Tubular Assembly Formation Induced by Leucine Alignment along the Hydrophobic Helix of Amphiphilic Polypeptides
Abstract
:1. Introduction
2. Results and Discussion
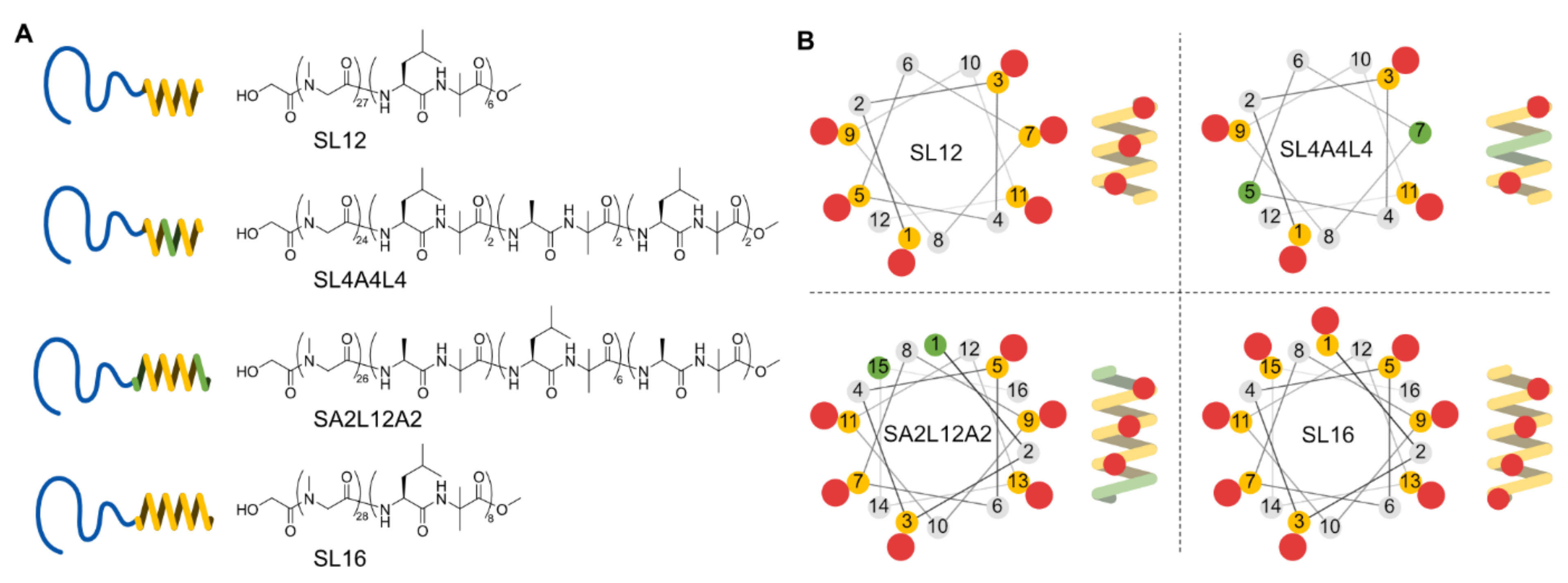
2.1. Amphiphilic Polypeptides
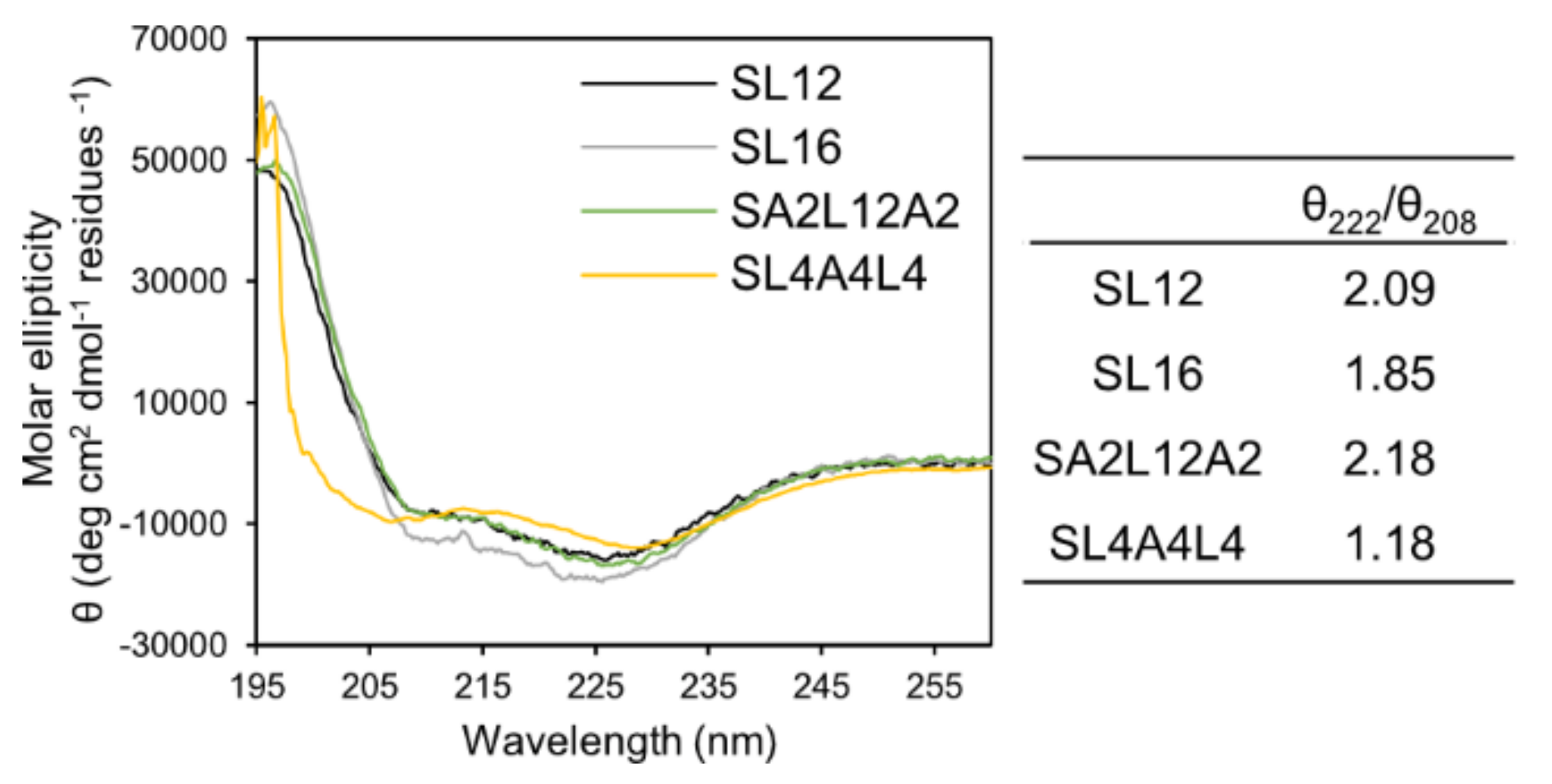
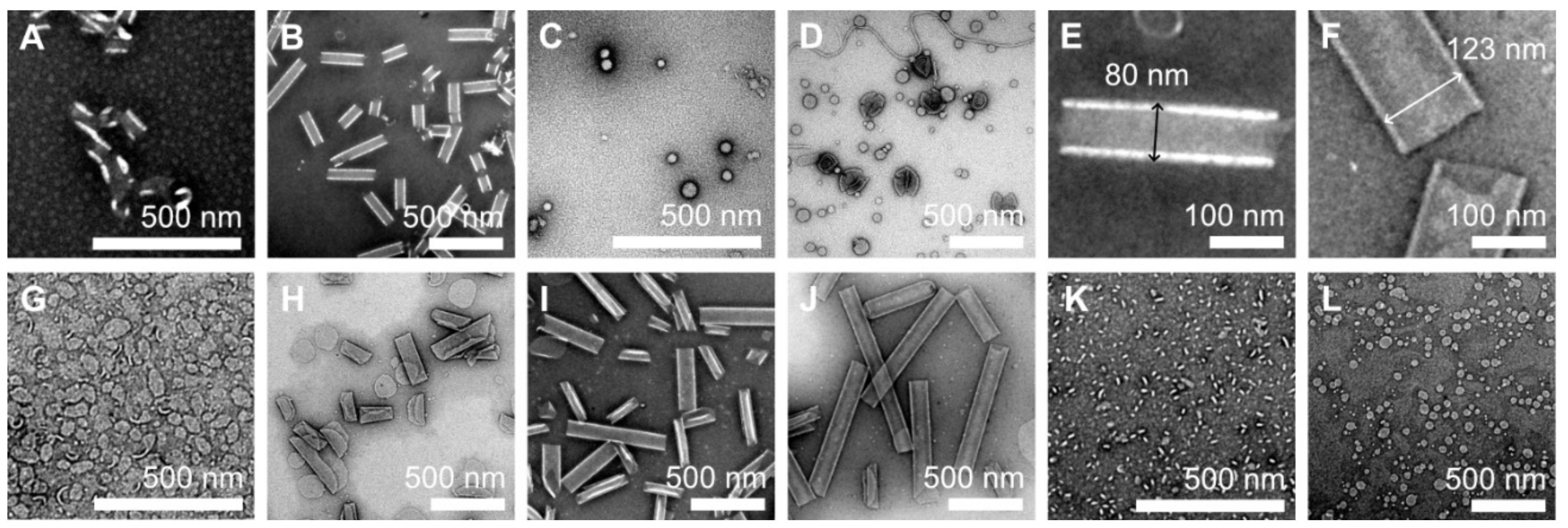
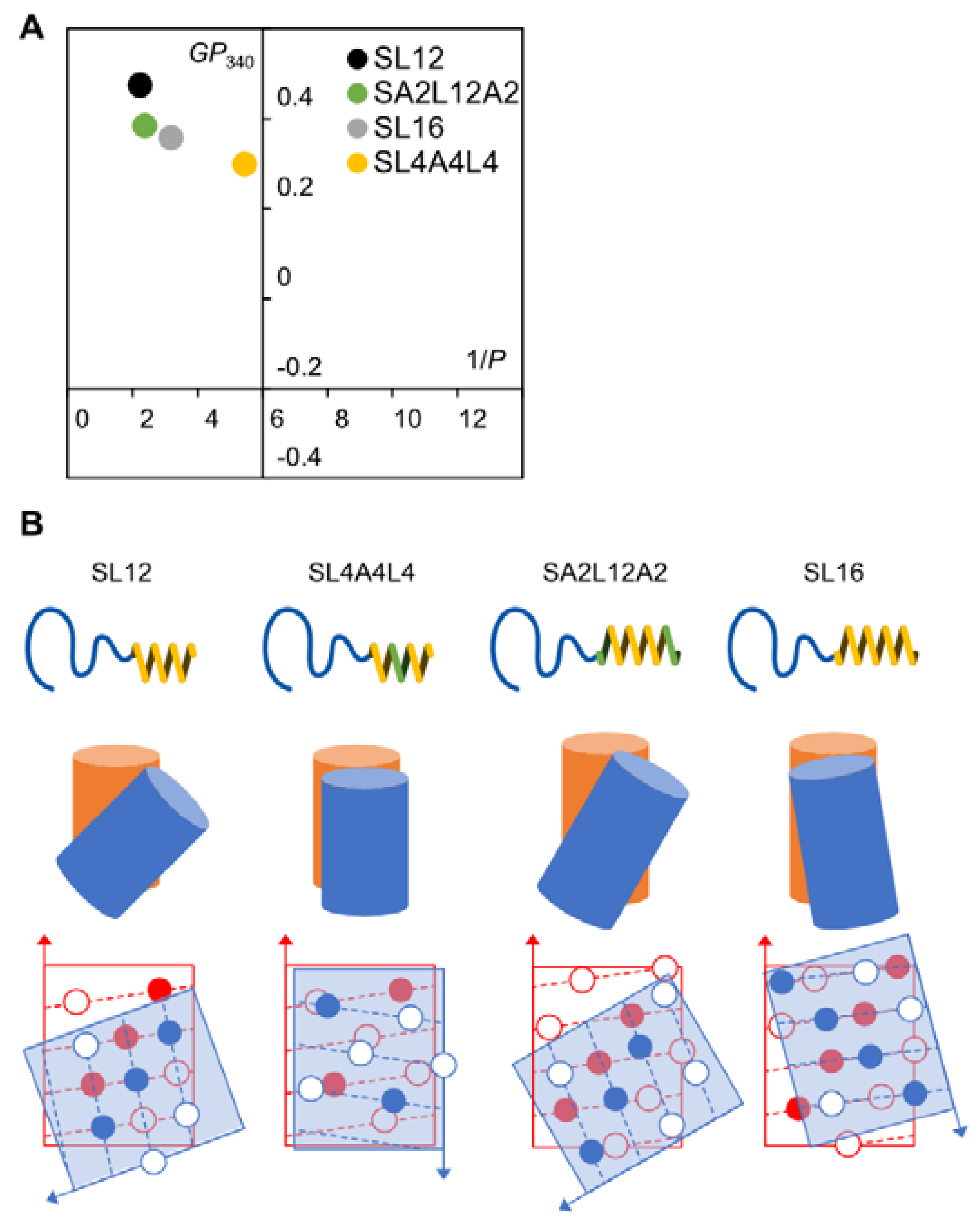
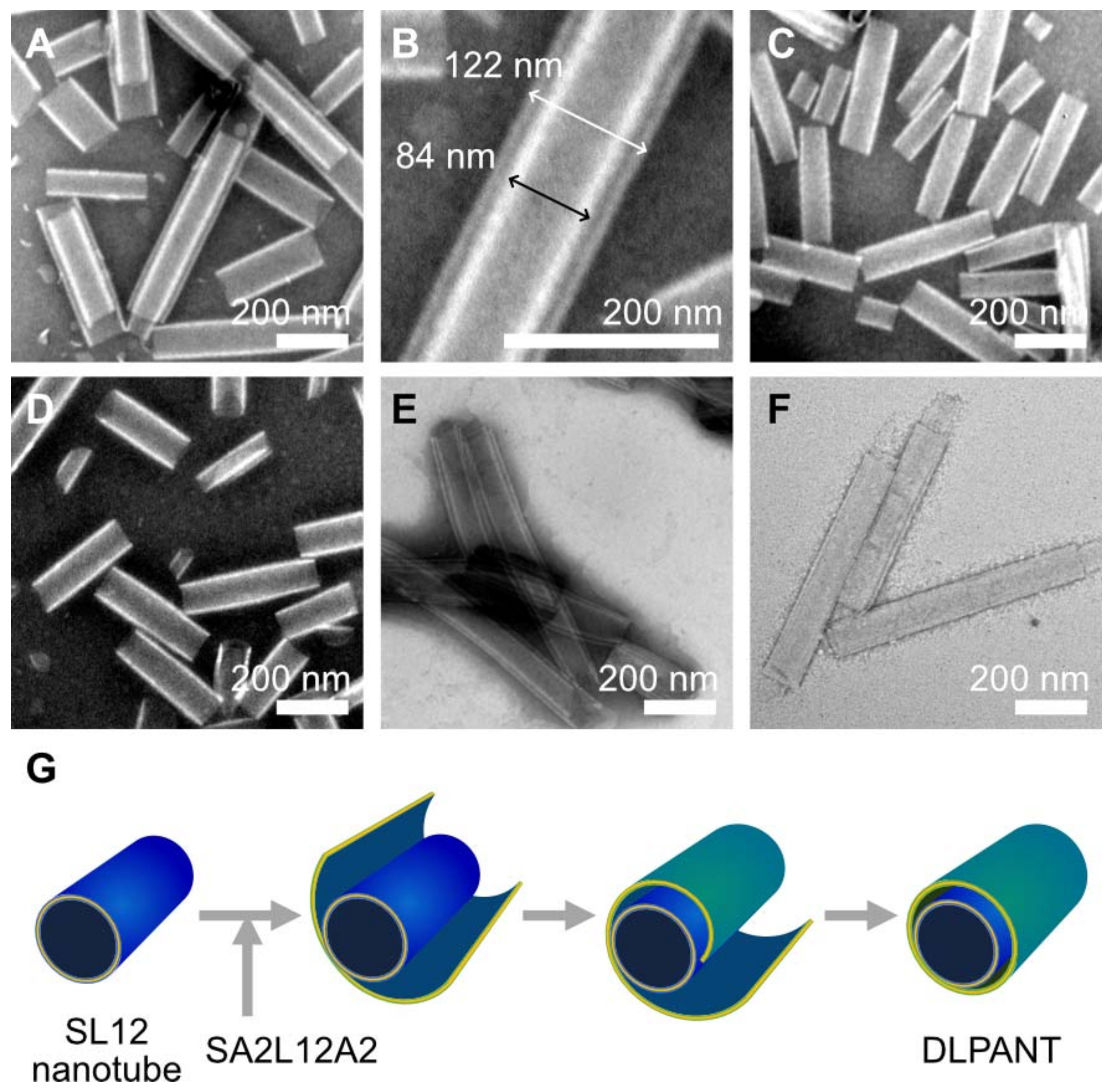
2.2. Tube Formation Induced by LxLxLxLxLxLx
2.3. Membrane Fluidity
2.4. Double Layer Nanotube Formation by SL12 and SA2L12A2 Nanotubes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Amphiphilic Polypeptides, SL12, SL16, SL4A4L4, and SA2L12A2
3.3. Preparation of Peptide Assemblies
3.4. Transmission Electron Microscopy (TEM)
3.5. Circular Dichroism (CD)
3.6. Membrane Fluidity by DPH
3.7. Membrane Fluidity by Laurdan
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberts, B. Molecular Biology of the Cell, 6th ed.; Garland Science, Taylor and Francis Group: New York, NY, USA, 2015; pp. 109–134. [Google Scholar]
- Fletcher, J.M.; Harniman, R.L.; Barnes, F.R.H.; Boyle, A.L.; Collins, A.; Mantell, J.; Sharp, T.H.; Antognozzi, M.; Booth, P.J.; Linden, N.; et al. Self-Assembling Cages from Coiled-Coil Peptide Modules. Science 2013, 340, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Glennie, S.J.; Lam, H.S.; Baum, H.E.; Kandage, D.; Williams, N.A.; Morgan, D.J.; Woolfson, D.N.; Davidson, A.D. A Modular Vaccine Platform Combining Self-Assembled Peptide Cages and Immunogenic Peptides. Adv. Funct. Mater. 2019, 29, 1807357. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.M.; Bray, H.E.V.; Shoemark, D.K.; Hodgson, L.R.; Coombs, J.; Mantell, J.M.; Rose, R.S.; Ross, J.F.; Morris, C.; Harniman, R.L.; et al. De Novo Designed Peptide and Protein Hairpins Self-Assemble into Sheets and Nanoparticles. Small 2021, 17, 2100472. [Google Scholar] [CrossRef]
