Urocortin Role in Ischemia Cardioprotection and the Adverse Cardiac Remodeling
Abstract
:1. Introduction
2. Structure and Expression of Urocortin
3. Corticotropin Releasing Factor Receptors and Signaling Pathways
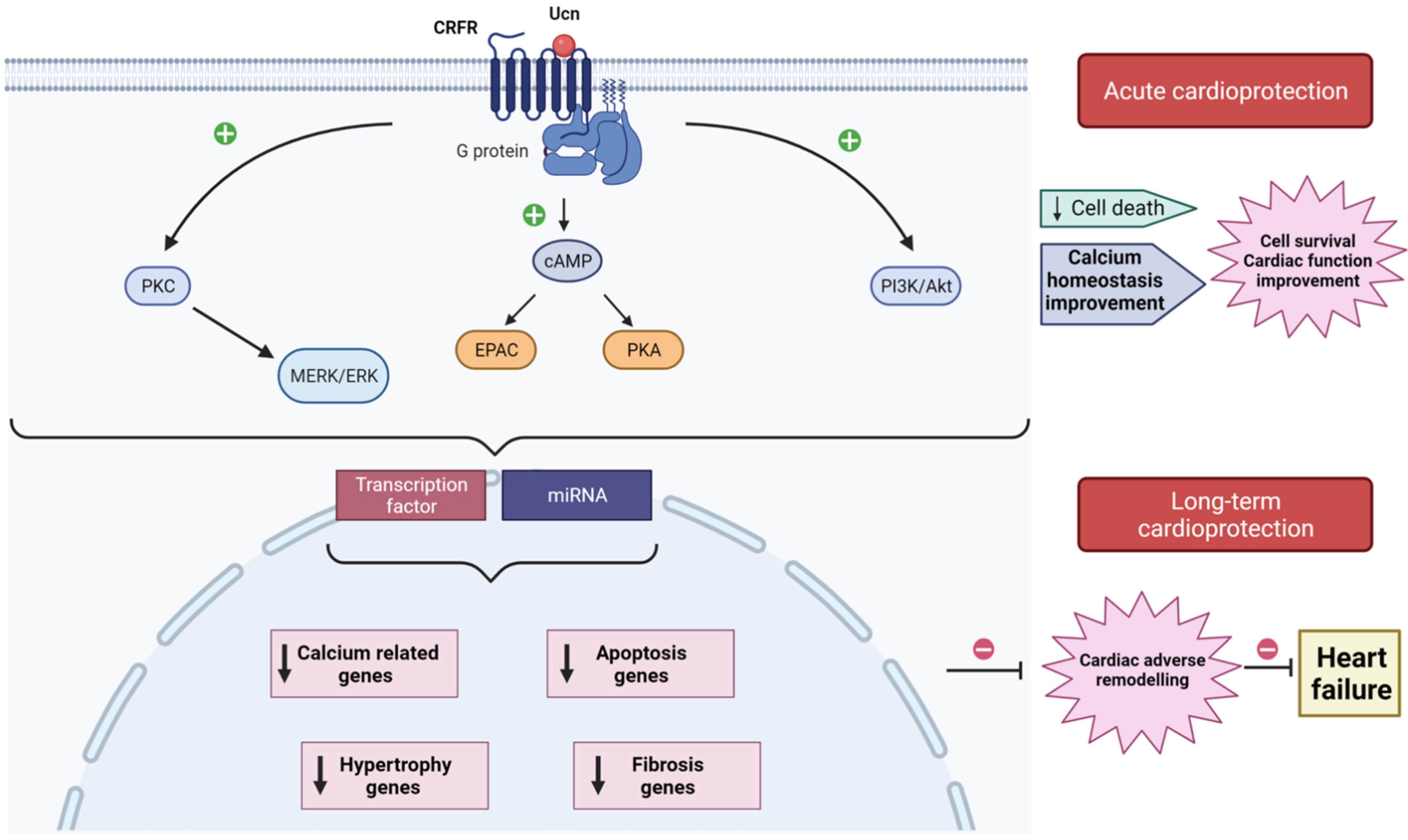
4. Acute Action of Urocortin in the Cardioprotection
5. Urocortin Role in the Adverse Cardiac Remodeling
6. Therapeutic Values of Urocortin in Heart Failure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| ACTH | corticotropin |
| ANP | atrial natriuretic peptide |
| AMI | acute myocardial infarct |
| AMPK | AMP-activated protein kinase |
| AAV | adeno-associated viruses |
| cAMP | cyclic AMP |
| BNP | brain natriuretic peptide |
| CHF | congestive heart failure |
| CRF | corticotropin-releasing factor |
| CRF-R1,2 | corticotropin-releasing factor-receptor 1,2 |
| CT-1 | cardiotrophin 1 |
| Epac | exchange protein directly activated by cAMP |
| ERK1/2 | extracellular signal–regulated kinases 1/2 |
| HF | heart failure |
| HFrEF | reduced ejection fraction |
| IHD | ischemic heart diseases |
| I/R | ischemia/reperfusion |
| LDH | lactate dehydrogenase |
| LVEF | left ventricular eyection fraction |
| LVDP | left ventricular developed pressure |
| LVEDP | left ventricular end diastolic pressure |
| MAPK | mitogen activated protein kinase |
| miRNAs | microRNAs |
| MPTP | mitochondrial permeability transition pore |
| NRVM | neonatal rat ventricular myocytes |
| NCX | Na+/Ca2+ exchanger |
| PI3-K | phosphatidylinositol 3-kinase |
| PKA | protein kinase A |
| PKC | protein kinase C |
| ROS | reactive oxygen species |
| SGK1 | glucocorticoid-responsive kinase-1 |
| SR | sarcoplasmic reticulum |
| STEMI | myocardial infarction with ST segment elevation |
| Ucn1, 2, 3 | urocortin 1, 2, 3 |
References
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribichini, F. Acute myocardial infarction: Reperfusion treatment. Heart 2002, 88, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Mathew, V.; Gersh, B.J. To open or not to open: That remains the question. Eur. Heart J. 2004, 25, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Pfeil, K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165768. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Sánchez, E.M.; Ávila-Medina, J.; Callejo-García, P.; Fernández-Velasco, M.; Ordóñez, A.; Smani, T. Role of Orai1 and L-type CaV1.2 channels in Endothelin-1 mediated coronary contraction under ischemia and reperfusion. Cell Calcium 2020, 86, 102157. [Google Scholar] [CrossRef]
- Eeckhout, E.; Kern, M.J. The coronary no-reflow phenomenon: A review of mechanisms and therapies. Eur. Heart J. 2001, 22, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial No-Reflow in Humans. J. Am. Coll. Cardiol. 2009, 54, 281–292. [Google Scholar] [CrossRef]
- Golden, J.; O’Dwyer, A.M.; Conroy, R.M. Depression and anxiety in patients with hepatitis C: Prevalence, detection rates and risk factors. Gen. Hosp. Psychiatry 2005, 27, 431–438. [Google Scholar] [CrossRef]
