Insulin-like Growth Factor Binding Protein-2 (IGFBP2) Is a Key Molecule in the MACC1-Mediated Platelet Communication and Metastasis of Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. The Impact of MACC1 on Tumor-Cell-Induced Platelet Activation
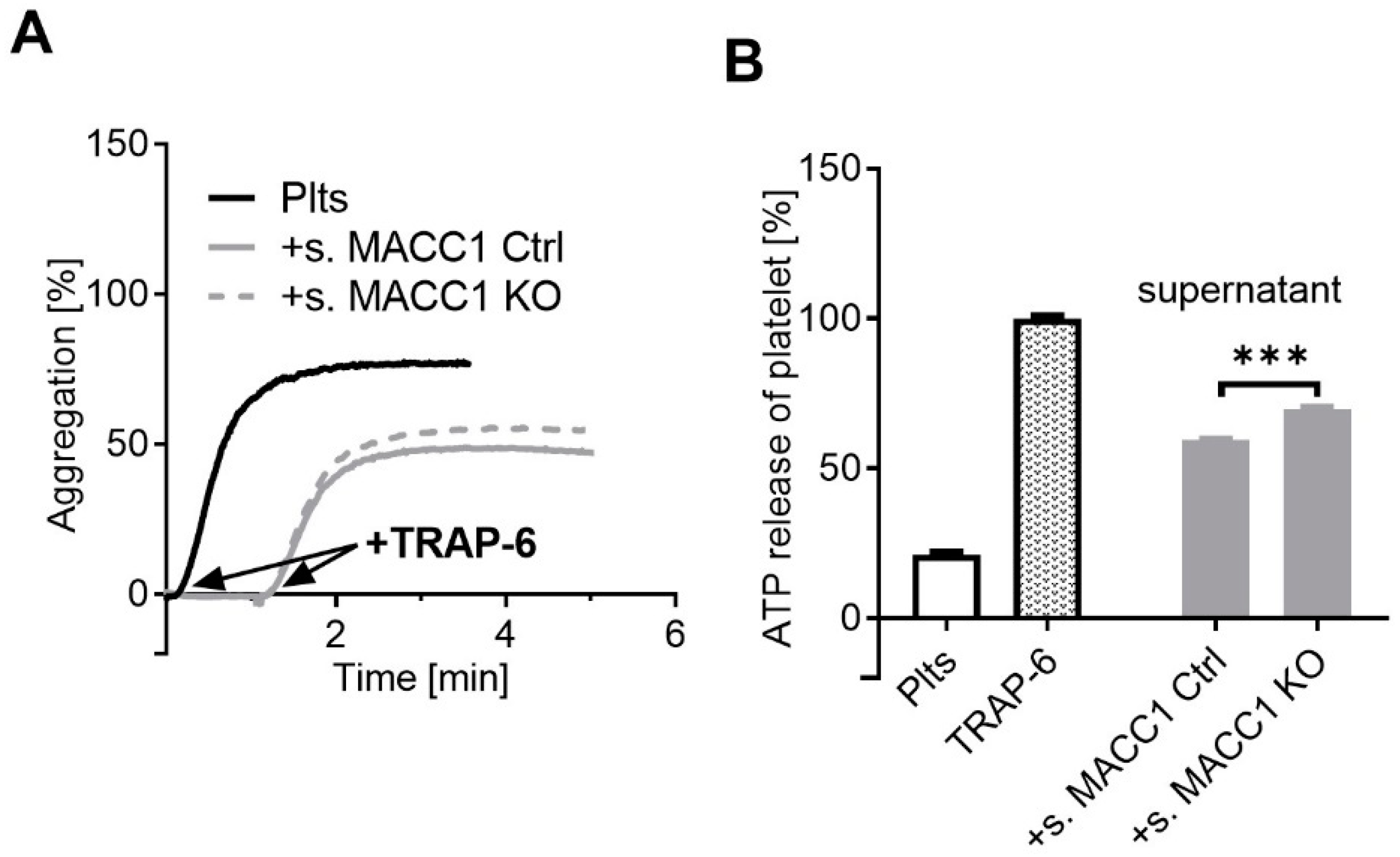
2.2. A Component of the Supernatant Is Responsible for Attenuated Platelet Activation by SW620 Cells
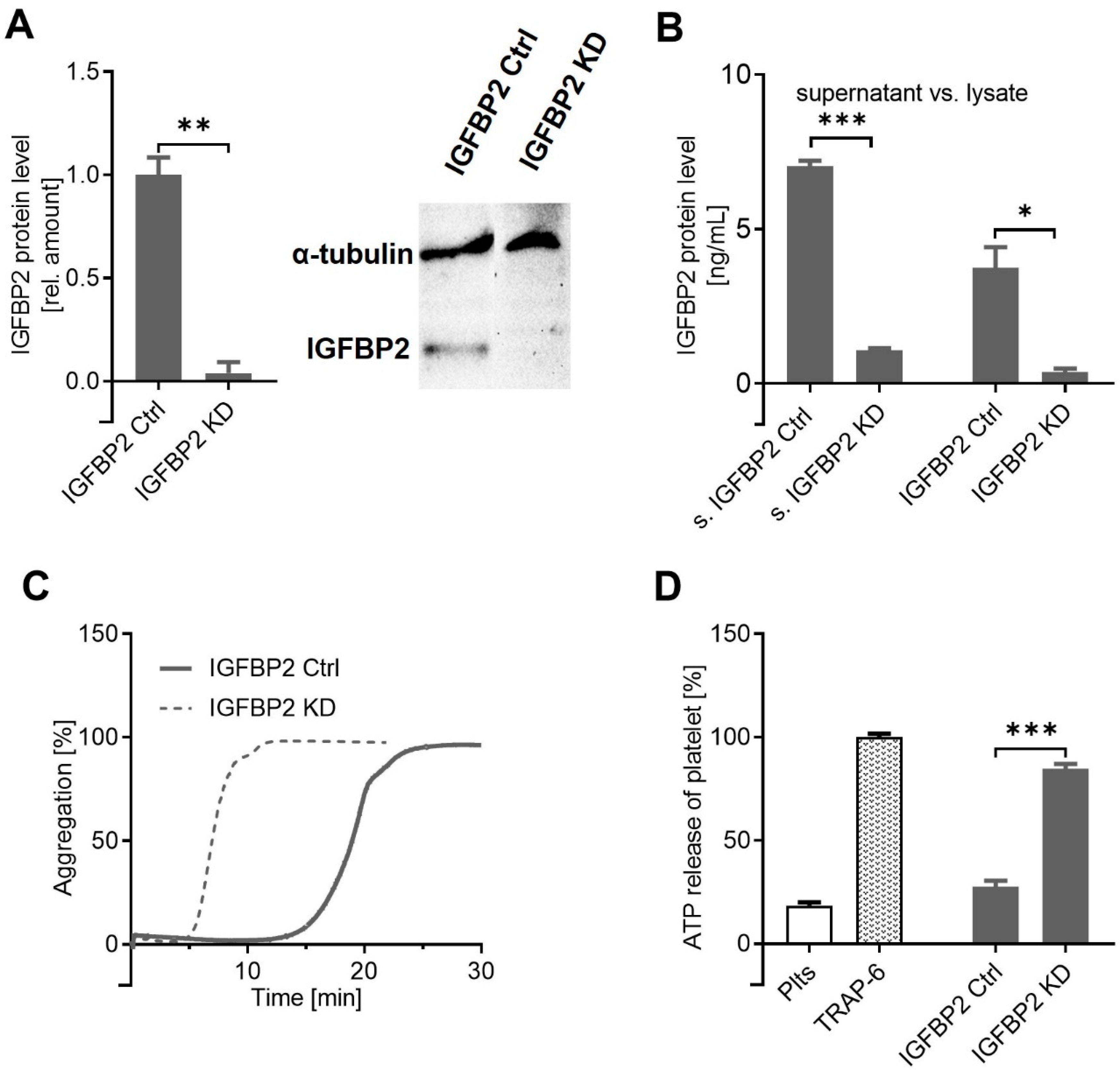
2.3. IGFBP2, Overexpressed by MACC1 Activity, Is Responsible for Attenuated Platelet Activation
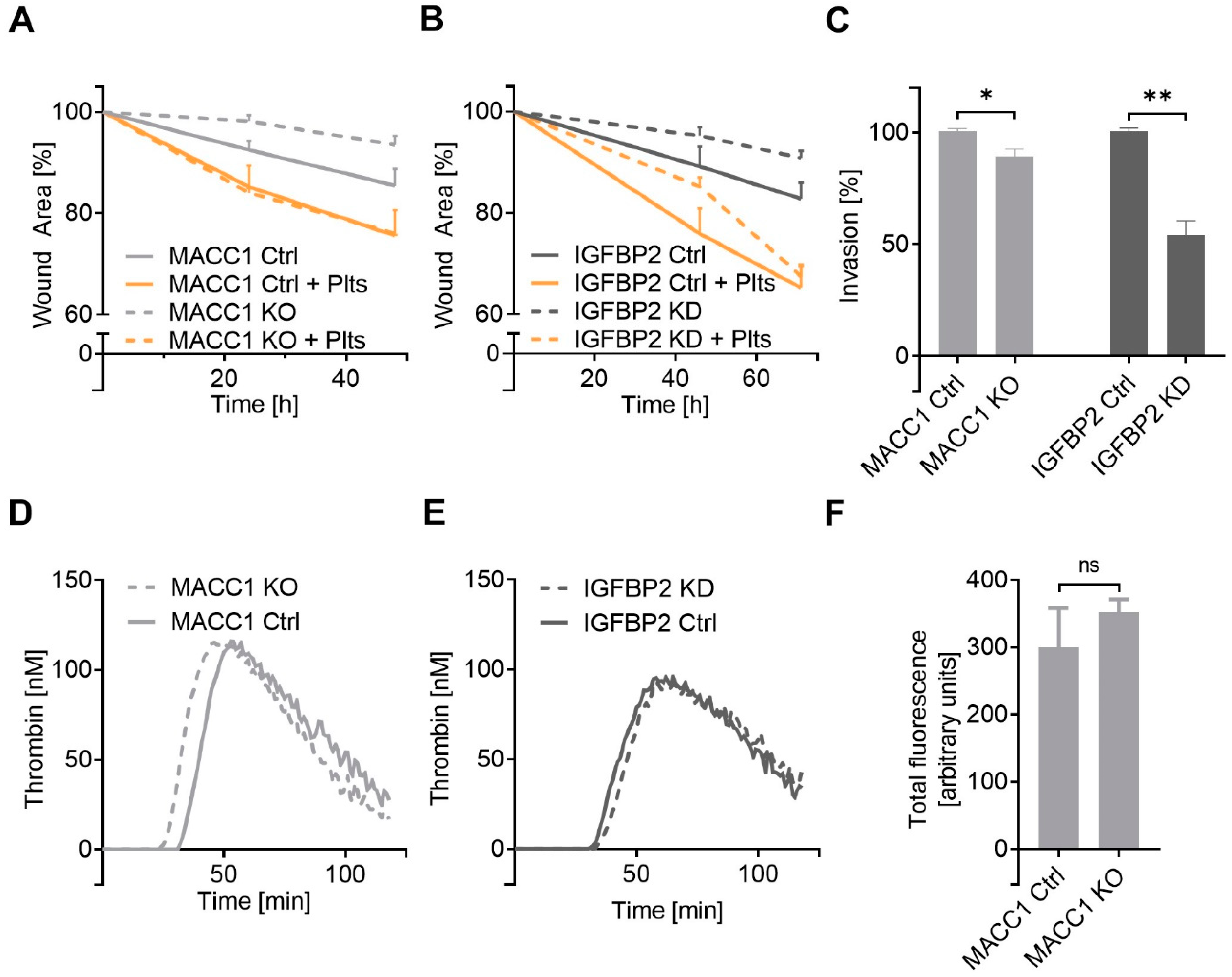
2.4. IGFBP2 Is a Functional Downstream Component of MACC1 with Impact on Cell Dynamics
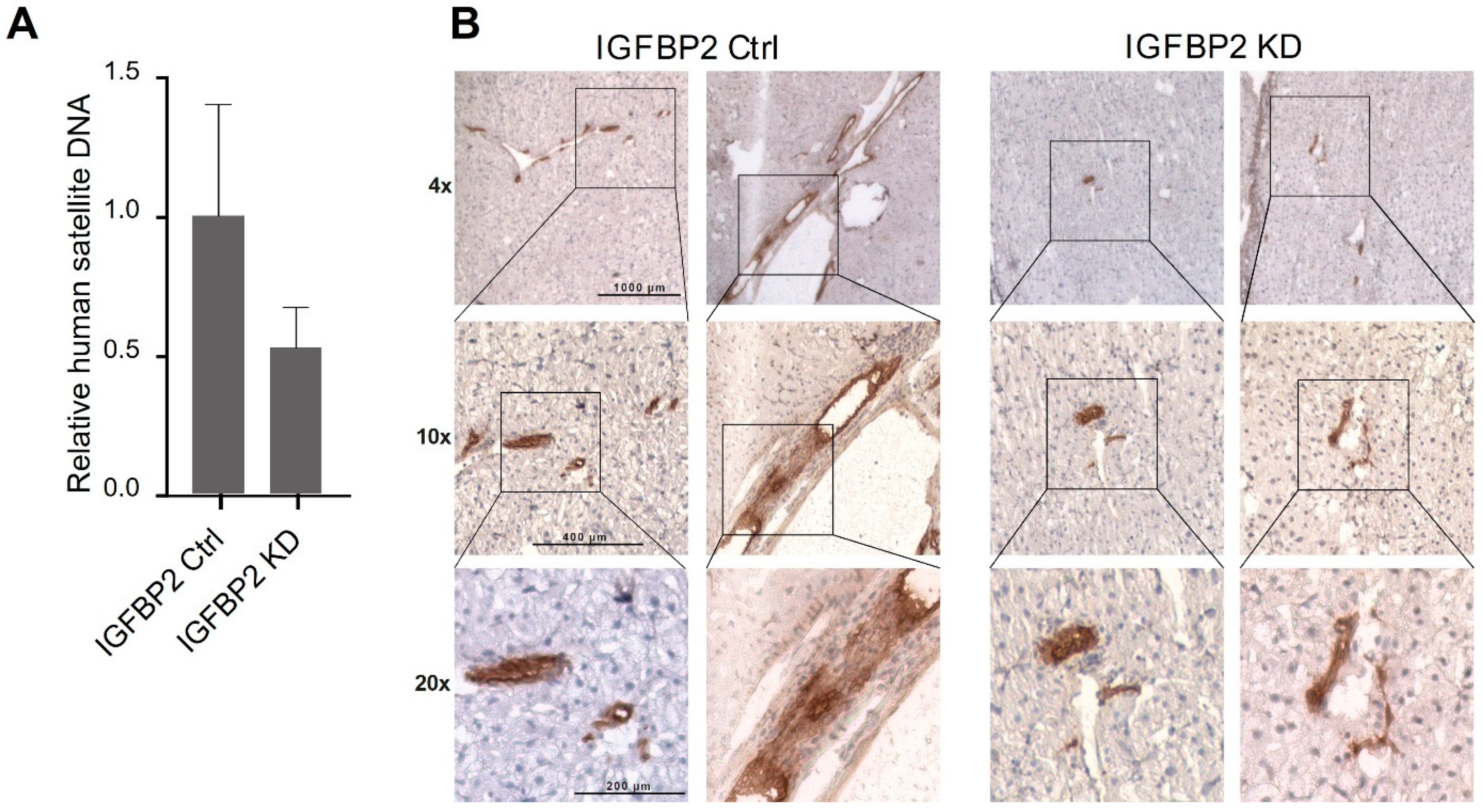
2.5. IGFBP2 Is the Metastatic Factor In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Preparation of Washed Platelets
4.3. Light Transmission Aggregometry
4.4. Platelet Dense Granule Secretion Assay
4.5. Platelet Tumor Cell Adhesion Assay
4.6. Reverse Transcription and qRT-PCR
4.7. Western Blot
4.8. Enzym-Linked Immunosorbent Assay
4.9. Flow Cytometry
4.10. Thrombin Generation Assay
4.11. Cell Migration Assay
4.12. Cell Invasion Assay
4.13. Genome-Wide Expression Analysis
4.14. Immunohistochemistry
4.15. Animal Experiments
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, U.; Walther, W.; Arlt, F.; Schwabe, H.; Smith, J.; Fichtner, I.; Birchmeier, W.; Schlag, P.M. MACC1, a Newly Identified Key Regulator of HGF-MET Signaling, Predicts Colon Cancer Metastasis. Nat. Med. 2009, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, H.; Walther, W.; Zincke, F.; Kobelt, D.; Imbastari, F.; Erdem, M.; Kortüm, B.; Dahlmann, M.; Stein, U. MACC1—The First Decade of a Key Metastasis Molecule from Gene Discovery to Clinical Translation. Cancer Metastasis Rev. 2018, 37, 805–820. [Google Scholar] [CrossRef]
