Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities
Abstract
:1. Introduction
2. Essential Oils
3. Annonaceae Ethnobotanics
4. Phytochemistry of Annonaceae Essential Oils
5. Biological Activities
5.1. Antimicrobian Activity
5.2. Anti-Inflammatory Activity
5.3. Antileishmanial Activity
5.4. Antioxidant Activity
5.5. Antiproliferative and Cytotoxic Activities
5.6. Larvicidal Activity
5.7. Trypanocidal and Antimalarial Activities
5.8. Other Activities
6. Methodology
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fechine, I.M.; Lima, M.A.; Navarro, V.R.; da Cunha, E.V.L.; Silva, M.S.; Barbosa-Filho, J.M.; Maia, J.G.S. Alcalóides de Duguetia trunciflora Maas (Annonaceae). Rev. Bras. Farmacogn. 2002, 12, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128094419. [Google Scholar]
- De Souza Araújo, C.; De Oliveira, A.P.; Lima, R.N.; Alves, P.B.; Diniz, T.C.; Da Silva Almeida, J.R.G. Chemical constituents and antioxidant activity of the essential oil from leaves of Annona vepretorum Mart. (Annonaceae). Pharmacogn. Mag. 2015, 11, 615–618. [Google Scholar] [CrossRef] [Green Version]
- Lobão, A.Q.; Lopes, J.C.; Erkens, R.H.J.; Mendes-Silva, I.; Pontes Pires, A.F.; Silva, L.V.; Oliveira, M.L.B.; Johnson, D.; Mello-Silva, R. Annonaceae in Flora do Brasil. 2020. Available online: http://reflora.jbrj.gov.br/reflora/floradobrasil/FB110760 (accessed on 9 September 2021).
- De Lemos, E.E.P. A Produçâo de Anonáceas no Brasil. Rev. Bras. Frutic. 2014, 36, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.; Martins, I.; Pereira, J.; Correia, A.; Sampaio, R.; Silva, M.; Costa, V.; Silva, M.; Cavalcante, F.; Silva, B. Tocolytic action of essential oil from Annona leptopetala R. E. Fries is mediated by oxytocin receptors and potassium channels. In Proceedings of the International Conference Series on Multidisciplinary Sciences, Puyo, Ecuador, 20 March–20 December 2019; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, S.V.; Quintans, J.; de Sousa Siqueira Quintans, J.; Costa, E.V.; Guedes da Silva Almeida, J.R.; Quintans, L.J. Annona species (Annonaceae) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 221–229. ISBN 9780124166448. [Google Scholar]
- Rajca Ferreira, A.K.; Lourenço, F.R.; Young, M.C.M.; Lima, M.E.L.; Cordeiro, I.; Suffredini, I.B.; Lopes, P.S.; Moreno, P.R.H. Chemical composition and biological activities of Guatteria elliptica R. E. Fries (Annonaceae) essential oils. J. Essent. Oil Res. 2018, 30, 69–76. [Google Scholar] [CrossRef]
- Shaaban, H.A.; El-Ghorab, A.H. Bioactivity of essential oils and their volatile aroma components: Review Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Fournier, G.; Leboeuf, M.; Cavé, A. Annonaceae essential oils: A review. J. Essent. Oil Res. 1999, 11, 131–142. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.C.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.A.; Dias, R.B.; Soares, M.B.P.; Prata, A.P.N.; Rocha, C.A.G.; Costa, E.V.; Bezerra, D.P. Antitumor Properties of the Essential Oil from the Leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar] [CrossRef]
- Moura, A.P.G.; Beltrão, D.M.; Pita, J.C.L.R.; Xavier, A.L.; Brito, M.T.; de Sousa, T.K.G.; Batista, L.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Della Torre, A.; et al. Essential oil from fruit of Xylopia langsdorffiana: Antitumour activity and toxicity. Pharm. Biol. 2016, 54, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, A.A.; Vieira, L.; de Azambuja Ribeiro, R.I.M.; Thomé, R.G.; dos Santos, H.B.; Silva, D.B.; Carollo, C.A.; de Oliveira, F.M.; de Oliveira Lopes, D.; de Siqueira, J.M.; et al. Chemical composition and evaluation of the anti-inflammatory and antinociceptive activities of Duguetia furfuracea essential oil: Effect on edema, leukocyte recruitment, tumor necrosis factor alpha production, iNOS expression, and adenosinergic and opioidergic systems. J. Ethnopharmacol. 2019, 231, 325–336. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Silva, S.G.; da Cruz, J.N.; Ortiz, E.; da Costa, W.A.; Bezerra, F.W.F.; Cunha, V.M.B.; Cordeiro, R.M.; de Jesus Chaves Neto, A.M.; de Aguiar Andrade, E.H.; et al. Supercritical CO2 Application in Essential Oil Extraction. In Industrial Applications of Green Solvents—Volume II; Inamuddin, R.M., Asiri, A.M., Eds.; Materials Research Foundations: Millersville, PA, USA, 2019; pp. 1–28. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Hamid, A.A.; Aiyelaagbe, O.; Usman, L.A. Essential oils: Its medicinal and pharmacological uses. Int. J. Curr. Res. 2011, 3, 86–98. [Google Scholar]
- De Oliveira, M.S.; Cruz, J.N.; Ferreira, O.O.; Pereira, D.S.; Pereira, N.S.; Oliveira, M.E.C.; Venturieri, G.C.; Guilhon, G.M.S.P.; da Silva Souza Filho, A.P.; de Aguiar Andrade, E.H. Chemical Composition of Volatile Compounds in Apis mellifera Propolis from the Northeast Region of Pará State, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef] [PubMed]
