Role of Hydrogen Sulfide, Substance P and Adhesion Molecules in Acute Pancreatitis
Abstract
1. Introduction
2. Acute Pancreatitis
3. Inflammatory Mediators
3.1. Hydrogen Sulfide (H2S)
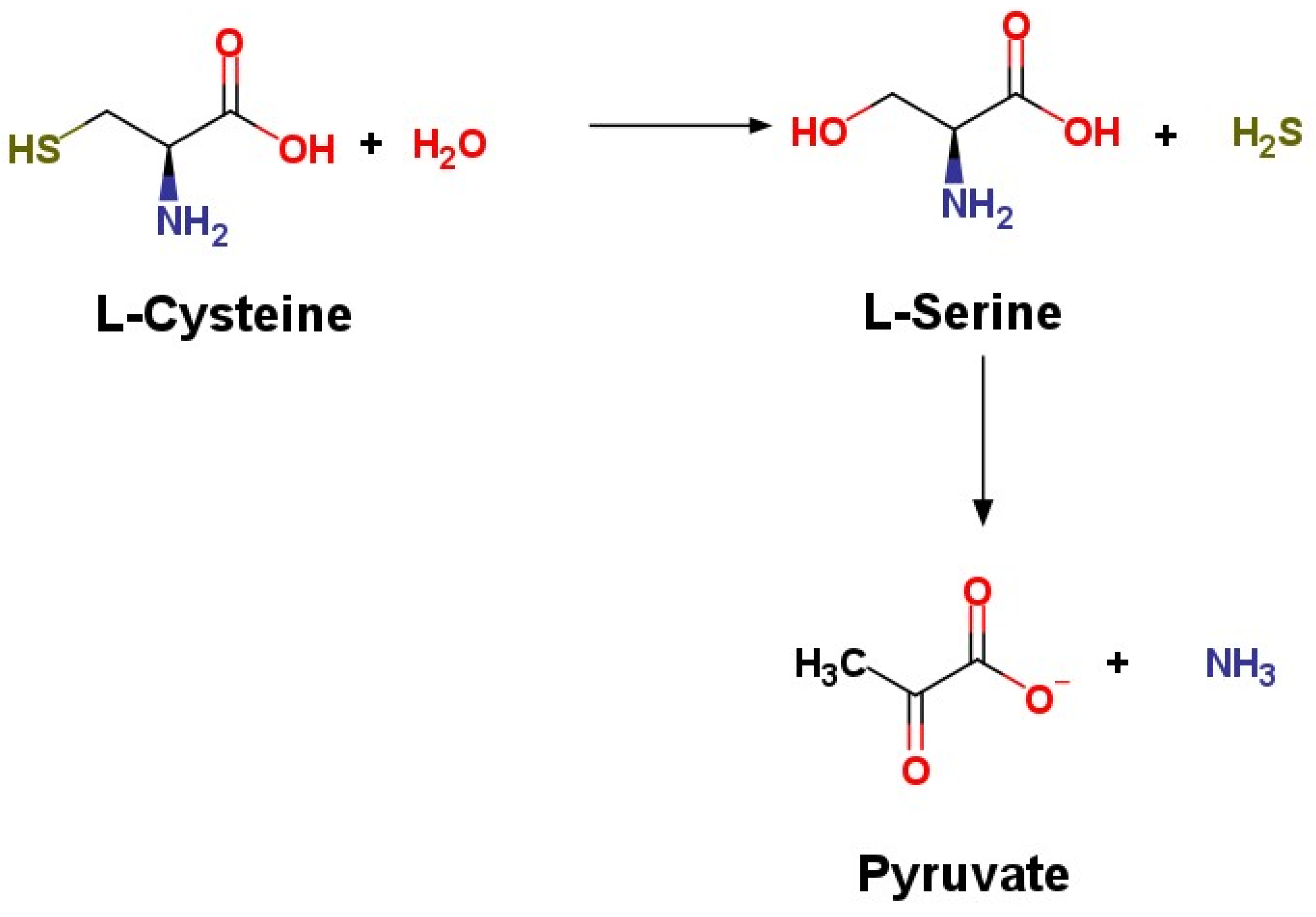
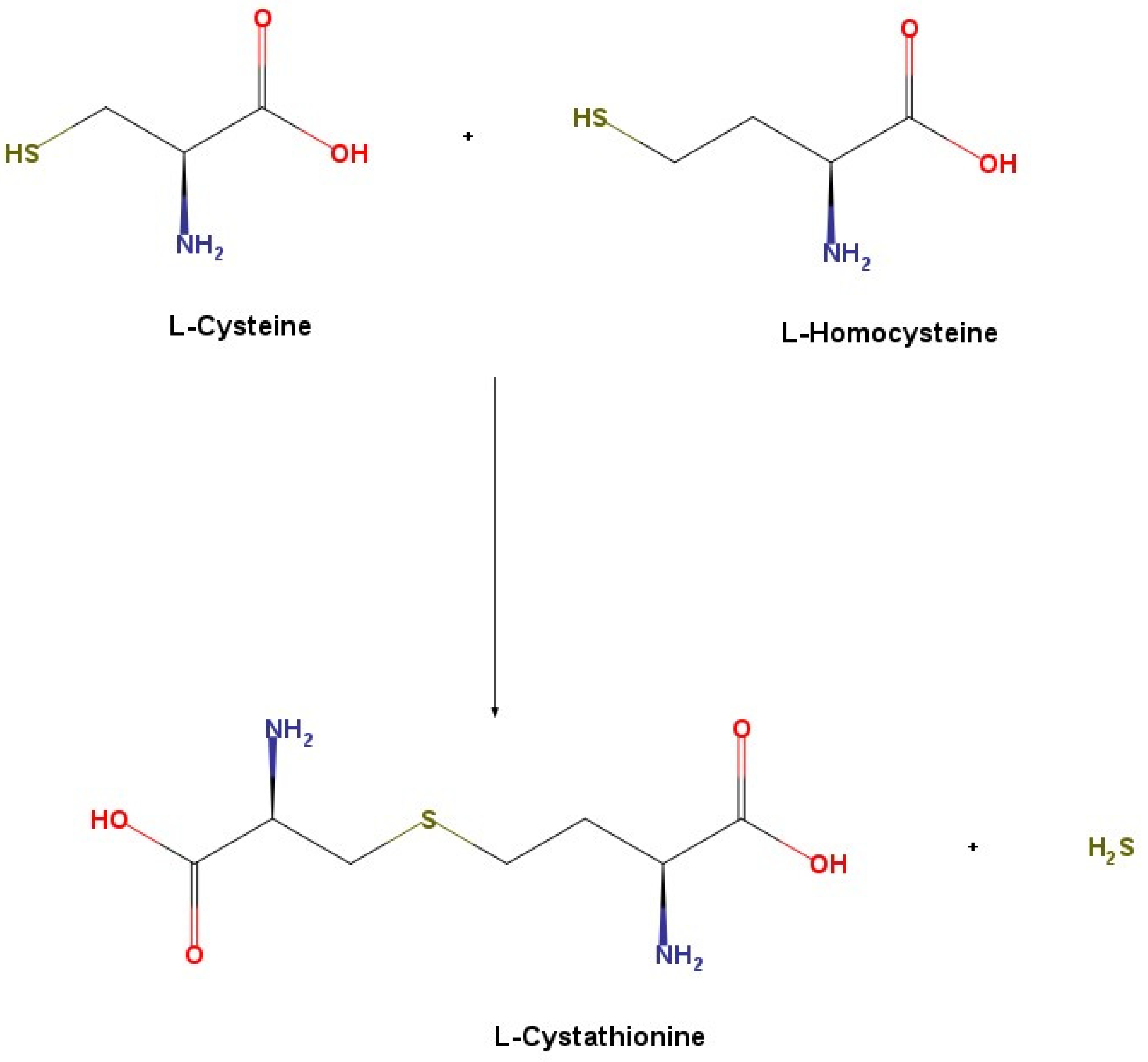
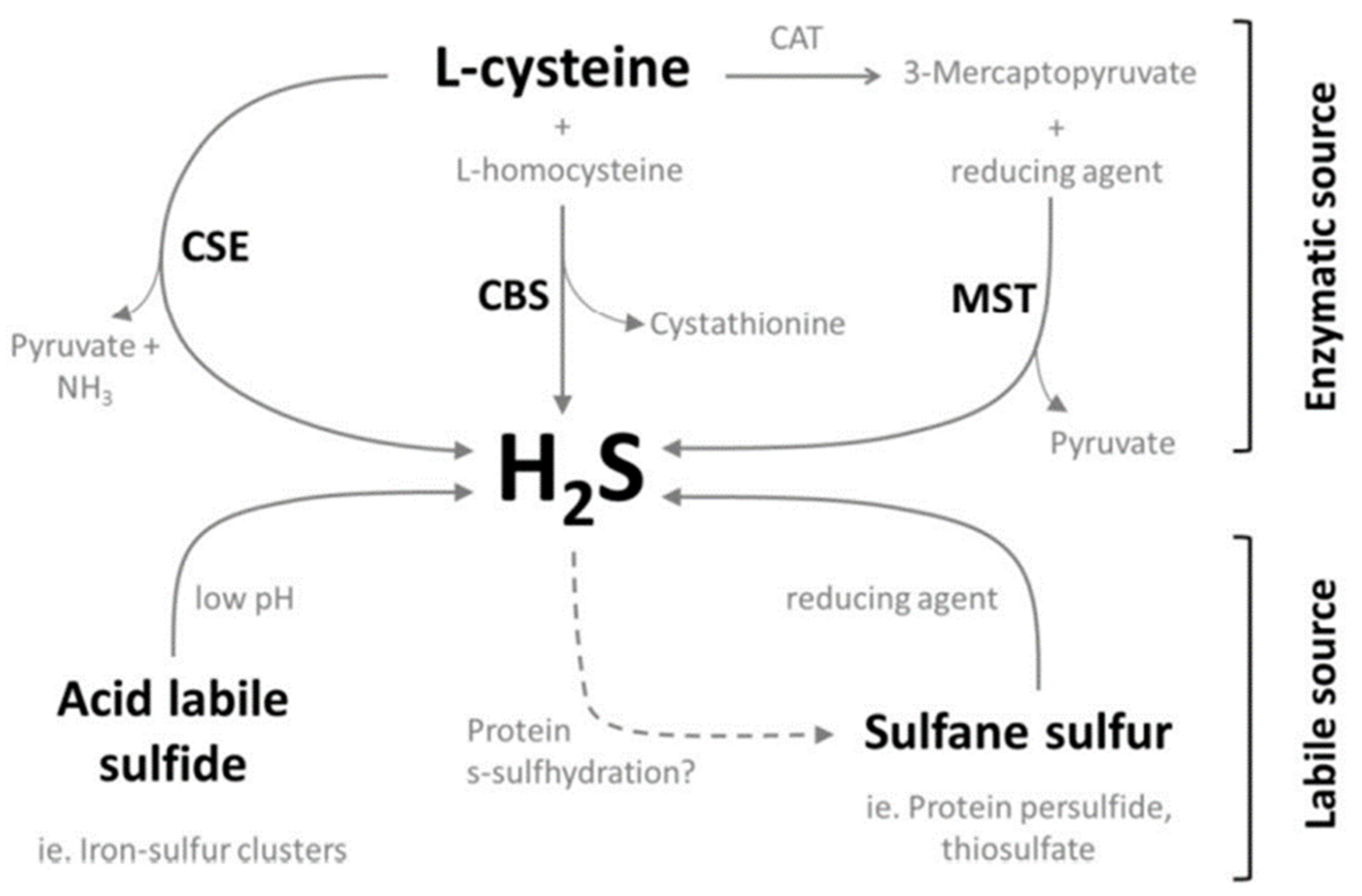
3.1.1. Synthesis
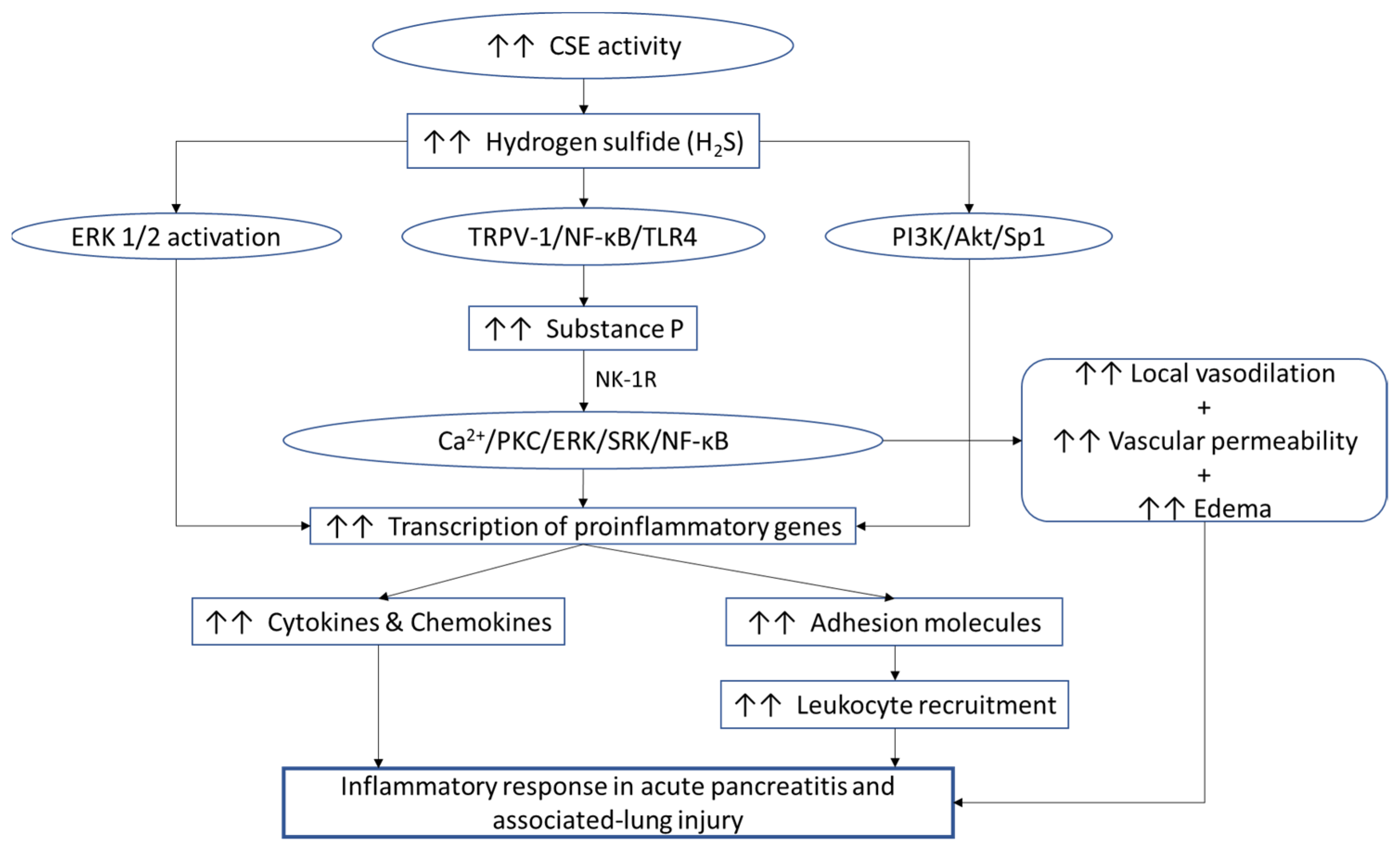
3.1.2. Role in Acute Pancreatitis
3.2. Substance P (SP)
Role in Acute Pancreatitis
3.3. Interaction between H2S and Substance P
3.4. Role of Endothelial Cells in Acute Pancreatitis
3.5. Adhesion Molecules
3.5.1. ICAM-1
3.5.2. VCAM-1
3.5.3. MAdCAM-1
3.5.4. VAP-1
3.5.5. Hyaluronic Acid or Hyaluronan
3.5.6. Selectins
3.5.7. Other Adhesion Molecules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Abbas, A.; Aster, J.; Turner, J.; Perkins, J.; Robbins, S. Robbins & Cotran Basic Pathology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 60–65, 680–683. [Google Scholar]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nature Reviews. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef]
- Wadhwa, V.; Patwardhan, S.; Garg, S.K.; Jobanputra, Y.; Lopez, R.; Sanaka, M.R. Health Care Utilization and Costs Associated with Acute Pancreatitis. Pancreas 2017, 46, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Vipperla, K.; Papachristou, G.I.; Easler, J.; Muddana, V.; Slivka, A.; Whitcomb, D.C.; Yadav, D. Risk of and factors associated with readmission after a sentinel attack of acute pancreatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1911–1919. [Google Scholar] [CrossRef]
- Ahmed Ali, U.; Issa, Y.; Hagenaars, J.C.; Bakker, O.J.; van Goor, H.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; Brink, M.A.; et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin. Gastroenterol. Hepatol. 2016, 14, 738–746. [Google Scholar] [CrossRef]
- Munigala, S.; Subramaniam, D.; Subramaniam, D.P.; Buchanan, P.; Xian, H.; Burroughs, T.; Trikudanathan, G. Predictors for early readmission in acute pancreatitis (AP) in the United States (US)—A nationwide population based study. Pancreatology 2017, 17, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Bhatia, M.; Gaddam, R.R. Hydrogen Sulfide in Inflammation: A Novel Mediator and Therapeutic Target. Antioxid. Redox Signal. 2021, 34, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef]