- Roe, A.; Frisk, M.; Louch, W. Targeting Cardiomyocyte Ca2+ Homeostasis in Heart Failure. Curr. Pharm. Des. 2014, 21, 431–448. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.-Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.-P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol. Circ. Physiol. 2003, 285, H579–H588. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.; Yellon, D. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis 2009, 204, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef]
- Hausenloy, D.; Botker, H.E.; Engstrom, T.; Erlinge, D.; Heusch, G.; Ibanez, B.; Kloner, R.A.; Ovize, M.; Yellon, D.; Garcia-Dorado, D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: Trials and tribulations. Eur. Heart J. 2016, 38, 935–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, G.; Rassaf, T. Left Ventricular Unloading in Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 700–702. [Google Scholar] [CrossRef]
- Brar, B.K.; Jonassen, A.K.; Stephanou, A.; Santilli, G.; Railson, J.; Knight, R.A.; Yellon, D.; Latchman, D.S. Urocortin Protects against Ischemic and Reperfusion Injury via a MAPK-dependent Pathway. J. Biol. Chem. 2000, 275, 8508–8514. [Google Scholar] [CrossRef] [Green Version]
- Brar, B.K.; Jonassen, A.K.; Egorina, E.M.; Chen, A.; Negro, A.; Perrin, M.H.; ΜjøsO, D.; Latchman, D.S.; Lee, K.-F.; Vale, W. Urocortin-II and Urocortin-III Are Cardioprotective against Ischemia Reperfusion Injury: An Essential Endogenous Cardioprotective Role for Corticotropin Releasing Factor Receptor Type 2 in the Murine Heart. Endocrinology 2004, 145, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, J.; Donaldson, C.J.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nat. Cell Biol. 1995, 378, 287–292. [Google Scholar] [CrossRef]
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A.; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.Y.; Hsueh, A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001, 7, 605–611. [Google Scholar] [CrossRef]
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848. [Google Scholar] [CrossRef] [Green Version]
- Kozicz, T.; Yanaihara, H.; Arimura, A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J. Comp. Neurol. 1998, 391, 1–10. [Google Scholar] [CrossRef]
- Morin, S.; Ling, N.; Liu, X.-J.; Kahl, S.; Gehlert, D. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience 1999, 92, 281–291. [Google Scholar] [CrossRef]
- Hillhouse, E.W.; Grammatopoulos, D. The Molecular Mechanisms Underlying the Regulation of the Biological Activity of Corticotropin-Releasing Hormone Receptors: Implications for Physiology and Pathophysiology. Endocr. Rev. 2006, 27, 260–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Takahashi, K.; Totsune, K.; Muramatsu, Y.; Kaneko, C.; Darnel, A.D.; Suzuki, T.; Ebina, M.; Nukiwa, T.; Sasano, H. Expression of Urocortin and Corticotropin-Releasing Factor Receptor Subtypes in the Human Heart. J. Clin. Endocrinol. Metab. 2002, 87, 340–346. [Google Scholar] [CrossRef]
- Nishikimi, T.; Miyata, A.; Horio, T.; Yoshihara, F.; Nagaya, N.; Takishita, S.; Yutani, C.; Matsuo, H.; Matsuoka, H.; Kangawa, K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am. J. Physiol. Circ. Physiol. 2000, 279, H3031–H3039. [Google Scholar] [CrossRef]
