Vascular Polyurethane Prostheses Modified with a Bioactive Coating—Physicochemical, Mechanical and Biological Properties
Abstract
:1. Introduction
2. Results
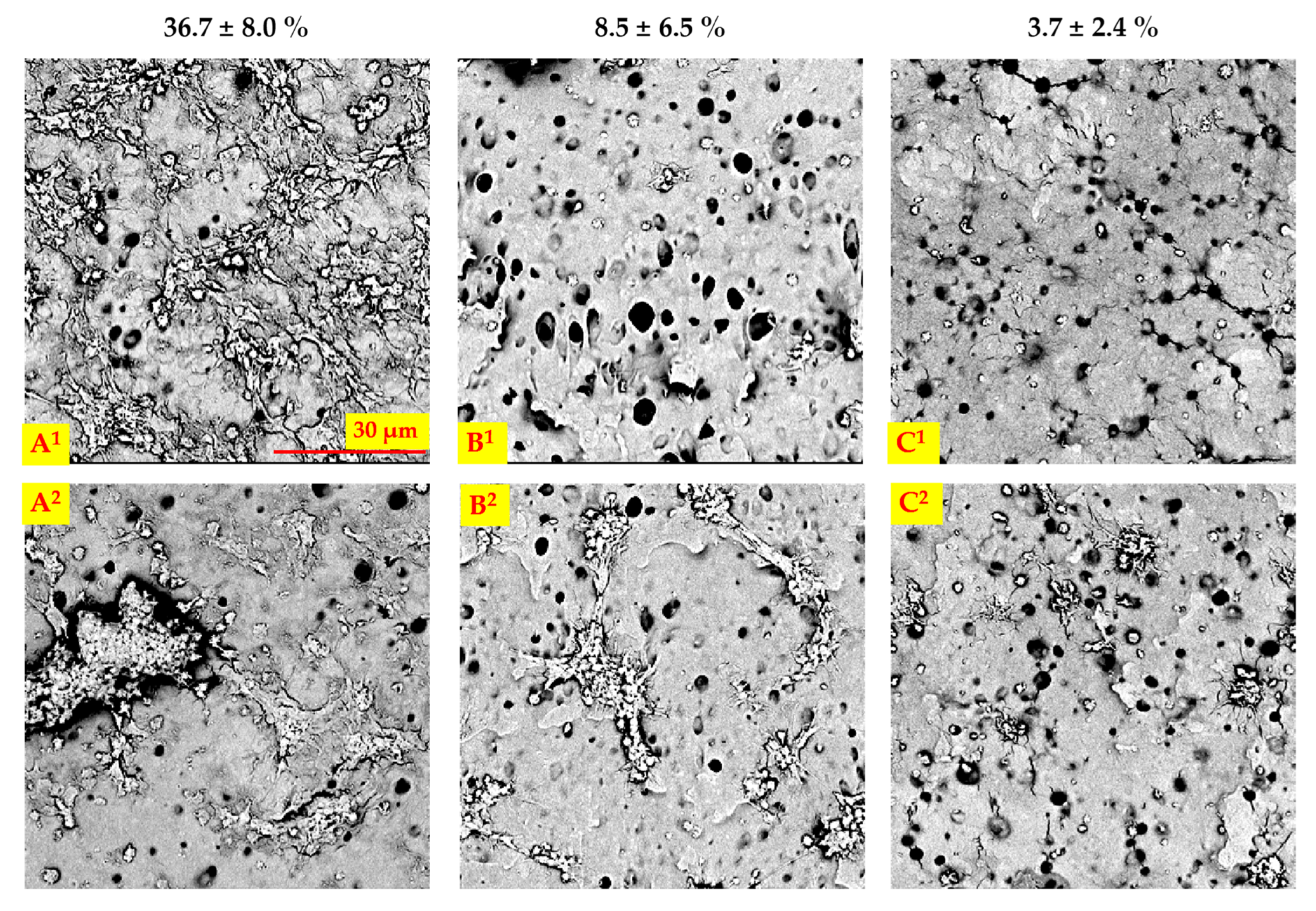
2.1. Surface Morphology
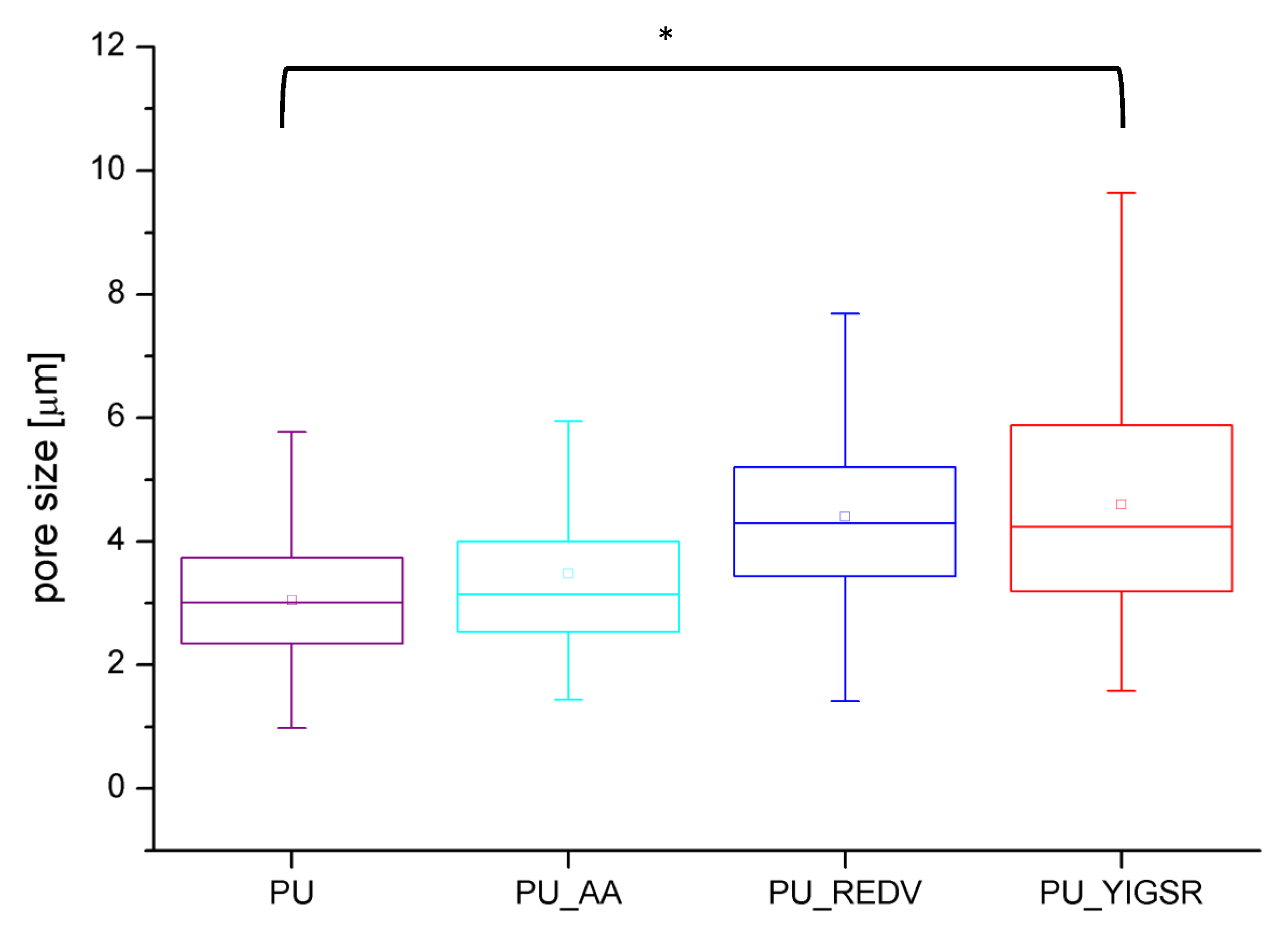
2.2. Porosity
2.3. Mechanical Testing
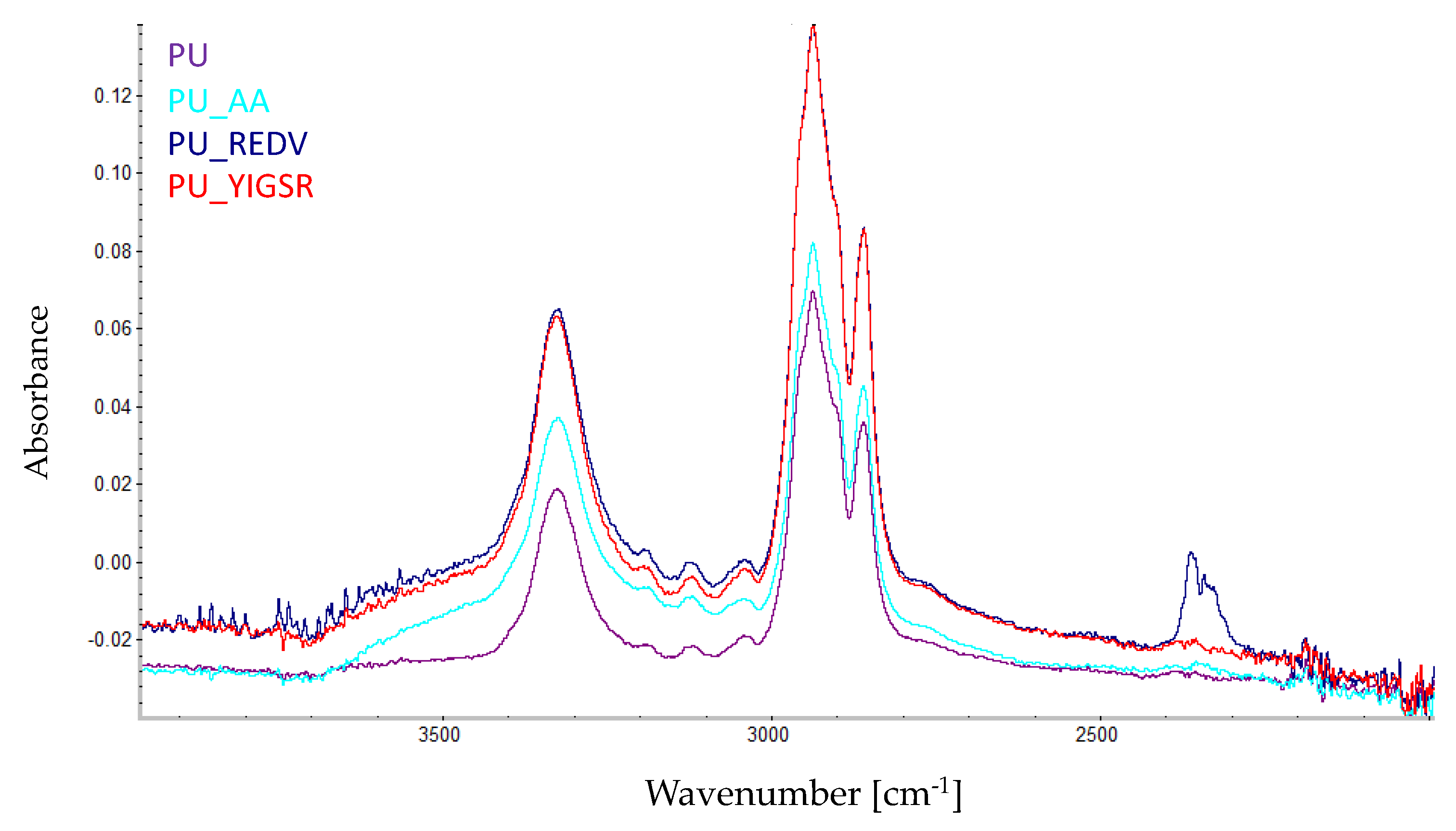
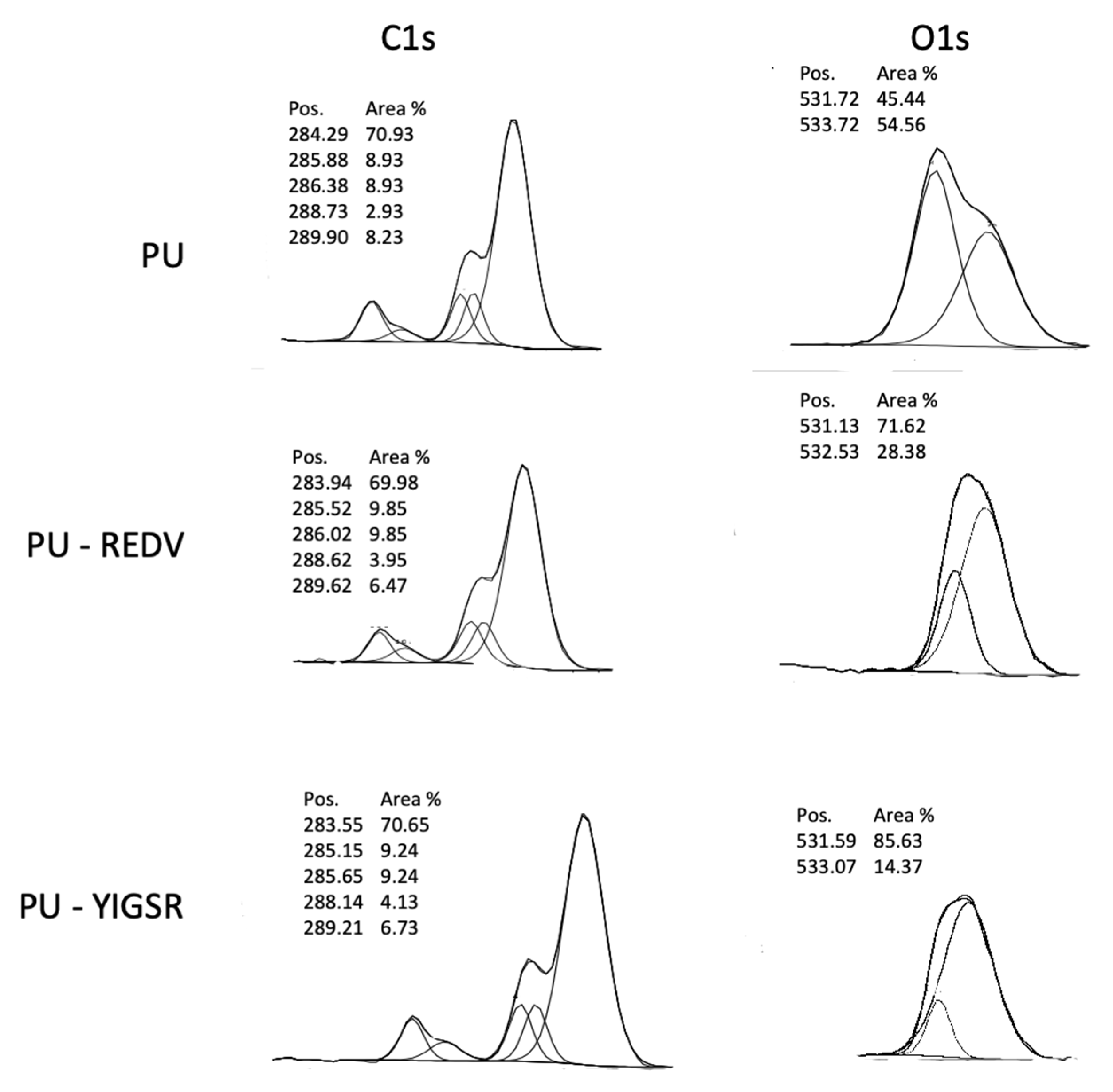
2.4. Chemical Characterization
