Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy
Abstract
1. Introduction
2. The Assessment of Nitric Oxide Delivery as Diagnostic Tool
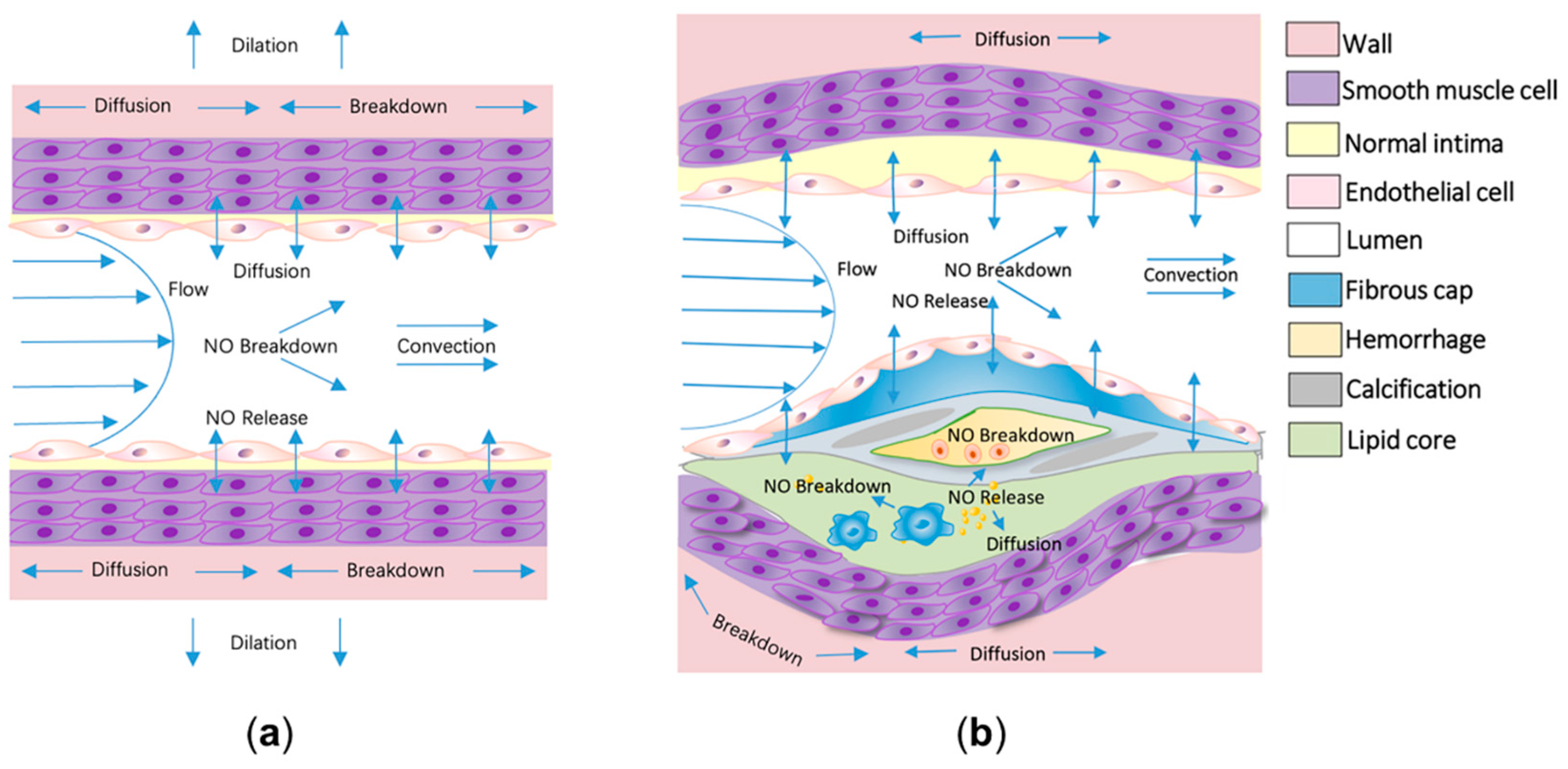
2.1. Diagnosing the Endothelial Function and Atherosclerosis Development through Computational Modeling of NO Delivery
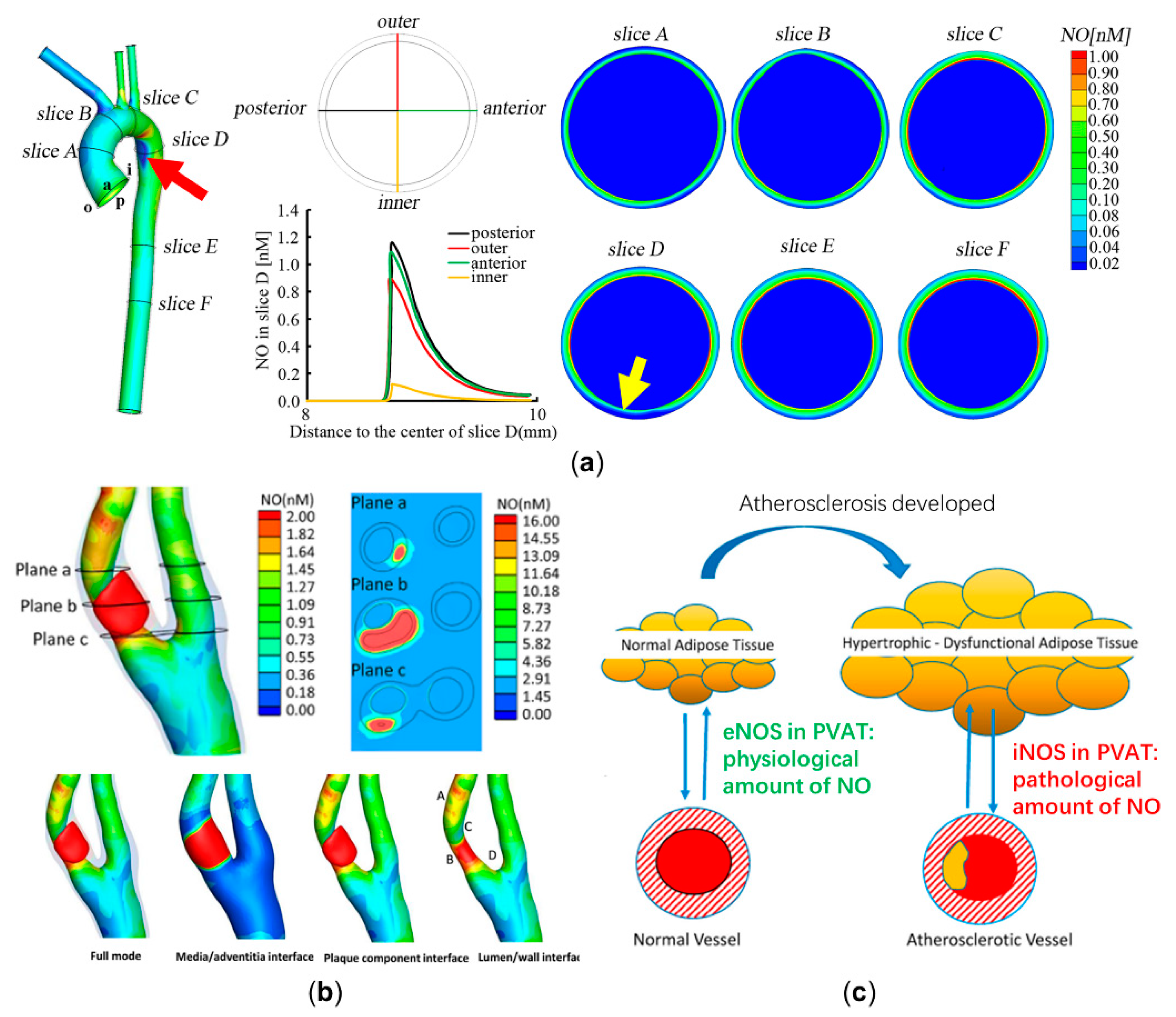
2.2. Improving the Specificity of the Flow-Mediated Dilation Test in Assessing Endothelial Function through Computational Modeling of Nitric Oxide Delivery
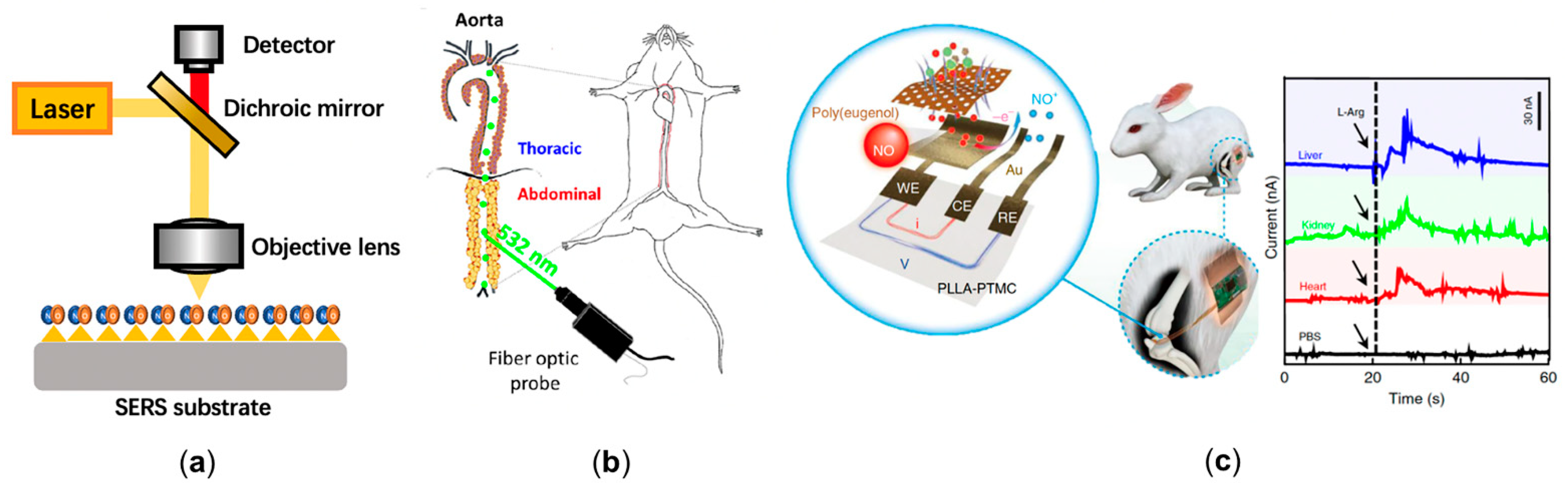
2.3. Experimental Bioimaging of Nitric Oxide in Cardiovascular System
| Name of Authors | Applications of the Technology | Findings | Computational/Experimental |
|---|---|---|---|
| Liu et al. [31] | Assessing the early occurrence of endothelial dysfunction by NO distribution. | NO concentration at the inner wall of the distal end of aortic arch is significantly hindered, corresponding to the atherosclerotic prone site. | Computational |
| Qian et al. [30] | Assessing the development and vulnerability of formed atherosclerotic plaque by NO distribution. | The average NO concentration around the lipid plaque is significantly higher than the plaque-free region, which potentially indicates the vulnerability of plaque. | Computational |
| Arzani et al. [49] | Assessing the occurrence and development of atherosclerosis with wall shear stress, which can indicate NO delivery at endothelium. | Wall shear stress dominates the NO delivery process at endothelium, and the low wall shear stress indicates the occurrence and development of atherosclerosis. | Computational |
| Yamazaki et al. [62] Brackle et al. [64] Jin et al. [65,66] | Excluding the interferences of individual differences in arterial stiffness, shear exposure, and blood pressure from flow-mediated dilation (FMD) test. | The result of FMD is not only determined by endothelial function but is also influenced by the confounding factors. The computational modeling is effective to reduce these interferences. | Computational |
| Cui et al. [71] | Designing a reaction-based surface-enhanced Raman spectroscopy (SERS) nanoprobe for the detection of intracellular NO with o-phenylenediamine-modified gold nanoparticles. | The probe reaches a temporal resolution of 30 s and a sensitivity of 100 nM. | Experimental |
| Xu et al. [72] | Designing a ratiometric SERS probe with compound 3,4-diaminobenzene-thiol. | The probe enhances the NO sensitivity to 54 nM. | Experimental |
| Chen et al. [73] | Designing a SERS probe with gold nanoparticles and synthesized 3,4-diaminophenylboronic acid pinacol ester. | The probe further increases the NO detection range to 0–105 nM. And it is capable of detecting peroxynitrite (ONOO−) synchronously. | Experimental |
| Takarada et al. [78] | Using the catheter-type NO sensor to measure NO concentration in human coronary circulation. | Measured the NO delivery in coronary circulation for the first time and found that NO concentration in the patients with severe left ventricular dysfunction (2.3 nM) was significantly lower than normal subjects (12.0 nM). | Experimental |
| Tang et al. [79] | Designing an acupuncture microsensor needle by gold film and iron porphyrin-functionalized graphene complex. | The microsensor needle achieved the detection of NO signal in rat via puncture. | Experimental |
| Li et al. [80] | Designing a flexible and degradable sensor to realize real time measurement of NO delivery in vivo. | The sensor has a low detection limit (3.97 nmol), high temporal resolution (350 ms), and high biocompatibility. | Experimental |
3. Nitric Oxide Delivery-Related Potential Therapeutic Approaches
3.1. Manipulating Nitric Oxide Delivery Process with NO Release Platform
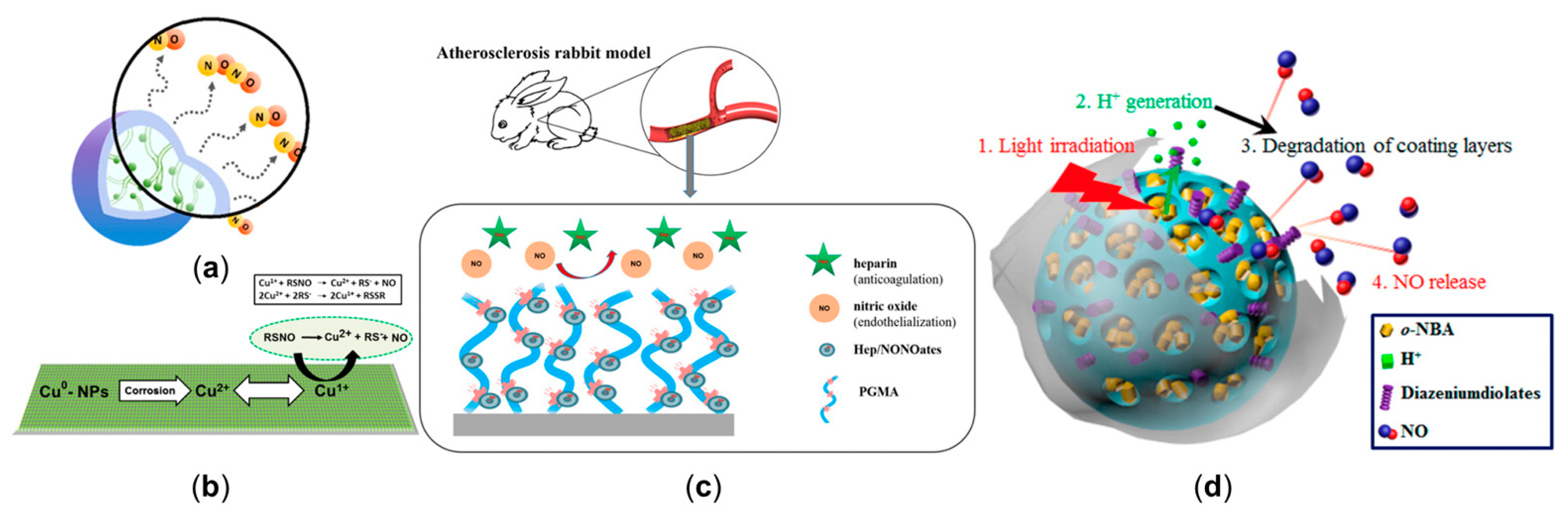
3.2. The Inhaled Nitric Oxide Therapy to Treat the Pulmonary Hypertension and Coronavirus (COVID-19)
3.3. Potential Methods to Enhance the Effectiveness of Nitric Oxide Therapy
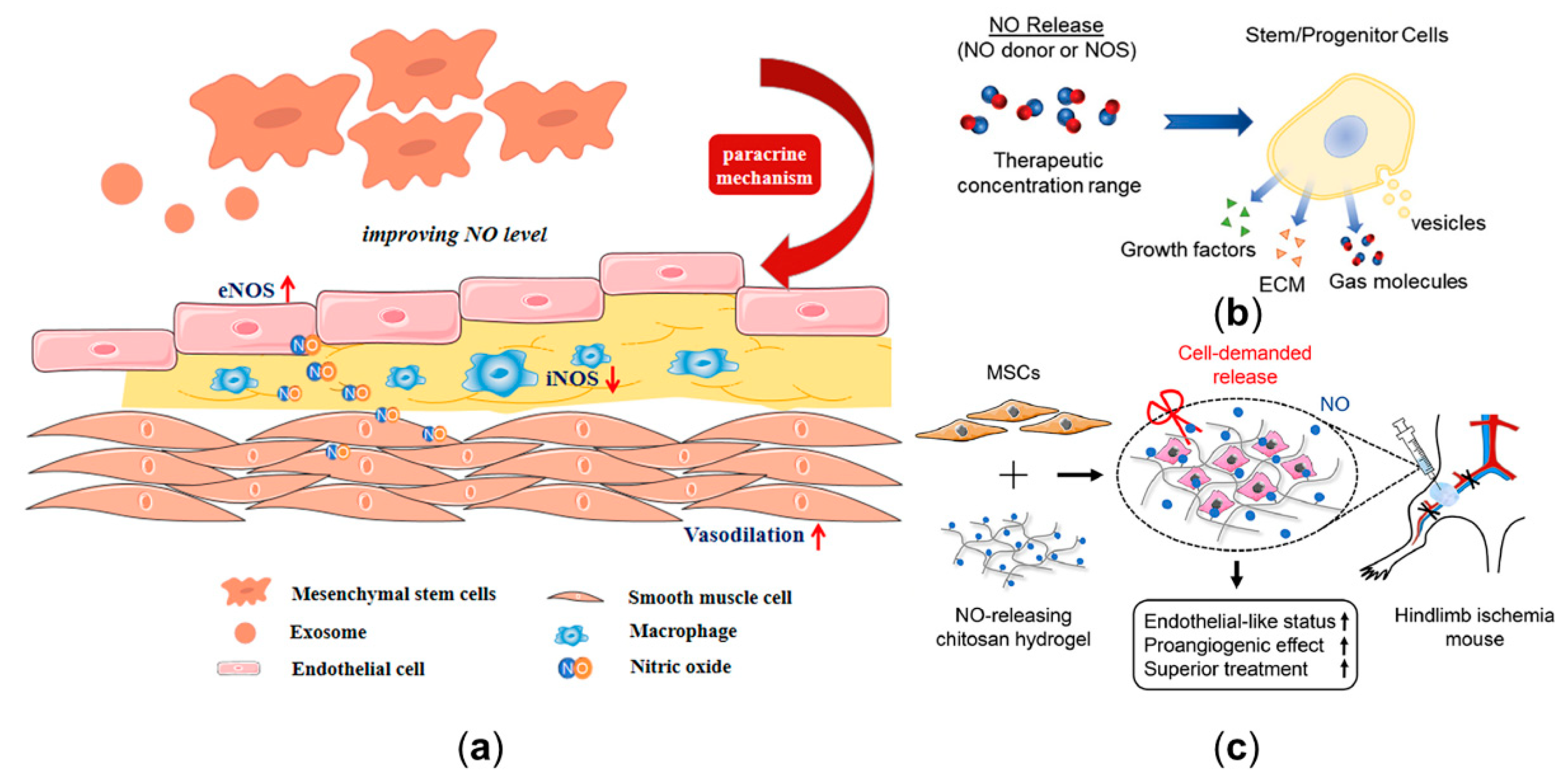
3.3.1. Combining Controllable NO Release Platform with Computational Modeling to Manipulate NO Delivery Process