- Dahlmann, M.; Werner, R.; Kortüm, B.; Kobelt, D.; Walther, W.; Stein, U. Restoring Treatment Response in Colorectal Cancer Cells by Targeting MACC1-Dependent ABCB1 Expression in Combination Therapy. Front. Oncol. 2020, 10, 599. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, H.; Ilm, K.; Walther, W.; Shirasawa, S.; Sasazuki, T.; Daniel, P.T.; Gillissen, B.; Stein, U. MACC1 Regulates Fas Mediated Apoptosis through STAT1/3—Mcl-1 Signaling in Solid Cancers. Cancer Lett. 2017, 403, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.; Wang, Q.; Huska, M.R.; Andrade-Navarro, M.A.; Lemm, M.; Fichtner, I.; Dahlmann, M.; Kobelt, D.; Walther, W.; Smith, J.; et al. SPON2, a Newly Identified Target Gene of MACC1, Drives Colorectal Cancer Metastasis in Mice and Is Prognostic for Colorectal Cancer Patient Survival. Oncogene 2016, 35, 5942–5952. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Herrmann, P.; Zlobec, I.; Karamitopoulou, E.; Lugli, A.; Stein, U. Heterogeneity Analysis of Metastasis Associated in Colon Cancer 1 (MACC1) for Survival Prognosis of Colorectal Cancer Patients: A Retrospective Cohort Study. BMC Cancer 2015, 15, 160. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, T.; Hohmann, U.; Kolbe, M.R.; Dahlmann, M.; Kobelt, D.; Stein, U.; Dehghani, F. MACC1 Driven Alterations in Cellular Biomechanics Facilitate Cell Motility in Glioblastoma. Cell Commun. Signal. 2020, 18, 85. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of Platelets to Tumour Metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Hynes, R.O. The Initial Hours of Metastasis: The Importance of Cooperative Host-Tumor Cell Interactions during Hematogenous Dissemination. Cancer Discov. 2012, 2, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Gay, L.J.; Felding-Habermann, B. Platelets Alter Tumor Cell Attributes to Propel Metastasis: Programming in Transit. Cancer Cell 2011, 20, 553–554. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, M. Role of Platelets and Platelet Receptors in Cancer Metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef]
- Maurer, S.; Kropp, K.N.; Klein, G.; Steinle, A.; Haen, S.P.; Walz, J.S.; Hinterleitner, C.; Märklin, M.; Kopp, H.-G.; Salih, H.R. Platelet-Mediated Shedding of NKG2D Ligands Impairs NK Cell Immune-Surveillance of Tumor Cells. Oncoimmunology 2018, 7, e1364827. [Google Scholar] [CrossRef] [Green Version]
- Kopp, H.-G.; Placke, T.; Salih, H.R. Platelet-Derived Transforming Growth Factor-Beta down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placke, T.; Örgel, M.; Schaller, M.; Jung, G.; Rammensee, H.-G.; Kopp, H.-G.; Salih, H.R. Platelet-Derived MHC Class I Confers a Pseudonormal Phenotype to Cancer Cells That Subverts the Antitumor Reactivity of Natural Killer Immune Cells. Cancer Res. 2012, 72, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets Guide the Formation of Early Metastatic Niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, R.J.; Boehme, B.; Podda, M.; Henschler, R.; Jager, E.; Tandi, C.; Boehncke, W.-H.; Zollner, T.M.; Kaufmann, R.; Gille, J. Endothelial P-Selectin as a Target of Heparin Action in Experimental Melanoma Lung Metastasis. Cancer Res. 2004, 64, 2743–2750. [Google Scholar] [CrossRef] [Green Version]