- Bao, L.; Vlcek, C.; Paces, V.; Kraus, J.P. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch. Biochem. Biophys. 1998, 350, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Kitamura, H.; Nishino, T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998, 110, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Akahoshi, N.; Yu, X.N.; Kobayashi, Y.; Namekata, K.; Komaki, G.; Kimura, H. Murine cystathionine gamma-lyase: Complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem. J. 2004, 381, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kimura, T.; Taniguchi, S.; Souma, M.; Kojima, Y.; Kimura, Y.; Kimura, H.; Niki, I. Glucoseinduced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett. 2009, 583, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Tangerman, A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J. Chromatogr. B 2009, 877, 3366–3377. [Google Scholar] [CrossRef]
- Shen, X.; Peter, E.A.; Bir, S.; Wang, R.; Kevil, C.G. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free. Radic. Biol. Med. 2012, 52, 2276–2283. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Isoda, S.; Tanabe, S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 1994, 17, 1535–1542. [Google Scholar] [CrossRef]
- Ubuka, T. Assay methods and biological roles of labile sulfur in animal tissues. J. Chromatogr. B 2002, 781, 227–249. [Google Scholar] [CrossRef]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H.A. source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, N.L.; Kreimier, E.L.; Verdial, F.C.; Skovgaard, N.; Olson, K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R1930–R1937. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Kabil, O.; Banerjee, R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid. Redox Signal. 2012, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tamizhselvi, R.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: In vitro studies using isolated mouse pancreatic acinar cells. J. Cell. Mol. Med. 2007, 11, 315–326. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Moore, P.K.; Bhatia, M. Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas 2008, 36, e24–e31. [Google Scholar] [CrossRef]
- Bhatia, M.; Wong, F.L.; Fu, D.; Lau, H.Y.; Moochhala, S.M.; Moore, P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005, 19, 623–625. [Google Scholar] [CrossRef]
- Ang, A.D.; Rivers-Auty, J.; Hegde, A.; Ishii, I.; Bhatia, M. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G712–G721. [Google Scholar] [CrossRef]
