Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW264.7 Cells
Abstract
:1. Introduction
2. Results
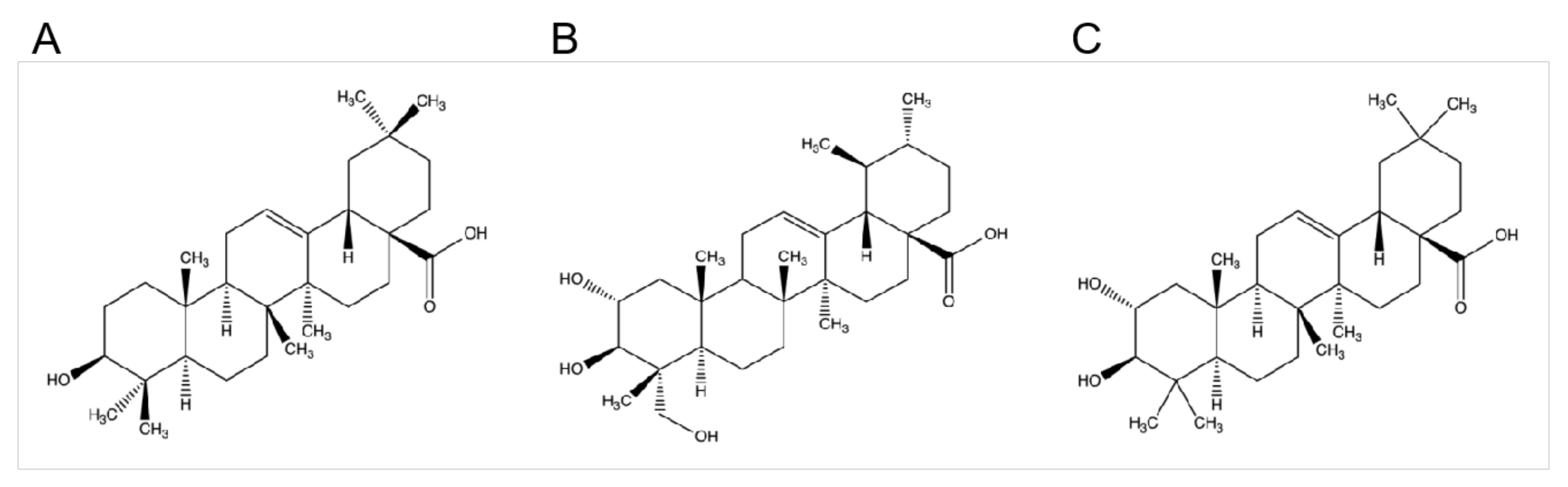
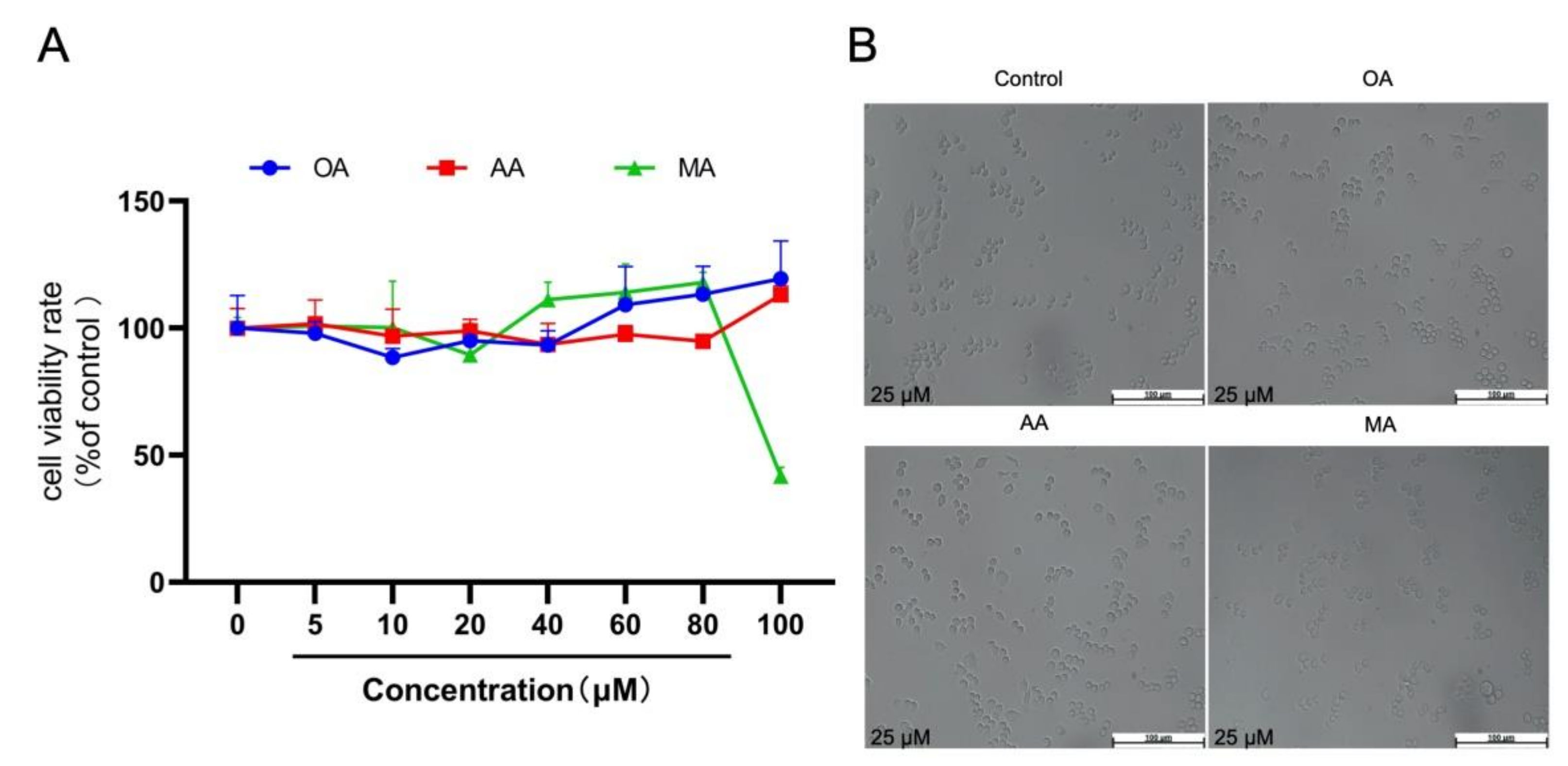
2.1. Cytotoxicity of OA, AA, and MA
2.2. Effects of OA, AA, and MA on the Production of Nitric Oxide (NO), Inducible Nitric Oxide Synthase (iNOS) and Inflammatory Factors in LPS-Stimulated RAW264.7 Cells
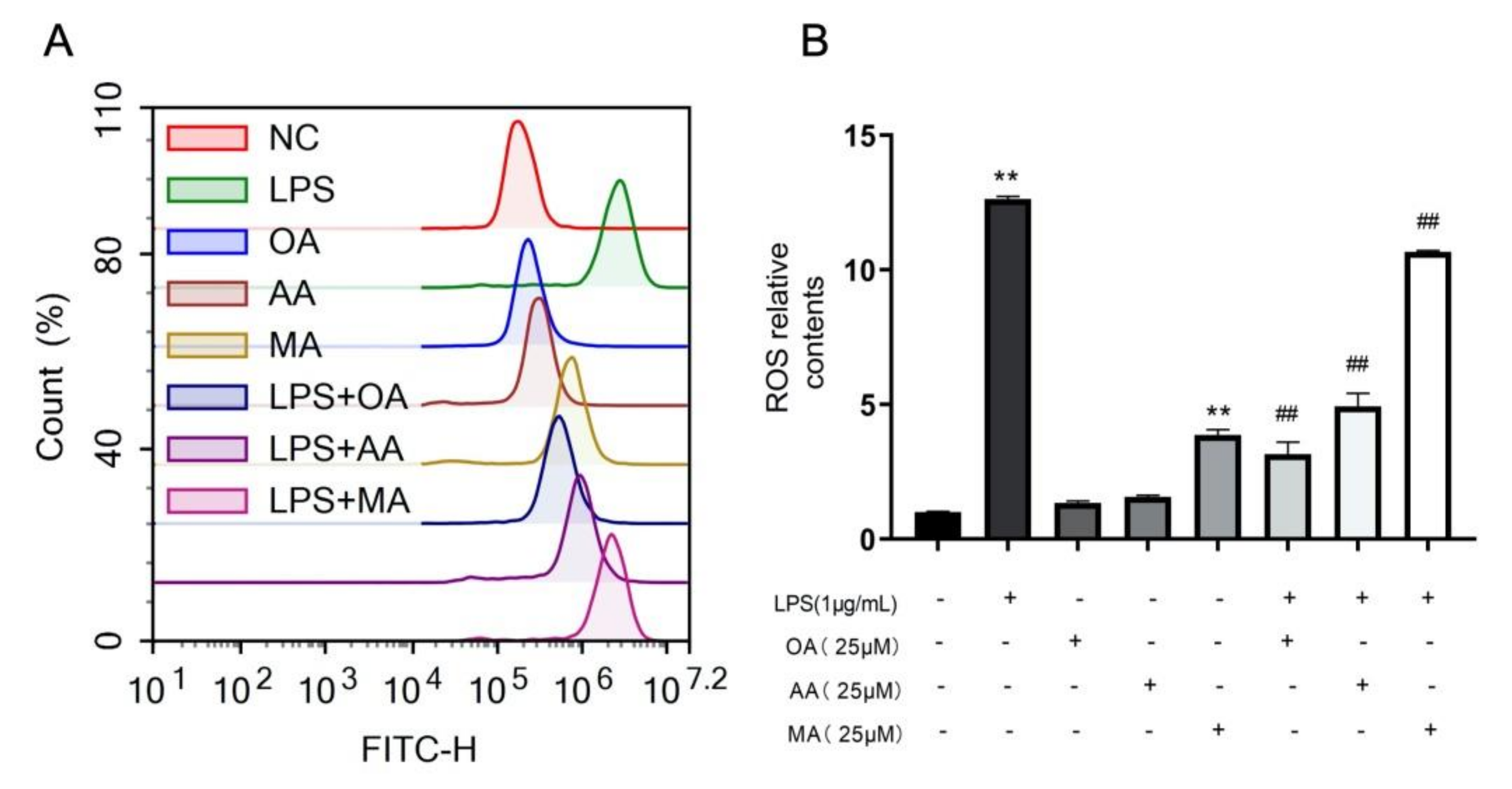
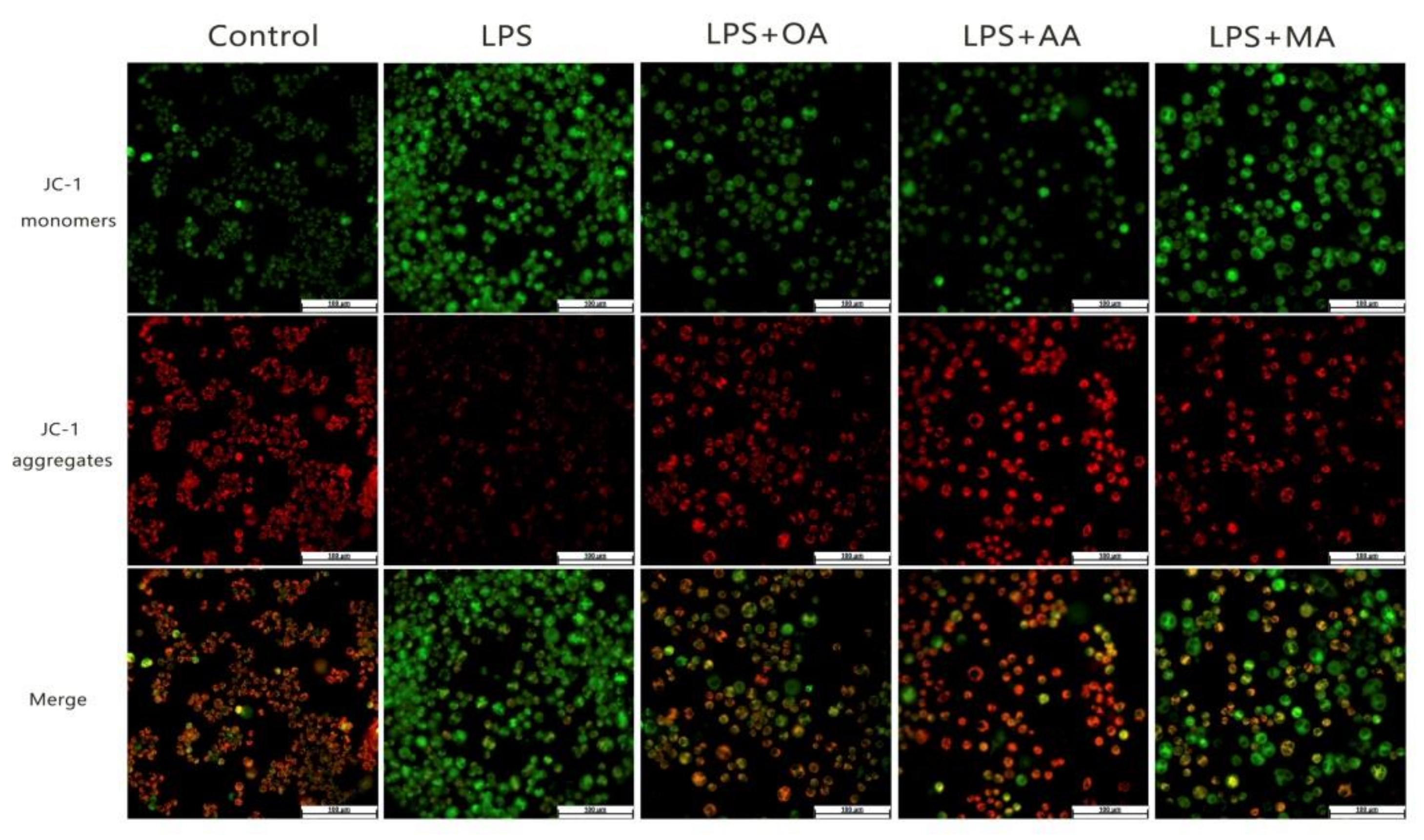
2.3. OA, AA, and MA Reduce ROS Content and Restore Mitochondrial Membrane Potential in LPS-Stimulated Macrophages to Varying Degrees
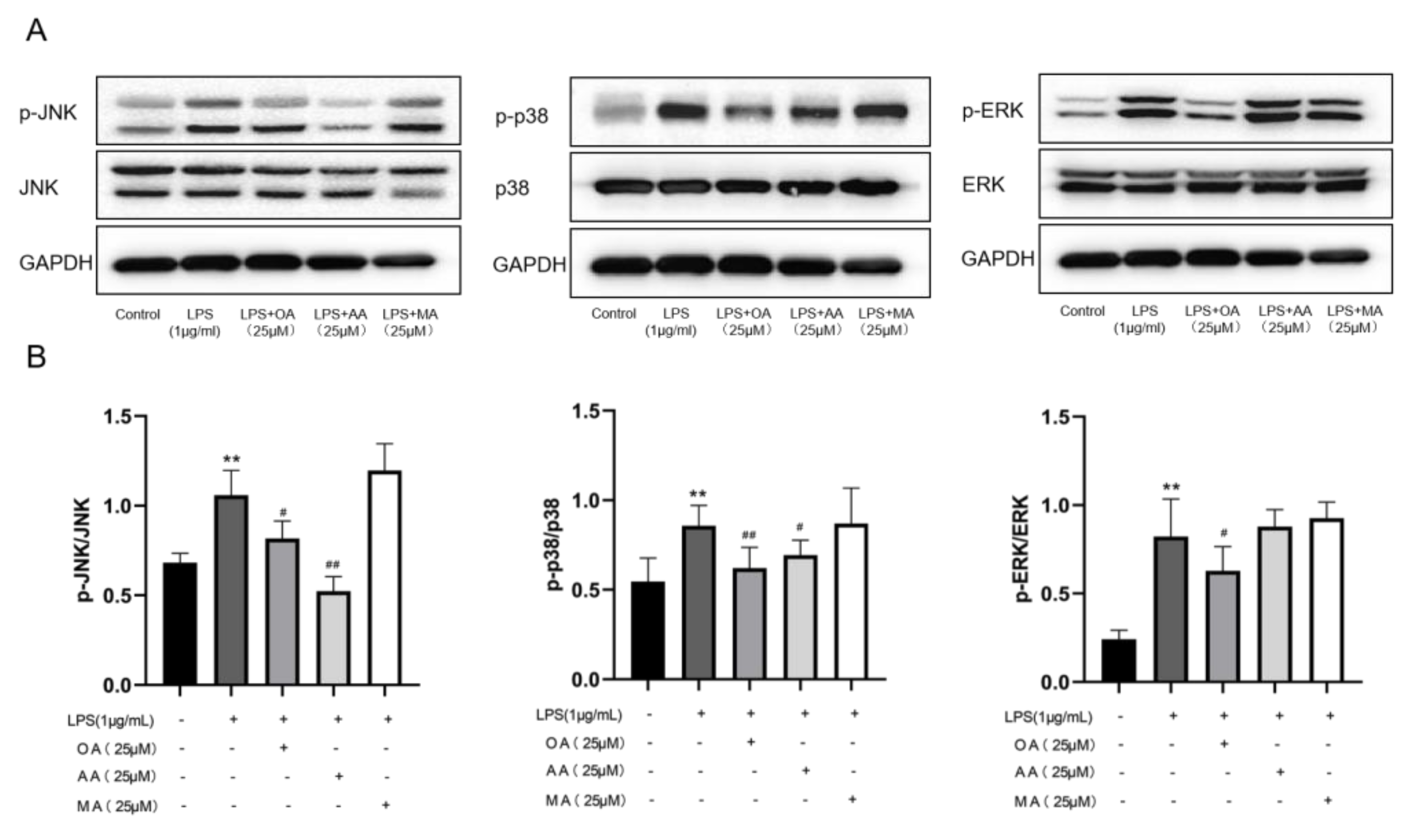
2.4. Effects of OA, AA, and MA on MAPK Pathways in LPS-Stimulated RAW264.7 Cells
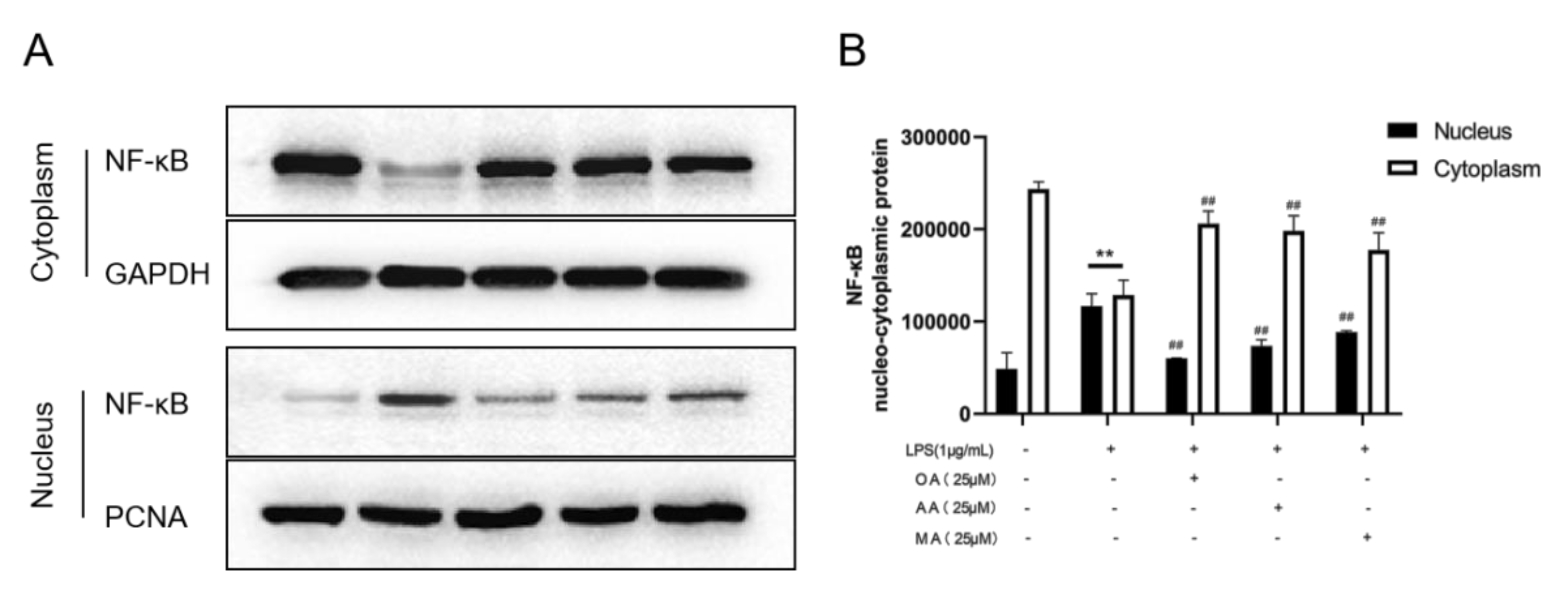
2.5. Effects of OA, AA, and MA on the NF-κB Pathway in LPS-Stimulated RAW264.7 Cells
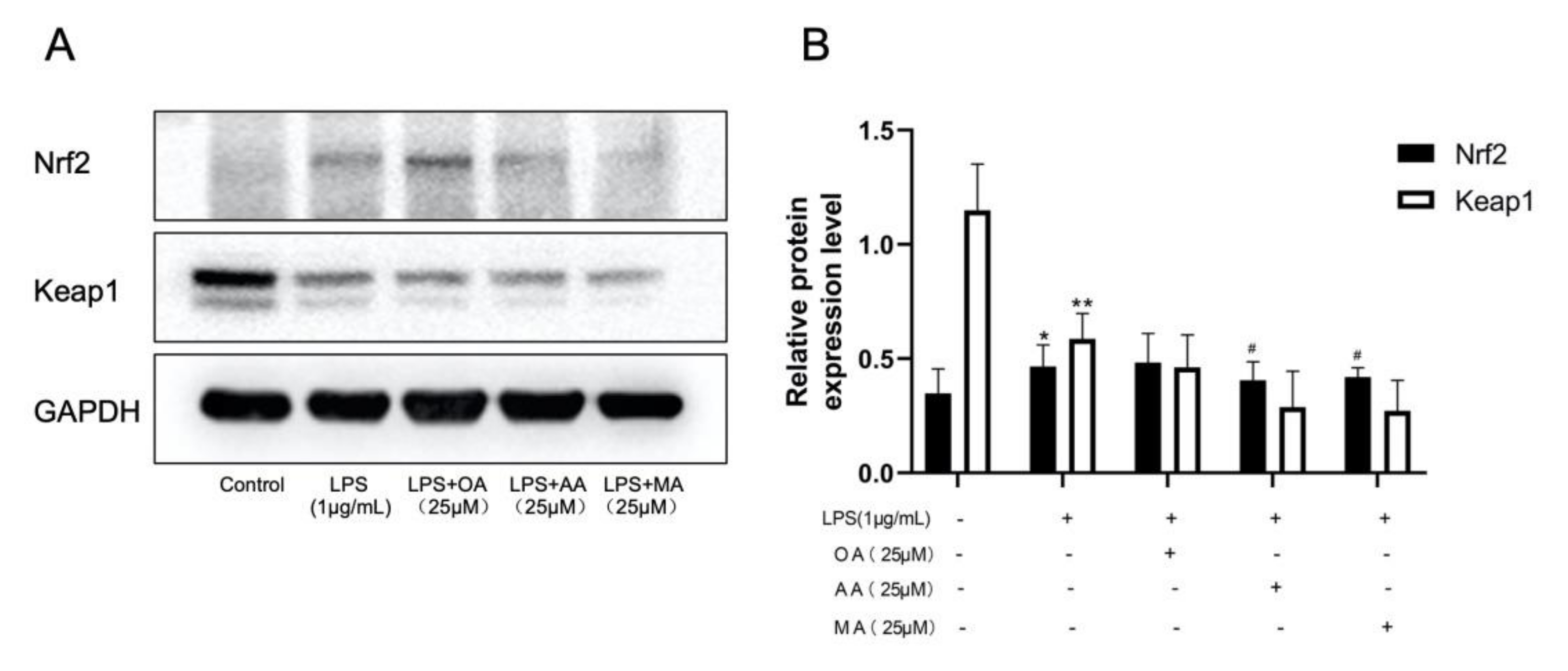
2.6. Effects of OA, AA, and MA on the Keap1-Nrf2 Pathway in LPS-Stimulated RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. NO Measurement
4.5. IL-6 and IL-10 Measurement
4.6. Flow Cytometry
4.7. Fluorescence Assay