- Grossi, I.M.; Fitzgerald, L.A.; Kendall, A.; Taylor, J.D.; Sloane, B.F.; Honn, K.V. Inhibition of Human Tumor Cell Induced Platelet Aggregation by Antibodies to Platelet Glycoproteins Ib and IIb/IIIa. Proc. Soc. Exp. Biol. Med. 1987, 186, 378–383. [Google Scholar] [CrossRef]
- Bastida, E.; Almirall, L.; Ordinas, A. Tumor-Cell-Induced Platelet Aggregation Is a Glycoprotein-Dependent and Lipoxygenase-Associated Process. Int. J. Cancer 1987, 39, 760–763. [Google Scholar] [CrossRef]
- Schumacher, D.; Strilic, B.; Sivaraj, K.K.; Wettschureck, N.; Offermanns, S. Platelet-Derived Nucleotides Promote Tumor-Cell Transendothelial Migration and Metastasis via P2Y2 Receptor. Cancer Cell 2013, 24, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Clezardin, P.; Drouin, J.; Morel-Kopp, M.C.; Hanss, M.; Kehrel, B.; Serre, C.M.; Kaplan, C.; Delmas, P.D. Role of Platelet Membrane Glycoproteins Ib/IX and IIb/IIIa, and of Platelet Alpha-Granule Proteins in Platelet Aggregation Induced by Human Osteosarcoma Cells. Cancer Res. 1993, 53, 4695–4700. [Google Scholar]
- Battinelli, E.M.; Markens, B.A.; Italiano, J.E. Release of Angiogenesis Regulatory Proteins from Platelet Alpha Granules: Modulation of Physiologic and Pathologic Angiogenesis. Blood 2011, 118, 1359–1369. [Google Scholar] [CrossRef]
- Janowska-Wieczorek, A.; Wysoczynski, M.; Kijowski, J.; Marquez-Curtis, L.; Machalinski, B.; Ratajczak, J.; Ratajczak, M.Z. Microvesicles Derived from Activated Platelets Induce Metastasis and Angiogenesis in Lung Cancer. Int. J. Cancer 2005, 113, 752–760. [Google Scholar] [CrossRef]
