The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma
Abstract
:1. Introduction
2. Malignant Pleural Mesothelioma
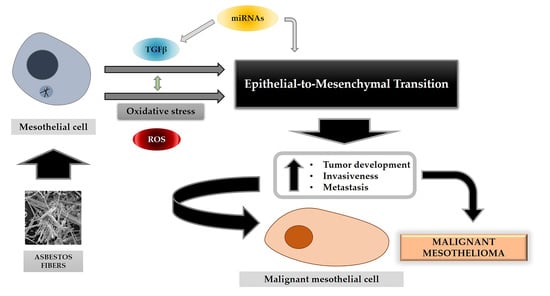
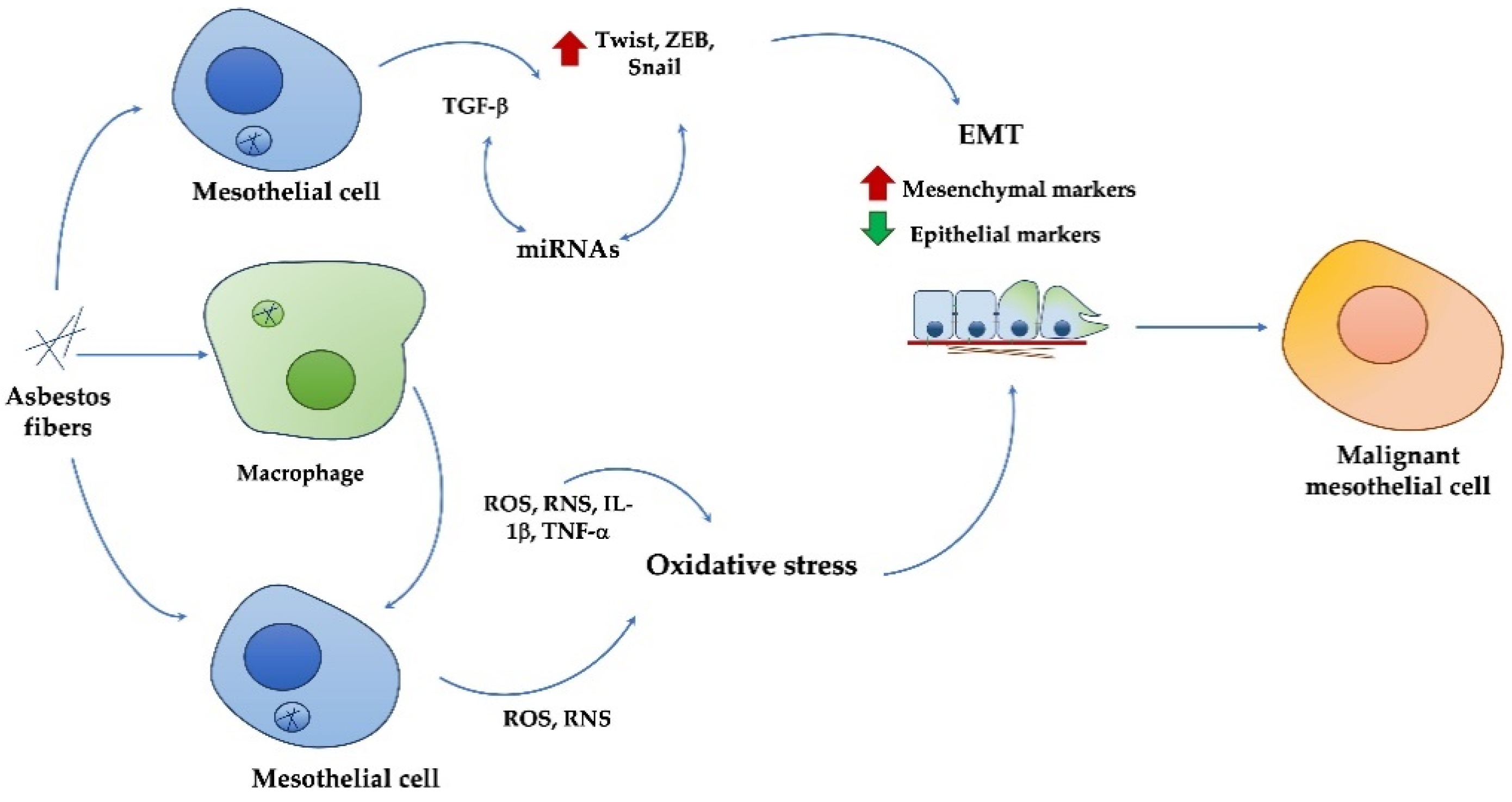
3. Asbestos Effects and MPM Development
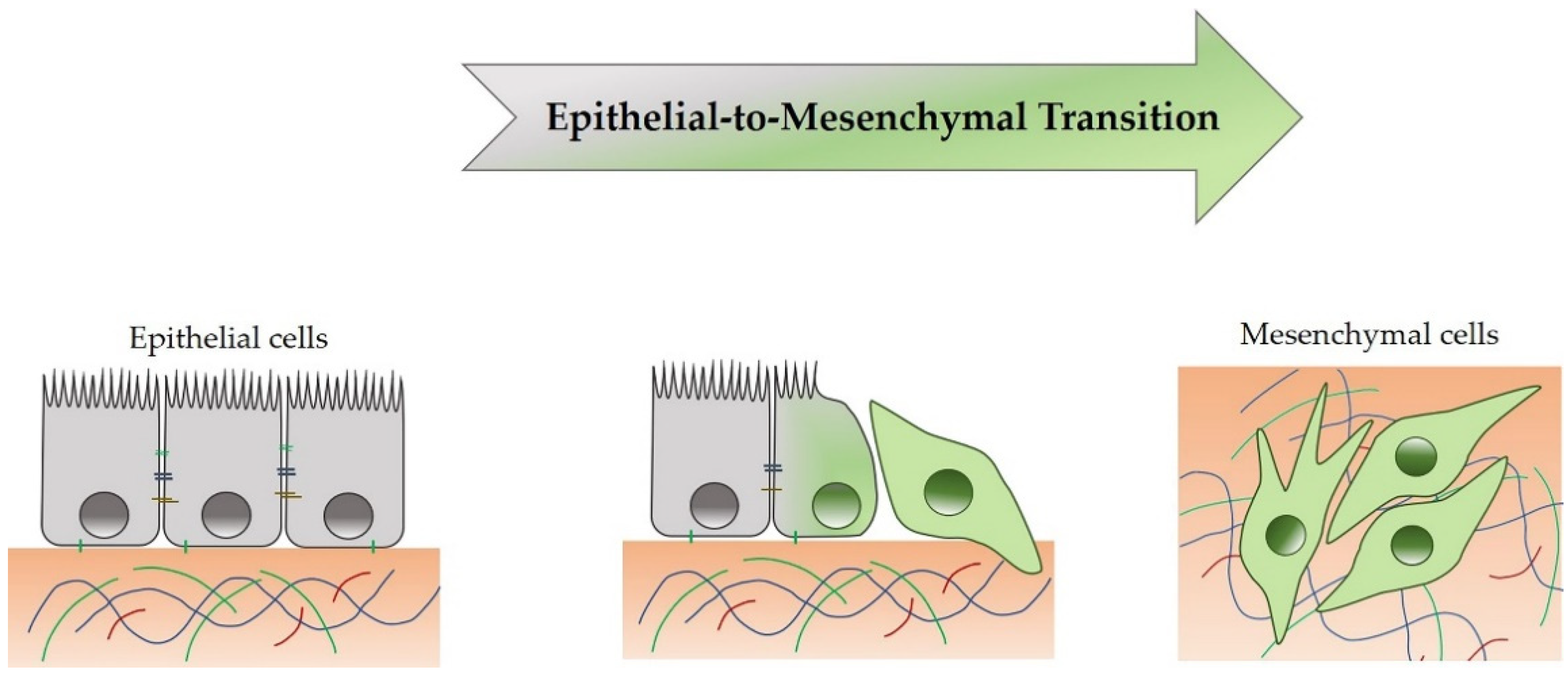
4. Epithelial-to-Mesenchymal Transition
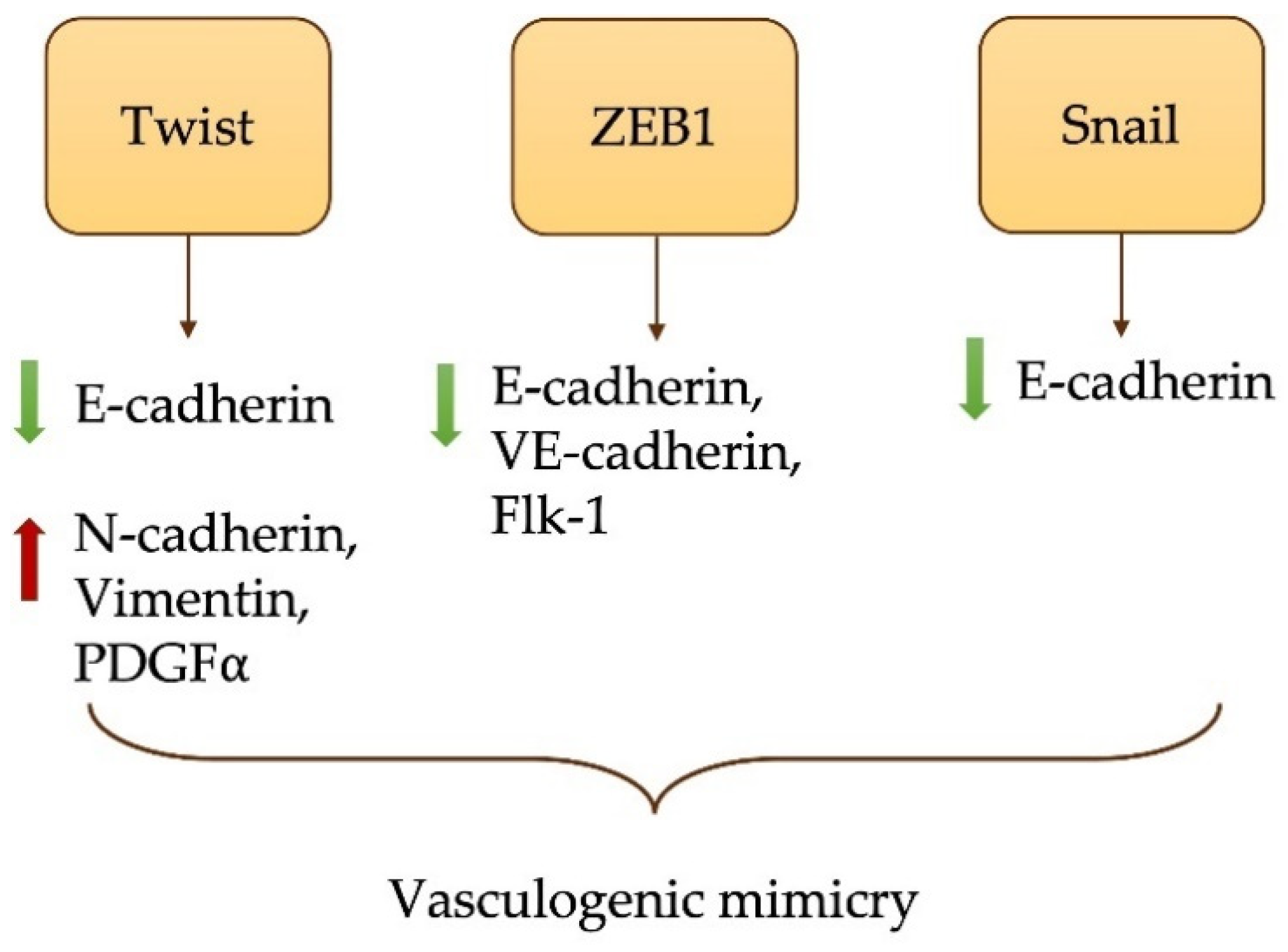
4.1. EMT in MPM Development and Metastasis
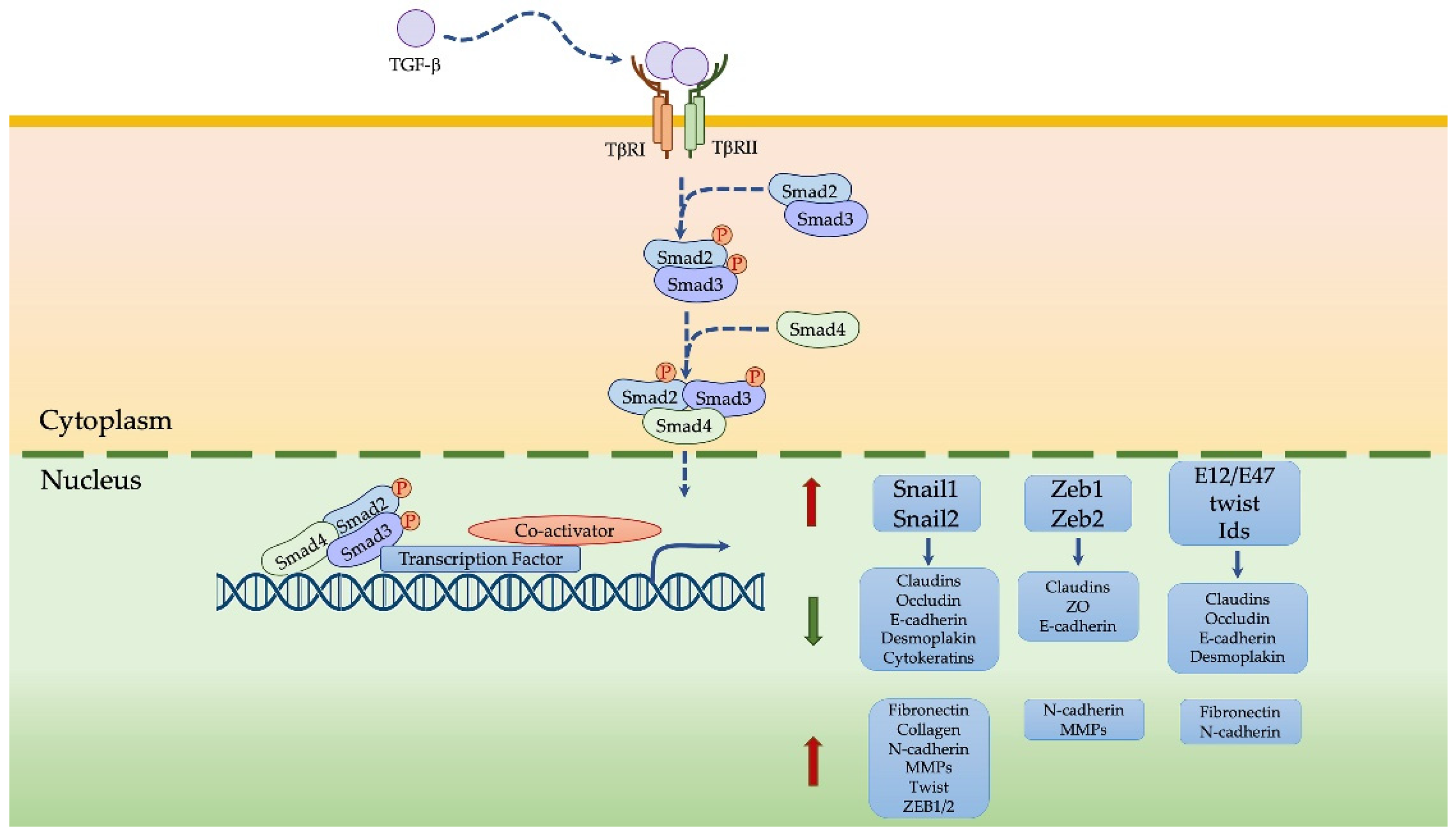
4.2. TGFβ-Induced EMT in MPM
4.3. Oxidative Stress-Induced EMT in MPM
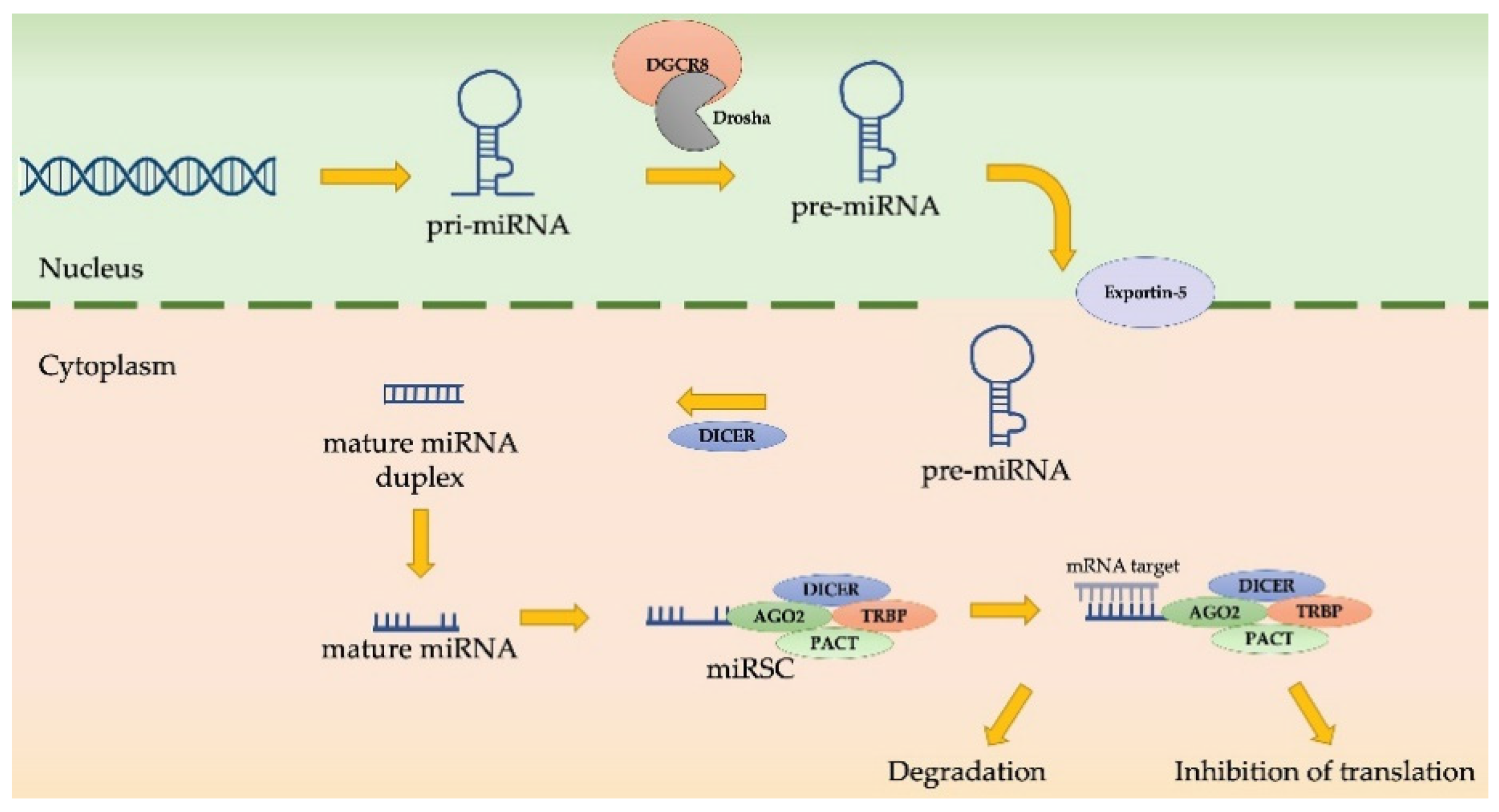
4.4. MiRNAs as EMT Regulators in MPM Progression
5. EMT and TME Crosstalk in MPM: A Possible Therapeutic Approach
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gaudino, G.; Xue, J.; Yang, H. How asbestos and other fibers cause mesothelioma. Trans. Lung Cancer Res. 2020, 9 (Suppl. 1), S39–S46. [Google Scholar] [CrossRef]
- Kamp, D.W. Asbestos-induced lung diseases: An update. Transl. Res. 2009, 153, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Cannito, S.; Novo, E.; Di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid. Redox Signal. 2010, 12, 1383–1430. [Google Scholar] [CrossRef]
- Giannoni, E.; Parri, M.; Chiarugi, P. EMT and oxidative stress: A bidirectional interplay affecting tumor malignancy. Antioxid. Redox Signal. 2012, 16, 1248–1263. [Google Scholar] [CrossRef]
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxid. Med. Cell Longev. 2015, 2015, 654594. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. MicroRNAs in mesothelioma: From tumour suppressors and biomarkers to therapeutic targets. J. Thorac. Dis. 2015, 7, 1031–1040. [Google Scholar] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur. Respir. Rev. 2016, 25, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; Demay, R.M.; Khan, G.; Tiirikainen, M.; Rinaudo, C.; et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am. J. Pathol. 2013, 183, 1654–1666.s. [Google Scholar] [CrossRef] [Green Version]
- Menis, J.; Pasello, G.; Remon, J. Immunotherapy in malignant pleural mesothelioma: A review of literature data. Transl. Lung. Cancer Res. 2021, 10, 2988–3000. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Beasley, M.B.; Galateau-Salle, F.; Dacic, S. Pleural mesothelioma classification update. Virchows Arch. 2021, 478, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Klebe, S.; Brownlee, N.A.; Mahar, A.; Burchette, J.L.; Sporn, T.A.; Vollmer, R.T.; Roggli, V.L. Sarcomatoid mesothelioma: A clinical-pathologic correlation of 326 cases. Mod. Pathol. 