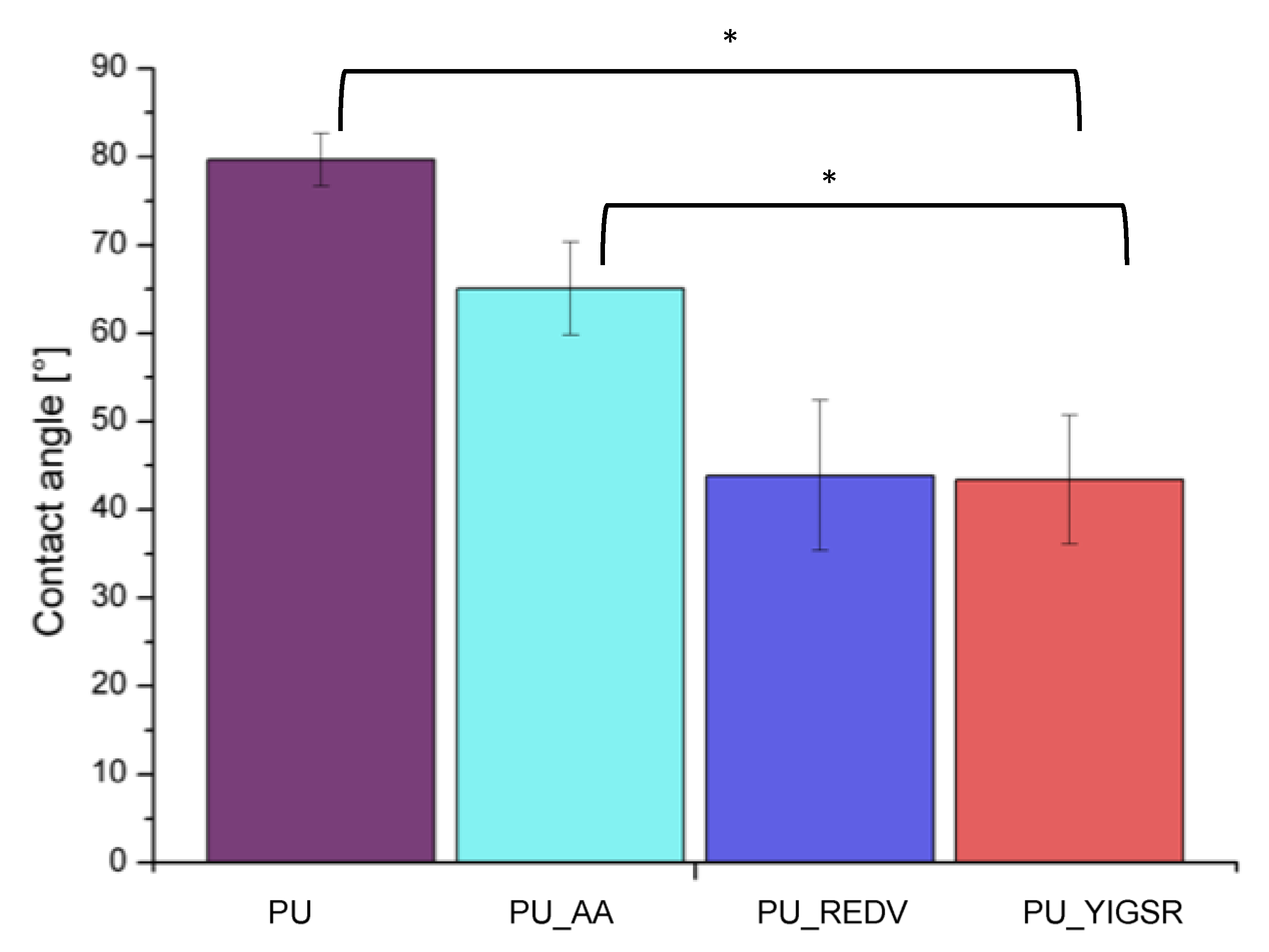
2.5. Water Contact Angle
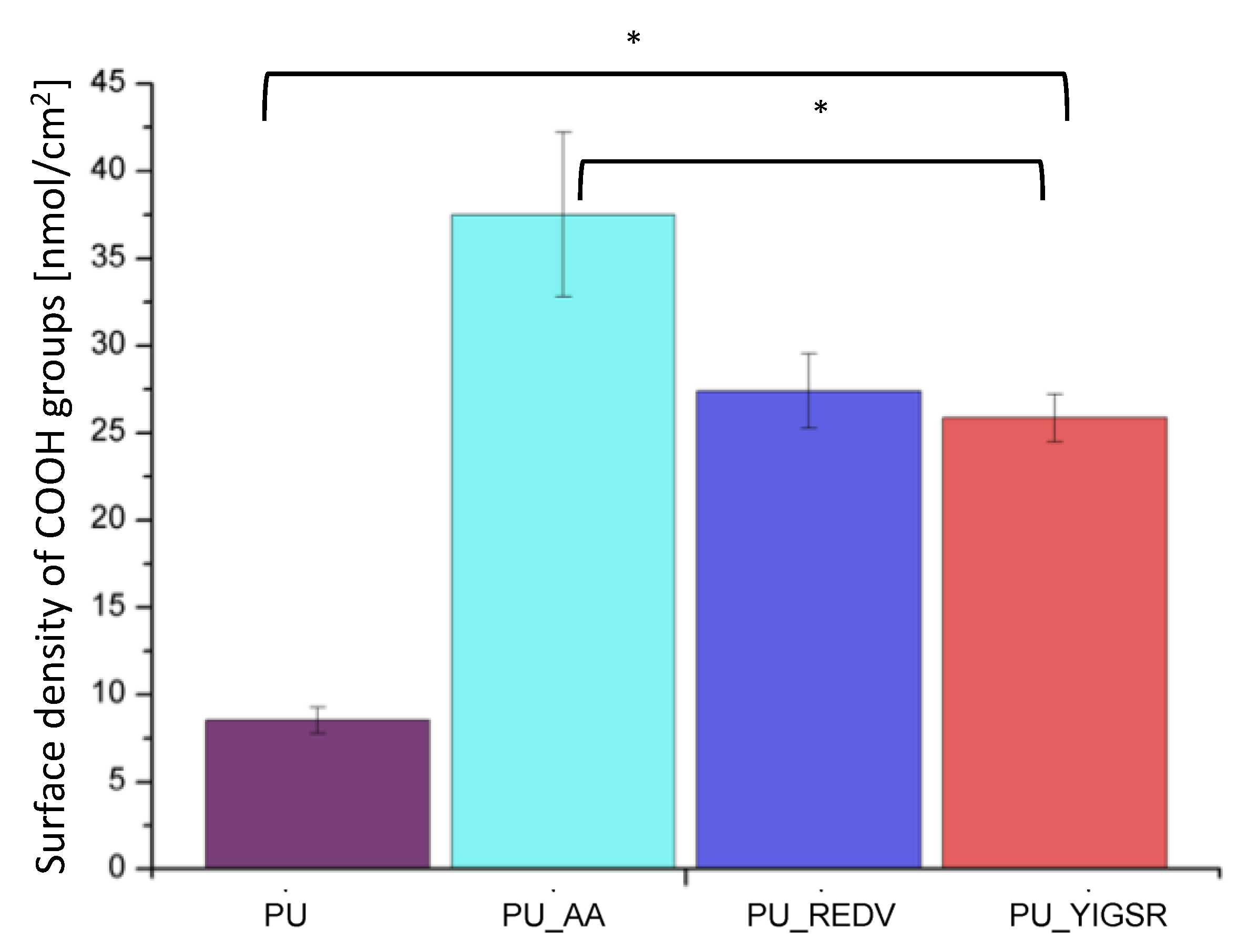
2.6. COOH Group Determination
2.7. Cytotoxicity
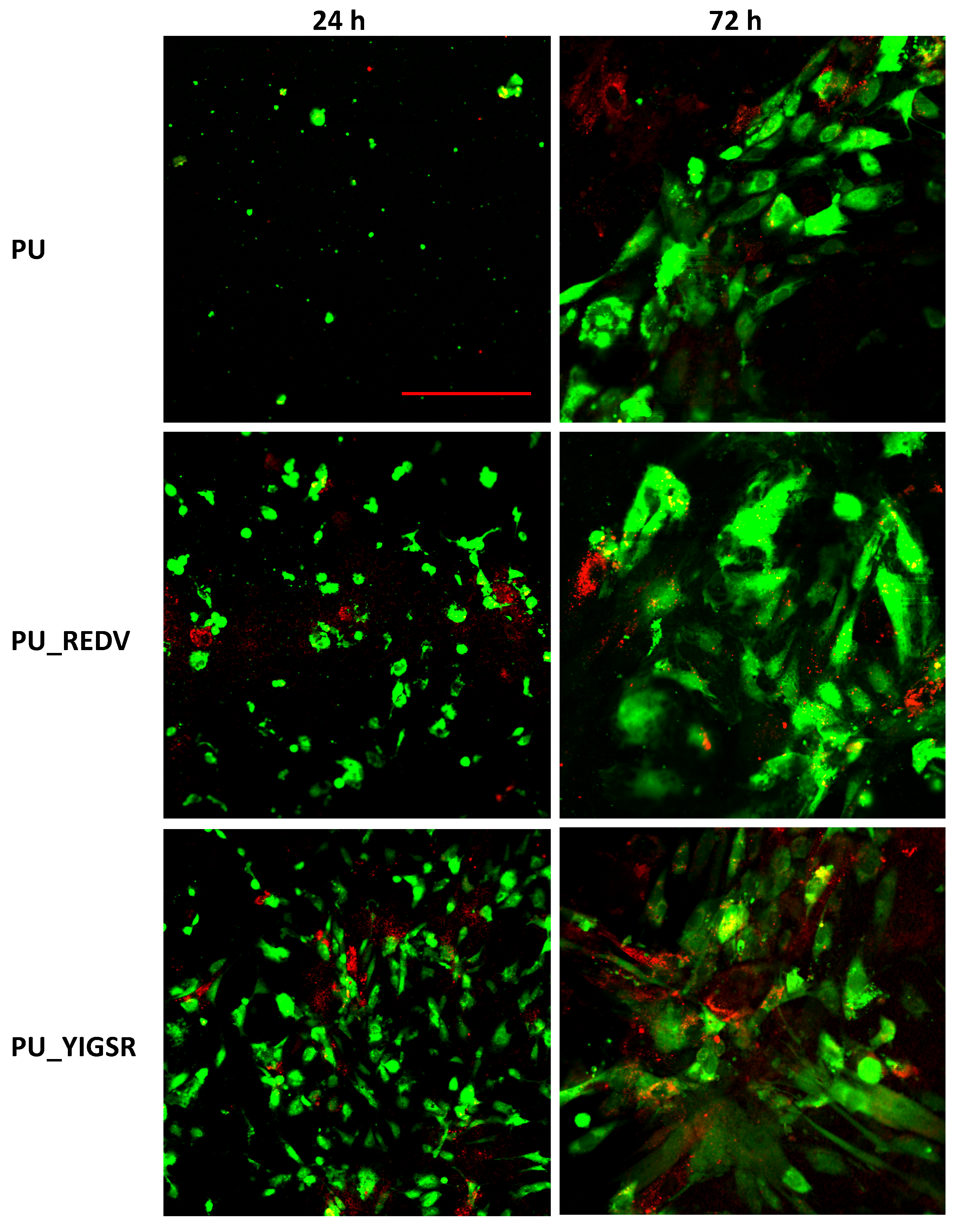
2.8. Endothelial and Smooth Muscle Cell Co-Culture
2.9. Blood-Material Interaction
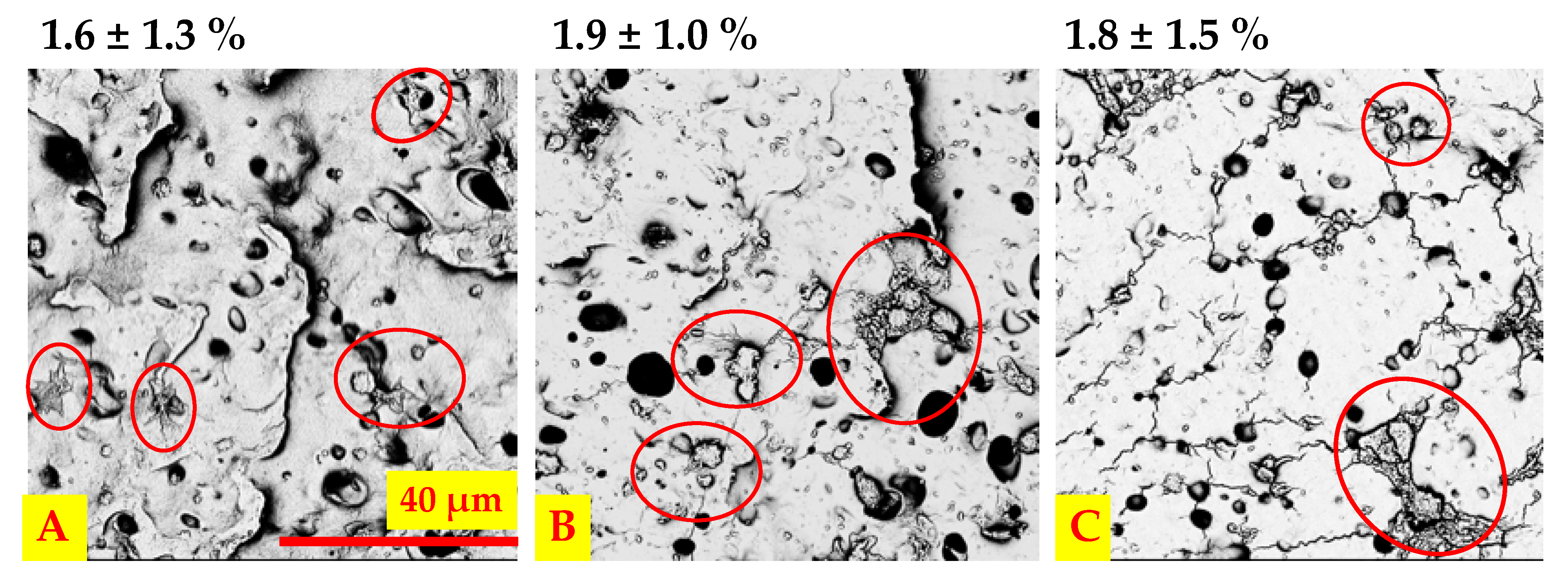
2.9.1. Platelet Adhesion and Activation under Static and Dynamic Conditions