- Janowska-Wieczorek, A.; Marquez-Curtis, L.A.; Wysoczynski, M.; Ratajczak, M.Z. Enhancing Effect of Platelet-Derived Microvesicles on the Invasive Potential of Breast Cancer Cells. Transfusion 2006, 46, 1199–1209. [Google Scholar] [CrossRef]
- Renehan, A.G.; Jones, J.; Potten, C.S.; Shalet, S.M.; O’Dwyer, S.T. Elevated Serum Insulin-like Growth Factor (IGF)-II and IGF Binding Protein-2 in Patients with Colorectal Cancer. Br. J. Cancer 2000, 83, 1344–1350. [Google Scholar] [CrossRef]
- Yao, X.; Sun, S.; Zhou, X.; Guo, W.; Zhang, L. IGF-Binding Protein 2 Is a Candidate Target of Therapeutic Potential in Cancer. Tumour Biol. 2016, 37, 1451–1459. [Google Scholar] [CrossRef]
- Russo, V.C.; Azar, W.J.; Yau, S.W.; Sabin, M.A.; Werther, G.A. IGFBP-2: The Dark Horse in Metabolism and Cancer. Cytokine Growth Factor Rev. 2015, 26, 329–346. [Google Scholar] [CrossRef]
- Pereira, J.J.; Meyer, T.; Docherty, S.E.; Reid, H.H.; Marshall, J.; Thompson, E.W.; Rossjohn, J.; Price, J.T. Bimolecular Interaction of Insulin-Like Growth Factor (IGF) Binding Protein-2 with Avβ3 Negatively Modulates IGF-I-Mediated Migration and Tumor Growth 1. Cancer Res. 2004, 64, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, L.; Chen, H.; Kong, R.; Pan, S.; Hu, J.; Wang, Y.; Li, Y.; Sun, B. Silencing IGFBP-2 Decreases Pancreatic Cancer Metastasis and Enhances Chemotherapeutic Sensitivity. Oncotarget 2017, 8, 61674–61686. [Google Scholar] [CrossRef] [Green Version]