2010, 23, 470–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quetel, L.; Meiller, C.; Assié, J.; Blum, Y.; Imbeaud, S.; Montagne, F.; Tranchant, R.; Wolf, J.; Caruso, S.; Copin, M.; et al. Genetic alterations of malignant pleural mesothelioma: Association with tumor heterogeneity and overall survival. Mol. Oncol. 2020, 14, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, C.A.; Grespin, M.; Best, E.A. Asbestos fiber length and its relation to disease risk. Inhal. Toxicol. 2017, 9, 541–554. [Google Scholar] [CrossRef]
- Manning, C.B.; Vallyathan, V.; Mossman, B.T. Diseases caused by asbestos: Mechanisms of injury and disease development. Int. Immunopharmacol. 2002, 2, 91–200. [Google Scholar] [CrossRef]
- MacCorkle, R.A.; Sluttery, S.D.; Nash, D.R.; Brinkley, B.R. Intracellular protein binding to asbestos induces aneuploidy in human lung fibroblasts. Cell Motil. Cytoskel. 2006, 63, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, V.C.; Everitt, J.I.; Black, B.; Kane, A.B. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 153–178. [Google Scholar] [CrossRef] [Green Version]
- Hiltbrunner, S.; Mannarino, L.; Kirschner, M.B.; Opitz, I.; Rigutto, A.; Laure, A.; Lia, M.; Nozza, P.; Maconi, A.; Marchini, S.; et al. Tumor Immune Microenvironment and Genetic Alterations in Mesothelioma. Front. Oncol. 2021, 11, 2223. [Google Scholar] [CrossRef]
- Tamminen, J.A.; Myllärniemi, M.; Hyytiäinen, M.; Keski-Oja, J.; Koli, K. Asbestos exposure induces alveolar epithelial cell plasticity through MAPK/Erk signaling. J. Cell. Biochem. 2012, 113, 2234–2247. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, Y.; Derynck, R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Dev. Cell 2021, 56, 726–746. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Opitz, I.; Thies, S.; Seifert, B.; Moch, H.; Weder, W.; Soltermann, A. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2010, 37, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Hosseinifard, H.; Cheng, J.; Wei, C.; Fu, J. Prognostic Value of EMT-inducing Transcription Factors (EMT-TFs) in Metastatic Breast Cancer: A Systematic Review and Meta-analysis. Sci. Rep. 2016, 6, 28587. [Google Scholar] [CrossRef]
- Caramel, J.; Papadogeorgakis, E.; Hill, L.; Browne, G.J.; Richard, G.; Wierinckx, A.; Saldanha, G.; Osborne, J.; Hutchinson, P.; Tse, G.; et al. A Switch in the Expression of Embryonic EMT-Inducers Drives the Development of Malignant Melanoma. Cancer Cell 2013, 24, 466–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, T.; Zhang, T.; Si, X.; Zhou, Y. Overexpression of EMT-inducing transcription factors as a potential poor prognostic factor for hepatocellular carcinoma in Asian populations: A meta-analysis. Oncotarget 2017, 8, 59500–59508. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, L.; Liang, N.; Xie, J.; Zhang, J.; Deng, G.; Luo, H.; Zhang, J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J. Cell. Mol. Med. 2016, 20, 1761–1769. [Google Scholar] [CrossRef]
- Pulford, E.; Hocking, A.; Griggs, K.; McEvoy, J.; Bonder, C.; Henderson, D.W.; Klebe, S. Vasculogenic mimicry in malignant mesothelioma: An experimental and immunohistochemical analysis. Pathology 2016, 48, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.K.; Brosseau, S.; Cook, A.; Zalcman, G. Antiangiogeneic Strategies in Mesothelioma. Front. Oncol. 2020, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Duda, D.; Duyverman, A.; Kohno, M.; Snuderl, M.; Steller, E.; Fukumura, D.; Jain, R.K. Malignant Cells Facilitate Lung Metastasis by Bringing Their Own Soil. Proc. Natl. Acad. Sci. USA 2010, 107, 21677–21682. [Google Scholar] [CrossRef] [Green Version]
- Casarsa, C.; Bassani, N.; Ambrogi, F.; Zabucchi, G.; Boracchi, P.; Biganzoli, E.; Coradini, D. Epithelial-to-mesenchymal transition, cell polarity and stemness-associated features in malignant pleural mesothelioma. Cancer Lett. 2011, 302, 136–143. [Google Scholar] [CrossRef]
- Ramesh, V.; Brabletz, T.; Ceppi, P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer 2020, 6, 942–950. [Google Scholar] [CrossRef]
- Adhikary, G.; Grun, D.; Alexander, H.R.; Friedberg, J.S.; Xu, W.; Keillor, J.W.; Kandasamy, S.; Eckert, R.L. Transglutaminase is a mesothelioma cancer stem cell survival protein that is required for tumor formation. Oncotarget 2018, 9, 34495–34505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Wang, L.; Riedel, H.; Wang, K.; Yang, Y.; Dinu, C.Z.; Rojanasakul, Y. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol. Cancer 2017, 16, 63. [Google Scholar] [CrossRef] [Green Version]
- Iwanami, T.; Uramoto, H.; Nakagawa, M.; Shimokawa, H.; Yamada, S.; Kohno, K.; Tanaka, F. Clinical significance of epithelial-mesenchymal transition-associated markers in malignant pleural mesothelioma. Oncology 2014, 86, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Merikallio, H.; Pääkkö, P.; Salmenkivi, K.; Kinnula, V.; Harju, T.; Soini, Y. Expression of snail, twist, and Zeb1 in malignant mesothelioma. APMIS 2013, 121, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Chew, S.H.; Nagai, H.; Yamashita, Y.; Ohara, H.; Jiang, L.; Akatsuka, S.; Takahashi, T.; Toyokuni, S. Overexpression