3.3.2. The Clinical Potential of Stem Cell in Modulating Nitric Oxide Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T. European Society of Cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.; Balligand, J.-L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Vita, J.A. Endothelial function. Circulation 2011, 124, e906–e912. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of nitric oxide in the cardiovascular and renal systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Carvajal, J.A.; Germain, A.M.; Huidobro-Toro, J.P.; Weiner, C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000, 184, 409–420. [Google Scholar] [CrossRef]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial Dysfunction and Cardiovascular Disease: History and Analysis of the Clinical Utility of the Relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tanabe, K.; Croker, B.P.; Johnson, R.J.; Grant, M.B.; Kosugi, T.; Li, Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 36–44. [Google Scholar] [CrossRef]
- Farghadan, A.; Arzani, A. The combined effect of wall shear stress topology and magnitude on cardiovascular mass transport. Int. J. Heat Mass Transf. 2019, 131, 252–260. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Key, C.-C.C.; Kavanagh, K. Adipose tissue macrophage polarization in healthy and unhealthy obesity. Front. Nutr. 2021, 8, 625331. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Xiong, K.; Li, X.; Qi, P.; Tu, Q.; Jing, F.; Weng, Y.; Wang, J.; Huang, N. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials 2015, 63, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Q.; Li, X.; Huang, N.; Zhao, X.; Yang, Z. Mussel-inspired dopamine-Cu(II) coatings for sustained in situ generation of nitric oxide for prevention of stent thrombosis and restenosis. Biomaterials 2019, 194, 117–129. [Google Scholar] [CrossRef]
- Elnaggar, M.A.; Seo, S.H.; Gobaa, S.; Lim, K.S.; Bae, I.H.; Jeong, M.H.; Han, D.K.; Joung, Y.K. Nitric Oxide Releasing Coronary Stent: A New Approach Using Layer-by-Layer Coating and Liposomal Encapsulation. Small 2016, 12, 6012–6023. [Google Scholar] [CrossRef]
- Naghavi, N.; de Mel, A.; Alavijeh, O.S.; Cousins, B.G.; Seifalian, A.M. Nitric oxide donors for cardiovascular implant applications. Small 2013, 9, 22–35. [Google Scholar] [CrossRef]
- Maruf, A.; Wang, Y.; Yin, T.; Huang, J.; Wang, N.; Durkan, C.; Tan, Y.; Wu, W.; Wang, G. Atherosclerosis Treatment with Stimuli-Responsive Nanoagents: Recent Advances and Future Perspectives. Adv. Healthc. Mater. 2019, 8, e1900036. [Google Scholar] [CrossRef]
- Sreejayan; Rao, M.N. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Gori, T. Exogenous NO Therapy for the Treatment and Prevention of Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 2703. [Google Scholar] [CrossRef]
- Víteček, J.; Lojek, A.; Valacchi, G.; Kubala, L. Arginine-Based Inhibitors of Nitric Oxide Synthase: Therapeutic Potential and Challenges. Mediat. Inflamm. 2012, 2012, 318087. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, X.; Chen, S.-Y. Function and therapeutic potential of mesenchymal stem cells in atherosclerosis. Front. Cardiovasc. Med. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.C.; Wei, Y.; Li, Z.; Kong, D.; Zhao, Q. Nitric-oxide-releasing biomaterial regulation of the stem cell microenvironment in regenerative medicine. Adv. Mater. 2020, 32, 1805818. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Qian, S.; Ma, T.; Zhang, N.; Liu, X.; Zhao, P.; Li, X.; Chen, D.; Hu, L.; Chang, L.; Xu, L. Spatiotemporal transfer of nitric oxide in patient-specific atherosclerotic carotid artery bifurcations with MRI and computational fluid dynamics modeling. Comput. Biol. Med. 2020, 125, 104015. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhao, P.; Fan, Z.; Sun, A.; Zhan, F.; Fan, Y.; Deng, X. Nitric oxide transport in normal human thoracic aorta: Effects of hemodynamics and nitric oxide scavengers. PLoS ONE 2014, 9, e112395. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Y.; Xu, X.Y.; Deng, X. Nitric oxide transport in an axisymmetric stenosis. J. R. Soc. Interface 2012, 9, 2468–2478. [Google Scholar] [CrossRef] [PubMed]
- Plank, M.J.; Wall, D.J.N.; David, T. The role of endothelial calcium and nitric oxide in the localisation of atherosclerosis. Math. Biosci. 2007, 207, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Vidanapathirana, A.K.; Psaltis, P.J.; Bursill, C.A.; Abell, A.D.; Nicholls, S.J. Cardiovascular bioimaging of nitric oxide: Achievements, challenges, and the future. Med. Res. Rev. 2021, 41, 435–463. [Google Scholar] [CrossRef]
