; Ueda, K.; Hirano, N. Comparative Biochemical Analysis of Cellulosomes Isolated from Clostridium clariflavum DSM 19732 and Clostridium thermocellum ATCC 27405 Grown on Plant Biomass. Appl. Biochem. Biotechnol. 2018, 187, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Chen, C.; Yan, F.; Feng, Y.; Bayer, E.A.; Kosugi, A.; Liu, Y.-J. Coordinated β-glucosidase activity with the cellulosome is effective for enhanced lignocellulose saccharification. Bioresour. Technol. 2021, 337, 125441. [Google Scholar] [CrossRef]
- Basak, B.; Ahn, Y.; Kumar, R.; Hwang, J.; Kim, K.; Jeon, B. Lignocellulolytic microbiomes for augmenting lignocellulose degradation in anaerobic digestion. Trends Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.A.F.; Chandel, A.K.; Terán-Hilares, R.; Ingle, A.P.; Rai, M.; dos Santos Milessi, T.S.; da Silva, S.S.; Dos Santos, J.C. Overcoming challenges in lignocellulosic biomass pretreatment for second-generation (2G) sugar production: Emerging role of nano, biotechnological and promising approaches. 3 Biotech. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, F.M.; Fatokun, C.O.; Oyelere, O.M. Biodelignification of some agro-residues by Stenotrophomonas sp. CFB-09 and enhanced production of ligninolytic enzymes. Biocatal. Agric. Biotechnol. 2018, 15, 120–130. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Biodelignification and hydrolysis of rice straw by novel bacteria isolated from wood feeding termite. 3 Biotech. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Hidayatullah, I.M.; Al Husna, M.D.; Radiyan, H.; Kresnowati, M.T.A.P.; Suhardi, S.H.; Setiadi, T.; Boopathy, R. Combining biodelignification and hydrothermal pretreatment of oil palm empty fruit bunches (OPEFB) for monomeric sugar production. Bioresour. Technol. Rep. 2021, 15, 100808. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yue, W.; Liu, J.; Li, G.L.; Miao, Y. Proteomic Analysis of the Silkworm (Bombyx mori L.) Hemolymph during Developmental Stage. J. Proteome Res. 2006, 5, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2016, 55, 182–196. [Google Scholar] [CrossRef]
- Twyman, R.M. Proteomics. Encycl. Appl. Ethics 2012, 642–649. [Google Scholar] [CrossRef]
- Cunha, B.; Aguiar, T.; Carvalho, S.; Silva, M.; Gomes, R.; Carrondo, M.; Gomes-Alves, P.; Peixoto, C.; Serra, M.; Alves, P.M. Bioprocess integration for human mesenchymal stem cells: From up to downstream processing scale-up to cell proteome characterization. J. Biotechnol. 2017, 248, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chang, Y.; Xie, S.; Song, J.; Wang, M. Impacts of bioprocess engineering on product formation by Acetobacter pasteurianus. Appl. Microbiol. Biotechnol. 2018, 102, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Raju, R.; Kshirsagar, R.; Ivanov, A.R.; Gilbert, A.; Zang, L.; Karger, B.L. Multi-Omics Study on the Impact of Cysteine Feed Level on Cell Viability and mAb Production in a CHO Bioprocess. Biotechnol. J. 2018, 14, 1800352. [Google Scholar] [CrossRef]
- Usai, G.; Cirrincione, S.; Re, A.; Manfredi, M.; Pagnani, A.; Pessione, E.; Mazzoli, R. Clostridium cellulovorans metabolism of cellulose as studied by comparative proteomic approach. J. Proteom. 2020, 216, 103667. [Google Scholar] [CrossRef]
- Saykhedkar, S.; Ray, A.; Ayoubi-Canaan, P.; Hartson, S.; Prade, R.; Mort, A. A time course analysis of the extracellular proteome of Aspergillus nidulans growing on sorghum stover. Biotechnol. Biofuels 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, L.; Wang, J.; Qiao, J.; Zhang, W. Quantitative proteomics reveals dynamic responses of Synechocystis sp. PCC 6803 to next-generation biofuel butanol. J. Proteom. 2013, 78, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Wang, J.; Qiao, J.; Zhang, W. Proteomic analysis reveals resistance mechanism against biofuel hexane in Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2012, 5, 68. [Google Scholar] [CrossRef]
- Jain, S.; Graham, C.; Graham, R.; McMullan, G.; Ternan, N. Quantitative Proteomic Analysis of the Heat Stress Response in Clostridium difficile Strain 630. J. Proteome Res. 2011, 10, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.; McLoughlin, N.; Milne, J.J.; Marison, I.W.; Bones, J. Application of Multi-Omics Techniques for Bioprocess Design and Optimization in Chinese Hamster Ovary Cells. J. Proteome Res. 2014, 13, 3144–3159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Xia, J.; Nielsen, J. The Impact of Systems Biology on Bioprocessing. Trends Biotechnol. 2017, 35, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Baycin-Hizal, D.; Tabb, D.L.; Chaerkady, R.; Chen, L.; Lewis, N.E.; Nagarajan, H.; Betenbaugh, M. Proteomic Analysis of Chinese Hamster Ovary Cells. J. Proteome Res. 2012, 11, 5265–5276. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, H.; Xiong, L.; Chen, X.; Wang, C.; Qi, G.; Chen, X. The hydrolytic efficiency and synergistic action of recombinant xylan-degrading enzymes on xylan isolated from sugarcane bagasse. Carbohydr. Polym. 2017, 175, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cagide, C.; Castro-Sowinski, S. Technological and biochemical features of lignin-degrading enzymes: A brief review. Environ. Sustain. 2020, 3, 371–389. [Google Scholar] [CrossRef]
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325. [Google Scholar] [CrossRef]
- Conway, J.M.; Crosby, J.R.; Hren, A.P.; Southerland, R.T.; Lee, L.L.; Lunin, V.V.; Alahuhta, P.; Himmel, M.E.; Bomble, Y.; Adams, M.W.W.; et al. Novel multidomain, multifunctional glycoside hydrolases from highly lignocellulolytic Caldicellulosiruptor species. AIChE J. 2018, 64, 4218–4228. [Google Scholar] [CrossRef]
- Babar, M.M.; Afzaal, H.; Pothineni, V.R.; Zaidi, N.-S.S.; Ali, Z.; Zahid, M.A.; Gul, A. Omics Approaches in Industrial Biotechnology and Bioprocess Engineering. In Omics Technologies and Bio-Engineering; Academic Press: Cambridge, MA, USA, 2018; pp. 251–269. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, S.; Stenoien, D.L.; Paša-Tolić, L. High-Throughput Proteomics. Annu. Rev. Anal. Chem. 2014, 7, 427–454. [Google Scholar] [CrossRef] [PubMed]