- Harada, S.; Imaki, T.; Naruse, M.; Chikada, N.; Nakajima, K.; Demura, H. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci. Lett. 1999, 267, 125–128. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Fukushima, K.; Iino, K.; Totsune, K.; Takahashi, K.; Suzuki, T.; Hirasawa, G.; Takeyama, J.; Ito, M.; Nose, M.; et al. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 2000, 21, 1799–1809. [Google Scholar] [CrossRef]
- Gutknecht, E.; Van der Linden, I.; Van Kolen, K.; Verhoeven, K.F.C.; Vauquelin, G.; Dautzenberg, F.M. Molecular Mechanisms of Corticotropin-Releasing Factor Receptor-Induced Calcium Signaling. Mol. Pharmacol. 2009, 75, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Asaba, K.; Makino, S.; Nishiyama, M.; Hashimoto, K. Regulation of Type-2 Corticotropin-Releasing Hormone Receptor mRNA in Rat Heart by Glucocorticoids and Urocortin. J. Cardiovasc. Pharmacol. 2000, 36, 493–497. [Google Scholar] [CrossRef]
- Li, J.; Qi, D.; Cheng, H.; Hu, X.; Miller, E.; Wu, X.; Russell, K.S.; Mikush, N.; Zhang, J.; Xiao, L.; et al. Urocortin 2 autocrine/paracrine and pharmacologic effects to activate AMP-activated protein kinase in the heart. Proc. Natl. Acad. Sci. USA 2013, 110, 16133–16138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Blount, A.; Vaughan, J.; Brar, B.; Vale, W. Urocortin II Gene Is Highly Expressed in Mouse Skin and Skeletal Muscle Tissues: Localization, Basal Expression in Corticotropin-Releasing Factor Receptor (CRFR) 1- and CRFR2-Null Mice, and Regulation by Glucocorticoids. Endocrinology 2004, 145, 2445–2457. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Totsune, K.; Murakami, O.; Saruta, M.; Nakabayashi, M.; Suzuki, T.; Sasano, H.; Shibahara, S. Expression of Urocortin III/Stresscopin in Human Heart and Kidney. J. Clin. Endocrinol. Metab. 2004, 89, 1897–1903. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K. Distribution of Urocortins and Corticotropin-Releasing Factor Receptors in the Cardiovascular System. Int. J. Endocrinol. 2012, 2012, 395284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, T.; Takefuji, M.; Wettschureck, N.; Kotani, K.; Morimoto, R.; Okumura, T.; Kaur, H.; Eguchi, S.; Sakaguchi, T.; Ishihama, S.; et al. Corticotropin releasing hormone receptor 2 exacerbates chronic cardiac dysfunction. J. Exp. Med. 2017, 214, 1877–1888. [Google Scholar] [CrossRef]
- Yarur, H.E.; Andrés, M.E.; Gysling, K. Type 2β Corticotrophin Releasing Factor Receptor Forms a Heteromeric Complex With Dopamine D1 Receptor in Living Cells. Front. Pharmacol. 2020, 10, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, T.; Pearse, R.; Lin, C.R.; Rosenfeld, M.G. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc. Natl. Acad. Sci. USA 1995, 92, 1108–1112. [Google Scholar] [CrossRef] [Green Version]
- Pilbrow, A.P.; Lewis, K.A.; Perrin, M.H.; Sweet, W.E.; Moravec, C.S.; Tang, W.H.W.; Huising, M.O.; Troughton, R.; Cameron, V. Cardiac CRFR1 Expression Is Elevated in Human Heart Failure and Modulated by Genetic Variation and Alternative Splicing. Endocrinology 2016, 157, 4865–4874. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, C.; Pelagalli, A.; Liguori, G.; Mirabella, N. Urocortins in the mammalian endocrine system. Acta Veter.-Scand. 2019, 61, 46. [Google Scholar] [CrossRef]