- Yang, N.; Vafai, K. Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int. J. Heat Mass Transf. 2006, 49, 850–867. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, L.; Zhou, W.; Chang, J.; Zhang, X.; He, C.; Chen, C.; Yan, R.; Yan, Y.; Lu, Y.; et al. Nitric oxide-releasing micelles with intelligent targeting for enhanced anti-tumor effect of cisplatin in hypoxia. J. Nanobiotechnol. 2021, 19, 246. [Google Scholar] [CrossRef]
- Yazdani, A.; Deng, Y.; Li, H.; Javadi, E.; Li, Z.; Jamali, S.; Lin, C.; Humphrey, J.D.; Mantzoros, C.S.; Em Karniadakis, G. Integrating blood cell mechanics, platelet adhesive dynamics and coagulation cascade for modelling thrombus formation in normal and diabetic blood. J. R. Soc. Interface 2021, 18, 20200834. [Google Scholar] [CrossRef]
- Menichini, C.; Cheng, Z.; Gibbs, R.G.; Xu, X.Y. Predicting false lumen thrombosis in patient-specific models of aortic dissection. J. R. Soc. Interface 2016, 13, 20160759. [Google Scholar] [CrossRef]
- Heijman, J.; Sutanto, H.; Crijns, H.J.; Nattel, S.; Trayanova, N.A. Computational models of atrial fibrillation: Achievements, challenges, and perspectives for improving clinical care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef]
- Smith, K.M.; Moore, L.C.; Layton, H.E. Advective transport of nitric oxide in a mathematical model of the afferent arteriole. Am. J. Physiol.-Ren. Physiol. 2003, 284, F1080–F1096. [Google Scholar] [CrossRef]
- Chen, X.; Buerk, D.G.; Barbee, K.A.; Kirby, P.; Jaron, D. 3D network model of NO transport in tissue. Med Biol. Eng. Comput. 2011, 49, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Vázquez, B.Y.S.; Yalcin, O.; Johnson, P.C.; Intaglietta, M.; Tartakovsky, D.M. The effect of small changes in hematocrit on nitric oxide transport in arterioles. Antioxid. Redox Signal. 2011, 14, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Fadel, A.; Barbee, K.; Jaron, D. A computational model of nitric oxide production and transport in a parallel plate flow chamber. Ann. Biomed. Eng. 2009, 37, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Plata, A.; Sherwin, S.; Krams, R. Endothelial nitric oxide production and transport in flow chambers: The importance of convection. Ann. Biomed. Eng. 2010, 38, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; He, Y.; Tang, Y.-l.; Mu, L.-Z. Finite element analysis of nitric oxide (NO) transport in system of permeable capillary and tissue. J. Hydrodyn. 2018, 30, 722–737. [Google Scholar] [CrossRef]
- Wei, Y.; Mu, L.; Tang, Y.; Shen, Z.; He, Y. Computational analysis of nitric oxide biotransport in a microvessel influenced by red blood cells. Microvasc. Res. 2019, 125, 103878. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, H.; Tajeddini, F.; Pakravan, H.A.; Mahzoon, M.; Yazdi, E.A.; Drissi, H.B. A validated reduced-order dynamic model of nitric oxide regulation in coronary arteries. Comput. Biol. Med. 2021, 139, 104958. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A.; Gambaruto, A.M.; Chen, G.; Shadden, S.C. Wall shear stress exposure time: A Lagrangian measure of near-wall stagnation and concentration in cardiovascular flows. Biomech. Modeling Mechanobiol. 2017, 16, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Farghadan, A.; McConnell, D.R.; Barker, A.J.; Wentzel, J.J.; Budoff, M.J.; Arzani, A. The story of wall shear stress in coronary artery atherosclerosis: Biochemical transport and mechanotransduction. J. Biomech. Eng. 2021, 143, 041002. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Lin, A.; Dey, D.; Baggiano, A.; Fusini, L.; Muscogiuri, G.; Pontone, G. Epicardial fat and coronary artery disease: Role of cardiac imaging. Atherosclerosis 2021, 321, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Sena, C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020, 59, 101040. [Google Scholar] [CrossRef]
- Barp, C.G.; Bonaventura, D.; Assreuy, J. NO, ROS, RAS, and PVAT: More than a soup of letters. Front. Physiol. 2021, 12, 108. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Cox, D.A.; Vita, J.A.; Treasure, C.B. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation 1989, 80, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A.; Treasure, C.B.; Nabel, E.G.; McLenachan, J.M.; Fish, R.D.; Yeung, A.C.; Vekshtein, V.I.; Selwyn, A.P.; Ganz, P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 1990, 81, 491–497. [Google Scholar] [CrossRef]