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Z.; YangBo, Z.; Xin, Z.; HaiYan, L. Effect of white tea extract on LPS-induced inflammation of RAW264.7 cells. Tea Commun. 2019, 46, 455–462. [Google Scholar]
- ZiRao, B. Preliminary Study on Anti-Inflammatory Effect and Molecular Mechanism of Triterpenoid Compounds in Ganoderma Lucidum; Inner Mongolia University: Hohhot, China, 2016. [Google Scholar]
- Yan-hua1, X.; San-hu1, Z.; Yu, W.; Ke-wen, C.; Ya-ting, L.; Na, Y.; Chun-yan, Z.; Jian-hua, Z.; Cheng-meng, W. Study on the Active Substances and Antioxidant Activity of Different Solvent Extracts of Seabuckthorn Leaves. Food Res. Dev. 2021, 42, 44–49. [Google Scholar]
- Haonan, Z.; Na, H.; Qi, D.; Honglun, W. Simultaneous determination of six triterpenic acids in Hippophae rhamnoides fruit by HPLC. West China J. Pharm. 2021, 36, 319–322. [Google Scholar]
- ZhiMin, Q. Study on Anti-Inflammatory and Anti-Oxidative Stress Effects of Asiatic Acid on Acute Liver Injury and Its Mechanism; Jilin University: Changchun, China, 2017. [Google Scholar]
- Jannus, F. Efficient In Vitro and In Vivo Anti-Inflammatory Activity of a Diamine-PEGylated Oleanolic Acid Derivative. Int. J. Mol. Sci. 2021, 22, 8158. [Google Scholar] [CrossRef]