2.9.2. Whole Blood Clotting Time
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Materials Preparation
4.2.1. Preparation of Polyurethane Scaffolds
4.2.2. Surface Modification
4.3. Physicochemical Properties
4.3.1. Surface Morphology
4.3.2. Chemical Characterization
4.3.3. Wettability
4.3.4. Carboxyl Groups Determination
4.4. Porosity
4.5. Mechanical Testing
4.6. Cytotoxicity
4.7. Endothelial and Smooth Muscle Cell Co-Culture
4.8. Interaction with Blood
4.8.1. Blood-Material Interaction
4.8.2. Whole Blood Clotting Time
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, E139–E596. [Google Scholar] [PubMed]
- Parikh, V.; Kadiwala, J.; Hidalgo Bastida, A.; Holt, C.; Sanami, M.; Miraftab, M.; Shakur, R.; Azzawi, M. Small diameter helical vascular scaffolds support endothelial cell survival. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric scaffolds for cardiac tissue engineering: Requirements and fabrication technologies. Polym. Int. 2014, 63, 2–11. [Google Scholar] [CrossRef]
- Yu, K.; Mei, Y.; Hadjesfandiari, N.; Kizhakkedathu, J.N. Engineering Biomaterials Surfaces to Modulate the Host Response. Colloids Surf. B Biointerfaces 2014. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25193153 (accessed on 10 August 2021).