- Pyke, K.E.; Tschakovsky, M.E. Peak vs. total reactive hyperemia: Which determines the magnitude of flow-mediated dilation? J. Appl. Physiol. 2007, 102, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Johnson, B.D.; Newcomer, S.C.; Wilhite, D.P.; Wallace, J.P. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc. Ultrasound 2008, 6, 44. [Google Scholar] [CrossRef]
- Atkinson, G.; Batterham, A.M.; Black, M.A.; Cable, N.T.; Hopkins, N.D.; Dawson, E.A.; Thijssen, D.H.J.; Jones, H.; Tinken, T.M.; Green, D.J. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J. Appl. Physiol. 2009, 107, 1893–1899. [Google Scholar] [CrossRef]
- Harris, R.A.; Padilla, J. Proper “normalization” of flow-mediated dilation for shear. J. Appl. Physiol. 2007, 103, 1108. [Google Scholar] [CrossRef]
- Atkinson, G.; Batterham, A.M.; Thijssen, D.H.J.; Green, D.J. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J. Hypertens 2013, 31, 287–291. [Google Scholar] [CrossRef]
- Atkinson, G.; Batterham, A.M. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis 2013, 226, 425–427. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kondo, Y.; Kamiyama, Y. Estimation of shear-stress-induced endothelial nitric oxide production from flow-mediated dilation. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4521–4524. [Google Scholar]
- Yamazaki, Y.; Kamiyama, Y. Mathematical model of wall shear stress-dependent vasomotor response based on physiological mechanisms. Comput. Biol. Med. 2014, 45, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Van Brackle, C.H.; Harris, R.A.; Hallow, K.M. Exposure-response modeling of flow-mediated dilation provides an unbiased and informative measure of endothelial function. J. Appl. Physiol. 2017, 122, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Chowienczyk, P.; Alastruey, J. An in silico simulation of flow-mediated dilation reveals that blood pressure and other factors may influence the response independent of endothelial function. Am. J. Physiol.-Heart Circ. Physiol. 2020, 318, H1337–H1345. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Cardiovascular Function Assessment Using Computational Blood Flow Modelling and Machine Learning. Ph.D. Thesis, King’s College London, London, UK, 2021. [Google Scholar]
- Sidnawi, B.; Chen, Z.; Sehgal, C.; Wu, Q. Characterization of arterial flow mediated dilation via a physics-based model. J. Mech. Behav. Biomed. Mater. 2020, 107, 103756. [Google Scholar] [CrossRef]
- Sidnawi, B.; Chen, Z.; Sehgal, C.; Santhanam, S.; Wu, Q. On the modeling of mechanotransduction in flow-mediated dilation. J. Mech. Behav. Biomed. Mater. 2021, 120, 104606. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Liu, X.; Ren, Q.; Zhang, Z.; Sun, X.; Zheng, Y.; Deng, X.; Yu, X.; Fan, Y. Flow-mediated dilation analysis coupled with nitric oxide transport to enhance the assessment of endothelial function. J. Appl. Physiol. 2021, 131, 1–14. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, C.; Zhao, H.; Feng, D.D. Model and verification of the NO distribution in curved blood vessel. IFAC-PapersOnLine 2018, 51, 237–240. [Google Scholar] [CrossRef]
- Cui, J.; Hu, K.; Sun, J.-J.; Qu, L.-L.; Li, D.-W. SERS nanoprobes for the monitoring of endogenous nitric oxide in living cells. Biosens. Bioelectron. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, W.; Li, L.; Zhou, F.; Zhou, J.; Tian, Y. Ratiometric SERS imaging and selective biosensing of nitric oxide in live cells based on trisoctahedral gold nanostructures. Chem. Commun. 2017, 53, 1880–1883. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Kouadio Fodjo, E.; Jiang, L.; Chang, S.; Li, J.-B.; Zhan, D.-S.; Gu, H.-X.; Li, D.-W. Simultaneous Detection of Intracellular Nitric Oxide and Peroxynitrite by a Surface-Enhanced Raman Scattering Nanosensor with Dual Reactivity. ACS Sens. 2019, 4, 3234–3239. [Google Scholar] [CrossRef]
- Majka, Z.; Czamara, K.; Wegrzyn, P.; Litwinowicz, R.; Janus, J.; Chlopicki, S.; Kaczor, A. A new approach to study human perivascular adipose tissue of the internal mammary artery by fiber-optic Raman spectroscopy supported by spectral modelling. Analyst 2021, 146, 270–276. [Google Scholar] [CrossRef]
- Czamara, K.; Majka, Z.; Sternak, M.; Koziol, M.; Kostogrys, R.B.; Chlopicki, S.; Kaczor, A. Distinct chemical changes in abdominal but not in thoracic aorta upon atherosclerosis studied using fiber optic raman spectroscopy. Int. J. Mol. Sci. 2020, 21, 4838. [Google Scholar] [CrossRef]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192. [Google Scholar] [CrossRef]
