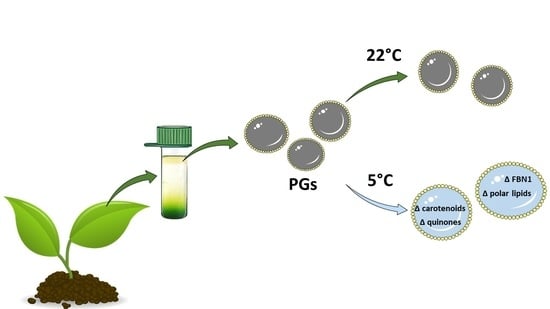
Bean and Pea Plastoglobules Change in Response to Chilling Stress
Abstract
1. Introduction
2. Results
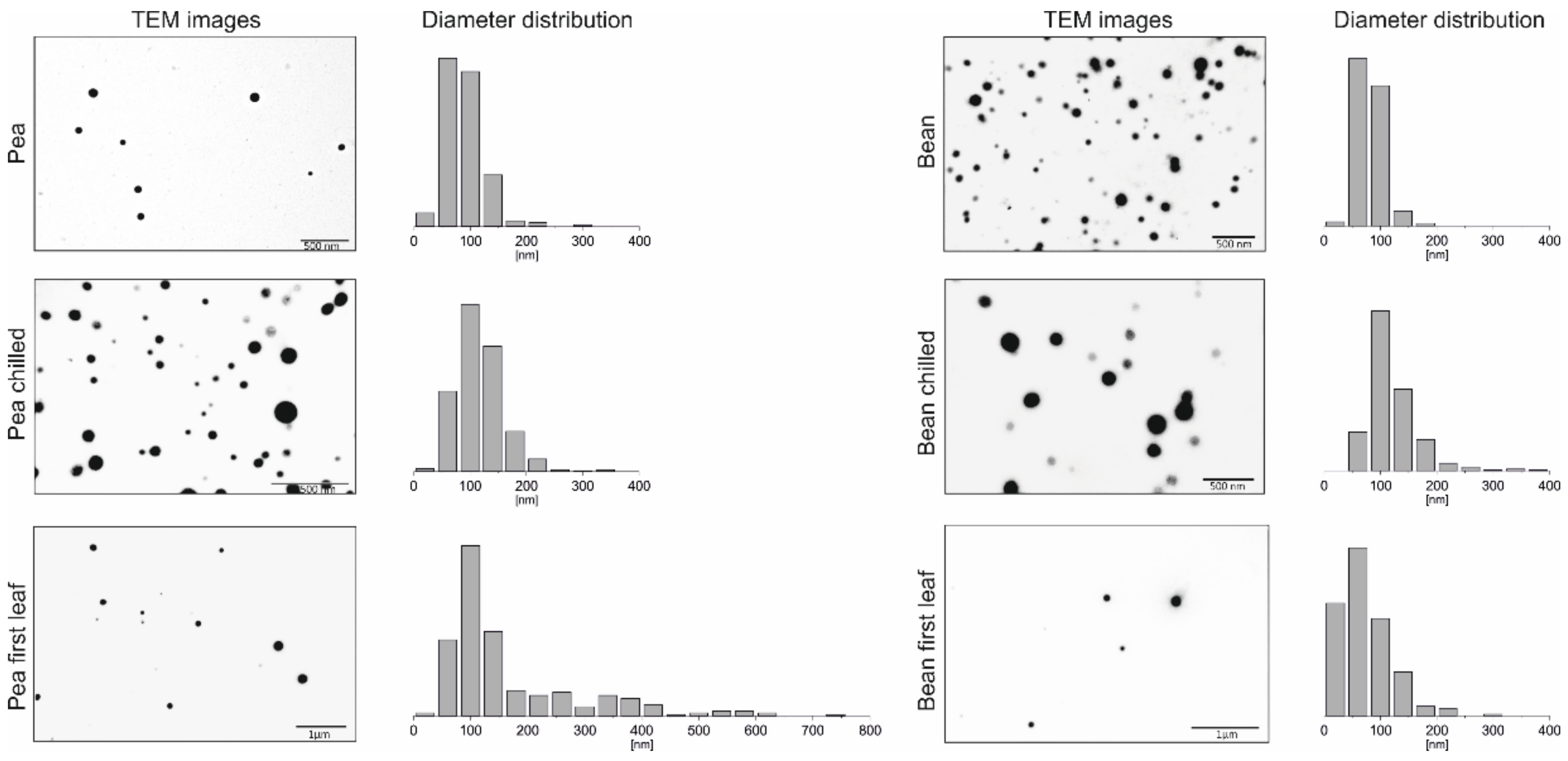
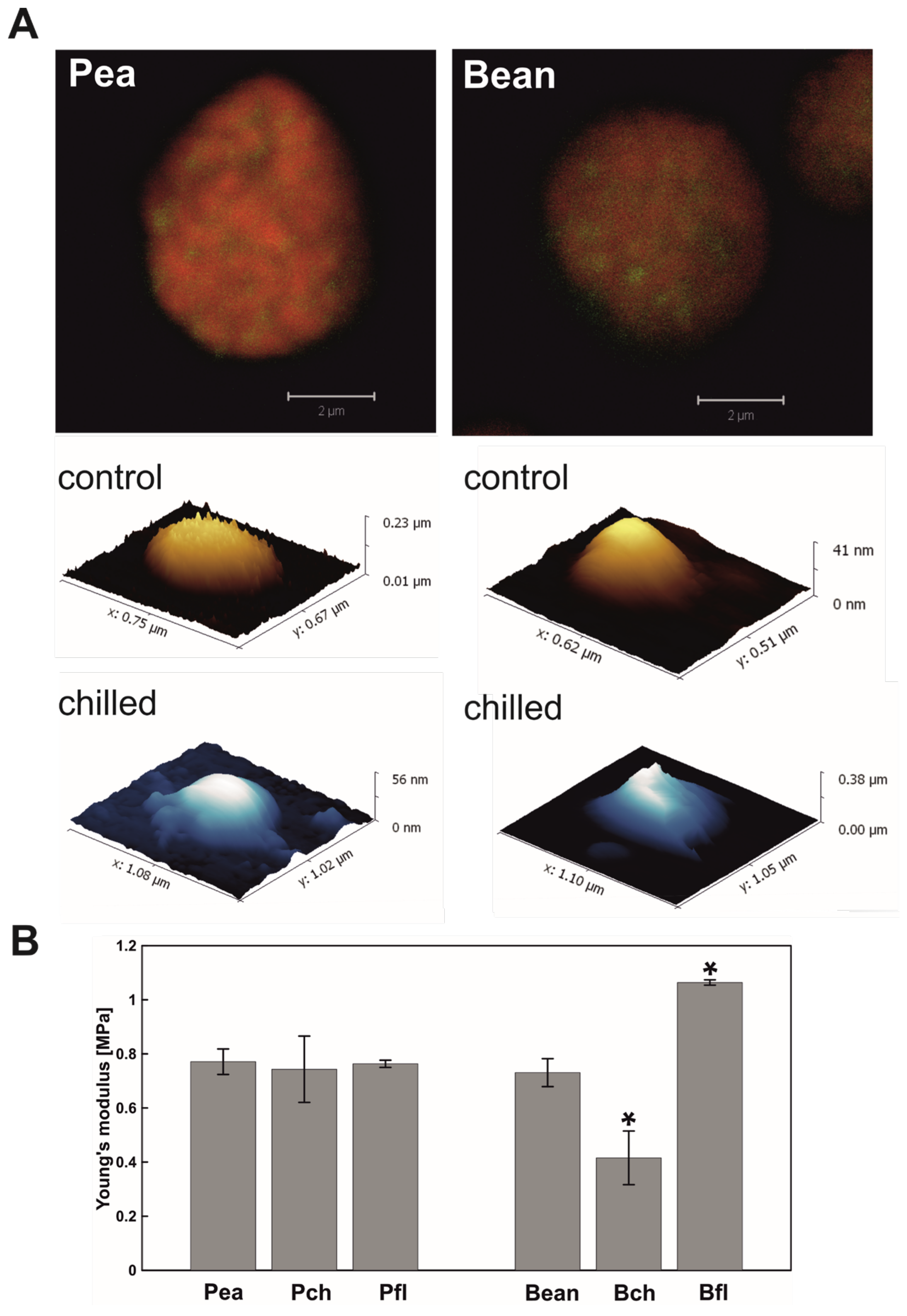
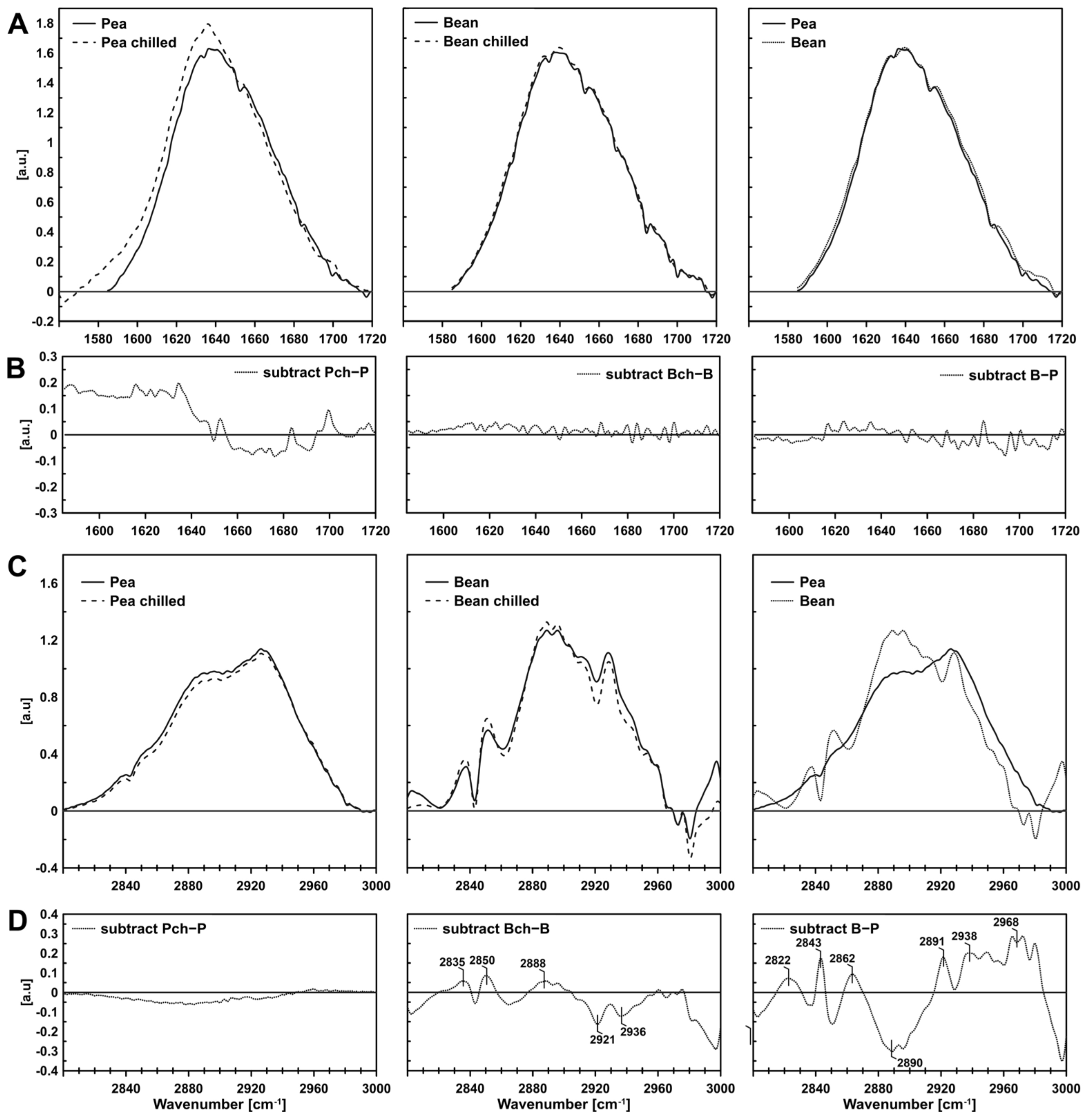
2.1. Visualisation of PGs
2.1.1. Transmission Electron Microscopy
2.1.2. Atomic Force Microscopy
2.1.3. FTIR (Fourier Transform Infrared Spectroscopy)
2.2. Composition of PGs
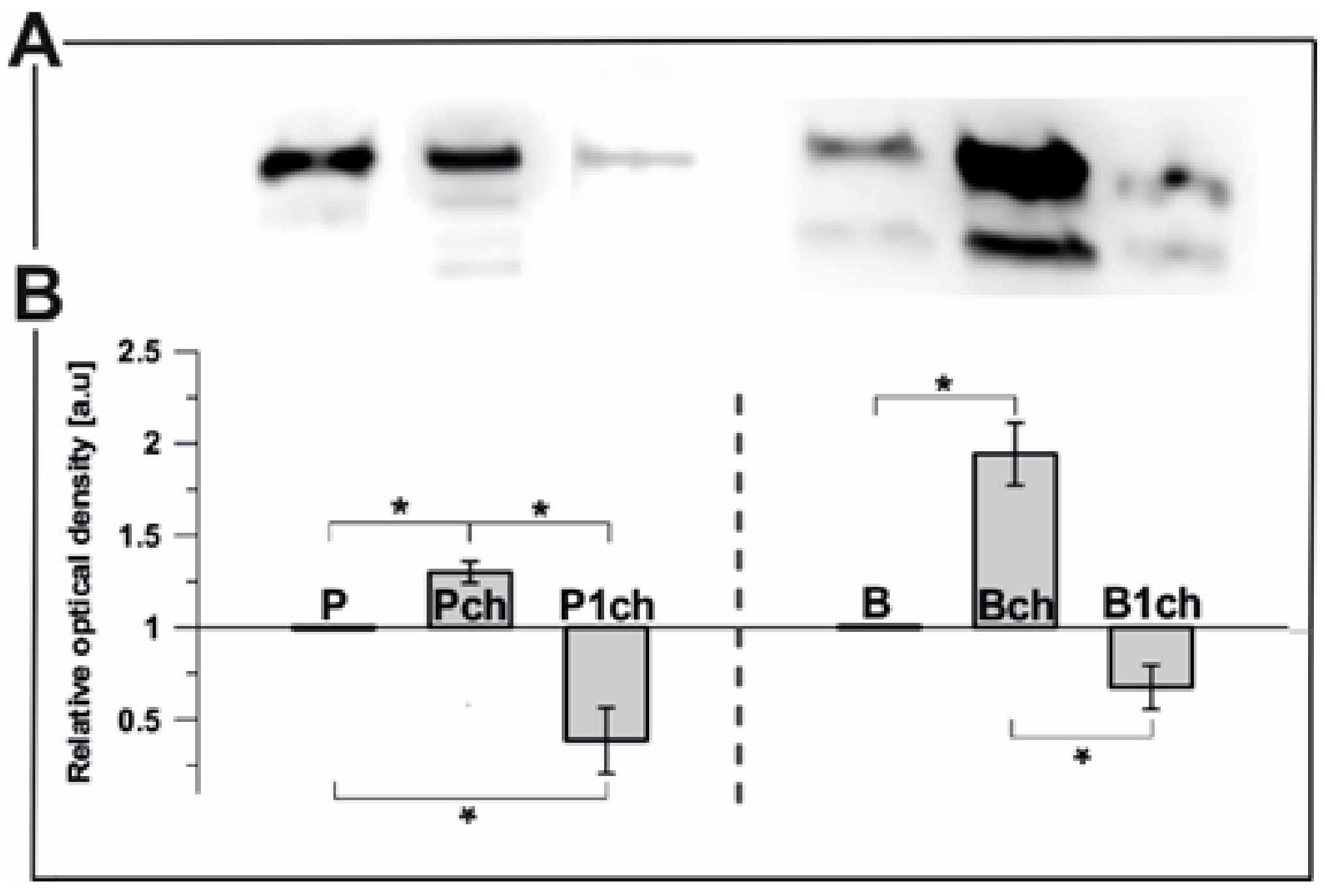
2.2.1. Plastoglobulin Content
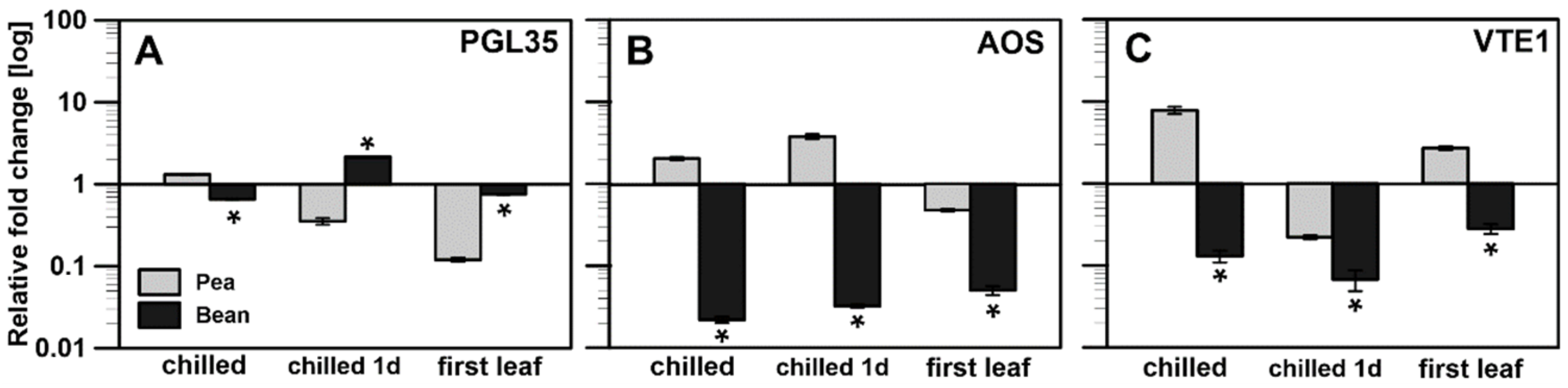
2.2.2. Transcriptional Analysis
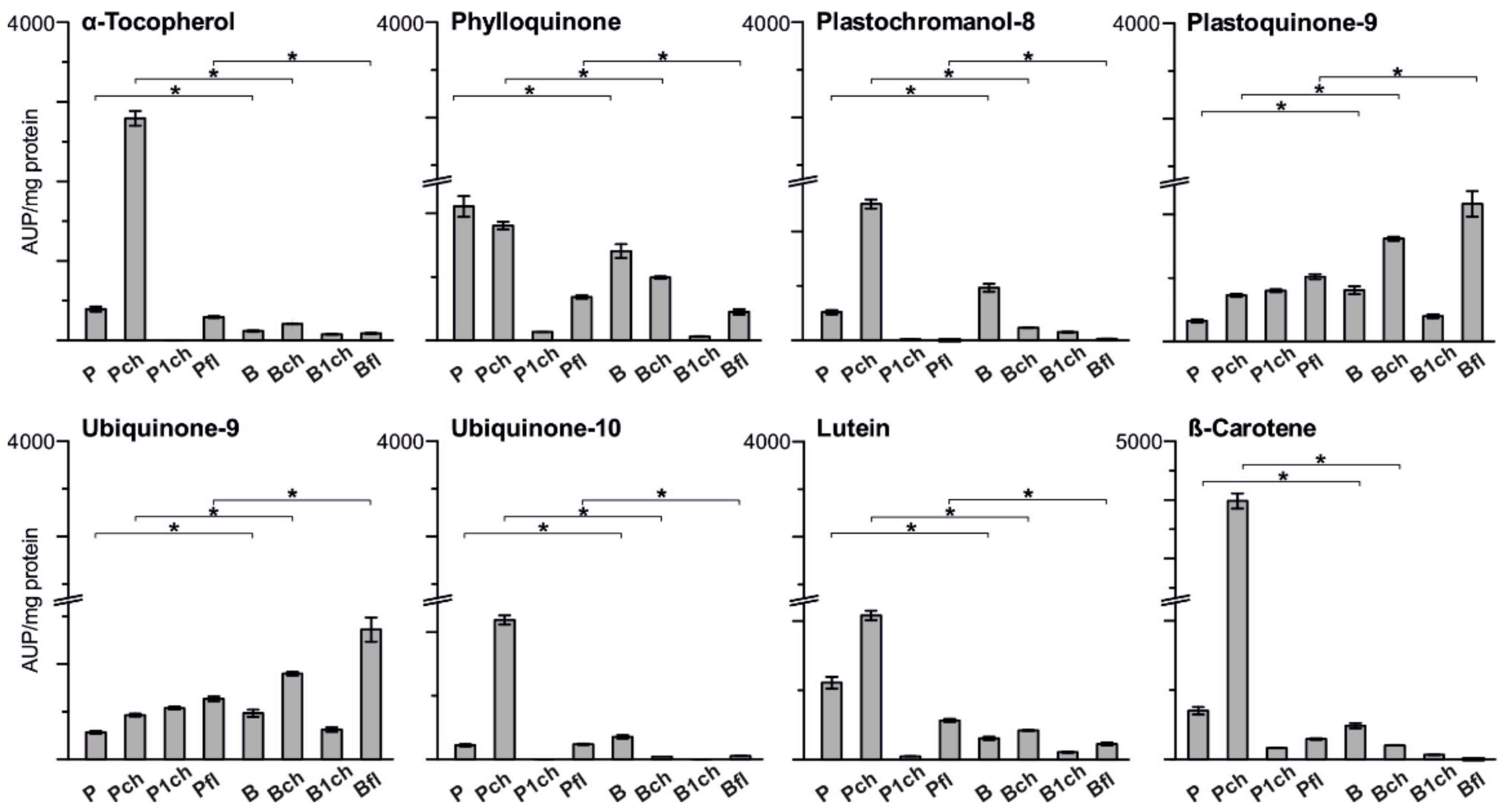
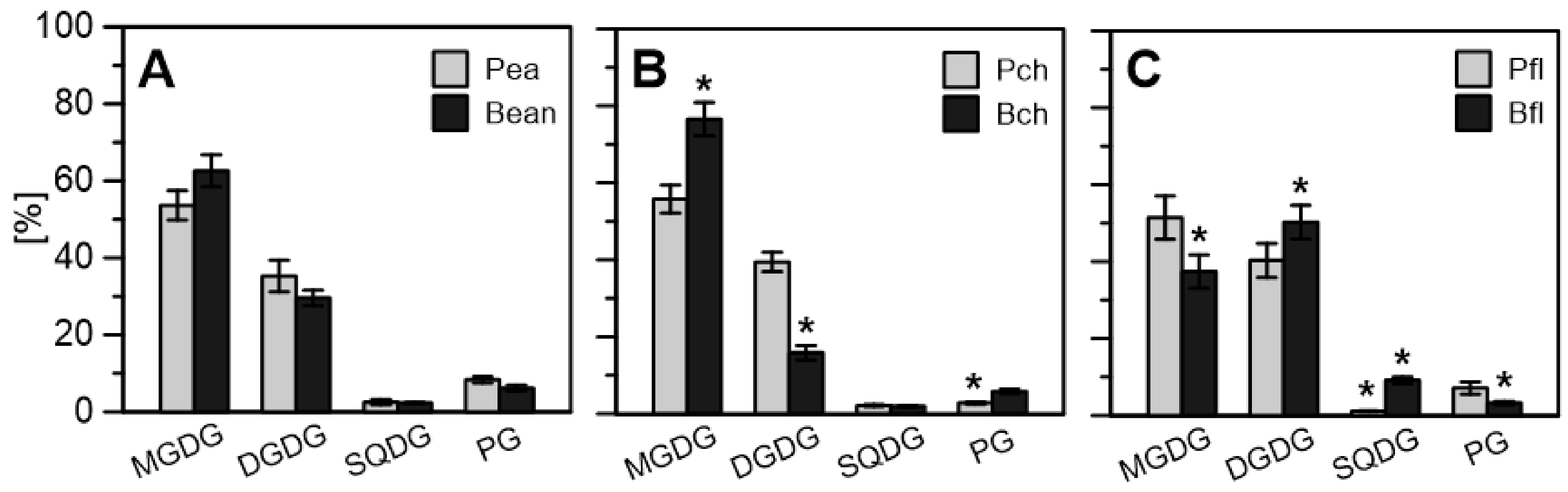
2.2.3. Lipid Enrichment of PGs
3. Discussion
3.1. PG Structural Alteration under Chilling Stress
3.2. Changes in PG Composition as a Physiological Response to Chilling Stress
3.3. PGs Structure and Composition Development from First Green Leaves
4. Materials and Methods
4.1. Plant Material and Stress Treatment
4.2. Isolation and Purification of Plastoglobules
4.3. Protein Concentration Determination
4.4. SDS-PAGE and Immunoblot Analysis
4.5. Electron Microscopy and CLSM
4.6. Atomic Force Microscopy
4.7. FTIR Analysis
4.8. Transcriptome Analysis
4.9. Lipidomic Analysis of Plastoglobules
4.10. Prenyl Lipid and Carotenoid Extraction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Chilling-sensitive |
| CT | Chilling-tolerant |
| PG | Phosphatidylglicerol |
| PGs | Plastoglobules |
References
- Van Wijk, K.J.; Kessler, F. Plastoglobuli: Plastid Microcompartments with Integrated Functions in Metabolism, Plastid Developmental Transitions, and Environmental Adaptation. Annu. Rev. Plant. Biol. 2017, 68, 253–289. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Plastoglobuli, thylakoids, chloroplast structure and Development of Plastids. In Plastid Development in Leaves During Growth and Senescence; Biswal, B., Krupinska, K., Biswal, U.C., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume Advances in Photosynthesis and Respiration 36, pp. 337–361. [Google Scholar]
- Austin, J.R., 2nd; Frost, E.; Vidi, P.A.; Kessler, F.; Staehelin, L.A. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant. Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef]
- Lundquist, P.K.; Poliakov, A.; Giacomelli, L.; Friso, G.; Appel, M.; McQuinn, R.P.; Krasnoff, S.B.; Rowland, E.; Ponnala, L.; Sun, Q.; et al. Loss of Plastoglobule Kinases ABC1K1 and ABC1K3 Causes Conditional Degreening, Modified Prenyl-Lipids, and Recruitment of the Jasmonic Acid Pathway. Plant. Cell 2013, 25, 1818–1839. [Google Scholar] [CrossRef]