- Santana de Oliveira, M.; Pereira da Silva, V.M.; Cantão Freitas, L.; Gomes Silva, S.; Nevez Cruz, J.; Aguiar Andrade, E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers. 2021, 18, e2000982. [Google Scholar] [CrossRef] [PubMed]
- Santana de Oliveira, M.; da Cruz, J.N.; Almeida da Costa, W.; Silva, S.G.; da Paz Brito, M.; de Menezes, S.A.F.; de Jesus Chaves Neto, A.M.; de Aguiar Andrade, E.H.; de Carvalho Junior, R.N. Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef]
- Bezerra, F.W.F.; de Oliveira, M.S.; Bezerra, P.N.; Cunha, V.M.B.; Silva, M.P.; da Costa, W.A.; Pinto, R.H.H.; Cordeiro, R.M.; da Cruz, J.N.; Chaves Neto, A.M.J.; et al. Extraction of bioactive compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–167. [Google Scholar]
- Silva, S.G.; de Oliveira, M.S.; Cruz, J.N.; da Costa, W.A.; da Silva, S.H.M.; Barreto Maia, A.A.; de Sousa, R.L.; Carvalho Junior, R.N.; de Aguiar Andrade, E.H. Supercritical CO2 extraction to obtain Lippia thymoides Mart. & Schauer (Verbenaceae) essential oil rich in thymol and evaluation of its antimicrobial activity. J. Supercrit. Fluids 2021, 168, 105064. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food. Macrobiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Kliszcz, A.; Danel, A.; Puła, J.; Barabasz-Krasny, B.; Mozdzen, K. Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules 2021, 26, 2473. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos Pereira, A.C.; das Graças Campolina Cunha, M. Medicina popular e saberes tradicionais sobre as propriedades medicinais da flora cerradeira. Hygeia-Rev. Bras. Geogr. Médica Saúde 2015, 67, 126–127. [Google Scholar]
- Santos, J.J.F.; Coelho-Ferreira, M.; Lima, P.G.C. Etnobotânica de plantas medicinais em mercados públicos da Região Metropolitana de Belém do Pará, Brasil. Biota Amaz. 2018, 8, 1–9. [Google Scholar]
- Vásquez, S.P.F.; de Mendonça, M.S.; do nascimento Noda, S. Etnobotânica de plantas medicinais em comunidades ribeirinhas do município de Manacapuru, Amazonas, Brasil. Acta Amaz. 2014, 44, 457–472. [Google Scholar] [CrossRef]
- Xavier, M.N.; Alves, J.M.; Carneiro, N.S.; Souchie, E.L.; Da Silva, E.A.J.; Martins, C.H.G.; Ambrosio, M.A.L.V.; Egea, M.B.; Alves, C.C.F.; Miranda, M.L.D. Chemical composition from essential oil of Cardiopetalum alophyllum Schltdl. (Annonaceae) and their antioxidant, antibacterial and antifungal activities. Rev. Virtual Quim. 2016, 8, 1433–1448. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef]
- Ferraz, R.P.C.; Cardoso, G.M.B.; Da Silva, T.B.; Fontes, J.E.D.N.; Prata, A.P.D.N.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumour properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013, 141, 542–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Quintans, J.S.S. Chemical Constituents and Anticancer Effects of the Essential Oil from Leaves of Xylopia laevigata. Planta Med. 2013, 79, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G.L. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.G.S.; Andrade, E.H.A.; Carreira, L.M.M.; Oliveira, J.; Araújo, J.S. Essential oils of the Amazon Guatteria and Guatteriopsis species. Flavour Fragr. J. 2005, 20, 478–480. [Google Scholar] [CrossRef]
- Da Silva, D.B.; Tulli, E.C.O.; Garcez, W.S.; Nascimento, E.A.; De Siqueira, J.M. Chemical constituents of the underground stem bark of Duguetia furfuracea (Annonaceae). J. Braz. Chem. Soc. 2007, 18, 1560–1565. [Google Scholar] [CrossRef] [Green Version]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Alves, M.S.; Araújo, A.A.L.; Pinto, M.A.O.; Amaral, M.P.H.; Rodarte, M.P.; Kaplan, M.A.C. Chemical composition and biological activities of the essential oils from Duguetia lanceolata St. Hil. barks. Molecules 2012, 17, 11056–11066. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, C.A.T.; Oliani, J.; Sartoratto, A.; Queiroga, C.L.; Moreno, P.R.H.; Reimão, J.Q.; Tempone, A.G.; Fischer, D.C.H. Chemical constituents of the volatile oil from leaves of Annona coriacea and in vitro antiprotozoal activity. Braz. J. Pharmacogn. 2011, 21, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, V.E.G.; Carvalho, D.A. Etnobotanical survey of medicinal plants in the dominion of meadows in the region of the alto rio grande—MINAS GERAIS. Cienc. Agrotec. 2001, 25, 102–123. [Google Scholar]
- Agra, M.D.F.; de Freitas, P.F.; Barbosa-Filho, J.M. Divulgação Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev. Bras. Farmacogn. 2007, 17, 114–140. [Google Scholar] [CrossRef] [Green Version]
- Brito, M.T.; Ferreira, R.C.; Beltrão, D.M.; Moura, A.P.G.; Xavier, A.L.; Pita, J.C.L.R.; Batista, T.M.; Longato, G.B.; Ruiz, A.L.T.G.; de Carvalho, J.E.; et al. Antitumor activity and toxicity of volatile oil from the leaves of Annona leptopetala. Braz. J. Pharmacogn. 2018, 28, 602–609. [Google Scholar] [CrossRef]