- Wang, F.B.; Gnewou, O.; Modlin, C.; Beltran, L.C.; Xu, C.F.; Su, Z.L.; Juneja, P.; Grigoryan, G.; Egelman, E.H.; Conticello, V.P. Structural analysis of cross alpha-helical nanotubes provides insight into the designability of filamentous peptide nanomaterials. Nat. Commun. 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Egelman, E.H.; Xu, C.; DiMaio, F.; Magnotti, E.; Modlin, C.; Yu, X.; Wright, E.; Baker, D.; Conticello, V.P. Structural Plasticity of Helical Nanotubes Based on Coiled-Coil Assemblies. Structure 2015, 23, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Xu, C.; Zuo, X.; Conticello, V.P. Structurally homogeneous nanosheets from self-assembly of a collagen-mimetic peptide. Angew. Chem. Int. Ed. 2014, 53, 8367–8371. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, C.F.; Liu, Y.; Liu, Z.; Wall, J.S.; Zuo, X.B.; Lian, T.Q.; Salaita, K.; Ni, C.Y.; Pochan, D.; et al. Structurally Defined Nanoscale Sheets from Self-Assembly of Collagen-Mimetic Peptides. J. Am. Chem. Soc. 2014, 136, 4300–4308. [Google Scholar] [CrossRef]
- Crick, F.H.C. The Packing of Alpha-Helices-Simple Coiled-Coils. Acta Crystallogr. 1953, 6, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Pratap, J.V.; Luisi, B.F.; Calladine, C.R. Geometric principles in the assembly of alpha-helical bundles. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20120369. [Google Scholar]
