Abstract
We have developed an in vitro system to easily examine the affinity for vitamin D receptor (VDR) and CYP24A1-mediated metabolism as two methods of assessing vitamin D derivatives. Vitamin D derivatives with high VDR affinity and resistance to CYP24A1-mediated metabolism could be good therapeutic agents. This system can effectively select vitamin D derivatives with these useful properties. We have also developed an in vivo system including a Cyp27b1-gene-deficient rat (a type I rickets model), a Vdr-gene-deficient rat (a type II rickets model), and a rat with a mutant Vdr (R270L) (another type II rickets model) using a genome editing method. For Cyp27b1-gene-deficient and Vdr mutant (R270L) rats, amelioration of rickets symptoms can be used as an index of the efficacy of vitamin D derivatives. Vdr-gene-deficient rats can be used to assess the activities of vitamin D derivatives specialized for actions not mediated by VDR. One of our original vitamin D derivatives, which displays high affinity VDR binding and resistance to CYP24A1-dependent metabolism, has shown good therapeutic effects in Vdr (R270L) rats, although further analysis is needed.
1. Introduction
The active form of vitamin D3 (1α,25(OH)2D3) plays essential roles in calcium and phosphate homeostasis, cellular proliferation and differentiation, and immune responses. Since it could cause hypercalcemia and hypercalciuria, its clinical utility is limited [1,2]. A huge number of vitamin D derivatives have been synthesized. Many of them have been studied in clinical trials for the treatment of type I rickets, osteoporosis, psoriasis, renal osteodystrophy, and also leukemia, pancreatic, prostate, and breast cancers [3,4,5,6,7,8,9]. A number of vitamin D derivatives have been approved by the FDA for clinical use in a variety of disorders, for example, 22-oxacalcitriol (Maxacalcitol) and calcipotriol (Dovonex) for treatment of psoriasis, 19-nor-1α,25(OH)2D2 (Zemplar), 26,26,26,27,27,27-hexafluoro-1α,25(OH)2D3 (Falecalcitriol), and doxercalciferol (Hectorol) for secondary hyperparathyroidism, and 1α(OH)D3 (alfacalcidol) and eldecalcitol (Edirol) for osteoporosis. Although many vitamin D derivatives have antiproliferative activity, none have been approved for cancer treatment. So far, only a small number of clinical studies have taken place, such as EB1089 in a phase II study for pancreatic cancer [6,10], and Hectorol and Zemplar in phase I/II advanced androgen-insensitive prostate cancer trials [7,11,12]. Unfortunately, neither have produced any significant objective responses. However, a new 1α,25(OH)2D3 analog, 19-nor-14-epi-23-yne-1α,25(OH)2D3 (inecalcitol), is being developed for prostate cancers and chronic leukemia [13,14].
The active form of vitamin D3 (1α,25(OH)2D3) plays essential roles in calcium and phosphate homeostasis, cellular proliferation and differentiation, and immune responses. Its clinical utility is limited because it can cause hypercalcemia and hypercalciuria. [1,2]. Several thousand vitamin D derivatives have been synthesized, and many have been studied in clinical trials to treat conditions, including type I rickets, osteoporosis, leukemia, psoriasis, renal osteodystrophy, and pancreatic, prostate, and breast cancers. [3,4,5,6,7,8,9]. A number of vitamin D derivatives have been approved by the FDA for clinical use in a variety of disorders. These derivatives include calcipotriol (Dovonex; Leo Pharmaceuticals) and 22-oxacalcitriol (Maxacalcitol; Chugai Pharmaceuticals) for treatment of psoriasis; 19-nor-1α,25(OH)2D2 (Zemplar; Abbot Laboratories; Chicago, IL, USA), 26,26,26,27,27,27-hexafluoro- (Falecalcitriol; Sumitomo Pharmaceuticals and Taisho Pharmaceuticals), and doxercalciferol (Hectorol; Bone Care Int.; Middleton, WI, USA) for secondary hyperparathyroidism; and 1α(OH)D3 (alfacalcidol; Chugai Pharmaceuticals Co., Ltd.; Tokyo, Japan) and eldecalcitol (Chugai Pharmaceuticals Co., Ltd.; Tokyo, Japan) for osteoporosis. Although many vitamin D derivatives, including those approved by the FDA for treating secondary hyperparathyroidism and renal osteodystrophy, have displayed antiproliferative activity, none have been approved for cancer treatment. To date, only a limited number of clinical studies have taken place, including a phase II study of EB1089 in pancreatic cancer. [6,10]. Hectorol and Zemplar have been studied in phase I/II advanced androgen-insensitive prostate cancer trials [7,11,12]. Unfortunately, neither produced any significant objective responses. Recently, a new 1α,25(OH)2D3 analog, inecalcitol, is being developed for prostate cancers and chronic leukemia [13,14].
In evaluating these vitamin D derivatives, (1) affinity for vitamin D receptor, (2) affinity for vitamin-D-binding protein (DBP), (3) resistance to metabolism by CYP24A1, and (4) ability to differentiate leukemia-derived HL-60 cells into macrophages are considered to be essential properties. In addition, they must show therapeutic efficacy in animal studies. In the case of derivatives under development for cancer treatment, therapeutic efficacy will be evaluated using tumor-bearing animals. Construction of appropriate evaluation models is indispensable for developing vitamin D derivatives for pharmaceutical use. We have developed in vitro systems that can easily measure vitamin D receptor (VDR) affinity [15,16,17,18,19] and CYP24A1-mediated metabolism [20,21,22,23,24]. We have also generated genetically modified rats using genome editing as follows: Cyp27b1-gene-deficient rats (a type 1 rickets model animal), vitamin D receptor-gene-deficient rats, and rats harboring a mutant vitamin D receptor (R270L) gene (type II rickets model animals) [25]. We have also generated Cyp24a1-gene-deficient rats to elucidate enzymes and metabolic pathways responsible for vitamin D derivative metabolism [26]. In this review, we describe the in vitro and in vivo systems we have developed for evaluation of vitamin D derivatives, and discuss the derivatives we have synthesized to date.
2. In Vitro System to Easily Examine the Affinity for VDR of Vitamin D Derivatives
2.1. Measurement of Binding Affinity of Vitamin D Derivatives for VDR
The widely used method for evaluating the binding ability of vitamin D derivatives for VDR in a cell-based assay system is a reporter assay that induces expression of luciferase (Luc) under the control of a promoter containing a vitamin D response element (VDRE) [27,28,29]. It is noted that it takes more than 12 h for the reporter protein to be expressed, and the direct binding between the receptor and the ligand cannot be evaluated. Although a competitive system using native VDR and tritium-labeled 1α,25(OH)2D3 was widely used, it is no longer commercially available. Thus, we tried to develop a new detection system that easily evaluates the affinity of vitamin D derivatives for VDR in a short time. We focused on the split-type luciferase technology [15,16,17,18,19,30,31,32,33,34,35,36,37]. This system can evaluate the affinity of the ligand by increasing or decreasing the luminescence of the split-type luciferase.