- Xuan, L.; YingChun, H.; FangLiang, Z. Research progress on the pharmacological action and mechanism of hawthoric acid. Chin. J. Mod. Med. 2021, 31, 49–53. [Google Scholar]
- Wanqing, L.; Hongxiang, Z.; Min, X.; Chenglong, H.; Linfen, T.; Jun, L.; Ting, Z.; Hong, C.; Jing, X.; Chunli, L.; et al. Oleanolic Acid Improves Obesity-Related Inflammation and Insulin Resistance by Regulating Macrophages Activation. Front. Pharmacol. 2021, 12, 697483. [Google Scholar]
- Mengyuan, F.; Wencui, W.; Qiuming, L.; Weiwei, W.; Yang, L.; Hongzhuo, L.; Xin, Y. Asiatic acid attenuates diabetic retinopathy through TLR4/MyD88/NF-κB p65 mediated modulation of microglia polarization. Life Sci. 2021, 277, 119567. [Google Scholar]
- Yan-Qiu, M.; He, T.; Xiao-Xiao, L.; Zhen-Yu, K.; Qian-Wen, L.; Chuan-Dong, X. Synthesis and anti-tumor activity of derivatives of ring A of asiatic acid. J. Asian Nat. Prod. Res. 2020, 22, 689–700. [Google Scholar]
- Xingfang, Y.; Gang, Z.; Zhichao, H.; Shangkun, T.; Jianchen, X.; Ping, S.; Qian, T.; Haixiao, L. Asiatic acid ameliorates obesity-related osteoarthritis by inhibiting myeloid differentiation protein-2. Food Funct. 2020, 11, 5513–5524. [Google Scholar]
- Jiuwei, C.; Lin, W. Maslinic Acid Inhibits Cervical Intraepithelial Neoplasia by Suppressing Interleukin-6 and Enhancing Apoptosis in a Mouse Model. Anti-Cancer Agents Med. Chem. 2021. [Google Scholar] [CrossRef]