- Brehelin, C.; Kessler, F. The plastoglobule: A bag full of lipid biochemistry tricks. Photochem. Photobiol. 2008, 84, 1388–1394. [Google Scholar] [CrossRef]
- Ytterberg, A.J.; Peltier, J.B.; van Wijk, K.J. Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant. Physiol. 2006, 140, 984–997. [Google Scholar] [CrossRef]
- Rottet, S.; Devillers, J.; Glauser, G.; Douet, V.; Besagni, C.; Kessler, F. Identification of Plastoglobules as a Site of Carotenoid Cleavage. Front. Plant. Sci. 2016, 7, 1855. [Google Scholar] [CrossRef]
- Basset, G.J.; Latimer, S.; Fatihi, A.; Soubeyrand, E.; Block, A. Phylloquinone (Vitamin K-1): Occurrence, Biosynthesis and Functions. Mini-Rev. Med. Chem. 2017, 17, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Spicher, L.; Kessler, F. Unexpected roles of plastoglobules (plastid lipid droplets) in vitamin K1 and E metabolism. Curr. Opin. Plant. Biol. 2015, 25, 123–129. [Google Scholar] [CrossRef][Green Version]
- Lohmann, A.; Schottler, M.A.; Brehelin, C.; Kessler, F.; Bock, R.; Cahoon, E.B.; Dormann, P. Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J. Biol. Chem. 2006, 281, 40461–40472. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Laizet, Y.; Block, M.A.; Marechal, E.; Alcaraz, J.P.; Larson, T.R.; Pontier, D.; Gaffe, J.; Kuntz, M. Plant lipid-associated fibrillin proteins condition jasmonate production under photosynthetic stress. Plant. J. 2010, 61, 436–445. [Google Scholar] [CrossRef]
- Kessler, F.; Schnell, D.; Blobel, G. Identification of proteins associated with plastoglobules isolated from pea (Pisum sativum L.) chloroplasts. Planta 1999, 208, 107–113. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Plastoglobuli and lipoquinone content of chloroplasts from Cereus peruvianus (L.) Mill. Planta 1969, 87, 304–310. [Google Scholar] [CrossRef]
- Tevini, M.; Steinmuller, D. Composition and function of plastoglobuli: II. Lipid composition of leaves and plastoglobuli during beech leaf senescence. Planta 1985, 163, 91–96. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Xu, S.; Liu, H. Membrane property and biofunction of phospholiposome incorporated with anomeric galactolipids. Springerplus 2016, 5, 655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaup, M.T.; Froese, C.D.; Thompson, J.E. A role for diacylglycerol acyltransferase during leaf senescence. Plant. Physiol. 2002, 129, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Kessler, F.; Vidi, P.A. Plastoglobule lipid bodies: Their functions in chloroplasts and their potential for applications. Adv. Biochem. Eng. Biotechnol. 2007, 107, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, J.M.; Solymosi, K.; Strzalka, K.; Mysliwa-Kurdziel, B. Visualization and characterization of prolamellar bodies with atomic force microscopy. J. Plant. Physiol. 2013, 170, 1217–1227. [Google Scholar] [CrossRef]