- Heffner, K.; Hizal, D.; Kumar, A.; Shiloach, J.; Zhu, J.; Bowen, M.; Betenbaugh, M. Exploiting the proteomics revolution in biotechnology: From disease and antibody targets to optimizing bioprocess development. Curr. Opin. Biotechnol. 2014, 30, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2017, 10, 235–251. [Google Scholar] [CrossRef]
- Pellegrini, V.; Sepulchro, A.; Polikarpov, I. Enzymes for lignocellulosic biomass polysaccharide valorization and production of nanomaterials. Curr. Opin. Green Sustain. Chem. 2020, 26, 100397. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, X.-D.; Lee, D.-J. Proteomic researches for lignocellulose-degrading enzymes: A mini-review. Bioresour. Technol. 2018, 265, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kulasinski, K.; Keten, S.; Churakov, S.V.; Derome, D.; Carmeliet, J. A comparative molecular dynamics study of crystalline, paracrystalline and amorphous states of cellulose. Cellulose 2014, 21, 1103–1116. [Google Scholar] [CrossRef]
- Li, X.; Griffin, K.; Langeveld, S.; Frommhagen, M.; Underlin, E.N.; Kabel, M.A.; de Vries, R.P.; Dilokpimol, A. Functional Validation of Two Fungal Subfamilies in Carbohydrate Esterase Family 1 by Biochemical Characterization of Esterases From Uncharacterized Branches. Front. Bioeng. Biotechnol. 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2013, 42, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, X.; Guo, Y.; Peng, S.; Liu, G.; Zhao, J. Effect of endoglucanases from different glycoside hydrolase families on enzymatic preparation of cellulose nanocrystal. Ind. Crop. Prod. 2020, 155, 112755. [Google Scholar] [CrossRef]
- Chuzel, L.; Ganatra, M.B.; Rapp, E.; Henrissat, B.; Taron, C.H. Functional metagenomics identifies an exosialidase with an inverting catalytic mechanism that defines a new glycoside hydrolase family (GH156). J. Biol. Chem. 2018, 293, 18138–18150. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, M.; Kitamura, M.; Hondoh, H.; Kang, M.-S.; Mori, H.; Kimura, A.; Yao, M. Catalytic Mechanism of Retaining α-Galactosidase Belonging to Glycoside Hydrolase Family 97. J. Mol. Biol. 2009, 392, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Turner, B.L.; Hopkins, D.W.; Haygarth, P.M.; Ostle, N. β-Glucosidase activity in pasture soils. Appl. Soil Ecol. 2002, 20, 157–162. [Google Scholar] [CrossRef]
- Zhu, N.; Yang, J.; Ji, L.; Liu, J.; Yang, Y.; Yuan, H. Metagenomic and metaproteomic analyses of a corn stover-adapted microbial consortium EMSD5 reveal its taxonomic and enzymatic basis for degrading lignocellulose. Biotechnol. Biofuels 2016, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.I.; Spicer, V.; Shamshurin, D.; Krokhin, O.V.; Wilkins, J.; Ramachandran, U.; Sparling, R.; Levin, B.D. Quantitative proteomic analysis of the cellulolytic system of Clostridium termitidis CT1112 reveals distinct protein expression profiles upon growth on α-cellulose and cellobiose. J. Proteom. 2015, 125, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, K.; Liao, H.; Hector, S.B.; Shi, X.; Li, J.; Liu, B.; Xu, T.; Tong, C.; Liu, X.; et al. Genomic and secretomic insight into lignocellulolytic system of an endophytic bacterium Pantoea ananatis Sd-1. Biotechnol. Biofuels 2016, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wongwilaiwalin, S.; Rattanachomsri, U.; Laothanachareon, T.; Eurwilaichitr, L.; Igarashi, Y.; Champreda, V. Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzym. Microb. Technol. 2010, 47, 283–290. [Google Scholar] [CrossRef]
- Lochner, A.; Giannone, R.J.; Rodriguez, M., Jr.; Shah, M.B.; Mielenz, J.R.; Keller, M.; Graham, D.E.; Hettich, R.L. Use of Label-Free Quantitative Proteomics To Distinguish the Secreted Cellulolytic Systems of Caldicellulosiruptor bescii and Caldicellulosiruptor obsidiansis. Appl. Environ. Microbiol. 2011, 77, 4042–4054. [Google Scholar] [CrossRef] [PubMed]
- Gold, N.D.; Martin, V.J.J. Global View of the Clostridium thermocellum Cellulosome Revealed by Quantitative Proteomic Analysis. J. Bacteriol. 2007, 189, 6787–6795. [Google Scholar] [CrossRef] [PubMed]
- Morisaka, H.; Matsui, K.; Tatsukami, Y.; Kuroda, K.; Miyake, H.; Tamaru, Y.; Ueda, M. Profile of native cellulosomal proteins of Clostridium cellulovorans adapted to various carbon sources. AMB Expr. 2012, 2, 37. [Google Scholar] [CrossRef]
- Blouzard, J.-C.; Coutinho, P.M.; Fierobe, H.-P.; Henrissat, B.; Lignon, S.; Tardif, C.; de Philip, P. Modulation of cellulosome composition in Clostridium cellulolyticum: Adaptation to the polysaccharide environment revealed by proteomic and carbohydrate-active enzyme analyses. Proteomics 2010, 10, 541–554. [Google Scholar] [CrossRef]
- Kumar, M.; Verma, S.; Gazara, R.K.; Kumar, M.; Pandey, A.; Verma, P.K.; Thakur, I.S. Genomic and proteomic analysis of lignin degrading and polyhydroxyalkanoate accumulating β-proteobacterium Pandoraea sp. ISTKB. Biotechnol. Biofuels 2018, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, X.; Feng, Q.; Yan, H. Extracellular differential proteome analysis of substrates of different lignin model compounds degraded by Aspergillus fumigatus G-13. J. Environ. Eng. Landsc. Manag. 2020, 28, 137–147. [Google Scholar] [CrossRef]
- Ravalason, H.; Jan, G.; Mollé, D.; Pasco, M.; Coutinho, P.M.; Lapierre, C.; Pollet, B.; Bertaud, F.; Petit-Conil, M.; Grisel, S.; et al. Secretome analysis of Phanerochaete chrysosporium strain CIRM-BRFM41 grown on softwood. Appl. Microbiol. Biotechnol. 2008, 80, 719. [Google Scholar] [CrossRef]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Nakamura, A.; Nascimento, A.; Polikarpov, I. Structural diversity of carbohydrate esterases. Biotechnol. Res. Innov. 2017, 1, 35–51. [Google Scholar] [CrossRef]