- Badiei, A.; Chambers, S.T.; Gaddam, R.R.; Bhatia, M. Cystathionine-γ-lyase gene silencing with siRNA in monocytes/ macrophages attenuates inflammation in cecal ligation and puncture-induced sepsis in the mouse. J. Biosci. 2016, 41, 87–95. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, R.; Qiang, Z.; Zhang, C. Pro-inflammatory cytokine-driven PI3K/Akt/Sp1 signalling and H2S production facilitates the pathogenesis of severe acute pancreatitis. Biosci. Rep. 2017, 37, BSR20160483. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Li, L.; Qu, F.; Zhang, G.; Wang, Y.; Bai, X.; Pan, S.; Xue, D.; Wang, G.; Sun, B. Hydrogen sulphide exacerbates acute pancreatitis by over-activating autophagy via AMPK/mTOR pathway. J. Cell. Mol. Med. 2016, 20, 2349–2361. [Google Scholar] [CrossRef]
- Klimaschewski, L. Neuropeptides in Autonomic Neurons. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 861–866. [Google Scholar] [CrossRef]
- Simmons, M.A. Substance P. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Koon, H.W.; Pothoulakis, C. Immunomodulatory Properties of Substance P. Ann. N. Y. Acad. Sci. 2006, 1088, 23–40. [Google Scholar] [CrossRef]
- Bhatia, M.; Slavin, J.; Cao, Y.; Basbaum, A.I.; Neoptolemos, J. Preprotachykinin—A gene deletion protects mice against acute pancreatitis and associated lung injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G830–G836. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Saluja, A.K.; Hofbauer, B.; Frossard, J.-L.; Lee, H.-S.; Castagliuolo, I.; Wang, C.C.; Gerard, N.; Pothoulakis, C.; Steer, M.L. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. USA 1998, 95, 4760–4765. [Google Scholar] [CrossRef] [PubMed]
- Patto, R.J.; Vinayek, R.; Jensen, R.T.; Gardner, J.D. Carbachol does not downregulate substance P receptors in pancreatic acini. Pancreas 1992, 7, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sjodin, L.; Gylfe, E. A selective and potent antagonist of substance P receptors on pancreatic acinar cells. Biochem. Int. 1992, 27, 145–153. [Google Scholar]
- Lau, H.Y.; Wong, F.L.; Bhatia, M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem. Biophys. Res. Commun. 2005, 327, 509–515. [Google Scholar] [CrossRef]
- Koh, Y.H.; Moochhala, S.; Bhatia, M. The role of neutral endopeptidase in caerulein-induced acute pancreatitis. J. Immunol. 2011, 187, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ramnath, R.D.; Bhatia, M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am. J. Physiol. Cell Physiol. 2007, 293, C696–C704. [Google Scholar] [CrossRef]
- Ramnath, R.D.; Sun, J.; Adhikari, S.; Bhatia, M. Effect of mitogen-activated protein kinases on chemokine synthesis induced by substance P in mouse pancreatic acinar cells. J. Cell. Mol. Med. 2007, 11, 1326–1341. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Zhi, L.; Zhang, H.; Ng, S.-W.; Moore, P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 291, L896–L904. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Chambers, S.; Fraser, R.; Cogger, V.C.; Le Couteur, D.G.; Ishii, I.; Bhatia, M. Cystathionine-Gamma-Lyase-Derived Hydrogen Sulfide-Regulated Substance P Modulates Liver Sieve Fenestrations in Caecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2019, 20, 3191. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Sidhapuriwala, J.N.; Wei Ng, S.; Tamizhselvi, R.; Moochhala, S.M. Pro-inflammatory effects of hydrogen sulphide on substance P in caerulein-induced acute pancreatitis. J. Cell. Mol. Med. 2008, 12, 580–590. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Shrivastava, P.; Koh, Y.-H.; Zhang, H.; Bhatia, M. Preprotachykinin-A gene deletion regulates hydrogen sulfide-induced toll-like receptor 4 signaling pathway in cerulein-treated pancreatic acinar cells. Pancreas 2011, 40, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.; Frade JM, R.; Sancho, D.; Mellado, M.; Martinez-A, C.; Sánchez-Madrid, F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J. Exp. Med. 1997, 186, 153–158. [Google Scholar] [CrossRef]
- Adams, D.H.; Shaw, S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet 1994, 343, 831–836. [Google Scholar] [CrossRef]
- Briskin, M.; Winsor-Hines, D.; Shyjan, A.; Cochran, N.; Bloom, S.; Wilson, J.; McEvoy, L.M.; Butcher, E.C.; Kassam, N.; Mackay, C.R.; et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1997, 151, 97–110. [Google Scholar]
- Gonzalez-Amaro, R.; Diaz-Gonzalez, F.; Sanchez-Madrid, F. Adhesion molecules in inflammatory diseases. Drugs 1998, 56, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Merinen, M.; Irjala, H.; Salmi, M.; Jaakkola, I.; Hänninen, A.; Jalkanen, S. Vascular adhesion protein-1 is involved in both acute and chronic inflammation in the mouse. Am. J. Pathol. 2005, 166, 793–800. [Google Scholar] [CrossRef]
- Sato, N.; Kohi, S.; Hirata, K.; Goggins, M. Role of hyaluronan in pancreatic cancer biology and therapy: Once again in the spotlight. Cancer Sci. 2016, 107, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, C.; Hällgren, R.; Tufveson, G. Role of hyaluronan in acute pancreatitis. Surgery 2000, 127, 650–658. [Google Scholar] [CrossRef]
- Sato, T.; Shibata, W.; Maeda, S. Adhesion molecules and pancreatitis. J. Gastroenterol. 2019, 54, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Ando, T.; Jordan, P.; Wang, Y.; Minagar, A.; Jennings, M.; Joh, T. TNF-α Regulated MAdCAM-1 Expression in Pancreatic Microvessel Endothelium: A Possible Role for MAdCAM-1 in Pancreatitis. Open Gastroenterol. J. 2008, 2, 1–8. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Sun, Z.; Börjesson, A.; Andersson, R. Inhibition of platelet-activating factor, intercellular adhesion molecule 1 and platelet endothelial cell adhesion molecule 1 reduces experimental pancreatitis-associated gut endothelial barrier dysfunction. Br. J. Surg. 1999, 86, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.; Salas, A.; Panés, J.; Gelpí, E.; Roselló-Catafau, J.; Anderson, D.C.; Navarro, S.; Piqué, J.M.; Fernández-Cruz, L.; Closa, D. Role of P-selectin and ICAM-1 in pancreatitis-induced lung inflammation in rats: Significance of oxidative stress. Ann. Surg. 1999, 230, 792–799. [Google Scholar] [CrossRef]
- Foitzik, T.; Eibl, G.; Buhr, H.J. Therapy for microcirculatory disorders in severe acute pancreatitis: Comparison of delayed therapy with ICAM-1 antibodies and a specific endothelin A receptor antagonist. J. Gastrointest. Surg. 2000, 4, 240–247. [Google Scholar] [CrossRef]
- Zhu, H.H.; Jiang, L.L. Serum inter-cellular adhesion molecule 1 is an early marker of diagnosis and prediction of severe acute pancreatitis. World J. Gastroenterol. 2012, 18, 2554–2560. [Google Scholar] [CrossRef]
- Frossard, J.L.; Saluja, A.; Bhagat, L.; Lee, H.S.; Bhatia, M.; Hofbauer, B.; Steer, M.L. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 1999, 116, 694–701. [Google Scholar] [CrossRef]
- Bhatia, M.; Brady, M.; Shokuhi, S.; Christmas, S.; Neoptolemos, J.P.; Slavin, J. Inflammatory mediators in acute pancreatitis. J. Pathol. 2000, 190, 117–125. [Google Scholar] [CrossRef]
- Wertheimer, S.J.; Myers, C.L.; Wallace, R.W.; Parks, T.P. Intercellular adhesion molecule-1 gene expression in human endothelial cells. Differential regulation by tumor necrosis factor-alpha and phorbol myristate acetate. J. Biol. Chem. 1992, 267, 12030–12035. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Koh, Y.-H.; Sun, J.; Zhang, H.; Bhatia, M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-κB and Src-family kinases pathway. Exp. Cell. Res. 2010, 316, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Carlos, T.M.; Harlan, J.M. Leukocyte-endothelial adhesion molecules. Blood 1994, 84, 2068–2101. [Google Scholar] [CrossRef] [PubMed]
- Callicutt, C.S.; Sabek, O.; Fukatsu, K.; Lundberg, A.H.; Gaber, L.; Wilcox, H.; Kotb, M.; Gaber, A.O. Diminished lung injury with vascular adhesion molecule-1 blockade in choline-deficient ethionine diet-induced pancreatitis. Surgery 2003, 133, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.H.; Granger, N.; Russell, J.; Callicutt, S.; Gaber, L.W.; Kotb, M.; Sabek, O.; Gaber, A.O. Temporal correlation of tumor necrosis factor-alpha release, upregulation of pulmonary ICAM-1 and VCAM-1, neutrophil sequestration, and lung injury in diet-induced pancreatitis. J. Gastrointest. Surg. 2000, 4, 248–257. [Google Scholar] [CrossRef]