- Costa, E.V.; Dutra, L.M.; Jesus, H.C.R.; Nogueira, P.C.L.; Moraes, V.R.S.; Salvador, M.J.; Cavalcanti, S.C.H.; Santos, R.C.; Prata, A.P.N. Chemical Composition and Antioxidant, Antimicrobial, and Larvicidal Activities of the Essential Oils of Annona salzmannii and A. pickelii (Annonaceae). Nat. Prod. Commun. 2011, 6, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Meira, C.S.; Guimarães, E.T.; MacEdo, T.S.; Da Silva, T.B.; Menezes, L.R.A.; Costa, E.V.; Soares, M.B.P. Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L. (Annonaceae) leaves and their antimalarial and trypanocidal activities. J. Essent. Oil Res. 2015, 27, 160–168. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Vieira, M.D.C.; Dos Santos, L.A.C.; Cardoso, C.A.L.; Foglio, M.A.; De Carvalho, J.E.; Andrade-Silva, M.; Kassuya, C.A.L. Composition and evaluation of the anti-inflammatory and anticancer activities of the essential oil from Annona sylvatica A. St.-Hil. J. Med. Food 2013, 16, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.M.; Bomfim, L.M.; Rocha, S.L.A.; Nepel, A.; Soares, M.B.P.; Barison, A.; Costa, E.V.; Bezerra, D.P. Ent-Kaurane diterpenes from the stem bark of Annona vepretorum (Annonaceae) and cytotoxic evaluation. Bioorganic Med. Chem. Lett. 2014, 24, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Valter, J.L.; Alencar, K.M.C.; Sartori, Â.L.B.; Nascimento, E.A.; Chang, R.; De Morais, S.A.L.; Laura, V.A.; Yoshida, N.C.; Carollo, C.A.; Da Silva, D.B.; et al. Variação química no óleo essencial das folhas de seis indivíduos de Duguetia furfuracea (Annonaceae). Braz. J. Pharmacogn. 2008, 18, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.G.S.; Andrade, E.H.A.; Carla, A.; Silva, M.; Oliveira, J.; Carreira, L.M.M.; Araújo, J.S. Leaf volatile oils from four Brazilian Xylopia species. Flavour Fragr. J. 2005, 20, 474–477. [Google Scholar] [CrossRef]
- Alcântara, J.M.; De Lucena, J.M.V.M.; Facanali, R.; Marques, M.O.M.; Da Paz Lima, M. Chemical composition and bactericidal activity of the essential oils of four species of annonaceae growing in brazilian amazon. Nat. Prod. Commun. 2017, 12, 619–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, R.d.F.; Pinto, d.C.C.; da Silva, J.M.; da Silva, J.B.; Hermisdorf, R.C.d.S.; Fabri, R.L.; Chedier, L.M.; Scio, E. The essential oil from the fruits of the Brazilian spice Xylopia sericea A. St.-Hil. presents expressive in-vitro antibacterial and antioxidant activity. J. Pharm. Pharmacol. 2017, 69, 341–348. [Google Scholar] [CrossRef]
- De Alencar, D.C.; Pinheiro, M.L.B.; Pereira, J.L.D.S.; De Carvalho, J.E.; Campos, F.R.; Serain, A.F.; Tirico, R.B.; Hernández-Tasco, A.J.; Costa, E.V.; Salvador, M.J. Chemical composition of the essential oil from the leaves of Anaxagorea brevipes (Annonaceae) and evaluation of its bioactivity. Nat. Prod. Res. 2016, 30, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Cascaes, M.M.; Silva, S.G.; Cruz, J.N.; Santana de Oliveira, M.; Oliveira, J.; de Moraes, A.A.B.; da Costa, F.A.M.; da Costa, K.S.; Diniz do Nascimento, L.; de Aguiar Andrade, E.H. First report on the Annona exsucca DC. Essential oil and in silico identification of potential biological targets of its major compounds. Nat. Prod. Res. 2021, 35, 1–4. [Google Scholar] [CrossRef]
- Costa, E.V.; Dutra, L.M.; Salvador, M.J.; Ribeiro, L.H.G.; Gadelha, F.R.; De Carvalho, J.E. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 2013, 27, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.B.P.; Neto, A.F.S.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.R.C.E.; et al. Antitumour Activity of the Microencapsulation of Annona vepretorum Essential Oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef]
- Diniz, T.C.; de Oliveira Júnior, R.G.; Miranda Bezerra Medeiros, M.A.; Gama e Silva, M.; de Andrade Teles, R.B.; dos Passos Menezes, P.; de Sousa, B.M.H.; Abrahão Frank, L.; de Souza Araújo, A.A.; Russo Serafini, M.; et al. Anticonvulsant, sedative, anxiolytic and antidepressant activities of the essential oil of Annona vepretorum in mice: Involvement of GABAergic and serotonergic systems. Biomed. Pharmacother. 2019, 111, 1074–1087. [Google Scholar] [CrossRef]
- Costa, E.V.; Dutra, L.M.; Nogueira, P.C.L.; Moraes, V.R.S.; Salvador, M.J.; Ribeiro, L.H.G.; Gadelha, F.R. Essential oil from the leaves of Annona vepretorum: Chemical composition and bioactivity. Nat. Prod. Commun. 2012, 7, 265–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bay, M.; Souza de Oliveira, J.V.; Sales Junior, P.A.; Fonseca Murta, S.M.; Rogério dos Santos, A.; dos Santos Bastos, I.; Puccinelli Orlandi, P.; Teixeira de Sousa Junior, P. In Vitro Trypanocidal and Antibacterial Activities of Essential Oils from Four Species of the Family Annonaceae. Chem. Biodivers. 2019, 16, e1900359. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.R.; Da Silva, F.M.A.; De Almeida, R.A.; De Lima, B.R.; Koolen, H.H.F.; Lourenço, C.C.; Salvador, M.J.; Flach, A.; Da Costa, L.A.M.A.; De Souza, A.Q.L.; et al. Chemical composition and antimicrobial evaluation of the essential oils of Bocageopsis pleiosperma Maas. Nat. Prod. Res. 2015, 29, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.N.; Alves, C.C.F.; Cazal, C.d.M.; Santos, N.H. Chemical composition of the volatile oil of Cardiopetalum calophyllum collected in the Cerrado area. Ciência Rural 2016, 46, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Maia, D.S.; Lopes, C.F.; Saldanha, A.A.; Silva, N.L.; Sartori, Â.L.B.; Carollo, C.A.; Sobral, M.G.; Alves, S.N.; Silva, D.B.; de Siqueira, J.M. Larvicidal effect from different Annonaceae species on Culex quinquefasciatus. Environ. Sci. Pollut. Res. 2020, 27, 36983–36993. [Google Scholar] [CrossRef]