- Mäkelä, M.; Dilokpimol, A.; Koskela, S.; Kuuskeri, J.; de Vries, R.; Hildén, K. Characterization of a feruloyl esterase from Aspergillus terreus facilitates the division of fungal enzymes from Carbohydrate Esterase family 1 of the carbohydrate-active enzymes (CAZy) database. Microb. Biotechnol. 2018, 11, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P. Role of various bacterial enzymes in complete depolymerization of lignin: A review. Biocatal. Agric. Biotechnol. 2020, 23, 101498. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Strittmatter, H.; Wiemann, L.O.; Schieder, D.; Sieber, V. Enzymatic cleavage of lignin β-O-4 aryl ether bonds via net internal hydrogen transfer. Green Chem. 2013, 15, 1373. [Google Scholar] [CrossRef]
- Picart, P.; de María, P.D.; Schallmey, A. From gene to biorefinery: Microbial β-etherases as promising biocatalysts for lignin valorization. Front. Microbiol. 2015, 6, 916. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Tanatani, K.; Kamimura, N.; Otsuka, Y.; Yamaguchi, M.; Nakamura, M.; Masai, E. Sphingobium sp. SYK-6 syringate O-demethylase gene is regulated by DesX, unlike other vanillate and syringate catabolic genes regulated by DesR. Appl. Environ. Microbiol. 2020, 86, e01712-20. [Google Scholar] [CrossRef] [PubMed]
- Gall, D.L.; Ralph, J.; Donohue, T.J.; Noguera, D.R. Benzoyl Coenzyme A Pathway-Mediated Metabolism of meta-Hydroxy-Aromatic Acids in Rhodopseudomonas palustris. J. Bacteriol. 2013, 195, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Philipp, B.; Kemmler, D.; Hellstern, J.; Gorny, N.; Caballero, A.; Schink, B. Anaerobic degradation of protocatechuate (3,4-dihydroxybenzoate) by Thauera aromaticastrain AR-1. FEMS Microbiol. Lett. 2002, 212, 139–143. [Google Scholar] [CrossRef]
- Boll, M. Key enzymes in the anaerobic aromatic metabolism catalysing Birch-like reductions. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1707, 34–50. [Google Scholar] [CrossRef]
- Wischgoll, S.; Heintz, D.; Peters, F.; Erxleben, A.; Sarnighausen, E.; Reski, R.; Dorsselaer, A.V.; Boll, M. Gene clusters involved in anaerobic benzoate degradation of Geobacter metallireducens. Mol. Microbiol. 2005, 58, 1238–1252. [Google Scholar] [CrossRef]
- Carmona, M.; Zamarro, M.T.; Blazquez, B.; Durante-Rodriguez, G.; Juarez, J.F.; Valderrama, J.A.; Barragan, M.J.L.; Garcia, J.L.; Diaz, E. Anaerobic Catabolism of Aromatic Compounds: A Genetic and Genomic View. Microbiol. Mol. Biol. Rev. 2009, 73, 71–133. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.S.; Parales, R.E. tThe β-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 1996, 50, 553–590. [Google Scholar] [CrossRef] [PubMed]
- JimenezJiménez, N.; Curiel, J.A.; Reveron, I.; de las Rivas, B.; Munoz, R. Uncovering the Lactobacillus plantarum WCFS1 Gallate Decarboxylase Involved in Tannin Degradation. Appl. Environ. Microbiol. 2013, 79, 4253–4263. [Google Scholar] [CrossRef] [PubMed]
- Reichenbecher, W.; Schink, B. Towards the reaction mechanism of pyrogallol–phloroglucinol transhydroxylase of Pelobacter acidigallici. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1999, 1430, 245–253. [Google Scholar] [CrossRef]
- Brune, A.; Schink, B. Phloroglucinol pathway in the strictly anaerobic Pelobacter acidigallici: Fermentation of trihydroxybenzenes to acetate via triacetic acid. Arch. Microbiol. 1992, 157, 417–424. [Google Scholar] [CrossRef]
- Otsuka, Y.; Sonoki, T.; Ikeda, S.; Kajita, S.; Nakamura, M.; Katayama, Y. Detection and characterization of a novel extracellular fungal enzyme that catalyzes the specific and hydrolytic cleavage of lignin guaiacylglycerol beta-aryl ether linkages. Eur. J. Biochem. 2003, 270, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Husarcíková, J.; Voß, H.; de María, P.D.; Schallmey, A. Microbial β-etherases and glutathione lyases for lignin valorization in biorefineries: Current state and future perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 5391–5401. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Chino, K.; Kamimura, N.; Masai, E.; Yumoto, I.; Kamagata, Y. Methanogenic degradation of lignin-derived monoaromatic compounds by microbial enrichments from rice paddy field soil. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.W.; Young, L.Y. Benzoyl-CoA, a Universal Biomarker for Anaerobic Degradation of Aromatic Compounds. Adv. Appl. Microbiol. 2014, 88, 167–203. [Google Scholar] [CrossRef]
- Wells, T.; Ragauskas, A. Biotechnological opportunities with the β-ketoadipate pathway. Trends Biotechnol. 2012, 30, 627–637. [Google Scholar] [CrossRef]
- Billings, A.F.; Fortney, J.L.; Hazen, T.C.; Simmons, B.; Davenport, K.W.; Goodwin, L.; Ivanova, N.; Mavromatis, K.; Woyke, T.; DeAngelis, K.M. Genome sequence and description of the anaerobic lignin-degrading bacterium Tolumonas lignolytica sp. nov. Stand. Genom. Sci. 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Hashimi, A.; Roccor, R.; Liu, L.Y.; Renneckar, S.; Eltis, W.; Mohn, W.W. Genomics and metatranscriptomics of biogeochemical cycling and degradation of lignin-derived aromatic compounds in thermal swamp sediment. ISME J. 2021, 15, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Stöcker, M. Biofuels and Biomass-To-Liquid Fuels in the Biorefinery: Catalytic Conversion of Lignocellulosic Biomass using Porous Materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Lo, Y.C.; Dong, C.D.; Nagarajan, D.; Chang, J.S.; Lee, D.J. Biobutanol production from lignocellulosic biomass using immobilized Clostridium acetobutylicum. Appl. Energy 2020, 277, 115531. [Google Scholar] [CrossRef]
- Wu, J.; Dong, L.; Liu, B.; Xing, D.; Zhou, C.; Wang, Q.; Wu, X.; Feng, L.; Cao, G. A novel integrated process to convert cellulose and hemicellulose in rice straw to biobutanol. Environ. Res. 2020, 186, 109580. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Khanna, S.; Moholkar, V.S. Feasibility of rice straw as alternate substrate for biobutanol production. Appl. Energy 2013, 103, 32–38. [Google Scholar] [CrossRef]
- Ko, J.; Enkh-Amgalan, T.; Gong, G.; Um, Y.; Lee, S. Improved bioconversion of lignocellulosic biomass by Saccharomyces cerevisiae engineered for tolerance to acetic acid. GCB Bioenergy 2019, 12, 90–100. [Google Scholar] [CrossRef]