- Brar, B.K.; Stephanou, A.; Knight, R.; Latchman, D.S. Activation of Protein Kinase B/Akt by Urocortin is Essential for its Ability to Protect Cardiac Cells Against Hypoxia/Reoxygenation-induced Cell Death. J. Mol. Cell. Cardiol. 2002, 34, 483–492. [Google Scholar] [CrossRef]
- Calderón-Sánchez, E.M.; Delgado, C.; Ruiz-Hurtado, G.; Domínguez-Rodríguez, A.; Cachofeiro, V.; Rodríguez-Moyano, M.; Gomez, A.M.; Ordonez, A.; Smani, T.; Hajami, T.S. Urocortin induces positive inotropic effect in rat heart. Cardiovasc. Res. 2009, 83, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Walther, S.; Pluteanu, F.; Renz, S.; Nikonova, Y.; Maxwell, J.T.; Yang, L.-Z.; Schmidt, K.; Edwards, J.N.; Wakula, P.; Groschner, K.; et al. Urocortin 2 stimulates nitric oxide production in ventricular myocytes via Akt- and PKA-mediated phosphorylation of eNOS at serine 1177. Am. J. Physiol. Circ. Physiol. 2014, 307, H689–H700. [Google Scholar] [CrossRef] [Green Version]
- Popov, S.V.; Prokudina, E.S.; Mukhomedzyanov, A.V.; Naryzhnaya, N.V.; Ma, H.; Zurmanova, J.M.; van der Ven, P.F.M.; Maslov, L.N. Cardioprotective and Vasoprotective Effects of Corticotropin-Releasing Hormone and Urocortins: Receptors and Signaling. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Okosi, A.; Brar, B.; Chan, M.; D’Souza, L.; Smith, E.; Stephanou, A.; Latchman, D.; Chowdrey, H.; Knight, R. Expression and protective effects of urocortin in cardiac myocytes. Neuropeptides 1998, 32, 167–171. [Google Scholar] [CrossRef]
- Scarabelli, T.M.; Pasini, E.; Stephanou, A.; Comini, L.; Curello, S.; Raddino, R.; Ferrari, R.; Knight, R.; Latchman, D.S. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J. Am. Coll. Cardiol. 2002, 40, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Janjua, S.; Lawrence, K.M.; Ng, L.L.; Latchman, D.S. The cardioprotective agent urocortin induces expression of CT-1. Cardiovasc. Toxicol. 2003, 3, 255–262. [Google Scholar] [CrossRef]
- Lawrence, K.M.; Kabir, A.M.N.; Bellahcene, M.; Davidson, S.; Mesquita, R.S.; Cao, X.; McCormick, J.; Carroll, C.; Chanalaris, A.; Townsend, P.A.; et al. Cardioprotection mediated by urocortin is dependent upon PKCε activation. FASEB J. 2005, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, A.; González, I.M.; Avila-Medina, J.; Pedro, E.S.D.R.-D.; Calderón-Sánchez, E.; Díaz, I.; Hmadcha, A.; Castellano, A.; Rosado, J.; Benitah, J.-P.; et al. Urocortin-2 Prevents Dysregulation of Ca2+ Homeostasis and Improves Early Cardiac Remodeling After Ischemia and Reperfusion. Front. Physiol. 2018, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Valentim, L.; Laurence, K.M.; Townsend, P.; Carroll, C.; Soond, S.; Scarabelli, T.M.; Knight, R.A.; Latchman, D.S.; Stephanou, A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J. Mol. Cell. Cardiol. 2006, 40, 846–852. [Google Scholar] [CrossRef]