- Al Qahtani, A.; Holly, J.; Perks, C. Hypoxia Negates Hyperglycaemia-Induced Chemo-Resistance in Breast Cancer Cells: The Role of Insulin-like Growth Factor Binding Protein 2. Oncotarget 2017, 8, 74635–74648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Zhang, Y.; Geng, Y.; Lu, W.; Yin, J.; Li, Z.; Huang, L.; Liu, H.; Xu, N. IGFBP2 Promotes the EMT of Colorectal Cancer Cells by Regulating E-Cadherin Expression. Int. J. Clin. Exp. Pathol. 2019, 12, 2559–2565. [Google Scholar] [PubMed]
- Cheriyamundath, S.; Ben-Ze’ev, A. Wnt/β-Catenin Target Genes in Colon Cancer Metastasis: The Special Case of L1CAM. Cancers 2020, 12, 3444. [Google Scholar] [CrossRef] [PubMed]
- Hers, I. Insulin-like Growth Factor-1 Potentiates Platelet Activation via the IRS/PI3Kalpha Pathway. Blood 2007, 110, 4243–4252. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hers, I. Insulin/IGF-1 Hybrid Receptor Expression on Human Platelets: Consequences for the Effect of Insulin on Platelet Function. J. Thromb. Haemost. 2009, 7, 2123–2130. [Google Scholar] [CrossRef]
- Kim, S.; Garcia, A.; Jackson, S.P.; Kunapuli, S.P. Insulin-like Growth Factor-1 Regulates Platelet Activation through PI3-Kalpha Isoform. Blood 2007, 110, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Forbes, M.E.; Fuller, G.N.; Li, J.; Yang, X.; Zhang, W. IGFBP2: Integrative Hub of Developmental and Oncogenic Signaling Network. Oncogene 2020, 39, 2243–2257. [Google Scholar] [CrossRef]
- Perks, C.M.; Vernon, E.G.; Rosendahl, A.H.; Tonge, D.; Holly, J.M.P. IGF-II and IGFBP-2 Differentially Regulate PTEN in Human Breast Cancer Cells. Oncogene 2007, 26, 5966–5972. [Google Scholar] [CrossRef] [Green Version]