- Menon, V.; Prakash, G.; Prabhune, A.; Rao, M. Biocatalytic approach for the utilization of hemicellulose for ethanol production from agricultural residue using thermostable xylanase and thermotolerant yeast. Bioresour. Technol. 2010, 101, 5366–5373. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Damilano, E.; Menezes, R.; de Morais, M., Jr. Second-generation ethanol from sugarcane and sweet sorghum bagasses using the yeast Dekkera bruxellensis. Ind. Crop. Prod. 2016, 92, 255–262. [Google Scholar] [CrossRef]
- Huang, C.; Guo, H.J.; Wang, C.; Xiong, L.; Luo, M.T.; Chen, X.F.; Zhang, H.-R.; Li, H.-L.; Chen, X.-D. Efficient continuous biogas production using lignocellulosic hydrolysates as substrate: A semi-pilot scale long-term study. Energy Convers. Manag. 2017, 151, 53–62. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Ojeda-Castillo, V.; Snell-Castro, R.; Corona-González, R.; Alatriste-Mondragón, F.; Méndez-Acosta, H. Methane production from acid hydrolysates of Agave tequilana bagasse: Evaluation of hydrolysis conditions and methane yield. Bioresour. Technol. 2015, 181, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, C. Enhanced coproduction of hydrogen and methane from cornstalks by a three-stage anaerobic fermentation process integrated with alkaline hydrolysis. Bioresour. Technol. 2012, 104, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, Y.; Xing, Y.; Pan, C.; Zhang, G.; Lay, J. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 2007, 31, 250–254. [Google Scholar] [CrossRef]
- Fan, Y.T.; Zhang, Y.H.; Zhang, S.F.; Hou, H.W.; Ren, B.Z. Efficient conversion of wheat straw wastes into biohydrogen gas by cow dung compost. Bioresour. Technol. 2006, 97, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Metabolic engineering and enzyme-mediated processing: A biotechnological venture towards biofuel production—A review. Renew. Sustain. Energy Rev. 2018, 82, 436–447. [Google Scholar] [CrossRef]
- Qian, E.W. Pretreatment and Saccharification of Lignocellulosic Biomass. Res. Approaches Sustain. Biomass Syst. 2014, 181–204. [Google Scholar] [CrossRef]
- Li, H.; Mei, X.; Liu, B.; Xie, G.; Ren, N.; Xing, D. Quantitative proteomic analysis reveals the ethanologenic metabolism regulation of Ethanoligenens harbinense by exogenous ethanol addition. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mei, X.; Liu, B.; Li, Z.; Wang, B.; Ren, N.; Xing, D. Insights on acetate-ethanol fermentation by hydrogen-producing Ethanoligenens under acetic acid accumulation based on quantitative proteomics. Environ. Int. 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Raut, M.P.; Couto, N.; Pham, T.K.; Evans, C.; Noirel, J.; Wright, P.C. Quantitative proteomic analysis of the influence of lignin on biofuel production by Clostridium acetobutylicum ATCC 824. Biotechnol. Biofuels 2016, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Giannone, R.J.; Basen, M.; Nookaew, I.; Poole, F.L.; Kelly, R.M.; Adams, M.W.W.; Hettich, R.L. The diversity and specificity of the extracellular proteome in the cellulolytic bacterium Caldicellulosiruptor bescii is driven by the nature of the cellulosic growth substrate. Biotechnol. Biofuels 2018, 11, 1–18. [Google Scholar] [CrossRef]
- Traut, T. Phosphofructokinase. Allosteric Regul. Enzym. 2008, 139–159. [Google Scholar] [CrossRef]
- Linton, K.J. Structure and Function of ABC Transporters. Physiology 2007, 22, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, J.V.; Conway, J.M.; Lee, L.L.; Simpson, H.J.; Izquierdo, J.A.; Blumer-Schuette, S.; Nookaew, I.; Adams, M.W.W.; Kelly, R.M. Comparative Analysis of Extremely Thermophilic Caldicellulosiruptor Species Reveals Common and Unique Cellular Strategies for Plant Biomass Utilization. Appl. Environ. Microbiol. 2015, 81, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Van Lis, R.; Popek, M.; Couté, Y.; Kosta, A.; Drapier, D.; Nitschke, W.; Atteia, A. Concerted Up-regulation of Aldehyde/Alcohol Dehydrogenase (ADHE) and Starch in Chlamydomonas reinhardtii Increases Survival under Dark Anoxia. J. Biol. Chem. 2017, 292, 2395–2410. [Google Scholar] [CrossRef]
- Dai, Z.; Dong, H.; Zhang, Y.; Li, Y. Elucidating the contributions of multiple aldehyde/alcohol dehydrogenases to butanol and ethanol production in Clostridium acetobutylicum. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lütke-Eversloh, T.; Bahl, H. Metabolic engineering of Clostridium acetobutylicum: Recent advances to improve butanol production. Curr. Opin. Biotechnol. 2011, 22, 634–647. [Google Scholar] [CrossRef]
- Groeger, C.; Wang, W.; Sabra, W.; Utesch, T.; Zeng, A.-P. Metabolic and proteomic analyses of product selectivity and redox regulation in Clostridium pasteurianum grown on glycerol under varied iron availability. Microb. Cell Factories 2017, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.; Döring, C.; Ehrenreich, A.; Voigt, B.; Hecker, M.; Bahl, H.; Fischer, R.J. A proteomic and transcriptional view of acidogenic and solventogenic steady-state cells of Clostridium acetobutylicum in a chemostat culture. Appl. Microbiol. Biotechnol. 2010, 87, 2209–2226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patakova, P.; Branska, B.; Sedlar, K.; Vasylkivska, M.; Jureckova, K.; Kolek, J.; Koscova, P.; Provaznik, I. Acidogenesis, solventogenesis, metabolic stress response and life cycle changes in Clostridium beijerinckii NRRL B-598 at the transcriptomic level. Sci. Rep. 2019, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, L.; Ke, C.; Pang, Z.; Liu, L. Pathway dissection, regulation, engineering and application: Lessons learned from biobutanol production by solventogenic clostridia. Biotechnol. Biofuels 2020, 13, 1–25. [Google Scholar] [CrossRef]
- Haus, S.; Jabbari, S.; Millat, T.; Janssen, H.; Fischer, R.-J.; Bahl, H.; King, J.R.; Wolkenhauer, O. A systems biology approach to investigate the effect of pH-induced gene regulation on solvent production by Clostridium acetobutylicum in continuous culture. BMC Syst. Biol. 2011, 5, 10. [Google Scholar] [CrossRef]