- De Sousa Orlando, V.; Glauciemar, D.V.V.; Bruna, C.S.S.; Ceacutelia, H.Y.; Santos de Matos Arajo, A.L.; da Luz, A.; Aparecida de Oliveira Pinto, M.; Pereira Rodarte, M.; Alves, M.S. In-vivo and vitro bioactivities of the essential oil of Duguetia lanceolata branches. Afr. J. Pharm. Pharmacol. 2016, 10, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.P.; Domingues, V.C.; Gonçalves, G.L.P.; Fernandes, J.B.; Glória, E.M.; Vendramim, J.D. Essential oil from Duguetia lanceolata St.-Hil. (Annonaceae): Suppression of spoilers of stored-grain. Food Biosci. 2020, 36, 100653. [Google Scholar] [CrossRef]
- Santos, A.R.; Benghi, T.G.S.; Nepel, A.; Marques, F.A.; Lobão, A.Q.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Carvalho, J.E.; Maia, B.H.L.N.S. In vitro Antiproliferative and Antibacterial Activities of Essential Oils from Four Species of Guatteria. Chem. Biodivers. 2017, 14, e1700097. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, C.A.T.; Serain, A.F.; Pascoal, A.C.R.F.; Andreazza, N.L.; De Lourenço, C.C.; Góis Ruiz, A.L.T.; De carvalho, J.E.; De Souza, A.C.O.; Tonini Mesquita, J.; Tempone, A.G.; et al. Bioactivity and chemical composition of the essential oil from the leaves of Guatteria australis A.St.-Hil. Nat. Prod. Res. 2015, 29, 1966–1969. [Google Scholar] [CrossRef]
- Aciole, S.D.G.; Piccoli, C.F.; Duque, J.E.L.; Costa, E.V.; Navarro-Silva, M.A.; Marques, F.A.; Maia, B.H.L.N.S.; Pinheiro, M.L.B.; Rebelo, M. Insecticidal activity of three species of Guatteria (Annonaceae) against Aedes aegypti (Diptera: Culicidae). Rev. Colomb. Entomol. 2011, 37, 262–268. [Google Scholar]
- Meira, C.S.; Menezes, L.R.A.; dos Santos, T.B.; Macedo, T.S.; Fontes, J.E.N.; Costa, E.V.; Pinheiro, M.L.B.; da Silva, T.B.; Teixeira Guimarães, E.; Soares, M.B.P. Chemical composition and antiparasitic activity of essential oils from leaves of Guatteria friesiana and Guatteria pogonopus (Annonaceae). J. Essent. Oil Res. 2017, 29, 156–162. [Google Scholar] [CrossRef]
- Costa, R.G.A.; Anunciação, T.A.D.; Araujo, M.d.S.; Souza, C.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Silva, F.M.A.D.; Koolen, H.H.F.; et al. In vitro and in vivo growth inhibition of human acute promyelocytic leukemia HL-60 cells by Guatteria megalophylla Diels (Annonaceae) leaf essential oil. Biomed. Pharmacother. 2020, 122, 109713. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.E.N.; Ferras, R.P.C.; Britto, A.C.S.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumor effect of the essential oil from leaves of Guatteria pogonopus (Annonaceae). Chem. Biodivers. 2013, 10, 722–729. [Google Scholar] [CrossRef] [PubMed]
- De Lima, B.R.; da Silva, F.M.A.; Soares, E.R.; de Almeida, R.A.; da Silva Filho, F.A.; Pereira Junior, R.C.; Hernandez Tasco, Á.J.; Salvador, M.J.; Koolen, H.H.F.; de Souza, A.D.L.; et al. Chemical composition and antimicrobial activity of the essential oils of Onychopetalum amazonicum R.E.Fr. Nat. Prod. Res. 2016, 30, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- De Lima, B.R.; da Silva, F.M.A.; Soares, E.R.; de Almeida, R.A.; Maciel, J.B.; Fernandes, C.C.; de Oliveira, A.C.; Tadei, W.P.; Koolen, H.H.F.; de Souza, A.D.L.; et al. Chemical composition and larvicidal activity of the essential oil from the leaves of Onychopetalum periquino (Rusby) D.M. Johnson & N.A. Murray. Nat. Prod. Res. 2019, 35, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.B.P.; Soares, M.G.; Mariane, B.; Vallim, M.A.; Pascon, R.C.; Sartorelli, P.; Lago, J.H.G. The Seasonal variation of the chemical composition of essential oils from Porcelia macrocarpa r.e. fries (annonaceae) and their antimicrobial activity. Molecules 2013, 18, 13574–13587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, N.C.; Saffran, F.P.; Lima, W.G.; Freire, T.V.; de Siqueira, J.M.; Garcez, W.S. Chemical characterization and bioherbicidal potential of the essential oil from the leaves of Unonopsis guatterioides (A.DC.) R.E.Fr. (Annonaceae). Nat. Prod. Res. 2019, 33, 3312–3316. [Google Scholar] [CrossRef]
- Nascimento, M.N.G.; Junqueira, J.G.M.; Terezan, A.P.; Severino, R.P.; De Souza Silva, T.; Martins, C.H.G.; Severino, V.G.P. Chemical composition and antimicrobial activity of essential oils from Xylopia aromatica (Annonaceae) flowers and leaves. Rev. Virtual Quim. 2018, 10, 1578–1590. [Google Scholar] [CrossRef]