2.2. Development of a Novel Bioluminescent Sensor to Detect and Discriminate between Vitamin D Receptor Agonists and Antagonists in Living Cells (1st Generation)
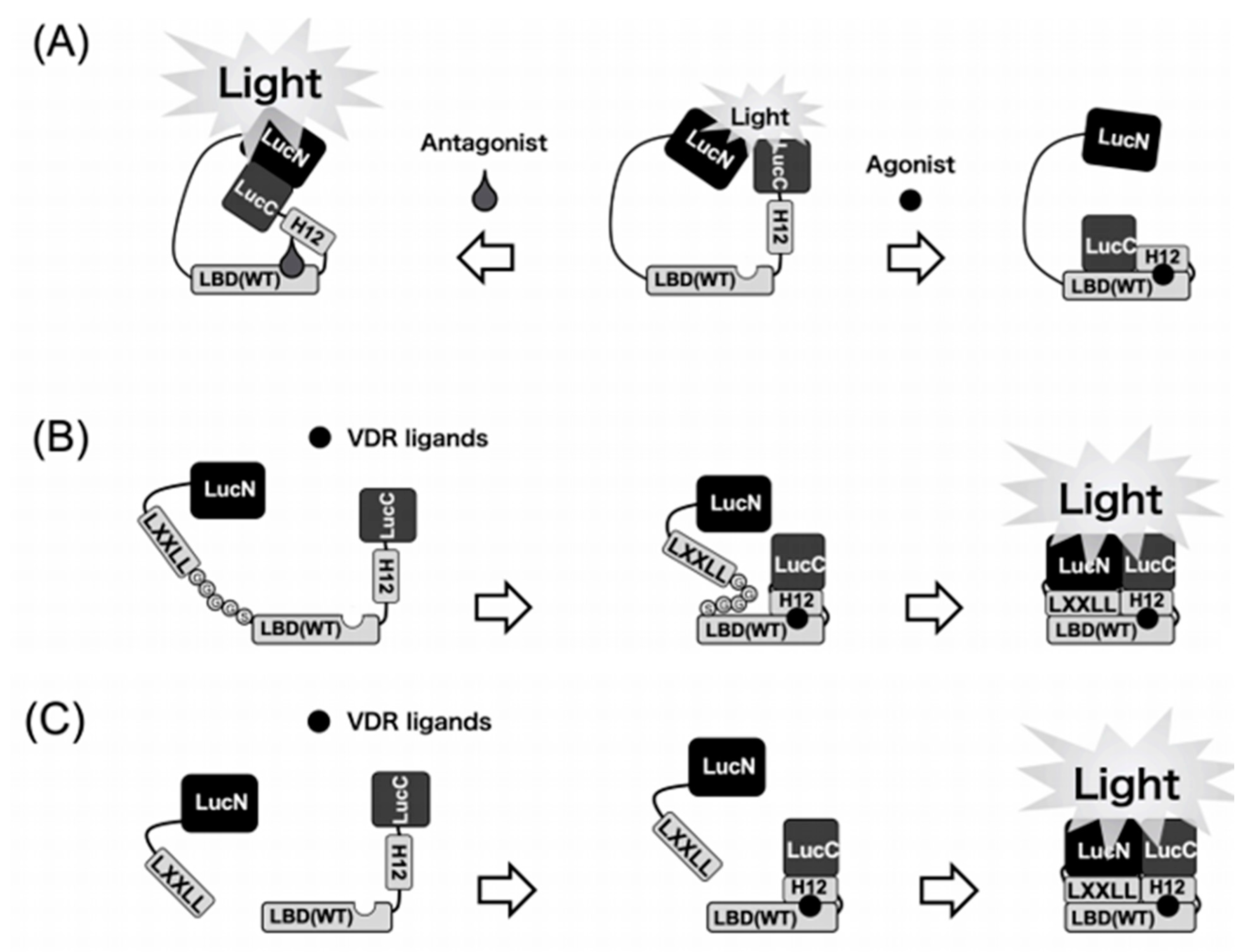
Two chimeric fusion proteins that contained both split-luciferase and the ligand binding domain (LBD) of the VDR were constructed. This fusion protein was labeled as LucN–LBD–LucC. It contained the N-terminal domain taken from luciferase (LucN), LBD, and C-terminal domain from luciferase (LucC) from N-terminus to C-terminus. LucC–LBD–LucN has the C-terminal domain of luciferase at the N-terminus of the fusion protein (Figure 1) [15]. Unexpectedly, the LucC–LBD–LucN worked better than LucN–LBD–LucC. Luciferase activity was significantly diminished by the addition of the VDR agonists to COS-7 cells that expressed LucC–LBD–LucN. On the other hand, the VDR antagonist notably enhanced the activity of the chimeric luciferase in a dose- and time-dependent manner. Our novel model for detecting and discriminating between VDR agonists and antagonists is very useful for testing synthetic analogs of vitamin D that show reasonable affinity for normal or mutant VDRs.
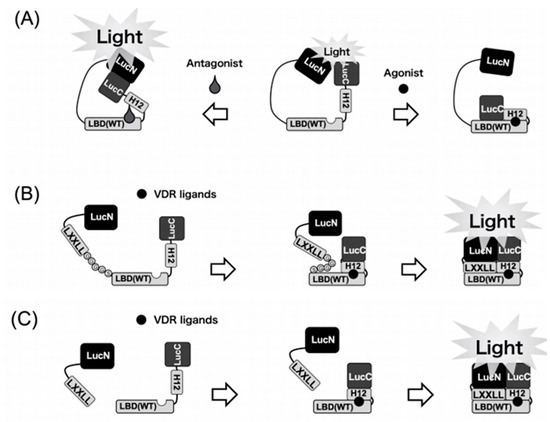
Figure 1.
Schematic diagrams of the biosensors to detect VDR ligands. (A) 1st generation. Binding of the VDR agonists to the LBD may cause a conformational change of the LBD that leads to disruption of the functional complex between N-terminal and C-terminal domains of the luciferase. In contrast, binding of the antagonist leads to the reassembly of N-terminal and C-terminal domains of the luciferase to increase the activity. (B) 2nd generation. Binding of VDR ligands to the biosensor may cause a conformational change of helix12 (H12) in LBD. After conformational change of LBD, the LXXLL motif interacts with LBD in the biosensor. Then, this intramolecular dynamic change of the WT biosensor leads to reconstitution of the functional complex between LucN and LucC fragments of the split luciferase. (C) 3rd generation. Binding of the VDR ligands to the LBD–LucC may cause a positional change of helix12 in LBD. Then, the LucN–LXXLL and LBD–LucC forms a functional complex to exhibit the luciferase activity.
Patients with type II rickets showing the R274L mutation caused a 1000-fold reduction in the binding activity for 1α,25(OH)2D3 and remarkably lowered vitamin-D-related gene expression [38]. It is Arg274, located in LBD of VDR, that is responsible for attaching 1α,25(OH)2D3. This happens by a formation of an additional hydrogen bond with 1α-hydroxyl of 1α,25(OH)2D3. LucC–LBD (R274L)–LucN was constructed to investigate vitamin D ligands of high affinity for the mutant VDR (R274L). A total of 5 out of the 33 vitamin D analogs tested showed much higher binding for the mutant VDR (R274L) than the vitamin D hormone. The highest binding activity was shown by 2α-(2-(tetrazol-2-yl)ethyl)-(AH-1). These analogs might be considered as future drug candidates against HVDRR that is caused by the mutant VDR (R274L) [16].