- Jing, Z.; Rui, W.; Ruihua, L.; Hao, Y.; Hengtong, F. Review of the biological activity of maslinic acid1. Curr. Drug Targets 2021. [Google Scholar] [CrossRef]
- Yong, Z.; XiaoKui, Z.; YuMei, S. Maslinic acid reduces cardiomyocyte injury and apoptosis mediated by high glucose and its mechanism. Heart Mag. 2017, 29, 276–280. [Google Scholar]
- Cai-xia, T.; Lei, C.; Yong-ting, H. Maslinic acid protects against LPS-induced inflammatory response in RAW264.7 cells by regulating phosphorylation of STST3. Food Mach. 2021, 37, 36–42. [Google Scholar]
- Martin-Navarro, C.M.; Lopez-Arencibia, A.; Sifaoui, I. Amoebicidal activityof caffeine and maslinic acid by the induction of programmed cell death in acanthamoeba. Antimicrob. Agents Chemother. 2017, 61, e02660-16. [Google Scholar]
- Guangren, X. Inhibition of chikusetsusaponin IVa on inflammatory responses in RAW264.7 cell line via MAPK pathway. Z. Für Nat. C 2020, 76, 103–110. [Google Scholar]
- XiaoYi, F.; Wei, Z.; PengLun, H.; WenHui, C.; Zheng, Y. Inhibitory functions of notoginseng on LPS-induced RAW264.7 cells inflammation via iNOS-NO-NFKB signaling pathways. Drug Eval. Study 2020, 43, 670–675. [Google Scholar]