- Rottet, S.; Besagni, C.; Kessler, F. The role of plastoglobules in thylakoid lipid remodeling during plant development. Biochim. Biophys. Acta 2015, 1847, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant. Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S. The role of alpha-tocopherol in plant stress tolerance. J. Plant. Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Vidi, P.A.; Kanwischer, M.; Baginsky, S.; Austin, J.R.; Csucs, G.; Dormann, P.; Kessler, F.; Brehelin, C. Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J. Biol. Chem. 2006, 281, 11225–11234. [Google Scholar] [CrossRef]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dormann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant. Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Song, W.; Sage, T.L.; DellaPenna, D. Tocopherols play a crucial role in low-temperature adaptation and Phloem loading in Arabidopsis. Plant. Cell 2006, 18, 2710–2732. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of Chilling on the Structure, Function and Development of Chloroplasts. Front. Plant. Sci. 2018, 9, 1715. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Weiler, E.W.; Alegre, L.; Muller, M.; Duchting, P.; Falk, J. Alpha-tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 2007, 225, 681–691. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Grennan, A.K. Plastoglobule proteome. Plant. Physiol. 2008, 147, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Garstka, M.; Drozak, A.; Rosiak, M.; Venema, J.H.; Kierdaszuk, B.; Simeonova, E.; van Hasselt, P.R.; Dobrucki, J.; Mostowska, A. Light-dependent reversal of dark-chilling induced changes in chloroplast structure and arrangement of chlorophyll-protein complexes in bean thylakoid membranes. Biochim. Biophys. Acta 2005, 1710, 13–23. [Google Scholar] [CrossRef]
- Garstka, M.; Venema, J.H.; Rumak, I.; Gieczewska, K.; Rosiak, M.; Koziol-Lipinska, J.; Kierdaszuk, B.; Vredenberg, W.J.; Mostowska, A. Contrasting effect of dark-chilling on chloroplast structure and arrangement of chlorophyll-protein complexes in pea and tomato: Plants with a different susceptibility to non-freezing temperature. Planta 2007, 226, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Rumak, I.; Gieczewska, K.; Kierdaszuk, B.; Gruszecki, W.I.; Mostowska, A.; Mazur, R.; Garstka, M. 3-D modelling of chloroplast structure under (Mg2+) magnesium ion treatment. Relationship between thylakoid membrane arrangement and stacking. Biochim. Biophys. Acta 2010, 1797, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Rudowska, L.; Gieczewska, K.; Mazur, R.; Garstka, M.; Mostowska, A. Chloroplast biogenesis—Correlation between structure and function. Biochim. Biophys. Acta 2012, 1817, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Laremore, T.N.; Smith, P.B.; Maximova, S.N.; McNellis, T.W. Knockdown of FIBRILLIN4 gene expression in apple decreases plastoglobule plastoquinone content. PLoS ONE 2012, 7, e47547. [Google Scholar] [CrossRef]
- Alonso, J.L.; Goldmann, W.H. Feeling the forces: Atomic force microscopy in cell biology. Life Sci. 2003, 72, 2553–2560. [Google Scholar] [CrossRef]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef]
- Mereghetti, P.; Corsetto, P.A.; Cremona, A.; Rizzo, A.M.; Doglia, S.M.; Ami, D. A Fourier transform infrared spectroscopy study of cell membrane domain modifications induced by docosahexaenoic acid. Biochim. Biophys. Acta 2014, 1840, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared Spectroscopy of Membrane Lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1074–1081. [Google Scholar] [CrossRef]
- Sherazi, S.T.; Mahesar, S.A.; Bhanger, M.I.; van de Voort, F.R.; Sedman, J. Rapid determination of free fatty acids in poultry feed lipid extracts by SB-ATR FTIR spectroscopy. J. Agric. Food Chem. 2007, 55, 4928–4932. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, L.; Rudella, A.; van Wijk, K.J. High light response of the thylakoid proteome in arabidopsis wild type and the ascorbate-deficient mutant vtc2-2. A comparative proteomics study. Plant. Physiol. 2006, 141, 685–701. [Google Scholar] [CrossRef]
- Martinis, J.; Glauser, G.; Valimareanu, S.; Kessler, F. A chloroplast ABC1-like kinase regulates vitamin E metabolism in Arabidopsis. Plant. Physiol. 2013, 162, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Martinis, J.; Glauser, G.; Valimareanu, S.; Stettler, M.; Zeeman, S.C.; Yamamoto, H.; Shikanai, T.; Kessler, F. ABC1K1/PGR6 kinase: A regulatory link between photosynthetic activity and chloroplast metabolism. Plant. J. 2014, 77, 269–283. [Google Scholar] [CrossRef]
- Brehelin, C.; Kessler, F.; van Wijk, K.J. Plastoglobules: Versatile lipoprotein particles in plastids. Trends Plant. Sci. 2007, 12, 260–266. [Google Scholar] [CrossRef]