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Zhang, C.; Cheng, M.; Huang, J.; Liu, Q.; Yuan, G.; Lin, K.; Yu, H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact. Mater. 2021, 6, 1791–1809. [Google Scholar] [CrossRef]
- Patterson, J.; Martino, M.M.; Hubbell, J.A. Biomimetic materials in tissue engineering. Mater. Today 2010, 13, 14–22. [Google Scholar] [CrossRef]
- Adipurnama, I.; Yang, M.C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2017, 5, 22–37. [Google Scholar] [CrossRef]
- Qi, P.; Maitz, M.F.; Huang, N. Surface modification of cardiovascular materials and implants. Surf. Coat. Technol. 2013, 233, 80–90. [Google Scholar] [CrossRef]
- Wu, J.; Hu, C.; Tang, Z.; Yu, Q.; Liu, X.; Chen, H. Tissue-engineered Vascular Grafts: Balance of the Four Major Requirements. Colloids Interface Sci. Commun. 2018, 23, 34–44. [Google Scholar] [CrossRef]
- Clauser, J.; Gester, K.; Steinseifer, U.; Sonntag, S.J. Regulating Blood Cell Adhesion via Surface Modification of Polyurethanes; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 287–318. [Google Scholar]
- Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 2019, 99, 53–71. [Google Scholar] [CrossRef]
- Navas-Gómez, K.; Valero, M.F. Why polyurethanes have been used in the manufacture and design of cardiovascular devices: A systematic review. Materials 2020, 13, 3250. [Google Scholar] [CrossRef]
- Ward, R.S.; Jones, R.L. Polyurethanes and Silicone Polyurethane Copolymers. Vol. 1, Comprehensive Biomaterials II; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 570–619. [Google Scholar]
- Knetsch, M.L.W.; Koole, L.H. VEGF-E enhances endothelialization and inhibits thrombus formation on polymeric surfaces. J. Biomed. Mater. Res.–Part A 2010, 93, 77–85. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12922148 (accessed on 8 August 2021). [CrossRef]
- Gupta, B.; Plummer, C.; Bisson, I.; Frey, P.; Hilborn, J. Plasma-induced graft polymerization of acrylic acid onto poly(ethylene terephthalate) films: Characterization and human smooth muscle cell growth on grafted films. Biomaterials 2002, 23, 863–871. [Google Scholar] [CrossRef]
- Lopez, L.C.; Gristina, R.; Ceccone, G.; Rossi, F.; Favia, P.; d’Agostino, R. Immobilization of RGD peptides on stable plasma-deposited acrylic acid coatings for biomedical devices. Surf. Coat. Technol. 2005, 200, 1000–1004. [Google Scholar] [CrossRef]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C 2017, 80, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, L.; Ren, X.K.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Co-immobilization of ACH11 antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization. Acta Biomater. 2019, 97, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Heilshorn, S.C. Designing ECM-mimetic materials using protein engineering. Acta Biomater. 2014, 10, 1751–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devalliere, J.; Chen, Y.; Dooley, K.; Yarmush, M.L.; Uygun, B.E. Improving functional re-endothelialization of acellular liver scaffold using REDV cell-binding domain. Acta Biomater. 2018, 78, 151–164. [Google Scholar] [CrossRef]
- Kanie, K.; Narita, Y.; Zhao, Y.; Kuwabara, F.; Satake, M.; Honda, S.; Kaneko, H.; Yoshioka, T.; Okochi, M.; Honda, H.; et al. Collagen type IV-specific tripeptides for selective adhesion of endothelial and smooth muscle cells. Biotechnol. Bioeng. 2012, 109, 1808–1816. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22359201 (accessed on 5 August 2021). [CrossRef]
- Munisso, M.C.; Yamaoka, T. Novel peptides for small-caliber graft functionalization selected by a phage display of endothelial-positive/platelet-negative combined selection. J. Mater. Chem. B 2013, 5, 9354–9364. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Yao, D.; Niu, Y.; Liu, H.; Fan, Y. Surface Modification of Multiple Bioactive Peptides to Improve Endothelialization of Vascular Grafts. Macromol. Biosci. 2019, 19, 1800368. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.I.; Zenses, A.S.; Grau, A.; Rodríguez-Cabello, J.C.; Gil, F.J.; Manero, J.M.; Pegueroles, M. Biofunctionalization of REDV elastin-like recombinamers improves endothelialization on CoCr alloy surfaces for cardiovascular applications. Colloids Surf. B Biointerfaces 2015, 127, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, K.R.; Tucker, B.S.; Thomas, V. Tissue Engineering of Small-Diameter Vascular Grafts [Internet]. Vol. 1957, Biointegration of Medical Implant Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 79–100. [Google Scholar]
- Natasha, G.; Tan, A.; Gundogan, B.; Farhatnia, Y.; Nayyer, L.; Mahdibeiraghdar, S.; Rajadas, J.; De Coppi, P.; Davies, A.H.; Seifalian, A.M. Tissue engineering vascular grafts a fortiori: Looking back and going forward. Expert Opin. Biol. Ther. 2015, 15, 231–244. [Google Scholar]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef]