- De Souza, I.L.L.; de Carvalho Correia, A.C.; da Cunha Araujo, L.C.; Vasconcelos, L.H.C.; da Conceicao Correia Silva, M.; de Oliveira Costa, V.C.; Tavares, J.F.; Paredes-Gamero, E.J.; de Andrade Cavalcante, F.; da Silva, B.A. Essential oil from Xylopia frutescens Aubl. reduces cytosolic calcium levels on guinea pig ileum: Mechanism underlying its spasmolytic potential. BMC Complement. Altern. Med. 2015, 15, 327. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, A.M.D.; Maia, T.D.S.; Soares, T.E.S.; Menezes, L.R.A.; Scher, R.; Costa, E.V.; Cavalcanti, S.C.H.; La Corte, R. Repellency and Larvicidal Activity of Essential oils from Xylopia laevigata, Xylopia frutescens, Lippia pedunculosa, and Their Individual Compounds against Aedes aegypti Linnaeus. Neotrop. Entomol. 2017, 46, 223–230. [Google Scholar] [CrossRef]
- Pereira, T.S.; Machado Esquissato, G.N.; Costa, E.V.; de Lima Nogueira, P.C.; de Castro-Prado, M.A.A. Mutagenic and cytostatic activities of the Xylopia laevigata essential oil in human lymphocytes. Nat. Prod. Res. 2019, 6419. [Google Scholar] [CrossRef]
- Costa, E.V.; Da Silva, T.B.; D’Souza Costa, C.O.; Soares, M.B.P.; Bezerra, D.P. Chemical composition of the essential oil from the fresh fruits of Xylopia laevigata and its cytotoxic evaluation. Nat. Prod. Commun. 2016, 11, 417–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gontijo, D.C.; do Nascimento, M.F.A.; Brandão, G.C.; de Oliveira, A.B. Phytochemistry and antiplasmodial activity of Xylopia sericea leaves. Nat. Prod. Res. 2019, 6419. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2019, 129, 108849. [Google Scholar] [CrossRef] [PubMed]
- Diniz Do Nascimento, L.; Antônio Barbosa De Moraes, A.; Santana Da Costa, K.; Marcos, J.; Galúcio, P.; Taube, P.S.; Leal Costa, M.; Neves Cruz, J.; Helena De Aguiar Andrade, E.; Guerreiro De Faria, L.J. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Alves, C.C.F.; Oliveira, J.D.; Estevam, E.B.B.; Xavier, M.N.; Nicolella, H.D.; Furtado, R.A.; Tavares, D.C.; Miranda, M.L.D. Antiproliferative activity of essential oils from three plants of the brazilian cerrado: Campomanesia adamantium (myrtaceae), Protium ovatum (burseraceae) and Cardiopetalum calophyllum (annonaceae). Braz. J. Biol. 2020, 80, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Cláudia, K.; Lidani, F.; Andrade, F.A.; Bavia, L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]


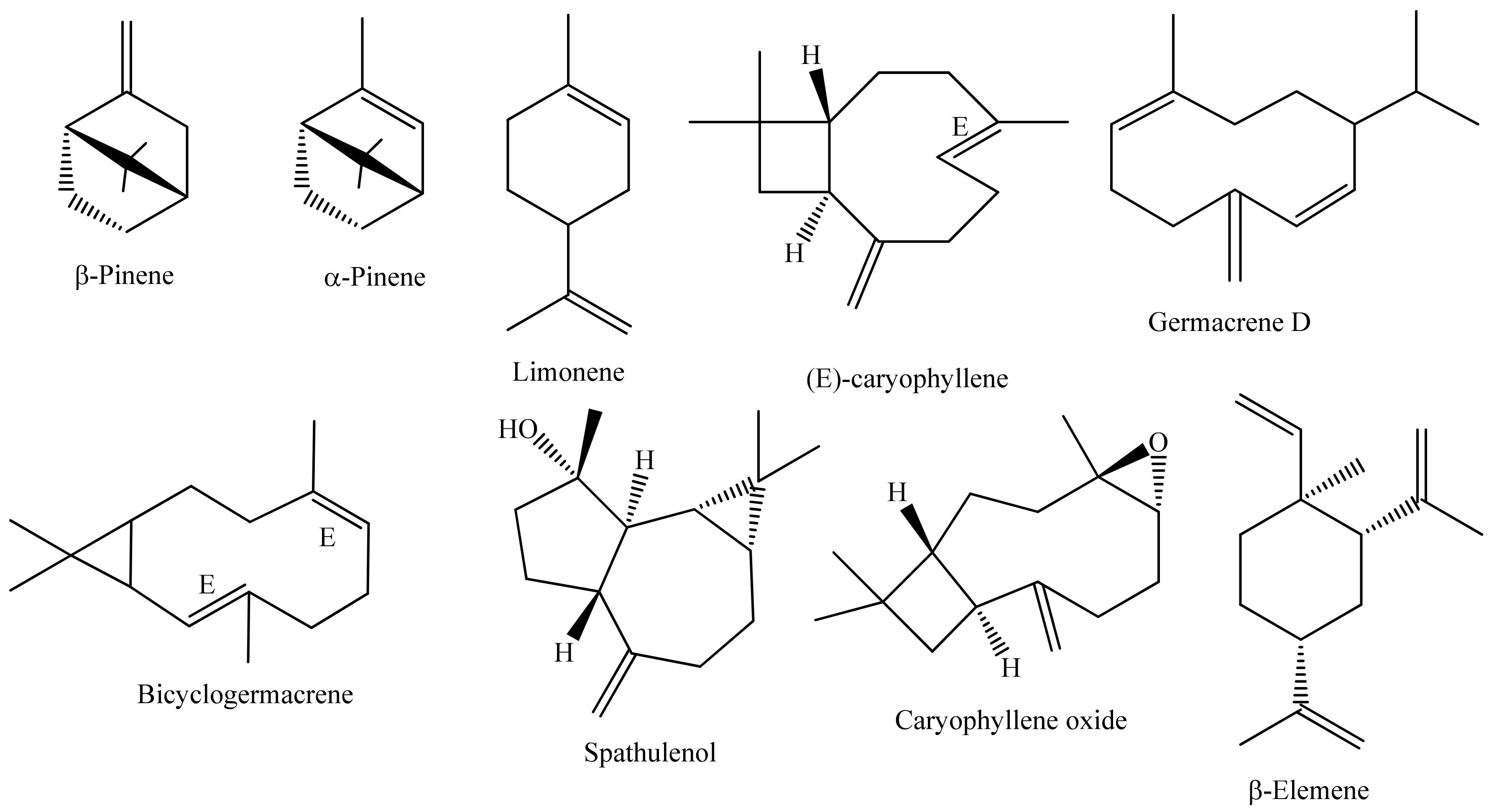

| Scientific Name | Popular Name | Brazil Region | Part of the Plant Used | Medicinal Use | Reference |
|---|---|---|---|---|---|
| Annona coriacea (Mart.) | Marolo, araticum and araticum-liso | Southeast | Not specified | Parasites, ulcers, inflammatory processes, rheumatism and anthelmintic | [39] |
| A. crassiflora Mart. | - | Not specified | Fruits and seeds (infusion) | Diarrhea | [31] |
| A. crassiflora | Marolo | Cerrado | Seeds | Chronic diarrhea | [40] |
| A. dioica St. Hil. | Araticum | Cerrado | Seeds, fruit and leaves | Chronic diarrhea, emollient and rheumatism | [40] |
| A. glabra L. | - | Not specified | Leaves | Rheumatism | [31] |
| A. glabra | Araticum and araticum do brejo | Northeast | Leaves | Rheumatism and vermifuge | [41] |
| A. leptopetala (R.E.Fr) H. Rainer | Pinha-brava | Northeast | Not specified | Anti-tumor and anti-inflammatory | [42] |
| A. montana Macfad | Graviola, araticum-grande and jaca-do-Pará | Northeast | Leaves | Snake bites and obesity | [41] |
| A. salzmannii A. DC. | - | Northeast | Leaves and bark | Diabetes, tumors, and inflammation | [43] |
| A. spinescens Mart | - | Not specified | Fruits and seeds | Ulcers | [31] |
| A. squamosa L. | - | Northeast | Leaves | Stimulants, antispasmodics, sweats, anthelmintics, and insecticides | [44] |
| A. squamosa | - | Not specified | Leaves | Boils and ulcers | [31] |
| A. squamosa | Pinha, ata and fruta-de-conde | Northeast | Seeds | Bath to remove lice | [41] |
| A. sylvatica A. St.-Hil | Araticum, araticum-do-mato, cortiça and cortiça-amarela | Southeast | Leaves | Fever, cough, ulcers caused by syphilis, muscle spasms, and diarrhea | [45] |
| A. vepretorum Mart. | Pinha da Caatinga | Northeast | Roots and Leaves | Bee and snake stings, inflammation, heart pain, bath for allergies, skin diseases, fungal and bacterial infections | [46] |
| Duguetia furfuracea (A.St.-Hil.) Saff | Araticum-seco | Cerrado | Seeds | Parasiticidal | [47] |
| D. furfuracea | Araticum-seco | Southeast | Stem bark | Bath to remove lice | [41] |
| D. furfuracea | Araticum seco | Cerrado | Branches with leaves | Rheumatism | [40] |
| D. lanceolata St. Hil. | - | Not specified | Leaves | Anti-inflammatory | [31] |
| D. lanceolata | Pindaíba, beribá and pinhão | Not specified | Not specified | Anti-inflammatory, healing, and antimicrobial | [38] |
| Guatteria ouregou (Aubl.) Dunal. | - | North | Seeds | Dyspepsia, stomach and uterine pain | [36] |
| Rollinia leptopetala R.E.Fr | Pinha-brava | Northeast | Stem bark | Stomachic | [41] |
| Xylopia aromatica (Lam.) Mart. | Pimenta-de-macaco | Cerrado | Fruits, leaves and stem bark | Digestive and anti- inflammatory | [40] |
| X. aromatica | Pimenta-de-macaco, pimenta-de-negro | North | Not specified | Carminative and stimulating | [48] |