- Liao, C.; Seo, S.O.; Lu, T. System-level modeling of acetone–butanol–ethanol fermentation. FEMS Microbiol. Lett. 2016, 363, 74. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rene, E.R.; Lens, P.N.L.; Veiga, M.C.; Kennes, C. Enrichment of a solventogenic anaerobic sludge converting carbon monoxide and syngas into acids and alcohols. Bioresour. Technol. 2019, 272, 130–136. [Google Scholar] [CrossRef]
- Jang, Y.S.; Han, M.J.; Lee, J.; Im, J.A.; Lee, Y.H.; Papoutsakis, E.T.; Bennett, G.; Lee, S.Y. Proteomic analyses of the phase transition from acidogenesis to solventogenesis using solventogenic and non-solventogenic Clostridium acetobutylicum strains. Appl. Microbiol. Biotechnol. 2014, 98, 5105–5115. [Google Scholar] [CrossRef]
- Nakayama, S.; Kosaka, T.; Hirakawa, H.; Matsuura, K.; Yoshino, S.; Furukawa, K. Metabolic engineering for solvent productivity by downregulation of the hydrogenase gene cluster hupCBA in Clostridium saccharoperbutylacetonicum strain N1-4. Appl. Microbiol. Biotechnol. 2008, 78, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, G.; Braunt, G.; Foresti, E.; Zaiat, M.; Guiot, S.R. Microbial Communities Performing Hydrogen Solventogenic Metabolism of Volatile Fatty Acids. bioRxiv 2021. [Google Scholar] [CrossRef]
- Yang, Y.; Nie, X.; Jiang, Y.; Yang, C.; Gu, Y.; Jiang, W. Metabolic regulation in solventogenic clostridia: Regulators, mechanisms and engineering. Biotechnol. Adv. 2018, 36, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xi, B.D.; Li, M.L.; Yang, Y.; Wang, Y. Meraptoreomic analysis of the functional insights into microbial communities of combined hydrogen and methane production by anaerobic fermentation from reed straw. PLoS ONE 2017, 12, e0183158. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Ziganshina, E.E.; Kleinstruber, S.; Nikolausz, M. Comparative Analysis of Methanogenic Communities in Different Laboratory-Scale Anaerobic Digesters. Archaea 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hanreich, A.; Heyer, R.; Benndorf, D.; Rapp, E.; Pioch, M.; Reichl, U.; Klocke, M. Metaproteome analysis to determine the metabolically active part of a thermophilic microbial community producing biogas from agricultural biomass. Can. J. Microbiol. 2012, 58, 917–922. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Yau, P.M.; Pandey, R.; Harpalani, S. A metaproteomic approach for identifying proteins in anaerobic bioreactors converting coal to methane. Int. J. Coal Geol. 2015, 146, 91–103. [Google Scholar] [CrossRef]
- Heyer, R.; Benndorf, D.; Kohrs, F.; De Vrieze, J.; Boon, N.; Hoffmann, M.; Rapp, E.; Schlüter, A.; Sczyrba, A.; Reichl, U. Proteotyping of biogas plant microbiomes separates biogas plants according to process temperature and reactor type. Biotechnol. Biofuels 2016, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Schauer-Gimenez, A.; Bahattad, U.; Kearney, C.; Struble, C.A.; Zitomer, D.; Maki, J.S. Methyl coenzyme M reductase (mcrA) gene abundance correlates with activity measurements of methanogenic H2/CO2-enriched anerobic biomass. Appl. Microbiol. 2013, 7, 77–84. [Google Scholar] [CrossRef]
- Aguinaga Casañas, M.A.; Rangkasenee, N.; Krattenmacher, N.; Thaller, G.; Metges, C.C.; Kuhla, B. Methyl-coenzyme M reductase A as an indicator to estimate methane production from dairy cows. J. Dairy Sci. 2015, 98, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.I.; Spicer, V.; Krokhin, O.V.; Shamshurin, D.; Zhang, X.; Taillefer, M.; Blunt, W.; Cicek, N.; Sparling, R.; Levin, D.B. Transcriptomic and proteomic analyses of core metabolism in Clostridium termitidis CT1112 during growth on α-cellulose, xylan, cellobiose and xylose. BMC Microbiol. 2016, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kruse, S.; Goris, T.; Westermann, M.; Adrian, L.; Diekert, G. Hydrogen production by Sulfurospirillum species enables syntrophic interactions of Epsilonproteobacteria. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Yan, Q.; Li, Y.; Huang, B.; Wang, A.; Zou, H.; Miao, H.; Li, R. Proteomic profiling of the acid tolerance response (ATR) during the enhanced biomethanation process from Taihu Blue Algae with butyrate stress on anaerobic sludge. J. Hazard. Mater. 2012, 235, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, D.; Dutta, M.; Das, D. Metabolically redirected biohydrogen pathway integrated with biomethanation for improved gaseous energy recovery. Fuel 2015, 158, 471–478. [Google Scholar] [CrossRef]
- Dev, S.; Saha, S.; Kurade, M.B.; Salama, E.-S.; El-Dalatony, M.M.; Ha, G.S.; Woong, S.; Jeon, B.H. Perspective on anaerobic digestion for biomethanation in cold environments. Renew. Sustain. Energy Rev. 2019, 103, 85–95. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pore, S.D.; Engineer, A.; Dagar, S.S.; Dhakephalkar, P.K. Meta-omics based analyses of microbiome involved in biomethanation of rice straw in a thermophilic anaerobic bioreactor under optimized conditions. Bioresour. Technol. 2019, 279, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Castellana, N.; Payne, S.; Shen, Z.; Stanke, M.; Bafna, V.; Briggs, S. Discovery and revision of Arabidopsis genes by proteogenomics. Proc. Natl. Acad. Sci. USA 2008, 105, 21034–21038. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Shiraya, T.; Kaneko, K.; Wada, K. Proteomics of rice grain under high temperature stress. Front. Plant Sci. 2013, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, J.; Chen, L.; Tian, X.; Huang, S.; Ren, X.; Zhang, W. Quantitative iTRAQ LC–MS/MS Proteomics Reveals Metabolic Responses to Biofuel Ethanol in Cyanobacterial Synechocystis sp. PCC 6803. J. Proteome Res. 2012, 11, 5286–5300. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.; Haas, W.; Chilaka, A.; Aach, J.; Gygi, S.; Church, G. Proteome-wide systems analysis of a cellulosic biofuel-producing microbe. Mol. Syst. Biol. 2011, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Taillefer, M.; Arntzen, M.; Henrissat, B.; Pope, P.; Larsbrink, J. Proteomic Dissection of the Cellulolytic Machineries Used by Soil-Dwelling Bacteroidetes. Msystems 2018, 3, e00240-18. [Google Scholar] [CrossRef] [PubMed]
| EC Number | Putative Function | Organism Source | Substrate | Activity or Function | References | |