- Zhang, R.; Wise, R.R.; Struck, K.R.; Sharkey, T.D. Moderate heat stress of Arabidopsis thaliana leaves causes chloroplast swelling and plastoglobule formation. Photosynth. Res. 2010, 105, 123–134. [Google Scholar] [CrossRef]
- Dumont, E.; Bahrman, N.; Goulas, E.; Valot, B.; Sellier, H.; Hilbert, J.L.; Vuylsteker, C.; Lejeune-Henaut, I.; Delbreil, B. A proteomic approach to decipher chilling response from cold acclimation in pea (Pisum sativum L.). Plant. Sci. 2011, 180, 86–98. [Google Scholar] [CrossRef]
- Farmer, E.E.; Goossens, A. Jasmonates: What ALLENE OXIDE SYNTHASE does for plants. J. Exp. Bot. 2019, 70, 3373–3378. [Google Scholar] [CrossRef]
- Eugeni Piller, L.; Glauser, G.; Kessler, F.; Besagni, C. Role of plastoglobules in metabolite repair in the tocopherol redox cycle. Front. Plant. Sci. 2014, 5, 298. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, I.; Dujardin, G.; Slonimski, P.P. ABC1, a novel yeast nuclear gene has a dual function in mitochondria: It suppresses a cytochrome b mRNA translation defect and is essential for the electron transfer in the bc 1 complex. EMBO J. 1991, 10, 2023–2031. [Google Scholar] [CrossRef]
- Lundquist, P.K.; Davis, J.I.; van Wijk, K.J. ABC1K atypical kinases in plants: Filling the organellar kinase void. Trends Plant. Sci 2012, 17, 546–555. [Google Scholar] [CrossRef]
- Lundquist, P.K.; Poliakov, A.; Bhuiyan, N.H.; Zybailov, B.; Sun, Q.; van Wijk, K.J. The Functional Network of the Arabidopsis Plastoglobule Proteome Based on Quantitative Proteomics and Genome-Wide Coexpression Analysis. Plant. Physiol. 2012, 158, 1172–1192. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Membrane lipids: It’s only a phase. Curr. Biol. 2000, 10, R377–R380. [Google Scholar] [CrossRef]
- Rumak, I.; Mazur, R.; Gieczewska, K.; Koziol-Lipinska, J.; Kierdaszuk, B.; Michalski, W.P.; Shiell, B.J.; Venema, J.H.; Vredenberg, W.J.; Mostowska, A.; et al. Correlation between spatial (3D) structure of pea and bean thylakoid membranes and arrangement of chlorophyll-protein complexes. BMC Plant. Biol. 2012, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Mazur, R.; Gieczewska, K.; Kowalewska, L.; Kuta, A.; Proboszcz, M.; Gruszecki, W.I.; Mostowska, A.; Garstka, M. Specific Composition of Lipid Phases Allows Retaining an Optimal Thylakoid Membrane Fluidity in Plant Response to Low-Temperature Treatment. Front. Plant. Sci. 2020, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Gaude, N.; Brehelin, C.; Tischendorf, G.; Kessler, F.; Dormann, P. Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant. J. 2007, 49, 729–739. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant. Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Kutik, J.; Hola, D.; Kocova, M.; Rothova, O.; Haisel, D.; Wilhelmova, N.; Ticha, I. Ultrastructure and dimensions of chloroplasts in leaves of three maize (Zea mays L.) inbred lines and their F-1 hybrids grown under moderate chilling stress. Photosynthetica 2004, 42, 447–455. [Google Scholar] [CrossRef]
- Zbierzak, A.M.; Kanwischer, M.; Wille, C.; Vidi, P.A.; Giavalisco, P.; Lohmann, A.; Briesen, I.; Porfirova, S.; Brehelin, C.; Kessler, F.; et al. Intersection of the tocopherol and plastoquinol metabolic pathways at the plastoglobule. Biochem. J. 2010, 425, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lohscheider, J.N.; Bartulos, C.R. Plastoglobules in algae: A comprehensive comparative study of the presence of major structural and functional components in complex plastids. Mar. Genom. 2016, 28, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Shanmugabalaji, V.; Besagni, C.; Piller, L.E.; Douet, V.; Ruf, S.; Bock, R.; Kessler, F. Dual targeting of a mature plastoglobulin/fibrillin fusion protein to chloroplast plastoglobules and thylakoids in transplastomic tobacco plants. Plant. Mol. Biol. 2013, 81, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Lohscheider, J.N.; Friso, G.; van Wijk, K.J. Phosphorylation of plastoglobular proteins in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 3975–3984. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Giavalisco, P.; Zuther, E.; Seiwert, B.; Hincha, D.K.; Willmitzer, L. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant. J. 2012, 72, 972–982. [Google Scholar] [CrossRef]
- Lippold, F.; vom Dorp, K.; Abraham, M.; Holzl, G.; Wewer, V.; Yilmaz, J.L.; Lager, I.; Montandon, C.; Besagni, C.; Kessler, F.; et al. Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant. Cell 2012, 24, 2001–2014. [Google Scholar] [CrossRef]
- Skupien, J.; Wojtowicz, J.; Kowalewska, L.; Mazur, R.; Garstka, M.; Gieczewska, K.; Mostowska, A. Dark-chilling induces substantial structural changes and modifies galactolipid and carotenoid composition during chloroplast biogenesis in cucumber (Cucumis sativus L.) cotyledons. Plant. Physiol. Biochem. PPB Soc. Fr. Physiol. Veg. 2017, 111, 107–118. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Falk, J. New insights into the function of tocopherols in plants. Planta 2004, 218, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Szymanska, R.; Nowicka, B.; Dluzewska, J. Function of isoprenoid quinones and chromanols during oxidative stress in plants. New Biotechnol. 2016, 33, 636–643. [Google Scholar] [CrossRef]