- Nakache, M.; Lakey Berg, E.; Streeter, P.R.; Butcher, E.C. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature 1989, 337, 179–181. [Google Scholar] [CrossRef]
- Butcher, E.C.; Picker, L.J. Lymphocyte homing and homeostasis. Science 1996, 272, 60–66. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. VAP-1: An adhesin and an enzyme. Trends in Immunology. Trends Immunol. 2001, 22, 211–216. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Developmental regulation of the adhesive and enzymatic activity of vascular adhesion protein-1 (VAP-1) in humans. Blood 2006, 108, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Bjermer, L.; Engström-Laurent, A.; Lundgren, R.; Rosenhall, L.; Hällgren, R. Hyaluronate and type III procollagen peptide concentrations in bronchoalveolar lavage fluid as markers of disease activity in farmer’s lung. Br. Med. J. 1987, 295, 803–806. [Google Scholar] [CrossRef]
- Waldenström, A.; Fohlman, J.; Ilbäcky, N.G.; Ronquist, G.; Hällgren, R.; Gerdin, B. Coxsackie B3 myocarditis induces a decrease in energy charge and accumulation of hyaluronan in the mouse heart. Eur. J. Clin. Investig. 1993, 23, 277–282. [Google Scholar] [CrossRef]
- Colombel, J.F.; Hällgren, R.; Engström-Laurent, A.; Rambaud, J.C. Hyaluronic acid and type III procollagen peptide in jejunal perfusion fluid as markers of connective tissue turnover. Gastroenterology 1989, 96, 68–73. [Google Scholar] [CrossRef]
- Nettelbladt, O.; Bergh, J.; Schenholm, M.; Tengblad, A.; Hällgren, R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am. Rev. Respir. Dis. 1989, 139, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Endo, S.; Sato, N.; Wakabayashi, G.; Inada, K.; Sato, S. Involvement of soluble adhesion molecules in acute pancreatitis. Eur. Surg. Res. 2001, 33, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Tsaroucha, A.K.; Schizas, D.; Vailas, M.G.; Rachmani, E.; Kanavidis, P.; Asimakopoulos, V.; Vlachos, S.; Sotiropoulou, M.; Pitiakoudis, M.S.; Simopoulos, C.E. E and P Selectins as Potential Markers in the Assessment of the Severity of Acute Pancreatitis. Pancreas 2018, 47, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ke, L.; Meng, L.; Yang, Q.; Tong, Z.; Pan, Y.; Li, W.; Li, J. Endothelial markers are associated with pancreatic necrosis and overall prognosis in acute pancreatitis: A preliminary cohort study. Pancreatology 2017, 17, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chooklin, S. Pathogenic aspects of pulmonary complications in acute pancreatitis patients. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 186–192. [Google Scholar] [PubMed]