- Walshaw, J.; Woolfson, D.N. Extended knobs-into-holes packing in classical and complex coiled-coil assemblies. J. Struct. Biol. 2003, 144, 349–361. [Google Scholar] [CrossRef]
- Efimov, A.V. Complementary packing of alpha-helices in proteins. FEBS Lett. 1999, 463, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Efimov, A.V. Standard Structures in Proteins. Prog. Biophys. Mol. Biol. 1993, 60, 201–239. [Google Scholar] [CrossRef]
- Kroeger, K.M.; Pfleger, K.D.G.; Eidne, K.A. G-protein coupled receptor oligomerization in neuroendocrine pathways. Front. Neuroendocrinol. 2003, 24, 254–278. [Google Scholar] [CrossRef] [PubMed]
- Hebert, T.E.; Moffett, S.; Morello, J.P.; Loisel, T.P.; Bichet, D.G.; Barret, C.; Bouvier, M. A peptide derived from a beta(2)-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 1996, 271, 16384–16392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Zeng, X.L.; Zhou, Z.C.; Zhao, J.; Pei, G. β-arrestin-1 contributes to brown fat function and directly interacts with PPAR alpha and PPAR gamma. Sci. Rep. 2016, 6, 26999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldring, N.; Pawson, T.; McDonnell, D.; Treuter, E.; Gustafsson, J.A.; Pike, A.C.W. Structural insights into corepressor recognition by antagonist-bound estrogen receptors. J. Biol. Chem. 2007, 282, 10449–10455. [Google Scholar] [CrossRef] [Green Version]
- Chothia, C.; Levitt, M.; Richardson, D. Structure of Proteins-Packing of Alpha-Helices and Pleated Sheets. Proc. Natl. Acad. Sci. USA 1977, 74, 4130–4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chothia, C.; Levitt, M.; Richardson, D. Helix to Helix Packing in Proteins. J. Mol. Biol. 1981, 145, 215–250. [Google Scholar] [CrossRef]