- Nowicka, B.; Trela-Makowej, A.; Latowski, D.; Strzalka, K.; Szymańska, R. Antioxidant and Signaling Role of Plastid-Derived Isoprenoid Quinones and Chromanols. Int. J. Mol. Sci. 2021, 22, 2950. [Google Scholar] [CrossRef]
- Kruk, J.; Trebst, A. Plastoquinol as a singlet oxygen scavenger in photosystem II. Biochim. Biophys. Acta 2008, 1777, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Raclaru, M.; Schusseler, T.; Gruber, J.; Sadre, R.; Luhs, W.; Zarhloul, K.M.; Friedt, W.; Enders, D.; Frentzen, M.; et al. Characterisation of plant tocopherol cyclases and their overexpression in transgenic Brassica napus seeds. FEBS Lett. 2005, 579, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Eugeni Piller, L.; Abraham, M.; Dormann, P.; Kessler, F.; Besagni, C. Plastid lipid droplets at the crossroads of prenylquinone metabolism. J. Exp. Bot. 2012, 63, 1609–1618. [Google Scholar] [CrossRef]
- Szymanska, R.; Kruk, J. Plastoquinol is the main prenyllipid synthesized during acclimation to high light conditions in Arabidopsis and is converted to plastochromanol by tocopherol cyclase. Plant. Cell Physiol. 2010, 51, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, C.L.; Misawa, N.; Perez-Fons, L.; Robertson, F.P.; Harada, H.; Bramley, P.M.; Fraser, P.D. The Formation and Sequestration of Nonendogenous Ketocarotenoids in Transgenic Nicotiana glauca. Plant. Physiol. 2017, 173, 1617–1635. [Google Scholar] [CrossRef]
- Fatihi, A.; Latimer, S.; Schmollinger, S.; Block, A.; Dussault, P.H.; Vermaas, W.F.J.; Merchant, S.S.; Basset, G.J. A Dedicated Type II NADPH Dehydrogenase Performs the Penultimate Step in the Biosynthesis of Vitamin K-1 in Synechocystis and Arabidopsis. Plant. Cell 2015, 27, 1730–1741. [Google Scholar] [CrossRef]
- Kowalewska, L.; Mazur, R.; Suski, S.; Garstka, M.; Mostowska, A. Three-Dimensional Visualization of the Tubular-Lamellar Transformation of the Internal Plastid Membrane Network during Runner Bean Chloroplast Biogenesis. Plant. Cell 2016, 28, 875–891. [Google Scholar] [CrossRef]
- Yang, Y.; Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotech. 2018, 49, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, L.A.; Ryberg, M.; Sundqvist, C. Proteomic analysis of highly purified prolamellar bodies reveals their significance in chloroplast development. Photosynth. Res. 2008, 96, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Nagata, N.; Masuda, T.; Wada, H.; Kobayashi, K. Galactolipids Are Essential for Internal Membrane Transformation during Etioplast-to-Chloroplast Differentiation. Plant. Cell Physiol. 2019, 60, 1224–1238. [Google Scholar] [CrossRef]