- Mehrian-Shai, R.; Chen, C.D.; Shi, T.; Horvath, S.; Nelson, S.F.; Reichardt, J.K.V.; Sawyers, C.L. Insulin Growth Factor-Binding Protein 2 Is a Candidate Biomarker for PTEN Status and PI3K/Akt Pathway Activation in Glioblastoma and Prostate Cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 5563–5568. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Sun, Y.; Zhang, X.; Hu, L.; Liu, Y.; Chua, C.Y.; Phillips, L.M.; Ren, H.; Fleming, J.B.; Wang, H.; et al. IGFBP2 Activates the NF-ΚB Pathway to Drive Epithelial-Mesenchymal Transition and Invasive Character in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2016, 76, 6543–6554. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.-S.; Huang, C.-Y.; Lee, C.-H.; Chen, W.-Y.; Huang, M.-T.; Wei, P.-L.; Chang, Y.-J. IGFBP2 Plays an Important Role in Heat Shock Protein 27-Mediated Cancer Progression and Metastasis. Oncotarget 2017, 8, 54978–54992. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shmuel, A.; Shvab, A.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. Global Analysis of L1-Transcriptomes Identified IGFBP-2 as a Target of Ezrin and NF-ΚB Signaling That Promotes Colon Cancer Progression. Oncogene 2013, 32, 3220–3230. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, S.; Schlesinger, M.; Bendas, G. Detection of Tumor Cell-Induced Platelet Aggregation and Granule Secretion. Methods Mol. Biol. 2021, 2294, 181–195. [Google Scholar] [CrossRef]
- Stein, U.; Arlt, F.; Smith, J.; Sack, U.; Herrmann, P.; Walther, W.; Lemm, M.; Fichtner, I.; Shoemaker, R.H.; Schlag, P.M. Intervening in β-Catenin Signaling by Sulindac Inhibits S100A4-Dependent Colon Cancer Metastasis. Neoplasia 2011, 13, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Nitsche, A.; Neumann, C.; Aumann, J.; Junghahn, I.; Fichtner, I. Sensitive PCR Method for the Detection and Real-Time Quantification of Human Cells in Xenotransplantation Systems. Br. J. Cancer 2002, 87, 1328–1335. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haschemi, R.; Kobelt, D.; Steinwarz, E.; Schlesinger, M.; Stein, U.; Bendas, G. Insulin-like Growth Factor Binding Protein-2 (IGFBP2) Is a Key Molecule in the MACC1-Mediated Platelet Communication and Metastasis of Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 12195. https://doi.org/10.3390/ijms222212195
Haschemi R, Kobelt D, Steinwarz E, Schlesinger M, Stein U, Bendas G. Insulin-like Growth Factor Binding Protein-2 (IGFBP2) Is a Key Molecule in the MACC1-Mediated Platelet Communication and Metastasis of Colorectal Cancer Cells. International Journal of Molecular Sciences. 2021; 22(22):12195. https://doi.org/10.3390/ijms222212195
Chicago/Turabian StyleHaschemi, Reza, Dennis Kobelt, Elisabeth Steinwarz, Martin Schlesinger, Ulrike Stein, and Gerd Bendas. 2021. "Insulin-like Growth Factor Binding Protein-2 (IGFBP2) Is a Key Molecule in the MACC1-Mediated Platelet Communication and Metastasis of Colorectal Cancer Cells" International Journal of Molecular Sciences 22, no. 22: 12195. https://doi.org/10.3390/ijms222212195
APA StyleHaschemi, R., Kobelt, D., Steinwarz, E., Schlesinger, M., Stein, U., & Bendas, G. (2021). Insulin-like Growth Factor Binding Protein-2 (IGFBP2) Is a Key Molecule in the MACC1-Mediated Platelet Communication and Metastasis of Colorectal Cancer Cells. International Journal of Molecular Sciences, 22(22), 12195. https://doi.org/10.3390/ijms222212195