- Fujita, S.; Roerig, D.L.; Chung, W.W.; Bosnjak, Z.J.; Stowe, D.F. Volatile anesthetics do not alter bradykinin-induced release of nitric oxide or L-citrulline in crystalloid perfused guinea pig hearts. J. Am. Soc. Anesthesiol. 1998, 89, 421–433. [Google Scholar] [CrossRef]
- Takarada, S.; Imanishi, T.; Goto, M.; Mochizuki, S.; Ikejima, H.; Tsujioka, H.; Kuroi, A.; Takeshita, T.; Akasaka, T. First evaluation of real-time nitric oxide changes in the coronary circulation in patients with non-ischaemic dilated cardiomyopathy using a catheter-type sensor. Eur. Heart J. 2010, 31, 2862–2870. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y.; Xie, H.; Shu, Q.; Yang, F.; Liu, Y.-l.; Liang, F.; Wang, H.; Huang, W.; Zhang, G.-J. A sensitive acupuncture needle microsensor for real-time monitoring of nitric oxide in acupoints of rats. Sci. Rep. 2017, 7, 6446. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Knorr, M.; Hausding, M.; Kröller-Schuhmacher, S.; Steven, S.; Oelze, M.; Heeren, T.; Scholz, A.; Gori, T.; Wenzel, P.; Schulz, E.; et al. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: Beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Daiber, A.; Di Stolfo, G.; Sicuro, S.; Dragoni, S.; Lisi, M.; Münzel, T.; Forconi, S.; Parker, J.D. Nitroglycerine causes mitochondrial reactive oxygen species production: In vitro mechanistic insights. Can. J. Cardiol. 2007, 23, 990–992. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef]
- Ramms, B.; Gordts, P.L. Dietary nitrate struggles in atherosclerosis. Atherosclerosis 2016, 245, 71–73. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Croft, K.D.; Hodgson, J.M.; Mori, T.; Ward, N.C. Mechanisms of the protective effects of nitrate and nitrite in cardiovascular and metabolic diseases. Nitric Oxide Biol. Chem. 2020, 96, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Kaun, C.; Krychtiuk, K.A.; Haider, P.; Brekalo, M.; Maier, N.; Goederle, L.; Binder, C.J.; Huber, K.; Hengstenberg, C.J.B. Effects of nicorandil on inflammation, apoptosis and atherosclerotic plaque progression. Biomedicines 2021, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Sato, M.; Funada, J.; Ohtani, T.; Akutsu, H.; Watanabe, K. Effects of the long-term administration of nicorandil on vascular endothelial function and the progression of arteriosclerosis. J. Cardiovasc. Pharmacol. 2005, 46, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Van der Donckt, C.; Emini Veseli, B.; Van Dam, D.; De Deyn, P.P.; Martinet, W.; Herman, A.G.; De Meyer, G.R.Y. Nitric oxide donor molsidomine favors features of atherosclerotic plaque stability and reduces myocardial infarction in mice. Vasc. Pharmacol. 2019, 118–119, 106561. [Google Scholar] [CrossRef] [PubMed]
- Fitzhugh, A.L.; Keefer, L.K. Diazeniumdiolates: Pro- and antioxidant applications of the “NONOates”. Free Radic. Biol. Med. 2000, 28, 1463–1469. [Google Scholar] [CrossRef]
- Yang, C.; Jeong, S.; Ku, S.; Lee, K.; Park, M.H. Use of gasotransmitters for the controlled release of polymer-based nitric oxide carriers in medical applications. J. Control. Release Off. J. Control. Release Soc. 2018, 279, 157–170. [Google Scholar] [CrossRef]
- Ding, Z.; He, K.; Duan, Y.; Shen, Z.; Cheng, J.; Zhang, G.; Hu, J. Photo-degradable micelles for co-delivery of nitric oxide and doxorubicin. J. Mater. Chem. B 2020, 8, 7009–7017. [Google Scholar] [CrossRef]
- Gao, M.; Liu, S.; Fan, A.; Wang, Z.; Zhao, Y. Nitric oxide-releasing graft polymer micelles with distinct pendant amphiphiles. RSC Adv. 2015, 5, 67041–67048. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Katsumi, H.; Takashima, R.; Suzuki, H.; Hirai, N.; Matsuura, S.; Kimura, H.; Morishita, M.; Yamamoto, A. S-nitrosylated l-serine-modified dendrimer as a kidney-targeting nitric oxide donor for prevention of renal ischaemia/reperfusion injury. Free Radic. Res. 2020, 54, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.V.; Soto, R.J.; Kinsley, P.C.; Schoenfisch, M.H. Active Release of Nitric Oxide-Releasing Dendrimers from Electrospun Polyurethane Fibers. ACS Biomater. Sci. Eng. 2016, 2, 426–437. [Google Scholar] [CrossRef]
- Duan, S.; Cai, S.; Xie, Y.; Bagby, T.; Ren, S.; Forrest, M.L. Synthesis and characterization of a multi-arm poly(acrylic acid) star polymer for application in sustained delivery of cisplatin and a nitric oxide prodrug. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Selvanayagam, R.; Ho, K.K.K.; Chen, R.; Kutty, S.K.; Rice, S.A.; Kumar, N.; Barraud, N.; Duong, H.T.T.; Boyer, C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7, 1016–1027. [Google Scholar] [CrossRef]