- Singh, D.K.; McNellis, T.W. Fibrillin protein function: The tip of the iceberg? Trends Plant. Sci. 2011, 16, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Besagni, C.; Piller, L.E.; Brehelin, C. Preparation of plastoglobules from Arabidopsis plastids for proteomic analysis and other studies. Methods Mol. Biol. 2011, 775, 223–239. [Google Scholar] [CrossRef]
- Necas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Klapetek, P.; Necas, D. Independent analysis of mechanical data from atomic force microscopy. Meas. Sci. Technol. 2014, 25, 044009. [Google Scholar] [CrossRef]
- Gil, J.H.; Hong, J.K.; Choe, J.C.; Kim, Y.H. Analysis of Fatty Acyl Groups of Diacyl Galactolipid Molecular Species by HPLC/ESI-MS with In-source Fragmentation. Bull. Korean Chem. Soc. 2003, 24, 1163–1168. [Google Scholar] [CrossRef][Green Version]
- Szalonek, M.; Sierpien, B.; Rymaszewski, W.; Gieczewska, K.; Garstka, M.; Lichocka, M.; Sass, L.; Paul, K.; Vass, I.; Vankova, R.; et al. Potato Annexin STANN1 Promotes Drought Tolerance and Mitigates Light Stress in Transgenic Solanum tuberosum L. Plants. PLoS ONE 2015, 10, e0132683. [Google Scholar] [CrossRef]
- Sztatelman, O.; Grzyb, J.; Gabrys, H.; Banas, A.K. The effect of UV-B on Arabidopsis leaves depends on light conditions after treatment. BMC Plant. Biol. 2015, 15, 281. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójtowicz, J.; Grzyb, J.; Szach, J.; Mazur, R.; Gieczewska, K.B. Bean and Pea Plastoglobules Change in Response to Chilling Stress. Int. J. Mol. Sci. 2021, 22, 11895. https://doi.org/10.3390/ijms222111895
Wójtowicz J, Grzyb J, Szach J, Mazur R, Gieczewska KB. Bean and Pea Plastoglobules Change in Response to Chilling Stress. International Journal of Molecular Sciences. 2021; 22(21):11895. https://doi.org/10.3390/ijms222111895
Chicago/Turabian StyleWójtowicz, Joanna, Joanna Grzyb, Joanna Szach, Radosław Mazur, and Katarzyna B. Gieczewska. 2021. "Bean and Pea Plastoglobules Change in Response to Chilling Stress" International Journal of Molecular Sciences 22, no. 21: 11895. https://doi.org/10.3390/ijms222111895
APA StyleWójtowicz, J., Grzyb, J., Szach, J., Mazur, R., & Gieczewska, K. B. (2021). Bean and Pea Plastoglobules Change in Response to Chilling Stress. International Journal of Molecular Sciences, 22(21), 11895. https://doi.org/10.3390/ijms222111895