|---|---|---|---|---|---|---|
| Cellulose | 3.2.1.21 | β-glucosidase | Bacteroides coprosuis Roseburia intestinails - Clostridium termitidi - Pantoea ananatis Sd-1 | Corn stover - α-cellulose and cellobiose - Rice straw | Cleavages β-1,4 linkages of cellobiose | [52] - [53] - [54] |
| 3.2.1.4 | Endo-β-1,4-glucanase -Endoglucanase | Cellulosilyticum lentocellum Clostridium cellobioparum Clostridium cellulolyticum Eubacterium cellulosolvens Clostridium saccharoperbutylacetonicum - Clostridium cellulolyticum Clostridium josui - Clostridium termitidi - Pantoea ananatis Sd-1 | Corn stover - Filter paper - α-cellulose and cellobiose - Rice straw | Hydrolyzes β-1,4 bonds in the amorphous regions of cellulose | [52] - [55] - [53] - [54] | |
| 3.2.1.91 | Cellobiohydrolase – Exoglucanase - 1,4- β-cellobiosidase | Clostridium saccharoperbutylacetonicum Clostridium cellulyticum Clostridium ruminicola - Clostridium termitidi - Clostridium josui - Pantoea ananatis Sd-1 - Caldicellulosiruptor bescii Caldicellulosiruptor obsidiansis | Corn stover - α-cellulose and cellobiose - Filter paper - Rice straw - Crystalline cellulose | Attack crystalline cellulose in the β-1,4 linkages | [52] - [53] - [55] - [54] - [56] | |
| 3.2. 1.86 | 6-phospho-β-glucosidase | Clostridium butyricum Enterococcus casseliflavus | Corn stover | Cleavage β-1, 4-linked cellobiose 6-phosphate | [52] | |
| 2.4.1.20 | Cellobiose phosphorylase | Clostridium phytofermentans | Corn stover | Catalyzes the reversible phosphorolysis of cellobiose | [52] | |
| NA | Cellulase | Clostridium cellobioparum Clostridium lentocellum Clostridium cellulolyticum - Caldicellulosiruptor bescii | Filter paper - Crystalline cellulose | Cleavage the β-1,4 linkages in cellulose | [55] - [56] | |
| 3.2.1.1 | α-amylase | Clostridium saccharoperbutylacetonicum - Caldicellulosiruptor bescii Caldicellulosiruptor obsidiansis | Corn stover - Crystalline cellulose | Hydrolyze the α-1,4-glucosidic bonds in α-glucans | [52] - [56] | |
| 3.2.1.39 | Endo-1,3-β-glucanase | Caldicellulosiruptor obsidiansis | Crystalline cellulose | Hydrolyzes β-1,3-bonds present in glucans | [56] | |
| Hemicellulose | 3.2.1.8 | Xylanase-Endoxylanase- Endo-β-1,4-xylanase | Cellulosilyticum lentocellum Roseburia intestinalis Ruminococcus sp. Cellulosilyticum ruminicola Lachnoclostridium phytofermentans Butyrivibrio fibrisolvens Clostridium cellulosi - Clostridium termitidi - Caldicellulosiruptor bescii Caldicellulosiruptor obsidiansis | Corn stover - α-cellulose - Crystalline cellulose | Attack β-1,4 bond of the xylan backbone | [52] - [53] - [56] |
| 3.2.1.23 | β-galactosidase | Clostridium sp. - Caldicellulosiruptor bescii Caldicellulosiruptor obsidiansis | Corn stover - Crystalline cellulose | Hydrolyze β-1,4-glycosidic linkage present in lactose | [52] - [56] | |
| 3.2.1.89 | arabinogalactan endo-1,4-β-galactosidase | Paenibacillus sp. - Caldicellulosiruptor obsidiansis | Corn stover | Hydrolyze β-1,4 linkages in arabinogalactans | [52] | |
| 3.2.1.25 | Endo-1,4-β- mannosidase | Clostridium clariflavum - Caldicellulosiruptor bescii Caldicellulosiruptor obsidiansis | Corn stover - Crystalline cellulose | Cleavage the β-1,4-manno-oligomers | [52] - [56] | |
| 3.2.1.131 | α-glucuronidase | Paenibacillus sp. | Corn stover | Hydrolyze α-1,2-glycosidic linkage between xylose and glucuronic acid | [52] | |
| 3.2.1.31 | β-glucuronidase | Clostridium cellulovorans | Corn stover | Exohydrolyze β-d-glucuronic acid residues of glycosaminoglycan | [52] | |
| 3.2.1.37 | β-xylosidase | Sphaerochaeta coccoides Clostridium saccharoperbutylacetonicum Clostridium ruminicola Flavobacterium johnsoniae Cellulosilyticum ruminicola | Corn stover | Exohydrolyze β-1,4 linkages of xylans, to removing xylose residues | [52] | |
| 3.2.1.6 | Endo -1,3(4)-β-α- Glucanase | Clostridium perfringens | Corn stover | Endohydrolysis of β -1,3 or β -1,4 linkages in β-D-glucans | [52] | |
| 3.2.1.78 | β-mannanase | Clostridium clariflavum Roseburia intestinalis Cellulosilyticum lentocellum - Clostridium termitidi | Corn stover α-cellulose | Attack the β-1,4 bond in D-mannan | [52] [53] | |
| 3.2.1.177 | α-xylosidase | Paenibacillus mucilaginosus | Corn stover | Hydrolyze α-1,6 linked xylose residues | [52] | |
| 3.2.1.55 | α-L-Arabinofuranosidase | -Enterococcus casseliflavus Enterococcus mundtii Klebsiella pneumoniae - Clostridium termitidi - Thermobacillus xylanolyticus - Caldicellulosiruptor obsidiansis | Corn stover - α-cellulose - Filter paper - Crystalline cellulose | Exohydrolyze α-L-1,5 and/or α-L-1,3 linkages of arabinofuranosyl-based oligomers | [52] - [53] - [55] - [56] | |
| 3.2.1.51 | α-L-fucosidase | Caldicellulosiruptor obsidiansis | Crystalline cellulose | Cleavage α-1,6-, α-1,3-, α-1,4-, and/or α-1,2 bonds in fucosylated oligosaccharides | [56] | |
| 3.1.1.72 | acetylxylan esterase | Enterococcus casseliflavus Pseudobutyrivibrio xylanivorans - Clostridium termitidi | Corn stover - α-cellulose | Remove the O-acetyl groups from the O-2 and/or O-3 positions | [52] - [53] | |
| 3.1.1.1 | Carboxylesterase | Caldicellulosiruptor obsidiansis | Crystalline cellulose | Hydrolyzes ester bonds, liberating alcohol and carboxylic acid | [56] | |
| NIA | Esterase | Clostridium clariflavum Clostridium josui - Clostrodium termitidi - Pantoea ananatis Sd-1 | Corn stover - α-cellulose - Rice straw | Cleavage ester bonds | [52] - [53] - [54] | |
| 3.5.1.41 | Chitin deacetylase | Clostridium termitidi | Cellobiose | Hydrolyze the N-acetoamido groups of N-acetyl-β-D-glucosaminide in chitin | [53] | |
| 3.2.1.14 | Chitinase | Clostridium termitidi | cellobiose | Endo-hydrolyzes N-acetyl-β-D-glucosaminide β-1,4 linkages in chitin and chitodextrins. | [53] | |
| 3.2.1.52 | β-N-acetylhexosaminidase | Pantoea ananatis Sd-1 | Rice straw | Hydrolyse the β-1,4 glycosidic bond between N-acetylglucosamine and anhydro-N-acetylmuramic acid | [54] | |
| NIA | Cellulosomal proteins | Clostridium termitidi - Clostridium josui Clostridium cellulolyticum | α-cellulose - Filter paper | Protein complex that achieves hydrolysis cellulose and hemicellulose | [53] - [55] | |