of miR-199/214 is a distinctive feature of iron-induced and asbestos-induced sarcomatoid mesothelioma in rats. Cancer Sci. 2020, 111, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Gulino, G.R.; Polimeni, M.; Prato, M.; Gazzano, E.; Kopecka, J.; Colombatto, S.; Ghigo, D.; Aldieri, E. Effects of Chrysotile Exposure in Human Bronchial Epithelial Cells: Insights into the Pathogenic Mechanisms of Asbestos-Related Diseases. Environ. Health Perspect. 2016, 124, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Schelch, K.; Wagner, C.; Hager, S.; Pirker, C.; Siess, K.; Lang, E.; Lin, R.; Kirschner, M.B.; Mohr, T.; Brcic, L.; et al. FGF2 and EGF induce epithelial–mesenchymal transition in malignant pleural mesothelioma cells via a MAPKinase/MMP1 signal. Carcinogenesis 2018, 39, 534–545. [Google Scholar] [CrossRef]
- Pociask, D.A.; Sime, P.J.; Brody, A.R. Asbestos-derived reactive oxygen species activate TGF-β1. Lab. Invest. 2004, 84, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Miyazono, K. Transforming growth factor-β signaling in epithelial–mesenchymal transition and progression of cancer. Proc. Jpn. Acad. 2009, 85, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Tsubakihara, Y.; Moustakas, A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β. Int. J. Mol. Sci. 2018, 19, 3672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial- mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, A.; Vizan, P.; Das, D.; Chakravarty, P.; Vogt, J.; Rogers, K.W.; Müller, P.; Hinck, A.P.; Sapkota, G.P.; Hill, C.S. TGF-β uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. eLife 2018, 7, e31756. [Google Scholar] [CrossRef]
- Simeone, P.; Trerotola, M.; Franck, J.; Cardon, T.; Marchisio, M.; Fournier, I.; Salzet, M.; Maffia, M.; Vergara, D. The multiverse nature of epithelial to mesenchymal transition. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic implications of cancer epithelial- mesenchymal transition (EMT). Arch. Pharm. Res. 2019, 42, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Tobar, N.; Villar, V.; Santibanez, J.F. ROS-NF𝜅Β mediates TGF-𝛽1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol. Cell Biochem. 2010, 340, 195–202. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-𝛽-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Trempolec, N.; Degavre, C.; Doix, B.; Brusa, D.; Corbet, C.; Feron, O. Acidosis-Induced TGF-β2 Production Promotes Lipid Droplet Formation in Dendritic Cells and Alters Their Potential to Support Anti-Mesothelioma T Cell Response. Cancers 2020, 12, 1284. [Google Scholar] [CrossRef]