- Kuźmińska, A.; Kwarta, D.; Ciach, T.; Butruk-Raszeja, B.A. Cylindrical Polyurethane Scaffold Fabricated Using the Phase Inversion Method: Influence of Process Parameters on Scaffolds’ Morphology and Mechanical Properties. Materials 2021, 14, 2977. [Google Scholar] [CrossRef]
- Butruk-Raszeja, B.A.; Kuźmińska, A.; Ciach, T.; Adipurnama, I.; Yang, M.C. Endothelial cell growth on polyurethane modified with acrylic acid and REDV peptide. Surf. Innov. 2020, 8, 89–104. [Google Scholar] [CrossRef]
- Butruk-Raszeja, B.A.; Dresler, M.S.; Kuźmińska, A.; Ciach, T. Endothelialization of polyurethanes: Surface silanization and immobilization of REDV peptide. Colloids Surf. B Biointerfaces 2016, 144, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: Physical properties and in vitro assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [Green Version]
- Auguścik, M.; Kurańska, M.; Prociak, A.; Karalus, W.; Lipert, K.; Ryszkowska, J. Production and characterization of poly(urea-urethane) elastomers synthetized from rapeseed oil-based polyols Part I. Structure and properties. Polimery 2016, 61, 490–498. [Google Scholar] [CrossRef]
- Qu, X.H.; Wu, Q.; Liang, J.; Qu, X.; Wang, S.G.; Chen, G.Q. Enhanced vascular-related cellular affinity on surface modified copolyesters of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx). Biomaterials 2005, 26, 6991–7001. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; PerkinElmer, Inc.: Waltham, MA, USA, 1992. [Google Scholar]
- Gardner, S.D.; Singamsetty, C.S.K.; Booth, G.L.; He, G.R.; Pittman, C.U. Surface characterization of carbon fibers using angle-resolved XPS and ISS. Carbon N. Y. 1995, 33, 587–595. [Google Scholar] [CrossRef]
- Tzoneva, R.; Faucheux, N.; Groth, T. Wettability of substrata controls cell-substrate and cell-cell adhesions. Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Enemchukwu, N.O.; García, A.J. Peptide- and Protein-Modified Surfaces. In Comprehensive Biomaterials II; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 200–220. [Google Scholar]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, J.; Li, Q.; Guo, J.; Ren, X.; Xia, S.; Zhang, W.; Feng, Y. Biofunctionalized Electrospun PCL-PIBMD/SF Vascular Grafts with PEG and Cell-Adhesive Peptides for Endothelialization. Macromol. Biosci. 2019, 19, 1800386. [Google Scholar] [CrossRef]
- Butruk-Raszeja, B.A.; Kuźmińska, A.; Wojasiński, M.; Piotrowska, Z. Physicochemical and Mechanical Properties of Blow Spun Nanofibrous Prostheses Modified with Acrylic Acid and REDV Peptide. Coatings 2020, 10, 1110. [Google Scholar] [CrossRef]
- Choi, W.S.; Joung, Y.K.; Lee, Y.; Bae, J.W.; Park, H.K.; Park, Y.H.; Park, J.C.; Park, K.D. Enhanced Patency and Endothelialization of Small-Caliber Vascular Grafts Fabricated by Coimmobilization of Heparin and Cell-Adhesive Peptides. ACS Appl. Mater. Interfaces 2016, 8, 4336–4346. [Google Scholar] [CrossRef]
- Seman, M.N.A.; Khayet, M.; Hilal, N. Comparison of two different UV-grafted nanofiltration membranes prepared for reduction of humic acid fouling using acrylic acid and N-vinylpyrrolidone. Desalination 2012, 287, 19–29. [Google Scholar] [CrossRef]
- Shi, S.; Zhou, Y.; Lu, X.; Ye, Y.; Huang, J.; Wang, X. Plasma-initiated DT graft polymerization of acrylic acid on surface of porous polypropylene membrane for pore size control. Plasma Chem. Plasma Process. 2014, 34, 1257–1269. [Google Scholar] [CrossRef]
- Contreras, M.A.; Quist, W.C.; LoGerfo, F.W. Effect of porosity on small-diameter vascular graft healing. Microsurgery 2000, 20, 15–21. [Google Scholar] [CrossRef]
- Yalcin Enis, I.; Gok Sadikoglu, T. Design parameters for electrospun biodegradable vascular grafts. J. Ind. Text. 2018, 47, 2205–2227. [Google Scholar] [CrossRef]
- Ji, Y.; Wei, Y.; Liu, X.; Wang, J.; Ren, K.; Ji, J.; Van Wachem, P.B.; Hogt, A.H.; Beugeling, T.; Bantjes, A.; et al. Tissue engineered vascular grafts—Preclinical aspects. Biomaterials 2011, 30, 38–47. [Google Scholar] [CrossRef]
- Zhou, F.; Wen, M.; Zhou, P.; Zhao, Y.; Jia, X.; Fan, Y.; Yuan, X. Electrospun membranes of PELCL/PCL-REDV loading with miRNA-126 for enhancement of vascular endothelial cell adhesion and proliferation. Mater. Sci. Eng. C 2018, 85, 37–46. [Google Scholar] [CrossRef]
- Ji, Y.; Wei, Y.; Liu, X.; Wang, J.; Ren, K.; Ji, J. Zwitterionic polycarboxybetaine coating functionalized with REDV peptide to improve selectivity for endothelial cells. J. Biomed. Mater. Res. A 2012, 100, 1387–1397. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22374807 (accessed on 10 August 2021). [CrossRef] [PubMed]