| NIA | Cellulosomal xylanase | Clostridium cellulolyticum | Filter paper | Hydrolyzes β-1,4 linkages in the xylan backbone | [55] |
| Microorganism | Substrate (Concentration) | Identified Enzymes | Number of Different Proteins a | Reference |
|---|---|---|---|---|
| Clostridium thermocellum ATCC 27405 | Avicel (2 g/L) | Exoglucanase Endoglucanase Xylanase Xyloglucanase Lichenase Mannanase Chitinase Endopygalactorunase Glycosyl hydrolase | 3 11 3 1 1 1 1 1 9 | [57] |
| Clostridium thermocellum ATCC 27405 | Cellobiose (2 g/L) | Xylanase Endoglucanase Exoglucanase Xyloglucanase Chitinase α-l-arabinofuranosidase B Glycoside hydrolase | 5 9 3 1 1 2 9 | [57] |
| Clostridium cellulovorans | Cellobiose (3 g/L) | Endoglucanase Mannanase Exocellulase | 5 4 1 | [58] |
| Clostridium cellulovorans | Avicel (3 g/L) | Endoglucanase Mannanase Xylanase Exocellulase | 6 4 1 1 | [58] |
| Clostridium cellulovorans | Xylan (3 g/L) | Endoglucanase Mannanase Xylanase Exocellulase | 8 4 2 1 | [58] |
| Clostridium cellulolyticum H10 | Washed hatched wheat straw (5 g/L) | Endoglucanase Acetyl xylan esterase Mannanase Rhamnogalacturonan lyase Xylanase Cellobiohydrolase Cellulase Feruloyl esterase Xyloglucanase Arabinosidase α-arabinofuranosidase α-galactosidase β-galactosidase | 17 2 2 1 10 3 1 2 1 1 1 2 1 | [59] |
| Pandoraea sp. ISTKB * | Kraft lignin (2 g/L) | Peroxidases Laccase Oxidases Oxidoreductases Vanillate-O-demethylase Dioxygenases Oxygenases Monooxygenase | 4 1 10 16 2 13 2 1 | [60] |
| Aspergillus fumigatus G-13 * | p-coumaric acid (0.1 mmol/L), sinapic acid (0.1 mmol/L), glucose (10 g/L) and cellulose (10 g/L) | Dioxygenase Glyoxylase Oxidoreductase Ferulic acid esterase Monooxygenase Catalase peroxidase Cellulase β-glucancellobiohydrolase Cellobiose dehydrogenase Peroxidase Methyltransferase Oxidase Ketoreductase Aldo keto reductase Catalase | 8 1 5 2 8 1 1 1 1 1 2 1 1 1 2 | [61] |
| Phanerochaete chrysosporium* | Softwood (30 g with 75% moisture content) | β-Glucosidase Mannanase Endoglucanase Exocellobiohydrolase Mannosidase Oxidase Lignin peroxidase | 3 1 2 3 1 1 1 | [62] |
| Reaction/Pathway | Enzyme | Microorganism | Gene | Reference |
|---|---|---|---|---|
| β-O-4 aryl ether | Cα-dehydrogenase | Sphingobium sp. SYK -6 | ligD ligL ligN ligO | [68,69] |
| β-etherase | Sphingobium sp. SYK -6 | ligF; ligE ligP | [68,69] | |
| Glutathione lyase | Sphingobium sp. SYK -6 | ligG | [68] | |
| O-demethylation | Syringate-O-demethylase | Sphingobium sp. SYK -6 | desA | [70] |
| Vanillate O-demethylase | Sphingobium sp. SYK -6 | ligM | [70] | |
| Benzoyl-CoA pathway | Ligase | Rhodopseudomonas palustris | hbaA | [71] |
| Reductase | Thauera aromatica | NIA | [72] | |
| pHB-CoA reductase | Rhodopseudomonas palustris | hbaBCD | [71] | |
| Benzoyl-CoA reductase class 1 | Thauera aromatica | bcrA bcrD bcrB bcrC | [73] | |
| Benzoyl-CoA reductase class 2 | Geobacter metallireducens | bamB bamC bamDE bamCF bamGHI | [74,75] | |
| Cyclohexadienoyl-CoA hydratase | Geobacter metallireducens | bamR | [74] | |
| Hydroxyenoyl-CoA dehydrogenase | Geobacter metallireducens | bamQ | [74] | |
| oxoacyll-CoA hydrolase | Geobacter metallireducens | bamA | [74] | |
| β-oxidation- Benzoyl-CoA pathway | Hydroxyacyl-CoA dehydrogenase | Geobacter metallireducens | pimE | [75] |
| Acyl-CoA acetyltransferase (β-Ketothiolase) | Geobacter metallireducens | pimB | [75] | |
| Glutaryl-CoA dehydrogenase | Geobacter metallireducens | gcdH | [75] | |
| 3-hydroxybutyryl-CoA dehydratase | Geobacter metallireducens | NIA | [75] | |
| 3-Hydroyibutyryl-CoA dehydrogenase | Geobacter metallireducens | NIA | [75] | |
| Acetoacetyl-CoA thiolase | Geobacter metallireducens | NIA | [75] | |
| β-Ketoadipate pathway | Protocatechuate 3,4-dioxygenase | Pseudomonas putida | pcaGH | [76] |
| Cycloisomerase | Pseudomonas putida | pcaB | [76] | |
| γ-Carboxy-muconolactone decarboxylase | Pseudomonas putida | pcaC | [76] | |
| β-ketoadipate enol-lactone hydrolase | Pseudomonas putida | pacD | [76] | |
| β-ketoadipate succinyl-CoA transferase | Pseudomonas putida | pcaIJ | [76] | |
| β-ketoadipate-CoA thiolase | Pseudomonas putida | pcaF | [76] | |
| Phloroglucinol pathway | Gallate decarboxylase | Lactobacillus plantarum | lpdB lpdC lpdD | [77] |
| Pyrogallol transhydroxylase | Pelobacter acidigallici | athL bthL | [78] | |
| Phloroglucinol reductase | Pelobacter acidigallici | NIA | [79] | |
| Dihydrophloroglucinol hydrolase | Pelobacter acidigallici | NIA | [79] | |
| β-oxidation- Phloroglucinol pathway | 3-hydroxyacyl-CoA dehydrogenase | Pelobacter acidigallici | NIA | [79] |
| Acetyl CoA transferase | Pelobacter acidigallici | NIA | [79] | |
| Triacetic acid β-ketothiolase | Pelobacter acidigallici | NIA | [79] | |
| Acetoacetyl-CoA β-ketothiolase | Pelobacter acidigallici | NIA | [79] | |
| Phosphotransacetylase | Pelobacter acidigallici | NIA | [79] | |
| Acetate kinase | Pelobacter acidigallici | NIA | [79] |
| Lignocellulosic Feedstock | Feedstock Preparation | Biofuel | Inoculum | Fermentation Method | Biofuel Yield | Biofuel Titer | Reference |
|---|---|---|---|---|---|---|---|
| Rice straw | Alkaline pretreatment and enzymatic hydrolysis | Biobutanol | Clostridium acetobutylicum ATCC 824 | PVA-immobilized | 0.23 g/g glucose | 13.8 g/L | [88] |
| Sugarcane bagasse | Alkaline pretreatment and enzymatic hydrolysis | Biobutanol | Clostridium acetobutylicum ATCC 824 | Suspended cell | 0.16 g/g glucose | 8.4 g/L | [88] |
| Rice straw | Alkaline and acid pretreatments and enzymatic hydrolysis | Biobutanol | Clostridium beijerinckii F-6 | ABE | 0.13 g/g | 4.22 g/L | [89] |
| Rice straw | Mechanic, thermal, and acid pretreatment | Biobutanol | Clostridium acetobutylicum NCIM 2337 | Batch | 0.34 g/g | 13.5 g/L | [90] |
| Sugarcane bagasse | Acid pretreatment and enzymatic hydrolysis | Ethanol | Saccharomyces cerevisiae XUSAE57 | NIA | 0.49 g/g | NIA | [91] |
| Oat spelt | Enzymatic hydrolysis | Ethanol | Debaryomyces hansenii | Immobilized | 0.46 g/g | 8.38 g/L | [92] |