| X. frutescens Aubl. | - | Not specified | Barks | Flu | [49] |
| X. frutescens | Embira and semente-de-embira | Northeast | Seeds and fruits | Digestive | [41] |
| X. frutescens | Embira, embira-vermelha and pau carne | Northeast | Seeds | Bladder stimulant, triggering menstruation, fighting rheumatism, for halitosis, for tooth decay, and for intestinal diseases | [33] |
| X. laevigata (Mart.) R. E. Fries | Meiú and pindaíba | Northeast | Leaves and flowers | Painful disorders, heart disease, and inflammatory conditions | [34] |
| X. sericea A. St.-Hil. | Embiriba and pindaíba | Southeast | Seeds and fruits | Analgesic and anti-inflammatory | [50] |
| X. sericea | Pindaíba, pindaíba-vermelha and/or pimenta-de-macaco | Southeast | Not specified | Antimalarial | [35] |
| Species | Collection Place | Collection Date | Part of Plant (Yield) | Extraction Technique | Majority Constituents (% >5); Substance Classes; Total | Reference |
|---|---|---|---|---|---|---|
| Anaxagorea Brevipes Benth | Amazonas | September 2009 | Leaves (0.52%) | HD | Guaiol, γ-eudesmol, β-eudesmol and α-eudesmol; M: 3.35%, SH: 13.56%; T: 75.69% | [51] |
| Annona coriacea Mart. | São Paulo | October 2009 | Leaves (0.05%) | HD | Bicyclogermacrene, γ-muurolene and δ-cadinene; M: 20.0%, S: 76.7%; O: 3.3%; T: 96.5% | [39] |
| A. exsucca DC. | Pará | March 2019 | Leaves (NI) | HD | Linalool, β-elemene, (E)-caryophyllene, α-humulene, germacrene D and bicyclogermacrene; HM: 2.3%, OM: 11.61%, SH: 80.52%, OS: 4.07%; T: 99.34% | [52] |
| A. exsucca | Pará | September 2019 | Leaves (NI) | HD | p-Cymene, sylvestrene, terpinolene, linalool, germacrene D and bicyclogermacrene; HM: 43.36%, OM: 19.39%, SH: 31.29%, OS: 5.10%; T: 99.14% | [52] |
| A. leptopetala (R.E.Fr.) H. Rainer | Paraíba | August 2016 | Leaves (0.04%) | HD | α-Limonene, linalool, α-terpineol, (E)-caryophyllene, bicyclogermacrene, spathulenol and guaiol; T: 98.1% | [42] |
| A. pickelii (Diels) H. Rainer | Sergipe | March 2010 | Leaves (0.2%) | HD | Bicyclogermacrene, (E)-caryophyllene, α-copaene and germacrene D; M: 0.6%, S: 97.7%; T: 98.3% | [43] |
| A. pickelii | Sergipe | September 2010 | Leaves (0.3%) | HD | Bicyclogermacrene, (E)-caryophyllene and α-copaene; T: 99.5% | [53] |
| A. salzmannii A. DC. | Sergipe | March 2010 | Leaves (0.1%) | HD | Bicyclogermacrene, (E)-caryophyllene, δ-cadinene, α-copaene, and allo-aromadendrene; M: 2.5%, S: 93.7%; T: 96.2% | [43] |
| A. salzmannii | Sergipe | September 2010 | Leaves (0.04%) | HD | δ-cadinene, (E)-caryophyllene, α-copaene, bicyclogermacrene and germacrene D; T: 98.7% | [53] |
| A. squamosa L. | Sergipe | September 2012 | Leaves | HD | (E)-Caryophyllene, germacrene D and bicyclogermacrene; M: 2.0%; S: 65.1%; T: 99.1% | [44] |
| A. sylvatica A. St.-Hil Anelise | Mato Grosso do Sul | September 2010 | Leaves (0.17%) | HD | Hinesol, (Z)-caryophyllene, β-malien, γ-gurjunene; T: 98.97% | [45] |
| A. vepretorum Mart. | Sergipe | April 2012 | Leaves (0.59%) | HD | α-Phellandrene, o-cymene, (E)-β-ocimene, bicyclogermacrene and spathulenol; M: 30.18%, S: 67.41%, T: 97.59% | [54] |
| A. vepretorum | Pernambuco | January 2012 | Leaves (0.09%) | HD | α-Pinene, limonene, spathulenol and caryophyllene oxide; T: 93.9% | [3] |
| A. vepretorum | Pernambuco | April 2015 | Leaves (NI) | HD | Limonene, (E)-β-ocimene, germacrene D and bicyclogermacrene | [55] |
| A. vepretorum | Sergipe | April 2010 | Leaves (NI) | HD | Bicyclogermacrene, spathulenol, α-phellandrene, α-pinene, (E)-β-ocimene, germacrene D and p-cymene; M: 29.2%, S: 68.9%; T: 98.1% | [56] |
| A. vepretorum | Sergipe | March 2012 | Leaves (0.76%) | HD | Bicyclogermacrene, spathulenol and α-phellandrene; M: 34.0%; S: 65.1%; T: 99.1% | [44] |
| Bocageopsis multiflora (Mart.) R.E. Fr. | Amazonas | June 2013 | Leaves (0.34%) | HD | α-trans-Bergamotene, β-bisabolene, spathulenol and β-copaen-4-α-ol; HM: 0.3%, OM: 1.0%, SH: 34.3%, OS: 49.5%, T: 95.0% | [49] |
| B. multiflora | Rondônia | July 2018 | Aerial parts (0.12%) | HD | cis-Linalool oxide (furanoid) and 1-epi-cubenol | [57] |
| B. pleiosperma Maas | Amazonas | NI | Leaves (0.28%) | HD | (E)-α-Bergamotene, (E)-β-farnesene and β-bisabolene; T: 87.64% | [58] |
| B. pleiosperma | Amazonas | NI | Barks (0.27%) | HD | β-Selinene, α-selinene, β-bisabolene and δ-cadinene; T: 97.11% | [58] |
| B. pleiosperma | Amazonas | NI | Twigs (0.25%) | HD | β-Bisabolene, (2Z,6Z)-farnesol and cryptomerone; T: 72.64% | [58] |
| Cardiopetalum calophyllum (Schltdl.) | Goiás | September 2014 | Flowers (NI) | HD | (E)-Caryophyllene, germacrene D and germacrene B; M: 0.51%, S: 70.11% | [59] |
| C. calophyllum | Goiás | December 2014 | Fruits (NI) | HD | Germacrene D, germacrene B and spathulenol; M: 0.55%, S: 73.29% | [59] |
| C. calophyllum | Goiás | March 2014 | Leaves (NI) | HD | Spathulenol, viridiflorol, (–)-isolongifolol acetate, and (Z,E)-farnesol; M: 0.43%, S: 66.04% | [59] |
| Duguetia furfuracea (A. St. -Hil.) Saff. | Minas Gerais | August 2016 | Stem bark (0.5%) | SD | Cyperene, α-gurjunene, bicyclogermacrene, 2,4,5-trimethoxystyrene and (E)-asarone; T: 99.5% | [13] |