- Wei, Y.; Ji, Y.; Xiao, L.L.; Lin, Q.K.; Xu, J.P.; Ren, K.F.; Ji, J. Surface engineering of cardiovascular stent with endothelial cell selectivity for in vivo re-endothelialisation. Biomaterials 2013, 34, 2588–2599. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23352039 (accessed on 5 August 2021). [CrossRef] [PubMed]
- Blok, S.L.J.; van Oeveren, W.; Engels, G.E. The optimal incubation time for in vitro hemocompatibility testing: Assessment using polymer reference materials under pulsatile flow with physiological wall shear stress conditions. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.S.; Guex, A.G.; Liu, S.S.; Müller, E.; Malini, R.I.; Zhao, H.J.; Rottmar, M.; Maniura-Weber, K.; Rossi, R.M.; et al. Corrigendum: A compliant and biomimetic three-layered vascular graft for small blood vessels. Biofabrication 2017, 9, 029501. [Google Scholar] [CrossRef]
- Cuenca-Zamora, E.J.; Ferrer-Marín, F.; Rivera, J.; Teruel-Montoya, R. Tubulin in Platelets: When the Shape Matters. Int. J. Mol. Sci. 2019, 20, 3484. [Google Scholar] [CrossRef] [Green Version]
- Kannan, M.; Ahmad, F.; Saxena, R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev. 2019, 37, 100583. [Google Scholar] [CrossRef]
- Zhang, E.; Shen, F. Blood compatibility of a ferulic acid (FA)-eluting PHBHHx system for biodegradable magnesium stent application. Mater. Sci. Eng. C 2015, 52, 37–45. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chollet, C.; Chanseau, C.; Brouillaud, B.; Durrieu, M.C. RGD peptides grafting onto poly(ethylene terephthalate) with well controlled densities. Biomol. Eng. 2007, 24, 477–482. Available online: https://www.sciencedirect.com/science/article/pii/S1389034407000810 (accessed on 10 August 2021). [CrossRef] [PubMed]
- Ahmed, M.; Ghanbari, H.; Cousins, B.G.; Hamilton, G.; Seifalian, A.M. Small calibre polyhedral oligomeric silsesquioxane nanocomposite cardiovascular grafts: Influence of porosity on the structure, haemocompatibility and mechanical properties. Acta Biomater. 2011, 7, 3857–3867. [Google Scholar] [CrossRef]
- Yadav, P.; Beniwal, G.; Saxena, K.K. A review on pore and porosity in tissue engineering. In Materials Today: Proceedings; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Shtilman, M.I. Polymeric Biomaterials. Part I. Polymer Implants; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- ISO. Biological Evaluation of Medical Devices BS EN ISO 10993-12:2004; European Committee for Standardization: Brussels, Belgium, 2004; pp. 1–17. [Google Scholar]
- Imai, Y.; Nose, Y. A new method for evalution of antithrombogenicity of materials. J. Biomed. Mater. Res. 1972, 6, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 2006, 27, 4315–4324. [Google Scholar] [CrossRef] [PubMed]



| Material | PU | PU_AA | PU_REDV | PU_YIGSR |
|---|---|---|---|---|
| wall thickness [μm] | ||||
| 230.8 ± 41.5 | 228.6 ± 35.4 | 227.1 ± 34.0 | 229.9 ± 33.2 | |
| average surface pore diameter [μm] | * | * | * | |
| 3.0 ± 1.0 | 3.5 ± 1.4 | 4.4 ± 1.5 | 4.6 ± 1.9 | |
| min surface pore diameter [μm] | ||||
| 1.0 | 1.4 | 1.4 | 1.6 | |
| max surface pore diameter [μm] | ||||
| 5.8 | 9.0 | 9.5 | 10.1 | |
| porosity [%] | * | * | * | |
| 56 ± 2 | 65 ± 5 | 62 ± 6 | 63 ± 3 | |
| Young’s modulus [MPa] | * | * | * | |
| 3.6 ± 1.5 | 9.1 ± 1.7 | 9.8 ± 1.8 | 10.6 ± 2.4 | |
| tensile strength [MPa] | * | * | * | |
| 11.2 ± 1.2 | 3.8 ± 1.1 | 5.7 ± 0.7 | 6.7 ± 1.1 | |
| elongation at break [mm/mm] | * | * | * | |
| 4.7 ± 0.4 | 1.4 ± 0.5 | 1.9 ± 0.3 | 2.1 ± 0.4 | |
| PU | PU_REDV | PU_YIGSR | ||
|---|---|---|---|---|
| Area coated with ECs [%] | 1 day | <1 | 4 ± 2 | 11 ± 8 *,# |
| 3 day | 8 ± 6 | 10 ± 7 | 12 ± 3 | |
| Area coated with SMCs [%] | 1 day | <1 | <1 | <1 |
| 3 day | <1 | 2 ± 2 | 3 ± 3 | |
| PU | PU_REDV | PU_YGSR | |
|---|---|---|---|
| % of area covered with platelets (anti-tubulin staining) | 3.2 ± 2.7 | 4.3 ± 8.8 | 3.8 ± 5.2 |
| % of area covered with activated platelets (CD62P staining) | 0.4 ± 0.5 | 0.1 ± 0.1 | 0.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźmińska, A.; Wojciechowska, A.; Butruk-Raszeja, B.A. Vascular Polyurethane Prostheses Modified with a Bioactive Coating—Physicochemical, Mechanical and Biological Properties. Int. J. Mol. Sci. 2021, 22, 12183. https://doi.org/10.3390/ijms222212183
Kuźmińska A, Wojciechowska A, Butruk-Raszeja BA. Vascular Polyurethane Prostheses Modified with a Bioactive Coating—Physicochemical, Mechanical and Biological Properties. International Journal of Molecular Sciences. 2021; 22(22):12183. https://doi.org/10.3390/ijms222212183
Chicago/Turabian StyleKuźmińska, Aleksandra, Aleksandra Wojciechowska, and Beata A. Butruk-Raszeja. 2021. "Vascular Polyurethane Prostheses Modified with a Bioactive Coating—Physicochemical, Mechanical and Biological Properties" International Journal of Molecular Sciences 22, no. 22: 12183. https://doi.org/10.3390/ijms222212183