- Xiong, L.; Ouyang, K.H.; Jiang, Y. Chemical composition of Cyclocarya paliurus polysaccharide and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophage. Int. J. Biol. Macromol. 2018, 107, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- ChengHua, C.; YaJing, H. The process and mechanism of inflammation mediated by LPS. J. Henan Univ. 2017, 36, 70–76. [Google Scholar]
- Liu, X.; Xie, J.; Jia, S. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Jin Kyu, K.; ChangGu, H. 4-Hydroxy-7-Methoxycoumarin Inhibits Inflammation in LPS-activated RAW264.7 Macrophages by Suppressing NF-κB and MAPK Activation. Molecules 2020, 25, 4424. [Google Scholar]
- Wu, H. Nuciferine Ameliorates Infammatory Responses by Inhibiting the TLR4-Mediated Pathway in LipopolysaccharideInduced Acute Lung Injury. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, P.V. Protective efect of gedunin on TLR-mediated infammation by modulation of infammasome activation and cytokine production: Evidence of a multitarget compound. Pharmacol. Res. 2017, 115, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Syama, H.P. Syzygium cumini seed attenuates LPS induced inflammatory response in murine macrophage cell line RAW264.7 through NF-κB translocation. J. Funct. Foods 2018, 44, 218–226. [Google Scholar]