- Kong, F.F.; Qu, Z.Q.; Yuan, H.H.; Wang, J.Y.; Zhao, M.; Guo, Y.H.; Shi, J.; Gong, X.D.; Zhu, Y.L.; Liu, F.; et al. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol. Rep. 2014, 31, 2660–2668. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.I. MicroRNA Control of TGF-β Signaling. Int. J. Mol. Sci. 2018, 19, 1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, E.Y.; Chu, C.S.; Goletz, T.J.; Schlienger, K.; Yeh, H.; Coukos, G.; Rubin, S.C.; Kaiser, L.R.; June, C.H. Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001, 61, 4766–4772. [Google Scholar]
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Cancer-Associated Fibroblasts and Tumor-Associated Ma1rcrophages in Cancer and Cancer Immunotherapy. Front. Oncol. 2021, 11, 668731. [Google Scholar] [CrossRef]
- Song, W.; Mazzieri, R.; Yang, T.; Gobe, G.C. Translational significance for tumor metastasis of tumor-associated macrophages and epithelial-mesenchymal transition. Front. Immunol. 2017, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Gerwin, B.I.; Lechner, J.F.; Reddel, R.R.; Roberts, A.B.; Robbins, K.C.; Gabrielson, E.W.; Harris, C.C. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987, 47, 6180–6184. [Google Scholar] [PubMed]
- DeLong, P.; Carroll, R.G.; Henry, A.C.; Tanaka, T.; Ahmad, S.; Leibowitz, M.S.; Sterman, D.H.; June, C.H.; Albelda, S.M.; Vonderheide, R. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol. 2005, 4, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, D.R.; Peroni, D.J.; Bielefeldt-Ohmann, H. The role of growth factors and cytokines in the tumorigenesis and immunobiology of malignant mesothelioma. Am. J. Respir. Cell Mol. Biol. 1995, 12, 455–460. [Google Scholar] [CrossRef]
- Tamminen, J.A.; Yin, M.; Rönty, M.; Sutinen, E.; Pasternack, A.; Ritvos, O.; Myllärniemi, M.; Koli, K. Overexpression of activin-A and -B in malignant mesothelioma—Attenuated Smad3 signaling responses and ERK activation promote cell migration and invasive growth. Exp. Cell Res. 2015, 332, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Makiko, F.; Takeshi, T.; Hayao, N.; Yasushi, Y.; Ayuko, S.; Yasue, M.; Hidemi, I.; Hideki, M.; Yutaka, K.; Eisaku, K.; et al. TGF-β synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J. Exp. Med. 2012, 209, 479–494. [Google Scholar]