- Folch, E.; Prats, N.; Hotter, G.; López, S.; Gelpi, E.; Roselló-Catafau, J.; Closa, D. P-selectin expression and Kupffer cell activation in rat acute pancreatitis. Dig. Dis. Sci. 2000, 45, 1535–1544. [Google Scholar] [CrossRef]
- Lundberg, A.H.; Granger, D.N.; Russell, J.; Sabek, O.; Henry, J.; Gaber, L.; Kotb, M.; Gaber, A.O. Quantitative measurement of P- and E-selectin adhesion molecules in acute pancreatitis: Correlation with distant organ injury. Ann. Surg. 2000, 231, 213–222. [Google Scholar] [CrossRef]
- Hartman, H.; Abdulla, A.; Awla, D.; Lindkvist, B.; Jeppsson, B.; Thorlacius, H.; Regnér, S. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br. J. Surg. 2012, 99, 246–255. [Google Scholar] [CrossRef]
- Hackert, T.; Sperber, R.; Hartwig, W.; Fritz, S.; Schneider, L.; Gebhard, M.-M.; Werner, J. P-selectin inhibition reduces severity of acute experimental pancreatitis. Pancreatology 2009, 9, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Bhatia, M. Effect of CP-96,345 on the expression of adhesion molecules in acute pancreatitis in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G1283–G1292. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Klonowski-Stumpe, H.; Eckert, M.; Lüthen, R.; Häussinger, D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas 2004, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Rahner, C.; Mitic, L.L.; Anderson, J.M. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001, 120, 411–422. [Google Scholar] [CrossRef]
- Meriläinen, S.; Mäkelä, J.; Anttila, V.; Koivukangas, V.; Kaakinen, H.; Niemelä, E.; Ohtonen, P.; Risteli, J.; Karttunen, T.; Soini, Y.; et al. Acute edematous and necrotic pancreatitis in a porcine model. Scand. J. Gastroenterol. 2008, 43, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Nakada, S.; Tsuneyama, K.; Kato, I.; Tabuchi, Y.; Takasaki, I.; Furusawa, Y.; Kawaguchi, H.; Fujimoto, M.; Goto, H.; Hikiami, H.; et al. Identification of candidate genes involved in endogenous protection mechanisms against acute pancreatitis in mice. Biochem. Biophys. Res. Commun. 2010, 391, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Aurrand-Lions, M.; Pastor, C.M.; Lamagna, C.; Hadengue, A.; Imhof, B.A.; Frossard, J.L. The role of junctional adhesion molecule C (JAM-C) in acute pancreatitis. J. Pathol. 2006, 209, 540–548. [Google Scholar] [CrossRef]