- Oliver, S.; Pham, T.T.P.; Li, Y.; Xu, F.J.; Boyer, C. More than skin deep: Using polymers to facilitate topical delivery of nitric oxide. Biomater. Sci. 2021, 9, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Lautner, G.; Meyerhoff, M.E.; Schwendeman, S.P. Biodegradable poly(lactic-co-glycolic acid) microspheres loaded with S-nitroso-N-acetyl-D-penicillamine for controlled nitric oxide delivery. J. Control. Release Off. J. Control. Release Soc. 2016, 225, 133–139. [Google Scholar] [CrossRef]
- Šírová, M.; Horková, V.; Etrych, T.; Chytil, P.; Říhová, B.; Studenovský, M. Polymer donors of nitric oxide improve the treatment of experimental solid tumours with nanosized polymer therapeutics. J. Drug Target. 2017, 25, 796–808. [Google Scholar] [CrossRef]
- Neha, D.; Momin, M.; Khan, T.; Gharat, S.; Ningthoujam, R.S.; Omri, A. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin. Drug Deliv. 2021, 18, 1261–1290. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pant, J.; Goudie, M.J.; Hopkins, S.P.; Brisbois, E.J.; Handa, H. Tunable nitric oxide release from S-nitroso-N-acetylpenicillamine via catalytic copper nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces 2017, 9, 15254–15264. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.J.; Yang, L.; Schoenfisch, M.H. Functionalized Mesoporous Silica via an Aminosilane Surfactant Ion Exchange Reaction: Controlled Scaffold Design and Nitric Oxide Release. ACS Appl. Mater. Interfaces 2016, 8, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.P.; Storm, W.L.; Koh, A.; Schoenfisch, M.H. Local delivery of nitric oxide: Targeted delivery of therapeutics to bone and connective tissues. Adv. Drug Deliv. Rev. 2012, 64, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Wu, Q.; Zhao, B.; Miao, J.; Su, L. The promotion effect of novel magnetic nanoparticles on atherosclerotic plaque vulnerability in apolipoprotein E(-/-) mice. Toxicology 2019, 419, 24–31. [Google Scholar] [CrossRef]
- Oh, Y.; Jeong, H.; Lim, S.; Hong, J. Controlled nitric oxide release using poly (lactic-co-glycolic acid) nanoparticles for anti-inflammatory effects. Biomacromolecules 2020, 21, 4972–4979. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, M.; Gao, W.; Fang, D.; Liu, Z.; Wu, G.; Wan, M.; Mao, C.; Shen, J.J.L. Coronary stents decorated by heparin/NONOate nanoparticles for anticoagulant and endothelialized effects. Langmuir 2020, 36, 2901–2910. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, J.; Kim, J.; Kim, Y.; Song, H.B.; Kim, J.H.; Kim, K.; Kim, W.J. Light-Induced Acid Generation on a Gatekeeper for Smart Nitric Oxide Delivery. ACS Nano 2016, 10, 4199–4208. [Google Scholar] [CrossRef]
- Roberts, J., Jr.; Polaner, D.M.; Zapol, W.; Lang, P. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992, 340, 818–819. [Google Scholar] [CrossRef]
- Germann, P.; Braschi, A.; Della Rocca, G.; Dinh-Xuan, A.T.; Falke, K.; Frostell, C.; Gustafsson, L.E.; Hervé, P.; Jolliet, P.; Kaisers, U. Inhaled nitric oxide therapy in adults: European expert recommendations. Intensive Care Med. 2005, 31, 1029–1041. [Google Scholar] [CrossRef]
- Lai, M.-Y.; Chu, S.-M.; Lakshminrusimha, S.; Lin, H.-C. Beyond the inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Pediatr. Neonatol. 2018, 59, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.G.; Swain, R.; Hislop, J.; Escudie, M.; Williams, R.H. Delivery of Inhaled Nitric Oxide During MRI to Ventilated Neonates and Infants. Respir. Care 2021, 66. [Google Scholar] [CrossRef]
- Schäfer, M.; Frank, B.S.; Ivy, D.D.; Abman, S.H.; Stenmark, K.R.; Mitchell, M.B.; Browne, L.P.; Barker, A.J.; Hunter, K.S.; Kheyfets, V.; et al. Short-Term Effects of Inhaled Nitric Oxide on Right Ventricular Flow Hemodynamics by 4-Dimensional-Flow Magnetic Resonance Imaging in Children With Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2021, 10, e020548. [Google Scholar] [CrossRef] [PubMed]
- Buess, A.; Van Muylem, A.; Nonclercq, A.; Haut, B. Modeling of the transport and exchange of a gas species in lungs with an asymmetric branching pattern. application to nitric oxide. Front. Physiol. 2020, 11, 570015. [Google Scholar] [CrossRef]