- Yin, M.; Tissari, M.; Tamminen, J.; Ylivinkka, I.; Rönty, M.; von Nandelstadh, P.; Lehti, K.; Hyytiäinen, M.; Myllärniemi, M.; Koli, K. Gremlin-1 is a key regulator of the invasive cell phenotype in mesothelioma. Oncotarget 2017, 8, 98280–98297. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Rezov, V.; Joensuu, E.; Vartiainen, V.; Rönty, M.; Yin, M.; Myllärniemi, M.; Koli, K. Pirfenidone decreases mesothelioma cell proliferation and migration via inhibition of ERK and AKT and regulates mesothelioma tumor microenvironment in vivo. Sci. Rep. 2018, 8, 10070. [Google Scholar] [CrossRef] [PubMed]
- Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int. J. Mol. Sci. 2019, 20, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockhammer, P.; Ploenes, T.; Theegarten, D.; Schuler, M.; Maier, S.; Aigner, C.; Hegedus, B. Detection of TGF-β in pleural effusions for diagnosis and prognosticstratification of malignant pleural mesothelioma. Lung Cancer 2020, 139, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Stockhammer, P.; Ploenes, T.; Schuler, M.; Langer, S.; Aigner, C.; Hegedus, B. 1849P—Pleural effusion TGF-beta is highly diagnostic and prognostic in malignant pleural mesothelioma. Ann. Oncol. 2019, 30, v750–v751. [Google Scholar] [CrossRef]
- Urso, L.; Silic-Benussi, M.; Boscolo, A.; Lorenzi, M.; Bonanno, L.; Lunardi, F.; Guarneri, V.; Calabrese, F.; Rea, F.; Conte, P.; et al. Detection of circulating immunosuppressive cytokines in malignant pleural mesothelioma patients for prognostic stratification. Cytokine 2021, 146, 155622. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Cui, F.J.; Kim, Y. Hydrogen peroxide promotes epithelial to mesenchymal transition and stemness in human malignant mesothelioma cells. Asian Pac. J. Cancer Prev. 2013, 14, 3625–3630. [Google Scholar] [CrossRef] [Green Version]
- Arsalane, K.; Dubois, C.M.; Muanza, T.; Bégin, R.; Boudreau, F.; Asselin, C.; Cantin, A.M. Transforming growth factor-𝛽1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: Transcriptional effect on the GSH rate-limiting enzyme 𝛾-glutamylcysteine synthetase. Am. J. Respir. Cell Mol. Biol. 1997, 17, 599–607. [Google Scholar] [CrossRef]
- Kim, M.C.; Hwang, S.H.; Kim, N.Y.; Lee, H.S.; Ji, S.; Yang, Y.; Kim, Y. Hypoxia promotes acquisition of aggressive phenotypes in human malignant mesothelioma. BMC Cancer 2018, 18, 819. [Google Scholar] [CrossRef]
- Sakai, K.; Inoue, M.; Mikami, S.; Nishimura, H.; Kuwabara, Y.; Kojima, A.; Toda, M.; Ogawa-Kobayashi, Y.; Kikuchi, S.; Hirata, Y.; et al. Functional inhibition of heat shock protein 70 by VER-155008 suppresses pleural mesothelioma cell proliferation via an autophagy mechanism. Thorac. Cancer 2021, 12, 491–503. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Bao, J.; Hao, J.; Peng, Y.; Hong, F. HSP70 inhibits high glucose-induced Smad3 activation and attenuates epithelial-to-mesenchymal transition of peritoneal mesothelial cells. Mol. Med. Rep. 2014, 10, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Park, K.S.; Heo, S.H.; Nam, H.S.; Cho, M.K.; Lee, S.H. Pifithrin-μ induces necroptosis through oxidative mitochondrial damage but accompanies epithelial–mesenchymal transition-like phenomenon in malignant mesothelioma cells under lactic acidosis. Arch. Pharm. Res. 2019, 42, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.S.; Guillon, J.; Petit, C.; Boissard, A.; Franconi, F.; Blandin, S.; Lambot, S.; Grégoire, M.; Verrièle, V.; Nawrocki-Raby, B.; et al. S100A4 Is a Biomarker of Tumorigenesis, EMT, Invasion, and Colonization of Host Organs in Experimental Malignant Mesothelioma. Cancers 2020, 12, 939. [Google Scholar] [CrossRef] [Green Version]
- Birnie, K.A.; Prêle, C.M.; Thompson, P.J.; Badrian, B.; Mutsaers, S.E. Targeting microRNA to improve diagnostic and therapeutic approaches for malignant mesothelioma. Oncotarget 2017, 8, 78193–78207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.S.; Heery, R.; Gray, S.G. In Silico and In Vitro Analyses of LncRNAs as Potential Regulators in the Transition from the Epithelioid to Sarcomatoid Histotype of Malignant Pleural Mesothelioma (MPM). Int. J. Mol. Sci. 2018, 19, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, S.; Nabeshima, K.; Hamasaki, M.; Shibuta, T.; Umemura, T. Upregulation of microRNA-31 associates with a poor prognosis of malignant pleural mesothelioma with sarcomatoid component. Med. Oncol. 2014, 31, 303. [Google Scholar] [CrossRef]
- Farrell, J.; Kelly, C.; Rauch, J.; Kida, K.; García-Muñoz, A.; Monsefi, N.; Turriziani, B.; Doherty, C.; Mehta, J.P.; Matallanas, D.; et al. HGF induces epithelial-to-mesenchymal transition by modulating the mammalian Hippo/MST2 and ISG15 pathways. J. Proteome Res. 2014, 13, 2874–2886. [Google Scholar] [CrossRef]
- Sohn, E.J.; Won, G.; Lee, J.; Yoon, S.W.; Lee, I.; Kim, H.J.; Kim, S.H. Blockage of epithelial to mesenchymal transition and upregulation of let 7b are critically involved in ursolic acid induced apoptosis in malignant mesothelioma cell. Int. J. Biol. Sci. 2016, 12, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Sturchio, E.; Berardinelli, M.G.; Boccia, P.; Zanellato, M.; Gioiosa, S. MicroRNAs diagnostic and prognostic value as predictive markers for malignant mesothelioma. Arch. Environ. Occup. Health 2020, 75, 471–482. [Google Scholar] [CrossRef]