| D. furfuracea | Minas Gerais | August 2016 | Leaves (0.8%) | HD | Spathulenol and bicyclogermacrene | [60] |
| D. furfuracea | Minas Gerais | August 2016 | Underground parts (wood) (0.7%) | HD | (E)-Asarone, cyperene, 2,4,5-trimethoxystyrene, bicyclogermacrene and α-gurjunene | [60] |
| D. furfuracea | Minas Gerais | August 2016 | Underground parts (trunk) (0.9%) | HD | (E)-Asarone and 2,4,5-trimethoxystyrene | [60] |
| D. lanceolata St. Hil. | Minas Gerais | April 2012 | Twigs (0.4%) | HD | β-Elemene, β-caryophyllene, β-selinene, δ-cadinene, caryophyllene oxide, humulene II epoxide, β-eudesmol and cadina-1,4-dien-3-ol; HM: 4.0%, OM: 3.8%, SH: 40.0%, OS: 44.9%; T: 92.9% | [61] |
| D. lanceolata | Minas Gerais | NI | Leaves (0.4%) | HD | α-Selinene, aristolochene, (E)-caryophyllene and (E)-calamenene | [60] |
| D. lanceolata | São Paulo | March 2012 | Leaves (0.3%) | HD | trans-Muurola-4(14),5-diene, β-bisabolene, 3,4,5- trimethoxy-styrene and 2,4,5-trimethoxy-styrene | [62] |
| D. lanceolata | Minas Gerais | NI | Barks (0.5%) | HD | β-elemene, caryophyllene oxide and β-selinene; HM: 1.6%, OM: 5.9%, SH: 31.9%, OS: 59.8%, H: 0.4%; T: 99.6% | [38] |
| D. quitarensis Benth. | Rondônia | June 2018 | Aerial parts (0.11%) | HD | 4-Heptanol, α-thujene, α-copaene, (E)-caryophyllene and germacrene D; M: 21.2%, OM: 2.5%, S: 37.8%, OS: 1.4%; T: 97.3% | [57] |
| D. gardneriana Mart. | Sergipe | November 2013 | Leaves (0.13%) | HD | β-Bisabolene and elemicin; S: 96.0%; T: 96.0% | [11] |
| Ephedranthus amazonicus R.E. Fr | Amazonas | September 2012 | Leaves (0.2%) | HD | Cyclosativene, α-muurolene, spathulenol, caryophyllene oxide and humulene epoxide II; OM: 0.6%, SH: 20.8%, OS: 74.2%; T: 98.0% | [49] |
| Fusaea longifolia Saff | Rondônia | July 2018 | Aerial parts (0.18%) | HD | (E)-Caryophyllene, β-selinene, cis-β-guayene and (Z)-α-bisabolene; M: 0.1%, S: 85.6%, OS: 2.0%; T: 88.5% | [57] |
| Guatteria australis A. ST.-HIL. | Rio de Janeiro | February 2011 | Aerial parts (0.1%) | HD | β-Pinene, trans-pinocarveol, trans-verbenol, myrtenol, spathulenol and caryophyllene oxide; M: 14.45%, OM: 27.47%, S: 0.76%, OS: 51.89%; T: 94.26% | [63] |
| G. australis | São Paulo | NI | Leaves (0.16%) | HD | (E)-Caryophyllene, germacrene D and germacrene B; M: 17.24%, S: 79.40%; T: 96.64% | [64] |
| G. blepharophylla Mart. | Amazonas | September 2012 | Leaves (0.16%) | HD | Palustrol, spathulenol and caryophyllene oxide; SH: 6.4%, OS: 88.0%; O: 4.6%; T: 99.0% | [49] |
| G. blepharophylla | Amazonas | January 2008 | Leaves (0.3%) | HD | Caryophyllene oxide; M: 0.1%, S: 91.2%; T: 91.3% | [65] |
| G. elliptica R. E. Fries | São Paulo | NI | Leaves (0.11%) | HD | Spathulenol and caryophyllene oxide; SH: 0.5%, OS: 99.5%; T: 100.0% | [9] |
| G. elliptica | São Paulo | NI | Leaves (0.21%) | HD | Spathulenol, caryophyllene oxide and β-copaen-α-ol; SH: 9.5%, OS: 91.5%, O: 0.5%; T: 100.0% | [9] |
| G. friesiana (W.A.Rodrigues) Erkens & Maas | Amazonas | NI | Leaves (1.17%) | HD | γ-Eudesmol, β-eudesmol and α-eudesmol; S: 93.0%; T: 93.0% | [66] |
| G. friesiana | Amazonas | January 2008 | Leaves (0.6%) | HD | β-Eudesmol, γ-eudesmol e α-eudesmol; S: 98.2%; T: 98.2% | [65] |
| G. hispida (R.E. Fries) | Amazonas | July 2008 | Leaves (0.5%) | HD | (E)-Caryophyllene; M: 68.4%, S: 31.0%; T: 99.4% | [65] |
| G. latifolia (Mart.) R.E.Fr. | Rio de Janeiro | February 2011 | Aerial parts (0.1%) | HD | Spathulenol and caryophyllene oxide; OM: 6.94%, S: 3.35%, OS: 64.46%; T: 73.24% | [63] |
| G. megalophylla Diels | Amazonas | September 2018 | Leaves (0.12%) | HD | δ-elemene, β-elemene, γ-muurolene, bicyclogermacrene and spathulenol; M: 1.41%, S: 87.30%; T: 88.71 | [67] |
| G. pogonopus Mart. | Sergipe | NI | Leaves (0.22%) | HD | Germacrene D, γ-amorphene and spathulenol; S: 88.4%; T: 88.4% | [66] |
| G. pogonopus | Sergipe | February 2012 | Leaves (0.28%) | HD | α-Pinene, β-pinene, (E)-caryophyllene, germacrene D, bicyclogermacrene and γ-patchoulene; M: 23.13%, S: 60.44%; T: 86.19% | [68] |
| G. punctata (Aubl.) R. A. Howard. | Rondônia | September 2018 | Aerial parts (0.39%) | HD | (E)-Caryophyllene, germacrene D, cis-β-guayene and (E)-nerolidol; HO: 2.8%; M: 0.6%; S: 56.8%; OS: 19.1%; T: 79.3% | [57] |
| G. sellowiana Schltdl | Rio de Janeiro | February 2011 | Aerial parts (0.1%) | HD | (Z)-β-Farnesene, β-bisabolene, cis-α-bisabolene, spathulenol and caryophyllene oxide; OM: 5.16%; S: 6.55%; OS: 78.28%; T: 89.99% | [63] |
| G. ferruginea A. St.-Hil. | Rio de Janeiro | February 2011 | Aerial parts (0.1%) | HD | trans-Pinocarveol, myrtenol, (E,E)-α-farnesene, spathulenol and caryophyllene oxide; M: 1.47%; OM: 24.54%; S: 1.91%; OS: 60.41%; T: 88.33% | [63] |
| Onychopetalum amazonicum R.E.Fr. | Amazonas | March 2015 | Leaves (0.18%) | HD | α-Copaene, (E)-caryophyllene, bicyclogermacrene, δ-cadinene, spathulenol and caryophyllene oxide; SH: 60.7%, OS: 27.1%; T: 87.8% | [69] |