- Kanzaki, T.; Horikawa, Y.; Makino, A.; Sugiyama, J.; Kimura, S. Nanotube and Three-Way Nanotube Formation with Nonionic Amphiphilic Block Peptides. Macromol. Biosci. 2008, 8, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Seo, S.; Nair, B.G.; Muller, S.; Takahashi, E.; Arai, T.; Iyoda, T.; Fujii, S.; Tsuneda, S.; Ito, Y. End-Sealed High Aspect Ratio Hollow Nanotubes Encapsulating an Anticancer Drug: Torpedo-Shaped Peptidic Nanocapsules. ACS Nano 2019, 13, 305–312. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ueda, M.; Son, K.; Seo, S.; Takeoka, S.; Hirose, T.; Ito, Y. Tubular Network Formation by Mixing Amphiphilic Polypeptides with Differing Hydrophilic Blocks. Biomacromolecules 2019, 20, 3908–3914. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Seo, S.; Muller, S.; Rahman, M.M.; Ito, Y. Integrated nanostructures based on self-assembled amphiphilic polypeptides. In Advances in Biosinpired and Biomedical Materials; Ito, Y., Chen, X., Kang, I., Eds.; American Chemical Society: Washington, DC, USA, 2017; Volume 1, pp. 19–30. [Google Scholar]
- Kim, C.J.; Ueda, M.; Imai, T.; Sugiyama, J.; Kimura, S. Tuning the Viscoelasticity of Peptide Vesicles by Adjusting Hydrophobic Helical Blocks Comprising Amphiphilic Polypeptides. Langmuir 2017, 33, 5423–5429. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Rational design of peptide nanotubes for varying diameters and lengths. J. Pept. Sci. 2011, 17, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Temperature-triggered fusion of vesicles composed of right-handed and left-handed amphiphilic helical peptides. Langmuir 2011, 27, 4300–4304. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, A.; Ito, Y.; Ueda, M. Solvent Effects on the Self-Assembly of an Amphiphilic Polypeptide Incorporating alpha-Helical Hydrophobic Blocks. J. Am. Chem. Soc. 2020, 142, 20994–21003. [Google Scholar] [CrossRef]
- Bromley, E.H.; Channon, K.J.; King, P.J.; Mahmoud, Z.N.; Banwell, E.F.; Butler, M.F.; Crump, M.P.; Dafforn, T.R.; Hicks, M.R.; Hirst, J.D.; et al. Assembly pathway of a designed alpha-helical protein fiber. Biophys. J. 2010, 98, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, D.W.; Yip, C.M.; Chakrabartty, A. Reversible assembly of helical filaments by de novo designed minimalist peptides. Biopolymers 2005, 80, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Potekhin, S.A.; Melnik, T.N.; Popov, V.; Lanina, N.F.; Vazina, A.A.; Rigler, P.; Verdini, A.S.; Corradin, G.; Kajava, A.V. De novo design of fibrils made of short alpha-helical coiled coil peptides. Chem. Biol. 2001, 8, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Pandya, M.J.; Spooner, G.M.; Sunde, M.; Thorpe, J.R.; Rodger, A.; Woolfson, D.N. Sticky-end assembly of a designed peptide fiber provides insight into protein fibrillogenesis. Biochemistry 2000, 39, 8728–8734. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Transformation of peptide nanotubes into a vesicle via fusion driven by stereo-complex formation. Chem. Commun. 2011, 47, 3204–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Tubulation on peptide vesicles by phase-separation of a binary mixture of amphiphilic right-handed and left-handed helical peptides. Soft Matter 2011, 7, 4143–4146. [Google Scholar] [CrossRef]
- Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Versatile peptide rafts for conjugate morphologies by self-assembling amphiphilic helical peptides. Polym. J. 2013, 45, 509–515. [Google Scholar] [CrossRef]
- Han, J.; Suga, K.; Hayashi, K.; Okamoto, Y.; Umakoshi, H. Multi-Level Characterization of the Membrane Properties of Resveratrol-Incorporated Liposomes. J. Phys. Chem. B 2017, 121, 4091–4098. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of Sterol Derivatives in Regulating the Properties of Phospholipid Bilayer Systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Krokosz, A.; Puchala, M. Membrane fluidity and activity of membrane ATPases in human erythrocytes under the influence of polyhydroxylated fullerene. Biochim. Biophys. Acta Biomembr. 2013, 1828, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef]
- Lentz, B.R.; Lee, J.K. Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: A mechanism in common with viral fusion and secretory vesicle release? Mol. Membr. Biol. 1999, 16, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Suga, K.; Yokoi, T.; Kondo, D.; Hayashi, K.; Morita, S.; Okamoto, Y.; Shimanouchi, T.; Umakoshi, H. Systematical characterization of phase behaviors and membrane properties of fatty acid/didecyldimethylammonium bromide vesicles. Langmuir 2014, 30, 12721–12728. [Google Scholar] [CrossRef] [PubMed]
- Suga, K.; Umakoshi, H. Detection of Nanosized Ordered Domains in DOPC/DPPC and DOPC/Ch Binary Lipid Mixture Systems of Large Unilamellar Vesicles Using a TEMPO Quenching Method. Langmuir 2013, 29, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Aleman, C.; Annereau, J.P.; Liang, X.J.; Cardarelli, C.O.; Taylor, B.; Yin, J.J.; Aszalos, A.; Gottesman, M.M. P-glycoprotein, expressed in multidrug resistant cells, is not responsible for alterations in membrane fluidity or membrane potential. Cancer Res. 2003, 63, 3084–3091. [Google Scholar] [PubMed]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta Biomembr. 2002, 1565, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of Lipid Phases in Phospholipid-Vesicles by the Generalized Polarization of Laurdan Fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abosheasha, M.A.; Itagaki, T.; Ito, Y.; Ueda, M. Tubular Assembly Formation Induced by Leucine Alignment along the Hydrophobic Helix of Amphiphilic Polypeptides. Int. J. Mol. Sci. 2021, 22, 12075. https://doi.org/10.3390/ijms222112075
Abosheasha MA, Itagaki T, Ito Y, Ueda M. Tubular Assembly Formation Induced by Leucine Alignment along the Hydrophobic Helix of Amphiphilic Polypeptides. International Journal of Molecular Sciences. 2021; 22(21):12075. https://doi.org/10.3390/ijms222112075
Chicago/Turabian StyleAbosheasha, Mohammed A., Toru Itagaki, Yoshihiro Ito, and Motoki Ueda. 2021. "Tubular Assembly Formation Induced by Leucine Alignment along the Hydrophobic Helix of Amphiphilic Polypeptides" International Journal of Molecular Sciences 22, no. 21: 12075. https://doi.org/10.3390/ijms222112075
APA StyleAbosheasha, M. A., Itagaki, T., Ito, Y., & Ueda, M. (2021). Tubular Assembly Formation Induced by Leucine Alignment along the Hydrophobic Helix of Amphiphilic Polypeptides. International Journal of Molecular Sciences, 22(21), 12075. https://doi.org/10.3390/ijms222112075