- I-Chuan, Y. Antrolone, a Novel Benzoid Derived from Antrodia cinnamomea, Inhibits the LPS-Induced Inflammatory Response in RAW264.7 Macrophage Cells by Balancing the NF-κB and Nrf2 Pathways. Am. J. Chin. Med. 2018, 46, 1297–1313. [Google Scholar]
- Deng, L.; Feng, J.; Cheng-song, H.E. Oleanolic Acid Inhibit Tumor Necrosis Factor Alpha-induced Inflammatory Cytokines Production of Synovial Cells and Its Mechanism. Nat. Prod. Res. Dev. 2018, 30, 1138–1142. [Google Scholar]
- Si-yun, C.; Zhong-wen, F.; Li-jun, P. Mechanism of asiatic acid in alcoholic hepatitis based on network pharmacology. Chin. Bull. Pharmacol. 2021, 37, 1145–1151. [Google Scholar]
- Song-bai, W.; Yuan-yuan, W.; Li-hua, Z. Mechanism of hawthorn acid reducing inflammatory response and oxidative stress in mice with acute liver injury. Int. J. Biomed. Eng. 2018, 41, 482–487, 508. [Google Scholar]
- Caiyun, Z. The anti-inflammatory effect of-kaur-15-en-17-al-18-oic acid on lipopolysaccharide-stimulated RAW264.7 cells associated with NF-κB and P38/MAPK pathways. J. Asian Nat. Prod. Res. 2021, 23, 570–583. [Google Scholar]