- Wu, D.; Zeng, Y.; Fan, Y.; Wu, J.; Mulatibieke, T.; Ni, J.; Yu, G.; Wan, R.; Wang, X.; Hu, G. Reverse-migrated neutrophils regulated by JAM-C are involved in acute pancreatitis-associated lung injury. Sci. Rep. 2016, 6, 20545. [Google Scholar] [CrossRef]
- Lerch, M.M.; Lutz, M.P.; Weidenbach, H.; Müller-Pillasch, F.; Gress, T.M.; Leser, J.; Adler, G. Dissociation and reassembly of adherens junctions during experimental acute pancreatitis. Gastroenterology 1997, 113, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Schnekenburger, J.; Mayerle, J.; Krüger, B.; Buchwalow, I.; Weiss, F.U.; Albrecht, E.; Samoilova, V.E.; Domschke, W.; Lerch, M.M. Protein tyrosine phosphatase kappa and SHP-1 are involved in the regulation of cell-cell contacts at adherens junctions in the exocrine pancreas. Gut 2005, 54, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Korompay, A.; Borka, K.; Lotz, G.; Somorácz, Á.; Törzsök, P.; Erdélyi-Belle, B.; Kenessey, I.; Baranyai, Z.; Zsoldos, F.; Kupcsulik, P.; et al. Tricellulin expression in normal and neoplastic human pancreas. Histopathology 2012, 60, E76–E86. [Google Scholar] [CrossRef] [PubMed]
| Adhesion Molecule | Biological Effects |
|---|---|
| ICAM-1 [41,64,65,66,67,68] | Important marker for early detection, key role in neutrophil migration |
| VCAM-1 [55,69,70,71] | Important role in leukocytic migration |
| MAdCAM-1 [54,60,72,73] | Possible role in lymphocytic migration |
| VAP-1 [56,74,75] | Important role in leukocytic migration |
| Hyaluronan or Hyaluronic acid [58,76,77,78,79] | Key role in interstitial edema, which leads to tissue necrosis |
| Selectins [62,80,81,82,83,84,85,86,87,88] | Important for leukocyte recruitment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Bhatia, M. Role of Hydrogen Sulfide, Substance P and Adhesion Molecules in Acute Pancreatitis. Int. J. Mol. Sci. 2021, 22, 12136. https://doi.org/10.3390/ijms222212136
Kumar A, Bhatia M. Role of Hydrogen Sulfide, Substance P and Adhesion Molecules in Acute Pancreatitis. International Journal of Molecular Sciences. 2021; 22(22):12136. https://doi.org/10.3390/ijms222212136
Chicago/Turabian StyleKumar, Ayush, and Madhav Bhatia. 2021. "Role of Hydrogen Sulfide, Substance P and Adhesion Molecules in Acute Pancreatitis" International Journal of Molecular Sciences 22, no. 22: 12136. https://doi.org/10.3390/ijms222212136
APA StyleKumar, A., & Bhatia, M. (2021). Role of Hydrogen Sulfide, Substance P and Adhesion Molecules in Acute Pancreatitis. International Journal of Molecular Sciences, 22(22), 12136. https://doi.org/10.3390/ijms222212136