| O. amazonicum | Amazonas | March 2015 | Trunk bark (0.37%) | HD | α-Gurjunene, allo-aromadendrene and α-epi-cadinol; SH: 56.9%; OS: 35.3%; T: 92.2% | [69] |
| O. amazonicum | Amazonas | March 2015 | Twigs (0.34%) | HD | α-Gurjunene, α-epi-cadinol and cyperotundone; SH: 27.5%; OS: 47.5%; T: 75.0% | [69] |
| O. periquino (Rusby) D.M. Johnson & N.A. Murray | Acre | March 2017 | Leaves (0.24%) | HD | β-elemene, β-selinene and spathulenol; SH: 78.86%; OS: 12.45%; T: 91.31% | [70] |
| Porcelia macrocarpa R.E. Fries | São Paulo | NI | Leaves | HD | Germacrene D, bicyclogermacrene and phytol; M: 0.39%; S: 76.0%; D: 7.3%; T: 84.0% | [71] |
| P. macrocarpa | São Paulo | November 2011 | Fruits | HD | Neril, geranil formate, γ-muurolene, δ-cadinene, dendrolasin, hexacosane; M: 44.8%; S: 37.1%; D: 0.51%; HC: 10.49%; O: 6.7%; T: 99.6% | [71] |
| Unonopsis guatterioides (A.DC.) R.E.Fr | Mato Grosso do Sul | March 2005 | Leaves (0.15%) | HD | α-Copaene, β-elemene, (E)-caryophyllene, α-humulene, allo-aromadendrene, germacrene D, bicyclogermacrene and spathulenol | [72] |
| Xylopia aromatica (Lam.) Mart. | Amazonas | September 2012 | Leaves (0.25%) | HD | trans-Pinocarveol, α-campholenal, camphor, dihydrocarveol, verbenone and spathulenol; HM: 2.2%; OM: 52.3%; SH: 14.6%; OS: 29.5%; T: 98.6% | [49] |
| X. aromatica | Goiás | February 2015 | Leaves (0.1%) | HD | Bicyclogermacrene, spathulenol, globulol, cis-guaia-3,9-dien-11-ol and khusinol; OM: 2.74%, SH: 9.62%, OS: 71.25%, D: 1.2%, O: 13.15%; T: 97.96% | [73] |
| X. aromatica | Goiás | October 2014 | Flowers (0.2%) | HD | Bicyclogermacrene, 7-epi-α-eudesmol, khusinol, pentadecan-2-one and n-tricosane; OM: 3.44%; SH: 17.24%; OS: 51.7%; D: 6.88%, O: 20.67%; T: 99.93% | [73] |
| X. frutescens Aubl. | Paraíba | April 2010 | Leaves (NI) | HD | (E)-Caryophyllene, γ-cadinene, β-ocimene and cadin-4-en-10-ol; T: 90.20% | [74] |
| X. frutescens | Sergipe | April 2013 | Leaves (NI) | HD | (E)-β-Ocimene, β-elemene, (E)-caryophyllene, germacrene D and bicyclogermacrene | [75] |
| X. frutescens | Sergipe | July 2011 | Leaves (1.0%) | HD | (E)-Caryophyllene, bicyclogermacrene, germacrene D, δ-cadinene, viridiflorene and α-copaene; M: 0.41%; S: 96.10%; T: 96.51% | [33] |
| X. laevigata (Mart.) R. E. Fries | Sergipe | NI | Leaves (1.4%) | HD | Germacrene D, bicyclogermacrene, (E)-caryophyllene and germacrene B; T: 98.68% | [76] |
| X. laevigata | Sergipe | November 2012 | Fresh fruits (0.4%) | HD | α-Pinene, β-pinene and limonene; M: 95.0%; S: 4.6%; T: 99.6% | [77] |
| X. laevigata | Sergipe | April 2013 | Leaves | HD | (E)-Caryophyllene, γ-muurolene, germacrene D, bicyclogermacrene, δ-cadinene and germacrene B | [75] |
| X. laevigata | Sergipe | April 2010 | Leaves (>1.0%) | HD | γ-Muurolene, δ-cadinene, germacrene D, bicyclogermacrene, α-copaene and (E)-caryophyllene; M: 2.14%, S: 95.35%; T: 97.49% | [34] |
| X. laevigata | Sergipe | March 2010 | Leaves (1.58%) | HD | Germacrene D, bicyclogermacrene and (E)-caryophyllene; M: 1.15%, S: 98.60%; T: 99.75% | [34] |
| X. laevigata | Sergipe | July 2010 | Leaves (>1.0%) | HD | Germacrene D, bicyclogermacrene, (E)-caryophyllene and germacrene B; M: 7.28%, S: 91.18%; D: 0.22 T: 98.68% | [34] |
| X. langsdorffiana St.-Hil. & Tul. | Paraíba | July 2012 | Fresh fruits (0.03%) | HD | α-Pinene, camphene, D-limonene, caryophyllene oxide and esclarene; T: 100.0% | [12] |
| X. sericea A. St.-Hil. | Minas Gerais | September 2011 | Fruits (0.93%) | HD | Germacrene D, spathulenol and guaiol; M: 9.65%; S: 81.5%; D: 7.79%; O: 0.1%; T: 99.04% | [50] |
| X. sericea | Minas Gerais | July 2012 | Leaves (0.5%) | HD | α-Pinene, β-pinene, o-cymene and D-limonene | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascaes, M.M.; Carneiro, O.d.S.; Nascimento, L.D.d.; de Moraes, Â.A.B.; de Oliveira, M.S.; Cruz, J.N.; Guilhon, G.M.S.P.; Andrade, E.H.d.A. Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. Int. J. Mol. Sci. 2021, 22, 12140. https://doi.org/10.3390/ijms222212140
Cascaes MM, Carneiro OdS, Nascimento LDd, de Moraes ÂAB, de Oliveira MS, Cruz JN, Guilhon GMSP, Andrade EHdA. Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. International Journal of Molecular Sciences. 2021; 22(22):12140. https://doi.org/10.3390/ijms222212140
Chicago/Turabian StyleCascaes, Márcia Moraes, Odirleny dos Santos Carneiro, Lidiane Diniz do Nascimento, Ângelo Antônio Barbosa de Moraes, Mozaniel Santana de Oliveira, Jorddy Neves Cruz, Giselle Maria Skelding Pinheiro Guilhon, and Eloisa Helena de Aguiar Andrade. 2021. "Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities" International Journal of Molecular Sciences 22, no. 22: 12140. https://doi.org/10.3390/ijms222212140
APA StyleCascaes, M. M., Carneiro, O. d. S., Nascimento, L. D. d., de Moraes, Â. A. B., de Oliveira, M. S., Cruz, J. N., Guilhon, G. M. S. P., & Andrade, E. H. d. A. (2021). Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. International Journal of Molecular Sciences, 22(22), 12140. https://doi.org/10.3390/ijms222212140