| Wheat bran | Enzymatic hydrolysis | Ethanol | Debaryomyces hansenii | Immobilized | 0.44 g/g | 6.89 g/L | [92] |
| Sugarcane bagasse | Alkaline pretreatment and enzymatic hydrolysis | Ethanol | Dekkera bruxellensis GDB248 | Anaerobic fermentation | 0.42 g/g | 4.5 g/g | [93] |
| Sweet sorghum bagasse | Alkaline pretreatment and enzymatic hydrolysis | Ethanol | Dekkera bruxellensis GDB248 | Anaerobic fermentation | 0.44 g/g | 4.85 g/g | [93] |
| Bagasse, rice straw, corncob | Acid pretreatment | Biogas | Granular anaerobic sludge from chemical plant | Continuous anaerobic digestion | 0.381 L/g COD (69.6 % CH4) | NIA | [94] |
| A. tequilana bagasse | Acid pretreatment | Methane | Granular anaerobic sludge from full-scale reactor | Batch anaerobic digestion | 0.26 L CH4/g COD | NIA | [95] |
| Cornstalks fermentation effluents | Alkaline pretreatment | Methane | Anaerobic sludge | Batch | 0.178 L CH4/g cornstalks | NIA | [96] |
| Cornstalks | Alkaline pretreatment | Hydrogen | Clostridium thermocellum 7072 | Two-stage batch fermentation | 0.074 L/g cornstalks | NIA | [96] |
| Cornstalks | Acid pretreatment | Hydrogen | Microbial consortium form cow dung compost | Batch | 0.149 L H2/g TVS | NIA | [97] |
| Wheat straw | Acid pretreatment | Hydrogen | Microbial consortium form cow dung compost | Batch | 0.068 L H2 g TVS | NIA | [98] |
| Microorganism | Conditions | Central Carbon Metabolism | Pyruvate Metabolism | Ethanol Production | References |
|---|---|---|---|---|---|
| Clostridium cellulovorans | Avicel | Upregulated | Upregulated | Upregulated | [22] |
| ATP-dependent 6-phosphofructokinase (Clocel_2901 *) | Pyruvate phosphate dikinase (Clocel_1454 **, Clocel_4349 **) Phosphoenolpyruvate carboxylase (Clocel_1149 **) | Alcohol dehydrogenase (Clocel_3817 ***) | |||
| Downregulated | Downregulated | NIA | |||
| Glyceraldehyde-3-phosphate dehydrogenase (Clocel_0719 *) | Malic enzyme (Clocel_0393 **) | ||||
| Glucose | Upregulated | Upregulated | Upregulated | [22] | |
| Glyceraldehyde-3-phosphate dehydrogenase (Clocel_0719 *) | Phosphoenolpyruvate carboxylase (Clocel_1149 **) | Pyruvate formate lyase (Clocel_1811 ***, Clocel_1812 ***) | |||
| Ethanoligenens harbinense (YUAN-3) | Ethanol stress 50 mM | NIA | NIA | Ethanologenesis Upregulated Enzymes | [102] |
| Acetaldehyde-CoA/alcohol dehydrogenase (ADU26923 ***) | |||||
| Ethanol stress 100 mM | Upregulated | NIA | Ethanologenesis Upregulated Enzymes | [102] | |
| Acetaldehyde-CoA/alcohol dehydrogenase (ADU26923 ***) | |||||
| Phosphoglycerate kinase (ADU27083 *) Triosephosphate isomerase (ADU27084 *) Glyceraldehyde-3-phosphate dehydrogenase (ADU28097 *) 2,3-diphosphoglycerate-dependent phosphoglycerate mutase (ADU26920 *) 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (ADU27085 *) | Ethanol tolerance Upregulated Enzymes | ||||
| Desulfoferrodoxin (ADU28196 ***) Glutathione peroxidase (ADU28264 ***) | |||||
| Ethanol stress 200 mM | Downregulated | NIA | Upregulated | [102] | |
| Carbon storage regulator protein (CsrA) (ADU28042 *) | Acetaldehyde-CoA/alcohol dehydrogenase (ADE, ADU26923 ***) | ||||
| Ethanol tolerance Upregulated Enzymes | |||||
| Desulfoferrodoxin (ADU28196 ***) | |||||
| Acetic acid stress | NIA | NIA | Upregulated | [103] | |
| Thioredoxin (ADU25713 ***, ADU26185 ***) Peroxiredoxin (ADU25886 ***) Alkyl hydroperoxide reductase (AhpC) subunit (ADU26936 ***) Glyceraldehyde-3-phosphate dehydrogenase (ADU27040 ***) | |||||
| Clostridium acetobutylicum (ATCC 824) | Cellobiose + Lignin | Upregulated in Stationary Phase | NIA | Downregulated in Stationary Phase | [104] |
| 2-keto-3-deoxy-6-phosphogluconate aldolase (CA_C2973 *) | Acetaldehyde dehydrogenase (CA_C0162 ***) Aldehyde/alcohol dehydrogenase (AdhE2 ***) | ||||
| Caldicellulosiruptor bescii (DSM 6725) | C5 substrates (xylose and xylan) | Upregulated in xylan | NIA | NIA | [105] |
| Extracellular solute binding proteins (ESBP) (Athe_0849 *) (Athe_0089 *) | |||||
| Upregulated in xylose and xylan | |||||
| ESBPs (Athe_0523 *) (Athe_2091 *) (Athe_2574 *) (Athe_0847 *) | |||||
| C6 substrates (glucose, cellobiose and avicel) | Upregulated in avicel | NIA | NIA | [105] | |
| Glycoside hydrolases (Athe_0459 *) (Athe_0460 *) | |||||
| Upregulated in glucose, cellobiose and avicel | |||||
| Xylose isomerase (Athe_0345 *) ABC transporter-related proteins (Athe_1109 *) (Athe_0106 *) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Mercado, M.I.; Talavera-Caro, A.G.; Escobedo-Uribe, K.M.; Sánchez-Muñoz, S.; Luévanos-Escareño, M.P.; Hernández-Terán, F.; Alvarado, A.; Balagurusamy, N. Bioconversion of Lignocellulosic Biomass into Value Added Products under Anaerobic Conditions: Insight into Proteomic Studies. Int. J. Mol. Sci. 2021, 22, 12249. https://doi.org/10.3390/ijms222212249
Vélez-Mercado MI, Talavera-Caro AG, Escobedo-Uribe KM, Sánchez-Muñoz S, Luévanos-Escareño MP, Hernández-Terán F, Alvarado A, Balagurusamy N. Bioconversion of Lignocellulosic Biomass into Value Added Products under Anaerobic Conditions: Insight into Proteomic Studies. International Journal of Molecular Sciences. 2021; 22(22):12249. https://doi.org/10.3390/ijms222212249
Chicago/Turabian StyleVélez-Mercado, Martha Inés, Alicia Guadalupe Talavera-Caro, Karla María Escobedo-Uribe, Salvador Sánchez-Muñoz, Miriam Paulina Luévanos-Escareño, Fernando Hernández-Terán, Alejandra Alvarado, and Nagamani Balagurusamy. 2021. "Bioconversion of Lignocellulosic Biomass into Value Added Products under Anaerobic Conditions: Insight into Proteomic Studies" International Journal of Molecular Sciences 22, no. 22: 12249. https://doi.org/10.3390/ijms222212249
APA StyleVélez-Mercado, M. I., Talavera-Caro, A. G., Escobedo-Uribe, K. M., Sánchez-Muñoz, S., Luévanos-Escareño, M. P., Hernández-Terán, F., Alvarado, A., & Balagurusamy, N. (2021). Bioconversion of Lignocellulosic Biomass into Value Added Products under Anaerobic Conditions: Insight into Proteomic Studies. International Journal of Molecular Sciences, 22(22), 12249. https://doi.org/10.3390/ijms222212249