2.3. Development of a Highly Sensitive In Vitro System to Detect and Discriminate between Vitamin D Receptor Agonists and Antagonists
We have established an in vitro screening system for VDR ligands using the LucC–LBD–LucN proteins expressed in Escherichia coli (E. coli) cells [17]. It should be noted that this system could be completed within 30 min, and its activity was unchanged after 10 freeze–thaw cycles. This highly sensitive and convenient system would be quite useful to screen VDR ligands with therapeutic potential for osteoporosis, renal osteodystrophy, cancers, and immune disorders.
2.4. Design of a Biosensor Based on Split Luciferase for Detection of VDR Ligands (2nd Generation)
The model we developed is very useful for a fast investigation of VDR ligands. However, the sensitivity of our biosensor (LucC–LBD–LucN) is not as high as expected. LBD is known to interact via the LXXLL motif with transcription coactivators, such as SRC-1, TIF-2, or DRIP-205 to initiate vitamin-D-related gene expression, when binding natural VDR ligands. This is why we anticipated that it is the LXXLL motif that changes the enzymatic profile of luciferase–LBD biosensors. This is why LucN–LBD–LucC and not LucC–LBD–LucN was used as a basing fragment. We created a new biosensor consisting of the LBD (121–427 aa) of VDR, N- and C-terminal of firefly luciferase fragments (LucN (1–415 aa) and LucC (416-550 aa)), the LXXLL peptide sequence, and peptide sequence (Gly-Gly-Gly-Gly-Ser (GGGGS)) × 3 as the flexible linker [18]. This construct we labeled as LucN–LXXLL–(GGGGS) × 3–LBD–LucC WT biosensor and WT means the wild-type of LBD (Figure 1). Light intensity of luciferase is low when natural VDR ligands are absent. The luciferase light intensity is immediately and remarkably increased when the ligand is bound to the WT biosensor. To sum up, we have successfully created a very sensitive biosensor which shows the increase in light intensity when binding VDR agonists.
To this end, we developed a novel and WT biosensor of high sensitivity by examining three types of LXXLL peptides (NHPMLMNLLKDN, LTEMHPILTSLLQNGVDHV, and LSETHPLLWTLLSSTEGDSM) that interact with the LBD in response to 1α,25(OH)2D3 or synthetic VDR agonists. The COS-7 cells that expressed each type of biosensor were treated with 1α,25(OH)2D3 (100 nM) and then the luminescence was measured 90 min later. Among the 10 biosensors we constructed, one showed a reduction in intensity of light in response to 1α,25(OH)2D3. Seven biosensors showed an excellent increase in light intensity. Our best biosensor showed the light intensity ca. one-third of that of full-length native luciferase of firefly. Quite unexpectedly, 25(OH)D3, as the low-affinity VDR ligand, also enhanced the intensity of light in a concentration-dependent manner. The half maximal relative intensity of light was recorded at 1 nM of 1α,25(OH)2D3 and at 20 nM of 25(OH)D3, respectively. We then compared the binding activity of 1α,25(OH)2D3 and 25(OH)D3 for the mutant VDR (R274L). As previously mentioned, the substitution of Arg274 to Leu causes a 1000-fold decrease in affinity of 1α,25(OH)2D3. As expected, in the R274L biosensor the concentration–response curve of 1α,25(OH)2D3 was very similar to that of 25(OH)D3. Thus, the biosensor system we developed may be very useful in elucidating novel vitamin D analogs as drug candidates against type II rickets resulting from VDR mutation, such as R274L.
2.5. Development of a Novel Two-Molecule System with a Highly Sensitive Biosensor (3rd Generation)
In the next step, we developed a two-molecule system named LXXLL + LBD biosensor, as shown in Figure 1, with a combination of two components [19]. The two plasmids were co-transfected and two proteins were co-expressed in COS-7 cells. The LXXLL + LBD biosensor-expressing COS-7 cells were treated with 100 nM of 1α,25(OH)2D3, and luciferase light intensity was measured at 90 min after treatment. Among all combinations of LXXLL + LBD biosensor, relative light intensity of A1 + B1 [19] was the highest in all combinations. The relative light intensity of combination A1 + B1 was approximately a 90- to 100-fold increase in response to 100 nM of 1α,25(OH)2D3. It should be noted that the detection limit was 0.005 nM (5 pM) of 1α,25(OH)2D3, indicating that the sensitivity of LXXLL + LBD biosensor is higher than that of our previous biosensors. [15,16,17,18]. Our LXXLL + LBD biosensor might be used for the measurement of 1α,25(OH)2D3 and 25(OH)D3 in the plasma.
3. In Vitro Evaluation of CYP24A1-mediated Metabolism of Vitamin D Derivatives
3.1. Expression of Rat or Human CYP24A1 in E. coli Cells
The rat Cyp24a1 cDNA was cloned from the rat kidney cDNA library [39], and the isolated cDNA clone contained the open reading frame consisting of 514 amino acids. Since the amino acid sequence showed less than 40 % homology with already known CYPs, the new CYP family name, CYP24, was given to this vitamin-D-24-hydroxylase.
The molecular mechanism of CYP24A1 gene regulation is quite complicated, and many factors are tissue-specifically involved in the expression of CYP24A1 [40,41,42,43,44]. These facts strongly suggest that CYP24A1 is a physiologically essential enzyme that regulates the level of the active form of vitamin D.
When the deduced amino acid sequence from its cDNA was compared to that amino-terminal amino acid sequence of the CYP24A1 purified from rat kidney, it was found that the mature form of rat CYP24A1 lacks amino-terminal 32 amino acids. These results suggest that amino-terminal 32 amino acids function as a mitochondrial targeting signal, which is removed after translocation of CYP24A1 to mitochondria. We have successfully expressed the mature forms of rat and human CYP24A1 in E. coli cells to reveal their enzymatic properties [20,21,22,23,24].