- Reid, G.; Johnson, T.G.; van Zandwijk, N. Manipulating microRNAs for the Treatment of Malignant Pleural Mesothelioma: Past, Present and Future. Front. Oncol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Dijke, P.T. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Harvard, C.; You, L.; Xu, Z.; Kuchenbecker, K.; Baehner, R.; Jablons, D. Stathmin is overexpressed in malignant mesothelioma. Anticancer Res. 2007, 27, 39–44. [Google Scholar] [PubMed]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.P.; Dupre, T.V.; Siskind, L.J.; Beverly, L.J. Common cytotoxic chemotherapeutics induce epithelial-mesenchymal transition (EMT) downstream of ER stress. Oncotarget 2017, 8, 22625–22639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taki, M.; Abiko, K.; Ukita, M.; Murakami, R.; Yamanoi, K.; Yamaguchi, K.; Hamanishi, J.; Baba, T.; Matsumura, N.; Mandai, M. Tumor Immune Microenvironment during Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2021, 27, 4669–4679. [Google Scholar] [CrossRef]
- Chintala, N.K.; Restle, D.; Quach, H.; Saini, J.; Bellis, R.; Offin, M.; Beattie, J.; Adusumilli, P.S. CAR T-cell therapy for pleural mesothelioma: Rationale, preclinical development, and clinical trials. Lung Cancer 2021, 157, 48–59. [Google Scholar] [CrossRef]
- Wirawan, A.; Tajima, K.; Takahashi, F.; Mitsuishi, Y.; Winardi, W.; Hidayat, M.; Hayakawa, D.; Matsumoto, N.; Izumi, K.; Asao, T.; et al. A novel therapeutic strategy targeting the mesenchymal phenotype of malignant pleural mesothelioma by suppressing LSD1. Mol. Cancer Res. 2021, 21, 230. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, P.; Aprile, V.; Ambrogi, M.; Mussi, A.; Lucchi, M. The role of intracavitary therapies in the treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2017, 10, 2. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramundo, V.; Zanirato, G.; Aldieri, E. The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2021, 22, 12216. https://doi.org/10.3390/ijms222212216
Ramundo V, Zanirato G, Aldieri E. The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma. International Journal of Molecular Sciences. 2021; 22(22):12216. https://doi.org/10.3390/ijms222212216
Chicago/Turabian StyleRamundo, Valeria, Giada Zanirato, and Elisabetta Aldieri. 2021. "The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma" International Journal of Molecular Sciences 22, no. 22: 12216. https://doi.org/10.3390/ijms222212216
APA StyleRamundo, V., Zanirato, G., & Aldieri, E. (2021). The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma. International Journal of Molecular Sciences, 22(22), 12216. https://doi.org/10.3390/ijms222212216