- Calderón-Sánchez, E.M.; Díaz, I.; Ordoñez, A.; Smani, T. Urocortin-1 Mediated Cardioprotection Involves XIAP and CD40-Ligand Recovery: Role of EPAC2 and ERK1/2. PLoS ONE 2016, 11, e0147375. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.-F.; Zhou, Y.; Wang, D.-Y.; Lew, K.-S.; Richards, A.M.; Wang, P. Urocortin-2 suppression of p38-MAPK signaling as an additional mechanism for ischemic cardioprotection. Mol. Cell. Biochem. 2014, 398, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Chanalaris, A.; Scarabelli, T.; Hubank, M.; Pasini, E.; Townsend, P.; Comini, L.; Ferrari, R.; Tinker, A.; Stephanou, A.; et al. K ATP Channel Gene Expression Is Induced by Urocortin and Mediates Its Cardioprotective Effect. Circulation 2002, 106, 1556–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, B.; Wang, L.; Zhu, X.; Li, X.; Liu, B.; Ni, X. SGK1 Is Involved in Cardioprotection of Urocortin-1 Against Hypoxia/Reoxygenation in Cardiomyocytes. Can. J. Cardiol. 2014, 30, 687–695. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Q.; Lew, K.S.; Richards, A.M.; Wang, P. Discovery of Potential Therapeutic miRNA Targets in Cardiac Ischemia–Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Calderón-Sánchez, E.M.; Del Toro, R.; Avila-Medina, J.; Pedro, E.S.D.R.-D.; Domínguez-Rodríguez, A.; Rosado, J.A.; Hmadcha, A.; Ordoñez, A.; Smani, T. miR-125a, miR-139 and miR-324 contribute to Urocortin protection against myocardial ischemia-reperfusion injury. Sci. Rep. 2017, 7, 8898. [Google Scholar] [CrossRef]
- Smani, T.; González, I.M.; Galeano-Otero, I.; Gallardo-Castillo, I.; Rosado, J.A.; Ordoñez, A.; Hmadcha, A. Non-coding RNAs and Ischemic Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1229, 259–271. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Ellmers, L.J.; Scott, N.J.A.; Cameron, V.A.; Richards, A.M.; Rademaker, M.T. Chronic Urocortin 2 Administration Improves Cardiac Function and Ameliorates Cardiac Remodeling After Experimental Myocardial Infarction. J. Cardiovasc. Pharmacol. 2015, 65, 269–275. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, C.-H.; Tsai, M.-S.; Hsu, N.-T.; Chiang, C.-Y.; Wang, T.-D.; Chang, W.-T.; Chen, H.-W.; Chen, W.-J. Urocortin Treatment Improves Acute Hemodynamic Instability and Reduces Myocardial Damage in Post-Cardiac Arrest Myocardial Dysfunction. PLoS ONE 2016, 11, e0166324. [Google Scholar] [CrossRef]
- Kennedy, S.G.; Kandel, E.S.; Cross, T.K.; Hay, N. Akt/Protein Kinase B Inhibits Cell Death by Preventing the Release of Cytochrome c from Mitochondria. Mol. Cell. Biol. 1999, 19, 5800–5810. [Google Scholar] [CrossRef] [Green Version]
- Giamouridis, D.; Gao, M.H.; Lai, N.C.; Tan, Z.; Kim, Y.C.; Guo, T.; Miyanohara, A.; Blankesteijn, M.W.; Biessen, E.A.L.; Hammond, H.K. Urocortin 3 Gene Transfer Increases Function of the Failing Murine Heart. Hum. Gene Ther. 2019, 30, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Pemberton, C.J.; Yandle, T.G.; Lainchbury, J.G.; Rademaker, M.T.; Nicholls, M.G.; Frampton, C.M.; Richards, A.M. Effect of urocortin 1 infusion in humans with stable congestive cardiac failure. Clin. Sci. 2005, 109, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.E.; Pemberton, C.J.; Yandle, T.G.; Fisher, S.F.; Lainchbury, J.G.; Frampton, C.M.; Rademaker, M.T.; Richards, A.M. Urocortin 2 Infusion in Healthy Humans: Hemodynamic, Neurohormonal, and Renal Responses. J. Am. Coll. Cardiol. 2007, 49, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Smani, T.; Calderon, E.; Rodriguez-Moyano, M.; Dominguez-Rodriguez, A.; Diaz, I.; Ordóñez, A. Urocortin-2 induces vasorelaxation of coronary arteries isolated from patients with heart failure. Clin. Exp. Pharmacol. Physiol. 2011, 38, 71–76. [Google Scholar] [CrossRef]