3.2. Construction of a CYP24A1 Enzyme System Containing Adrenodoxin (ADX) and NADPH-Adrenodoxin Reductase (ADR)
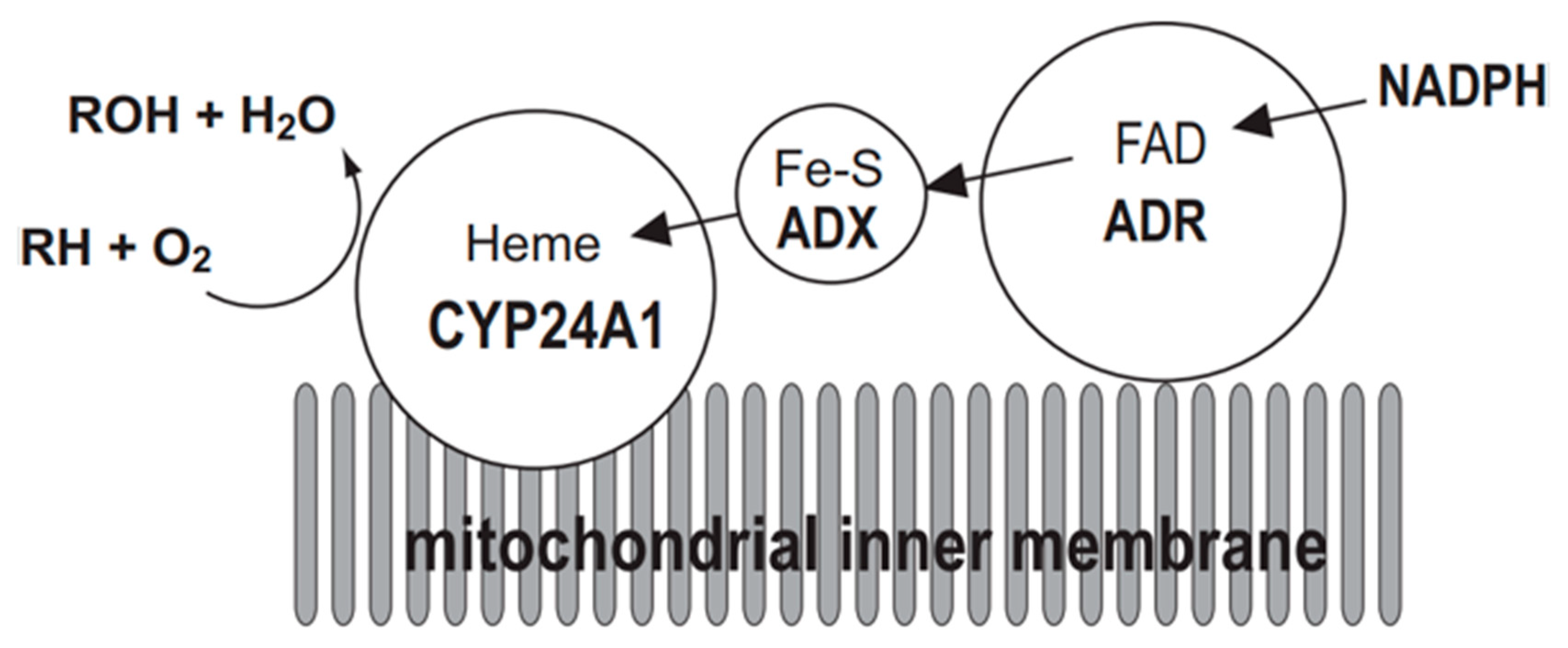
The mitochondrial P450 system consists of three components: CYP, ADX, and ADR. Electrons are sequentially transferred from NADPH through ADR and ADX to CYP24A1 (Figure 2). Thus, CYP24A1-dependent activity was measured in an in vitro reconstituted system containing purified ADX and ADR proteins. On the other hand, in a whole-cell system, co-expression of mature forms of CYP24A1, ADX, and ADR in E. coli is required. We have demonstrated that the E. coli expression system is quite useful to investigate enzymatic properties of CYP24A1. Using this E. coli expression system, we have determined kinetic parameters of CYP24A1 in the metabolism of the native vitamin D and various vitamin D derivatives, and revealed their metabolic pathways [45,46,47,48,49,50,51,52,53,54,55,56,57,58].
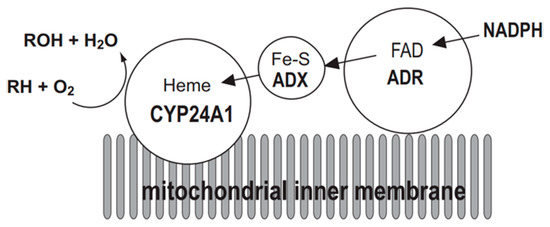
Figure 2.
Mitochondrial electron transport chain of CYP24A1. CYP24A1-dependent mono-oxygenase activity requires the electron transfer from NADPH via NADPH-adrenodoxin oxidoreductase (ADR) and adrenodoxin (ADX) to the heme iron of CYP24A1 situated on the inner membrane of mitochondria. RH represents substrate of CYP24A1.
3.3. CYP24A1-Dependent Multi-Step Reaction toward the Active form of Vitamin D3
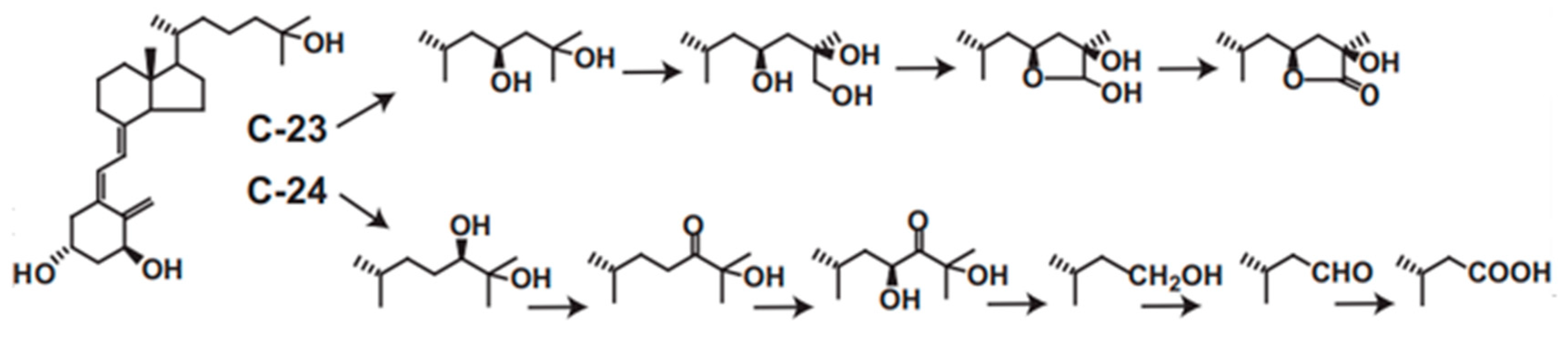
CYP24A1 plays central roles in vitamin D metabolism and produces a wide variety of metabolites. We revealed that rat or human CYP24A1 catalyzes a six-step reaction, starting with hydroxylation at the 24R position of 1α,25(OH)2D3 to produce the final metabolite, calcitroic acid (Figure 3). In addition, human CYP24A1 catalyzes a four-step reaction, starting with hydroxylation at the 23S position to produce the 26,23-lactone form (Figure 3). In the reaction of P450, it is often seen that the reaction product is not released from the substrate binding pocket and the reaction proceeds further. Thus, the two- or three-step reaction is not special in the P450 reaction; however, there is no other P450 that catalyzes such a multi-step reaction for one substrate. Moreover, it is noted that the reaction by human CYP24A1 proceeds in a dual pathway, the C-24 pathway and the C-23 pathway. Interestingly, the ratio of the C-24 to C-23 pathways varies among animal species. In human CYP24A1, it is about 4:1, but, in rat CYP24A1, about 25:1; however, in animal species such as guinea pig and opossum, the C-23 pathway is major. In rat and human CYP24A1, the 326th amino acid residue from the N-terminus is Ala, whereas it is Gly in guinea pigs and opossum, and, when the Ala326 in rat and human CYP24A1 is replaced by Gly, it changes to the guinea pig type [59]. Given that inactivating the active form of vitamin D is the physiological role of CYP24A1, it may be less important whether the C-24 or C-23 pathway is predominant.