- Qi, Z.L.; Qi, S.M.; Ling, L.F. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int. Immunopharmacol. 2016, 35, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, L.X.; Wang, Y.E. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NFkappa B and MAPK activation to reduce inflammation in LPSactivated RAW264.7 cells. Biomed Pharm. 2019, 109, 555–562. [Google Scholar] [CrossRef]
- Shan, H. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 315–322. [Google Scholar]
- AlSaeedi Fatma, J. Mangiferin protect oxidative stress against deoxynivalenol induced damages through Nrf2 signalling pathways in endothelial cells. Clin. Exp. Pharmacol. Physiol. 2020, 48, 389–400. [Google Scholar] [CrossRef]
- Cheng-Cao, S.; Shu-Jun, L.; Cui-Li, Y.; Rui-Lin, X.; Yong-Yong, X.; Liang, W.; Qian-Long, Z.; De-Jia, L. Sulforaphane Attenuates Muscle Inflammation in Dystrophin-deficient mdx Mice via NF-E2-related Factor 2 (Nrf2)-mediated Inhibition of NF-κB Signaling Pathway. J. Biol. Chem. 2015, 290, 17784–17795. [Google Scholar]
- Balsa, E.; Perry, E.A.; Bennett, C.F. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun. 2020, 11, 2714. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; Chow, S.H.; Han, M.L. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 2020, 5, 1418–1427. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Yuan, C.; Zhou, X.; Han, Y.; He, Y.; Ouyang, J.; Zhou, W.; Wang, Z.; Wang, H.; Li, G. Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW264.7 Cells. Int. J. Mol. Sci. 2021, 22, 12009. https://doi.org/10.3390/ijms222112009
Han Y, Yuan C, Zhou X, Han Y, He Y, Ouyang J, Zhou W, Wang Z, Wang H, Li G. Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW264.7 Cells. International Journal of Molecular Sciences. 2021; 22(21):12009. https://doi.org/10.3390/ijms222112009
Chicago/Turabian StyleHan, Yu, Chen Yuan, Xiaowei Zhou, Yingjie Han, Yanhao He, Jian Ouyang, Wenna Zhou, Zhenhua Wang, Honglun Wang, and Gang Li. 2021. "Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW264.7 Cells" International Journal of Molecular Sciences 22, no. 21: 12009. https://doi.org/10.3390/ijms222112009
APA StyleHan, Y., Yuan, C., Zhou, X., Han, Y., He, Y., Ouyang, J., Zhou, W., Wang, Z., Wang, H., & Li, G. (2021). Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW264.7 Cells. International Journal of Molecular Sciences, 22(21), 12009. https://doi.org/10.3390/ijms222112009