- Monteiro-Pinto, C.; Adão, R.; Leite-Moreira, A.; Brás-Silva, C. Cardiovascular Effects of Urocortin-2: Pathophysiological Mechanisms and Therapeutic Potential. Cardiovasc. Drugs Ther. 2019, 33, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Liew, O.W.; Yandle, T.G.; Chong, J.P.C.; Ng, Y.X.; Frampton, C.M.; Ng, T.P.; Lam, C.S.P.; Richards, A.M. High-Sensitivity Sandwich ELISA for Plasma NT-proUcn2: Plasma Concentrations and Relationship to Mortality in Heart Failure. Clin. Chem. 2016, 62, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.P.; Doughty, R.N.; Frampton, C.M.; Gamble, G.D.; Yandle, T.G.; Richards, A.M. Plasma Urocortin 1 in Human Heart Failure. Circ. Heart Fail. 2009, 2, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, D.K.; Farkas, N.; Soós, A.; Hegyi, P.; Kelava, L.; Eitmann, S.; Schekk, A.; Molnár, Z.; Erőss, B.; Balaskó, M. Assessment of clinical data on urocortins and their therapeutic potential in cardiovascular diseases: A systematic review and meta-analysis. Clin. Transl. Sci. 2021, 1–13. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Sivasinprasasn, S.; Lailerd, N.; Chattipakorn, S.; Kuanprasert, S.; Chattipakorn, N. Plasma urocortin in acute myocardial infarction patients. Eur. J. Clin. Investig. 2010, 40, 874–882. [Google Scholar] [CrossRef]
- Ng, L.L.; Loke, I.W.; O’Brien, R.J.; Squire, I.B.; Davies, J.E. Plasma urocortin in human systolic heart failure. Clin. Sci. 2004, 106, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Topal, E.; Yagmur, J.; Otlu, B.; Atas, H.; Cansel, M.; Acikgoz, N.; Ermis, N. Relationship of urocortin-2 with systolic and diastolic functions and coronary artery disease: An observational study. Anadolu Kardiyol. Derg 2012, 12, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Pemberton, C.J.; Yandle, T.G.; Lainchbury, J.G.; Rademaker, M.T.; Nicholls, M.G.; Frampton, C.M.; Richards, A.M. Urocortin-1 Infusion in Normal Humans. J. Clin. Endocrinol. Metab. 2004, 89, 1402–1409. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Francis, G.S. Exploring new drugs for heart failure: The case of urocortin. Eur. Heart J. 2007, 28, 2561–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.Y.W.; Frampton, C.M.; Crozier, I.G.; Troughton, R.; Richards, A.M. Urocortin-2 Infusion in Acute Decompensated Heart Failure. JACC Heart Fail. 2013, 1, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Stirrat, C.G.; Venkatasubramanian, S.; Pawade, T.; Mitchell, A.J.; Shah, A.; Lang, N.; Newby, D.E. Cardiovascular effects of urocortin 2 and urocortin 3 in patients with chronic heart failure. Br. J. Clin. Pharmacol. 2016, 82, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Greene, S.J.; Ponikowski, P.; Maggioni, A.P.; Korewicki, J.; Macarie, C.; Metra, M.; Grzybowski, J.; Bubenek-Turconi, S.-I.; Radziszewski, W.; et al. Haemodynamic effects, safety, and pharmacokinetics of human stresscopin in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2013, 15, 679–689. [Google Scholar] [CrossRef] [Green Version]