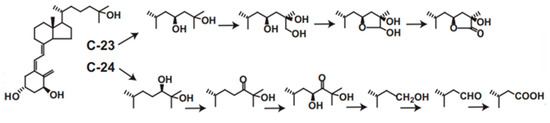
Figure 3.
C-23 and C-24 oxidative pathways of 1α,25(OH)2D3 catalyzed by human CYP24A1. Human CYP24A1 catalyzes 6-step mono-oxygenation from C-24 hydroxylation to produce calcitroic acid, and 4-step mono-oxygenation from C-23 hydroxylation to the lactone formation.
3.4. Metabolism of Vitamin D Derivatives by CYP24A1
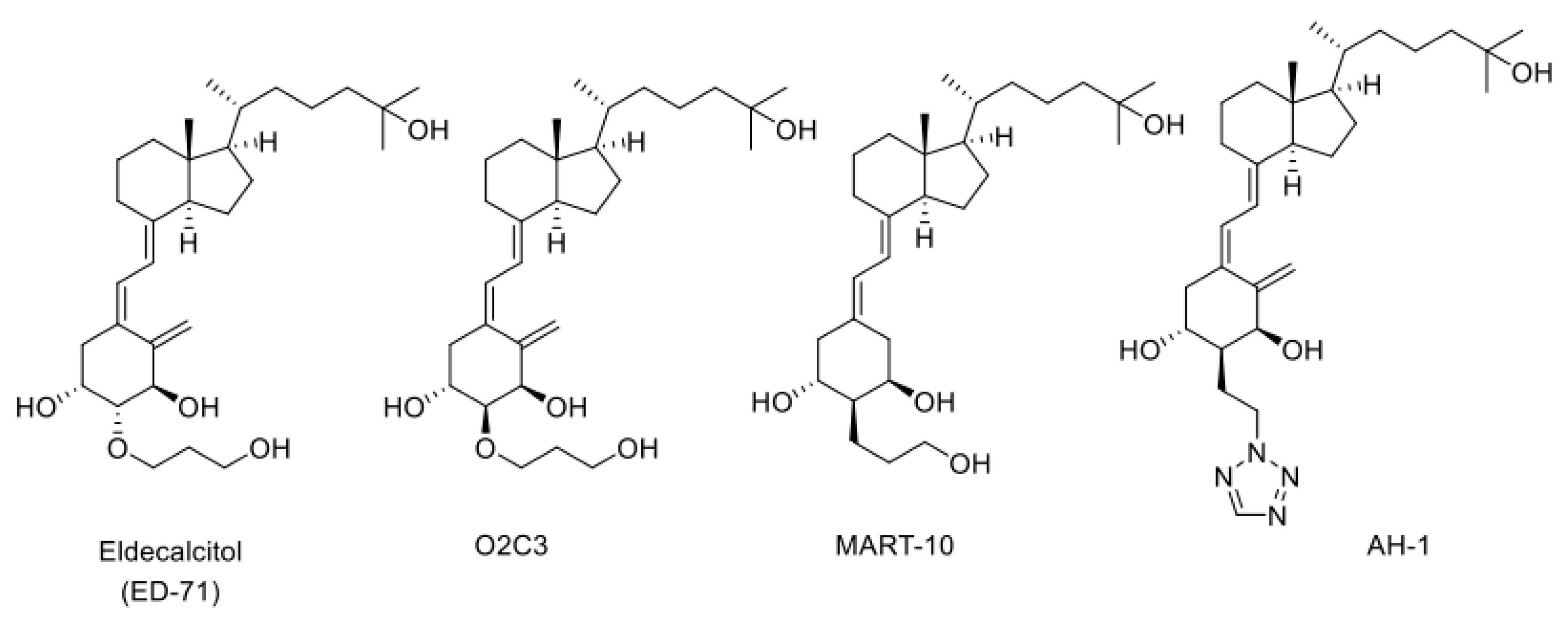
The CYP24A1 gene has two VDREs in the promoter region [40,41,60] and, when the active form of vitamin D binds to VDR, remarkable transcriptional induction of CYP24A1 occurs. When a large amount of CYP24A1 protein is expressed in the cell, the active form of vitamin D is inactivated via the metabolic pathways described above. This mechanism appears to be crucial for keeping the level of the active form of vitamin D. However, when a vitamin D derivative with a high affinity for VDR is developed as a drug, the drug binds to VDR to induce CYP24A1. Therefore, vitamin D derivatives that are not easily metabolized by CYP24A1 could be excellent drugs with long-lasting efficacy. Eldecalcitol, an osteoporosis treatment drug developed by Chugai Pharmaceutical Co., Ltd., has a 3-hydroxy-propyloxy group at the 2β position of 1α,25(OH)2D3 (Figure 4). We revealed that CYP24A1 hardly metabolizes Eldecalcitol [53] and suggest that the resistance to CYP24A1-dependent metabolism may be a key factor that keeps its efficacy for a long time [53,61,62,63]. We have investigated the metabolism of many vitamin D derivatives by CYP24A1 and have clearly demonstrated the importance of CYP24A1-dependent metabolism. In addition, as mentioned above, the fact that there are animal species differences in the metabolic mode of 1α,25(OH)2D3 by CYP24A1 suggests that there are also animal species differences in the metabolism of vitamin D derivatives. Therefore, the development of vitamin D derivatives requires not only animal studies, but also metabolic studies using human CYP24A1 enzyme.
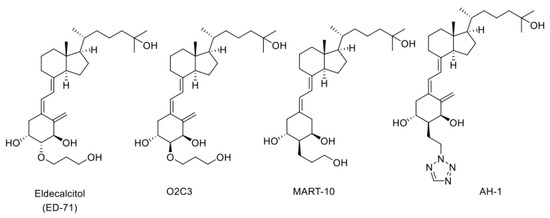
Figure 4.
Structures of three CYP24A1-resistant VDR agonists: Eldecalcitol (ED-71), O2C3, MART-10, and AH-1.
3.5. CYP24A1-Resistant Vitamin D Derivatives with a Substituent at C2α Position
We have synthesized many of A-ring-modified derivatives with a substituent at the C2α position, which have unique biological activities [64,65,66,67,68]. Of these derivatives, 2α-(3-hydroxypropoxy)-1α,25(OH)2D3 (O2C3), which is a C2-epimer of Eldecalcitol, was examined for the metabolism by CYP24A1. Five metabolites were detected in its metabolism by human CYP24A1, including both C-23 and C-24 oxidation pathways [48]. The Km and kcat values of human CYP24A1 for O2C3 were estimated to be approximately 16 times greater and 3 times lower than those for 1α,25(OH)2D3, respectively [48]. Accordingly, the catalytic efficiency (kcat/Km) of human CYP24A1 for O2C3 is only about 3% of 1α,25(OH)2D3. These results strongly suggest that O2C3 is much more resistant to CYP24A1-dependent metabolism than 1α,25(OH)2D3. It is noted that another C-2-substituted derivative, 19-nor-2α-(3-hydroxypropyl)-1α,25(OH)2D3 (MART-10) (Figure 4), was more resistant to CYP24A1-dependent degradation than O2C3 [69,70,71,72,73,74]. The kcat/Km values of human CYP24A1 for MART-10 were about 0.3 % of those for 1α,25(OH)2D3.