- Bhagavathula, A.S.; Aldhaleei, W.A.; Rovetta, A.; Rahmani, J. Vaccines and drug therapeutics to lock down novel coronavirus disease 2019 (COVID-19): A systematic review of clinical trials. Cureus 2020, 12, e8342. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Wilson, C.; Weinberg, J.; Gavin, D.; Murphy, J.; Reardon, C.C. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther. Adv. Respir. Dis. 2020, 14, 1753466620933510. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Marco, P.; Mongodi, S.; Dammassa, V.; Romito, G.; Mojoli, F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit. Care 2020, 24, 508. [Google Scholar] [CrossRef]
- Lotz, C.; Muellenbach, R.M.; Meybohm, P.; Mutlak, H.; Lepper, P.M.; Rolfes, C.-B.; Peivandi, A.; Stumpner, J.; Kredel, M.; Kranke, P.; et al. Effects of inhaled nitric oxide in COVID-19–induced ARDS—Is it worthwhile? Acta Anaesthesiol. Scand. 2021, 65, 629–632. [Google Scholar] [CrossRef]
- Bagate, F.; Tuffet, S.; Masi, P.; Perier, F.; Razazi, K.; de Prost, N.; Carteaux, G.; Payen, D.; Mekontso Dessap, A. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann. Intensive Care 2020, 10, 151. [Google Scholar] [CrossRef]
- Abou-Arab, O.; Huette, P.; Debouvries, F.; Dupont, H.; Jounieaux, V.; Mahjoub, Y. Inhaled nitric oxide for critically ill COVID-19 patients: A prospective study. Crit. Care 2020, 24, 645. [Google Scholar] [CrossRef]
- Hedenstierna, G.; Chen, L.; Hedenstierna, M.; Lieberman, R.; Fine, D.H. Nitric oxide dosed in short bursts at high concentrations may protect against COVID 19. Nitric Oxide Biol. Chem. 2020, 103, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, P.; Wang, J.; Tu, Q.; Bai, L.; Xiong, K.; Qiu, H.; Zhao, X.; Maitz, M.F.; Wang, H.; et al. Endothelium-Mimicking Multifunctional Coating Modified Cardiovascular Stents via a Stepwise Metal-Catechol-(Amine) Surface Engineering Strategy. Research 2020, 2020, 9203906. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, T.; Liang, K.; Chandrawati, R. Metal-organic frameworks for therapeutic gas delivery. Adv. Drug Deliv. Rev. 2021, 171, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.V.; Antunes, F.; Pires, J.; Graça, V.; Brandão, P.; Pinto, M.L. Vitamin B(3) metal-organic frameworks as potential delivery vehicles for therapeutic nitric oxide. Acta Biomater. 2017, 51, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, C.; Ji, K.; Aghoghovbia, R.E.; Pan, Z.; Chittavong, V.; Ke, B.; Wang, B. Click and Release: A Chemical Strategy toward Developing Gasotransmitter Prodrugs by Using an Intramolecular Diels-Alder Reaction. Angew. Chem. 2016, 55, 15846–15851. [Google Scholar] [CrossRef]
- Chandrawati, R.; Chang, J.Y.H.; Reina-Torres, E.; Jumeaux, C.; Sherwood, J.M.; Stamer, W.D.; Zelikin, A.N.; Overby, D.R.; Stevens, M.M. Localized and Controlled Delivery of Nitric Oxide to the Conventional Outflow Pathway via Enzyme Biocatalysis: Toward Therapy for Glaucoma. Adv. Mater. 2017, 29, 1604932. [Google Scholar] [CrossRef]
- Hou, J.; Pan, Y.; Zhu, D.; Fan, Y.; Feng, G.; Wei, Y.; Wang, H.; Qin, K.; Zhao, T.; Yang, Q. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme–prodrug pair. Nat. Chem. Biol. 2019, 15, 151–160. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Qin, K.; Wu, Y.; Tian, Y.; Wang, J.; Zhang, J.; Hou, J.; Cui, Y.; Wang, K.; et al. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Control. Release Off. J. Control. Release Soc. 2015, 210, 179–188. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, Y.; Cheng, J.; Shen, J.; Huang, H.; Liu, M.; Chen, Z.; Liu, Y. Nitric oxide-releasing platinum(IV) prodrug efficiently inhibits proliferation and metastasis of cancer cells. Chem. Commun. 2020, 56, 14051–14054. [Google Scholar] [CrossRef]
- Dang, Y.; Ruan, L.; Tian, Y.; Xu, Z.; Zhang, W. Nitric Oxide Prodrug Delivery and Release Monitoring Based on a Galactose-Modified Multifunctional Nanoprobe. Anal. Chem. 2021, 93, 7625–7634. [Google Scholar] [CrossRef] [PubMed]
- Hewlin, R.L.; Ciero, A.; Kizito, J.P. Development of a Two-Way Coupled Eulerian–Lagrangian Computational Magnetic Nanoparticle Targeting Model for Pulsatile Flow in a Patient-Specific Diseased Left Carotid Bifurcation Artery. Cardiovasc. Eng. Technol. 2019, 10, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Forouzandehmehr, M. Personalised deposition maps for micro- and nanoparticles targeting an atherosclerotic plaque: Attributions to the receptor-mediated adsorption on the inflamed endothelial cells. Biomech. Modeling Mechanobiol. 2019, 18, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Meschi, S.S.; Farghadan, A.; Arzani, A. Flow topology and targeted drug delivery in cardiovascular disease. J. Biomech. 2021, 119, 110307. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem cells applications in regenerative medicine and disease therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, W.; Chen, X. The involving progress of MSCs based therapy in atherosclerosis. Stem Cell Res. Ther. 2020, 11, 216. [Google Scholar] [CrossRef]