| Animal Model | Dosage | End Points | Reference |
|---|---|---|---|
| Mice post-AMI | 415 μg/Kg/d Ucn2 (30 days) |
| [58] |
| Preclinical cardiac arrest rat model | 10 μg/Kg i.v. Ucn2 |
| [59] |
| Rat model of I/R | 150 µg/Kg i.v. Ucn2 |
| [48] |
| Rat model of I/R | 50, 150, or 300 µg/Kg Ucn2 |
| [55] |
| Mice post-AMI | 1.9 × 1013 gc/Kg * AAV8.UCn3 |
| [61] |
| Clinical Study Design and State | Patients | Dosage | Outcomes | Negative Outcomes | Reference |
|---|---|---|---|---|---|
| Ucn1 vs. placebo in a balanced, randomized, single-blind, cross-over design | 8 healthy unmedicated men | Ucn1, 50 μg (1 μg/mL) |
| Temporal ACTH and cortisol increase | [72] |
| Ucn1 vs. placebo in a balanced, randomized, single-blind, cross-over design | 8 males with stable congestive HF (LVEF ≥40%), NYHA class II–III and creatine <0.15 mM. With medication | Ucn1, 50 μg (1 μg/mL) |
| Temporal ACTH and cortisol increase | [62] |
| Single-blind dose-escalation design | 8 males with stable congestive HF (LVEF ≥40%), NYHA class II–III. With medication | Ucn2, 25–100 μg (0.5–2 μg/mL) |
| Temporal flushed during drug infusion | [63] |
| Single-blind dose-escalation design | 8 healthy unmedicated men | Ucn2, 25–100 μg (0.5–2 μg/mL) |
| Urine Na+, K+, and CrCl decrease | [73] |
| Single-center, randomized, double-blind, placebo-controlled trial | 53 HF patients (LVEF <40%) | Ucn2, 400 μg (2 μg/mL) |
| Temporal flushed during infusion Hypotension Urine volume and CrCl reduced | [74] |
| Randomized study | 8 healthy patients and 8 HF patients (LVEF <35%), NYHA functional class II–III | Ucn2, (3.6–36 pmol/min) Ucn3, (360–3600 pmol/min) |
| Tachycardia at Ucn3 high dose | [75] |
| Randomized, double-blind, placebo-controlled, parallel-group, ascending dose study | 15 healthy patients and 45 HF patients (LVEF ≤35%), NYHA functional class II–IV | Human stresscopin (5, 15, 30 ng/Kg/min) |
| Erythema and hot feeling | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Sánchez, E.M.; Falcón, D.; Martín-Bórnez, M.; Ordoñez, A.; Smani, T. Urocortin Role in Ischemia Cardioprotection and the Adverse Cardiac Remodeling. Int. J. Mol. Sci. 2021, 22, 12115. https://doi.org/10.3390/ijms222212115
Calderón-Sánchez EM, Falcón D, Martín-Bórnez M, Ordoñez A, Smani T. Urocortin Role in Ischemia Cardioprotection and the Adverse Cardiac Remodeling. International Journal of Molecular Sciences. 2021; 22(22):12115. https://doi.org/10.3390/ijms222212115
Chicago/Turabian StyleCalderón-Sánchez, Eva M., Débora Falcón, Marta Martín-Bórnez, Antonio Ordoñez, and Tarik Smani. 2021. "Urocortin Role in Ischemia Cardioprotection and the Adverse Cardiac Remodeling" International Journal of Molecular Sciences 22, no. 22: 12115. https://doi.org/10.3390/ijms222212115
APA StyleCalderón-Sánchez, E. M., Falcón, D., Martín-Bórnez, M., Ordoñez, A., & Smani, T. (2021). Urocortin Role in Ischemia Cardioprotection and the Adverse Cardiac Remodeling. International Journal of Molecular Sciences, 22(22), 12115. https://doi.org/10.3390/ijms222212115