Our in vivo studies using rats revealed that MART-10 had a potent anticancer effect, with a low calcemic effect, which is a suitable property as an anticancer drug. The resistance to CYP24A1 is also a suitable property of MART-10 as an anticancer drug.
4. In Vivo Evaluation System for Vitamin D Derivatives Using Genetically Modified Rats Generated by Genome Editing
4.1. Appearance and Growth of Genetically Modified (GM) Rats
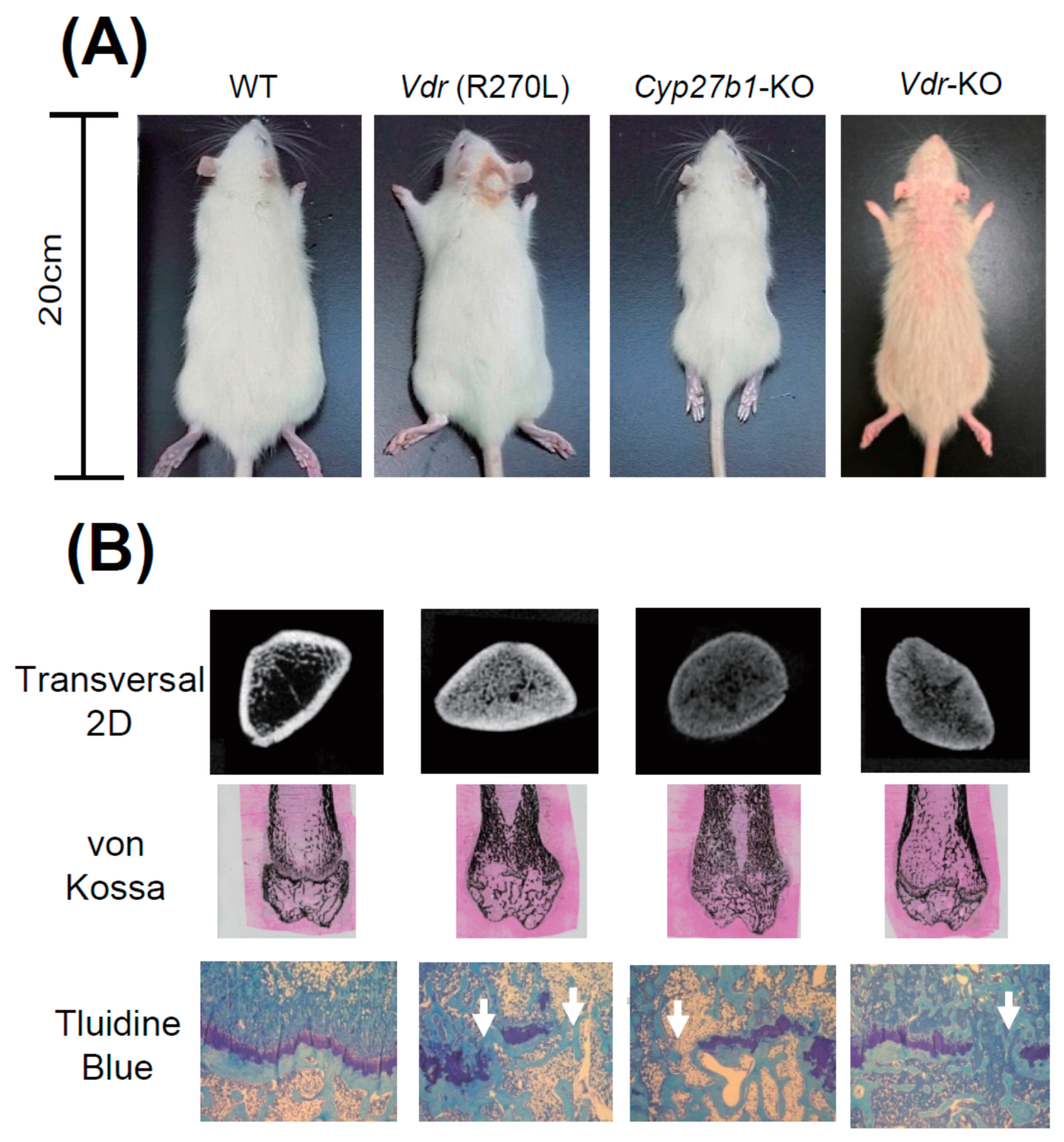
Figure 5A shows WT, Vdr (R270L), and Vdr-KO rats fed an F-2 diet containing 0.75% Ca, and Cyp27b1-KO rats fed a diet containing 1.15% Ca at 15 weeks after birth. Although Cyp27b1-KO rats were much smaller than WT rats, body sizes of Vdr (R270L) and Vdr-KO rats were not so different from that of the WT rats. Figure 5A shows the Vdr- abnormal skin and alopecia of KO rats. Elasticity and softness of the skin of Vdr-KO rats were substantially lowered and the wavy skin was formed [25]. Keratinization was elevated and follicles decreased, and formation of cysts appeared in the dorsal skin of Vdr-KO rats [25].
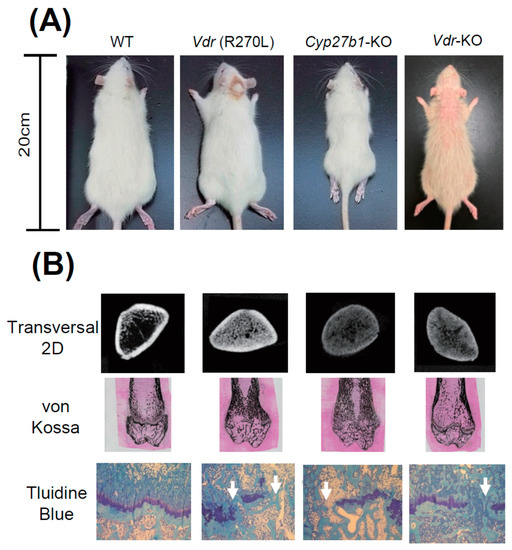
Figure 5.
The appearance of GM rats and their abnormal bone formation [25]. (A) Comparison of body size and skin phenotype at 15 weeks of age. (B) First panels, 2D μ-CT images of horizontal section at distal femur; second panels, von Kossa staining of distal femur; bottom panels, toluidine blue staining of epiphyseal cartilage.
Figure 5A shows that growth was substantially diminished in Cyp27b1-KO rats compared to WT rats. However, only a slight decrease was observed in Vdr (R270L) and Vdr-KO rats. It was noted that approximately a half of male Cyp27b1-KO rats fed with the diet containing 0.75 % Ca died prior to 9 weeks of age, and none survived to 10 weeks of age (data not shown), whereas no animals had died at 15 weeks of age in the Cyp27b1-KO rats fed with the diet containing 1.15% Ca. Thus, the diet that contained 0.75% Ca was used for mutant Vdr (R270L) and Vdr-KO rats, while the diet containing 1.15 % Ca was used for Cyp27b1-KO rats.