- Salvolini, E.; Orciani, M.; Vignini, A.; Mattioli-Belmonte, M.; Mazzanti, L.; Di Primio, R.J.E.d. Skin-derived mesenchymal stem cells (S-MSCs) induce endothelial cell activation by paracrine mechanisms. Exp. Dermatol. 2010, 19, 848–850. [Google Scholar] [CrossRef]
- Kirwin, T.; Gomes, A.; Amin, R.; Sufi, A.; Goswami, S.; Wang, B.J.R.M. Mechanisms underlying the therapeutic potential of mesenchymal stem cells in atherosclerosis. Regen. Med. 2021, 16, 669–682. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Yet, S.-F.; Hsu, Y.-T.; Wang, G.-J.; Hung, S.-C. Mesenchymal stem cells ameliorate atherosclerotic lesions via restoring endothelial function. Stem Cells Transl. Med. 2015, 4, 44–55. [Google Scholar] [CrossRef]
- Abdel-Kawi, S.H.; Hashem, K.S. Possible therapeutic effect of stem cell in atherosclerosis in albino rats. A histological and immunohistochemical study. Int. J. Stem Cells 2015, 8, 200. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Zhu, X.; Li, Q.; Hu, L.; Li, H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Int. J. Stem Cells 2021, 53, 1227–1236. [Google Scholar]
- Yun, C.W.; Lee, S.H. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y.J.A. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Yin, R.H.; Zhu, X.Y.; Yang, S.N.; Wang, J.; Zhou, Z.F.; Pan, X.D.; Ma, A.J. Mesenchymal stem-cell-derived exosomal miR-145 inhibits atherosclerosis by targeting JAM-A. Mol. Ther.-Nucleic Acids 2021, 23, 119–131. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Afshari, J.T.; Esmaeili, S.A.; Saburi, E.; Joneidi, Z.; Momtazi-Borojeni, A.A. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis 2019, 285, 1–9. [Google Scholar] [CrossRef]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef]
- Ling, H.; Guo, Z.Y.; Tan, L.L.; Cao, Q.D.; Song, C.L. Stem cell-derived exosomes: Role in the pathogenesis and treatment of atherosclerosis. Int. J. Biochem. Cell Biol. 2021, 130, 105884. [Google Scholar] [CrossRef]
- Lu, X.J. The Role of Exosomes and Exosome-derived microRNAs in Atherosclerosis. Curr. Pharm. Des. 2017, 23, 6182–6193. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Regmi, S.; Pathak, S.; Kim, J.O.; Yong, C.S.; Jeong, J.H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef] [PubMed]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Cao, J.; Pathak, S.; Gupta, B.; Kumar Poudel, B.; Tung, P.T.; Yook, S.; Park, J.-B.; Yong, C.S.; Kim, J.O.; et al. A three-dimensional assemblage of gingiva-derived mesenchymal stem cells and NO-releasing microspheres for improved differentiation. Int. J. Pharm. 2017, 520, 163–172. [Google Scholar] [CrossRef]
- Sato, K.; Maeda, M.; Kamata, E.; Ishii, S.; Yanagisawa, K.; Kitajima, K.; Hara, T. Nitric Oxide and a Conditioned Medium Affect the Hematopoietic Development in a Microfluidic Mouse Embryonic Stem Cell/OP9 Co-Cultivation System. Micromachines 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, X.; Li, H.; Feng, G.; Nie, Y.; Wei, Y.; Li, N.; Han, Z.; Han, Z.-c.; Kong, D.; et al. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Acta Biomater. 2020, 113, 289–304. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Zhang, Z.; Chen, Y.; Su, H.; Deng, X.; Liu, X.; Fan, Y. Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 12166. https://doi.org/10.3390/ijms222212166
Ma T, Zhang Z, Chen Y, Su H, Deng X, Liu X, Fan Y. Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy. International Journal of Molecular Sciences. 2021; 22(22):12166. https://doi.org/10.3390/ijms222212166
Chicago/Turabian StyleMa, Tianxiang, Zhexi Zhang, Yu Chen, Haoran Su, Xiaoyan Deng, Xiao Liu, and Yubo Fan. 2021. "Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy" International Journal of Molecular Sciences 22, no. 22: 12166. https://doi.org/10.3390/ijms222212166
APA StyleMa, T., Zhang, Z., Chen, Y., Su, H., Deng, X., Liu, X., & Fan, Y. (2021). Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy. International Journal of Molecular Sciences, 22(22), 12166. https://doi.org/10.3390/ijms222212166