4.2. Osteogenesis and Plasma Ca, PTH, and 1α,25(OH)2D3 Levels in the GM Rats
It is noted that Cyp27b1-KO rats are remarkably smaller than other rats. Figure 5B shows the middle region of the femur in 2D μCT scan images. The femur lengths of Cyp27b1-KO, Vdr (R270L), and Vdr-KO rats were found to be remarkably shorter than those of WT rats. The μCT scanning and von Kossa staining of femurs showed hyperplasia of calcified trabecular bones with a narrow medullary cavity in all the Vdr (R270L), Cyp27b1-KO, and Vdr-KO rats (Figure 5B). The Vdr (R270L) and Vdr-KO rats expressed no clear differences in total bone mineral density (BMD). In contrast, the BMD of cortical bone in Cyp27b1-KO rats was substantially diminished [25].
Histological analysis of the epiphyseal cartilage demonstrated structural disorder of the growth plate in all the Vdr (R270L), Cyp27b1-KO, and Vdr-KO rats. Whereas WT growth plates contained aligned cartilage cells in the layered structure, growth plates in all three GM rats lost the sequential plate structure and cartilage cell alignment (Figure 5). Thus, the morphology of bone was abnormal in all three GM rats, and, in Cyp27b1-KO rats, the most significant disorders of bone were observed.
It is well known that rickets type I model Cyp27b1-KO mice, and rickets type II model Vdr-KO mice, have significantly lower plasma Ca levels than WT mice [75,76]. Expectedly, the plasma Ca level was substantially reduced, and the level of parathyroid hormone (PTH) in plasma was greatly increased in Vdr (R270L) rats and Cyp27b1-KO rats [25]. Unexpectedly, the plasma Ca level in Vdr-KO rats was normal at 15 weeks. In Vdr-KO rats, until 10 weeks, the plasma level of Ca was significantly lower than that in WT rats, and PTH level was substantially higher than that in WT rats [25]. Plasma PTH level in Vdr-KO rats was remarkably higher than that in WT rats; although, at 15 weeks, the level of Ca in plasma in Vdr-KO rats returns to normal. These findings might indicate that hyperparathyroidism occurred in Vdr-KO rats [25]. In addition, the putative incomplete formation of intercellular barriers in epithelial tissues, including the small intestine, in VDR-KO rats might cause the increased calcium permeability to result in the normal level of plasma Ca concentration [77].
Although plasma 1α,25(OH)2D3 level was significantly increased in Vdr (R270L) and Vdr-KO rats, it was significantly decreased in Cyp27b1-KO rats (8.0 ± 3.2 pg/mL (mean ± SEM, n = 7)) compared to WT rats (24.8 ± 5.2 pg/mL, (mean ± SEM, n = 7)) [25].
4.3. Effects of 25(OH)D3 Administration on Cyp27b1- KO Rats
As described previously [76], dietary administration of 25(OH)D3 recovered growth failure, skeletal disorders, and hypocalcemia of Cyp27b1-KO mice. Dietary administration of 25(OH)D3 to Cyp27b1-KO rats at 200 μg·kg−1·day−1 also significantly reversed growth failure [25]. The 25(OH)D3 administration normalized BMD of the cortex and trabecular bone of Cyp27b1-KO rats. Histological analysis of the femur clearly indicated a normal structure of the cortex and trabecular bone in Cyp27b1-KO rats [25]. The growth plate and chondrocytes were also normalized, and the plasma Ca and PTH levels of Cyp27b1-KO rats were fully normalized after 25(OH)D3 administration [25].
Plasma 1α,25(OH)2D3 level in Cyp27b1-KO rats was normalized by 25(OH)D3 administration. The 1α-hydroxylation activity toward 25(OH)D3 was observed in the liver mitochondrial fraction prepared from Cyp27b1-KO rats. It is noted that these results were similar to those obtained in our previous study using Cyp27b1-KO mice [76]. Because hepatic Cyp27a1 has a weak 1α-hydroxylation activity toward 25(OH)D3, Cyp27a1 is the most probable candidate to produce 1α,25(OH)2D3 from 25(OH)D3 in Cyp27b1-KO rats.
It is noted that 25(OH)D3 administration is highly effective in type I rickets model mice and rats. Because human CYP27A1 can convert 25(OH)D3 into 1α,25(OH)2D3, similar effects might be expected in humans.
4.4. Effects of 25(OH)D3 Administration on Vdr (R270L) Rats
The 25(OH)D3 administration also normalized bone disorders with increased cortical BMD of Vdr (R270L) rats [25]. The reduced plasma Ca level in Vdr (R270L) rats was normalized by 25(OH)D3 diet, and the elevated plasma PTH and 1α,25(OH)2D3 levels observed before 25(OH)D3 administration were reduced to the normal levels.
The plasma concentration of 25(OH)D3 in Vdr (R270L) rats fed a 25(OH)D3-containing diet was about 500 nM. This concentration was 20 times higher than that in WT rats. It is noted that the affinity of 1α,25(OH)2D3 for Vdr (R270L) is nearly the same as that of 25(OH)D3. Thus, 25(OH)D3 is thought to be a leading ligand of Vdr (R270L) in these rats, because plasma 1α,25(OH)2D3 level in the Vdr (R270L) rats after the 25(OH)D3 treatment was much lower than that of 25(OH)D3 [25]. The remarkably higher levels of 24,25(OH)2D3 and 24-oxo-25(OH)D3 were consistent with the induction of Cyp24a1 expression, which indicates the “Vdr (R270L)-dependent effects of 25(OH)D3”. The remarkable effects of 25(OH)D3 administration on rickets symptoms in Vdr (R270L) rats indicate that 25(OH)D3 might be efficacious in the treatment of patients with type II rickets caused by the human VDR mutant (R274L).
4.5. Predicted Effects of the Vitamin D Derivative AH-1 towards Patients with Type II Rickets Harboring VDR (R274L) cDNA
As described in the previous sections, AH-1 showed a high binding ability to VDR (R274L) and a high resistance to CYP24A1-dependent metabolism. These results suggest that AH-1 could demonstrate therapeutic effects on type II rickets caused by VDR (R274L). Currently, we administered AH-1 to VDR (R270L) rats, and the expected results have been obtained (data not shown).
4.6. Elucidation of Molecular Mechanism Vitamin D Actions by Comparison among the GM Rats
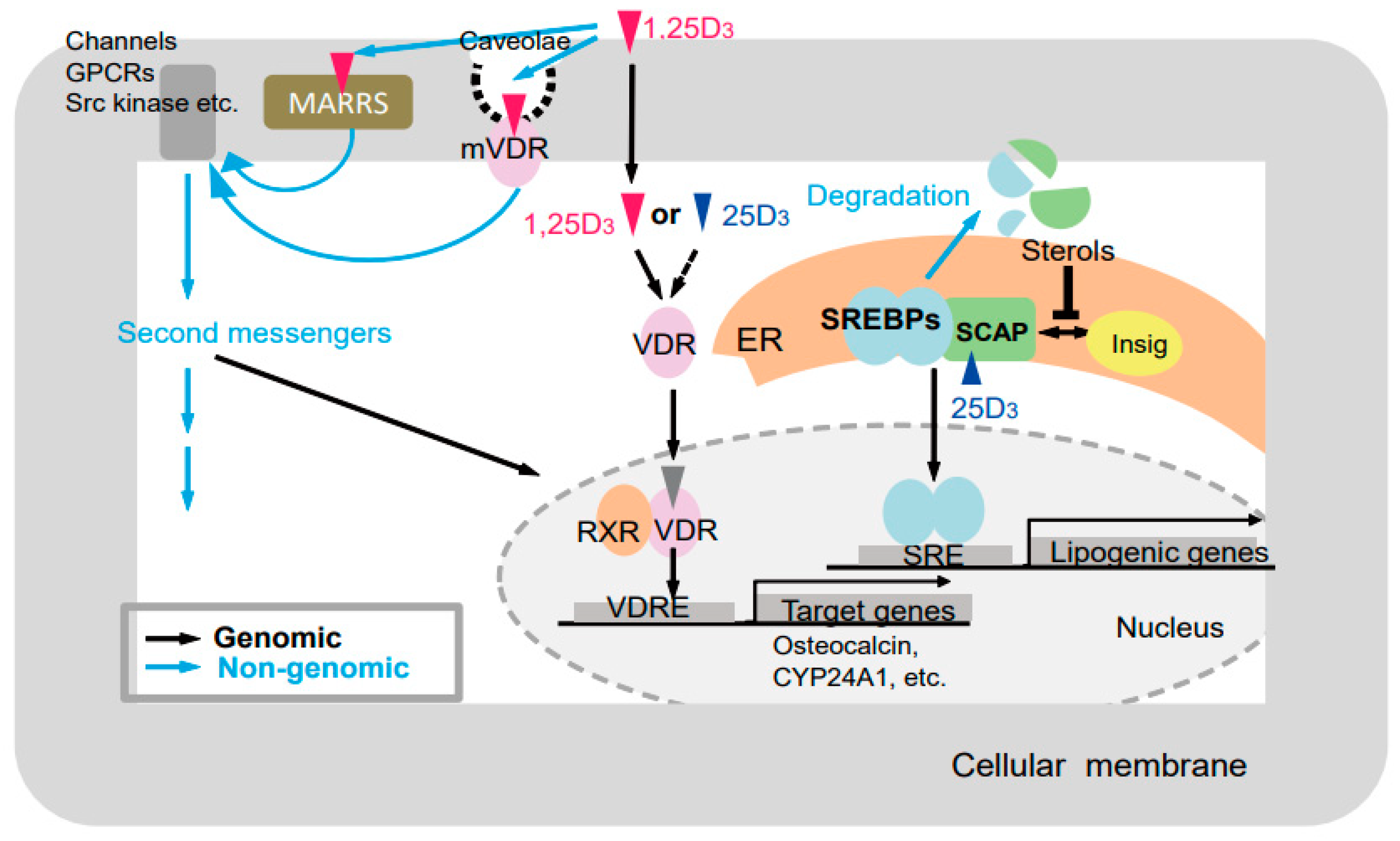
Various vitamin D actions could be elucidated by comparing physiological conditions, such as bone and skin formation, and multiple serum parameters, such as Ca, P, 25(OH)D3, and PTH, in the GM rats generated in this study (Figure 6). Previous studies have showed genomic and nongenomic actions of vitamin D mediated by VDR [78,79], and VDR-independent actions of vitamin D [80]. The VDR-independent effect of 25(OH)D3 on lipid metabolism by inducing degradation of SREBP/SCAP was recently reported. In addition, ligand-independent effects of VDR have been reported [81]. Thus, at least five types of effects of vitamin D and/or the VDR should be considered, namely: (1) VDR-dependent effects of 1α,25(OH)2D3 [19,20], (2) VDR-independent effects of 1α,25(OH)2D3 [80], (3) VDR-dependent effects of 25(OH)D3 (VDR-25(OH)D3) [82], (4) VDR-independent effects of 25(OH)D3 [83], and (5) ligand-independent effects of VDR (Table 1) [81].
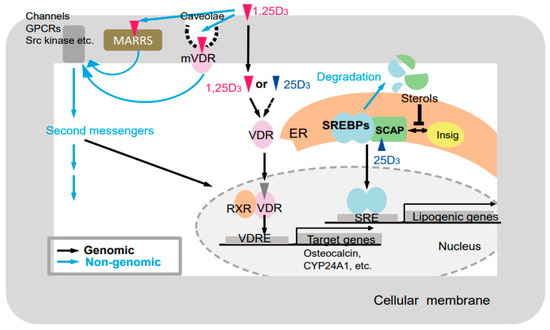
Figure 6.
Putative modes of action of vitamin D [25]. Black and blue arrows indicate genomic and nongenomic pathways, respectively. GPCRs, G-protein-coupled receptor; MARRS, (membrane-associated, rapid response steroid-binding) receptor; VDR, vitamin D receptor; mVDR, membrane-bound vitamin D receptor; RXR, retinoid X receptor; VDRE, vitamin D response element; ER, endoplasmic reticulum; SREBPs, sterol regulatory-element–binding proteins; SCAP, SREBP cleavage-activating protein; SRE, sterol regulatory element.

Table 1.
Vitamin D and/or VDR actions observed in WT and GM rats [25].
Comparison between wild-type and Vdr (R270L) rats could reveal (1) VDR-dependent 1α,25(OH)2D3 effects (Table 1). Comparison between Vdr (R270L) and Cyp27b1-KO rats may reveal (2) VDR-independent effects of 1α,25(OH)2D3. In addition, comparison between Vdr (R270L) and Vdr-KO rats may reveal (3) VDR-dependent effects of 25(OH)D3 or (5) ligand-independent effects of the VDR. Thus, our GM rats appear to be useful for the elucidation of molecular mechanism vitamin D actions and the development of vitamin D derivatives for clinical treatment.
5. Conclusions
The vitamin D derivative evaluation systems we have developed in this study are quite useful. They can readily measure VDR affinity and CYP24A1-mediated metabolism. In addition, the GM rats we have generated by genome editing are highly useful for evaluating the efficacy, safety, and pharmacokinetics of vitamin D derivatives. The reasons rats were used in this study instead of mice include their much larger body size and greater blood volume relative to mice, rendering rats more suitable for pharmacokinetic studies. We hope these evaluation systems will contribute to the near-future development of drugs with excellent therapeutic potential.
Author Contributions
Conceptualization, T.S.; methodology, K.Y., M.N., H.M., M.T., A.K.; validation, K.Y., M.N., H.M., A.K. formal analysis, K.Y., T.S.; investigation, K.Y., M.N., H.M., M.T., A.K.; data curation, K.Y., T.S.; writing—original draft preparation, K.Y., T.S.; writing—review and editing, S.I., T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
Japan Society for the Promotion of Science: 16K14904, 16H04912, 19H02889.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Toyama Prefectural University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Osborn, J.L.; Schwartz, G.G.; Smith, D.; Bahnson, R.; Day, R.; Trump, D.L. Phase II trial of oral 1,25-dihydroxyvitamin D (calcitriol) in hormone refractory prostate cancer. Urol. Oncol. Semin. Orig. Investig. 1995, 1, 195–198. [Google Scholar] [CrossRef]
- Gross, C.; Stamey, T.; Hancock, S.; Feldman, D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol). J. Urol. 1998, 15, 2035–2039. [Google Scholar] [CrossRef]
- Binderup, L.; Latini, S.; Bretting, C.; Calverley, M.; Hansen, K. 20-EPI-vitamin D3 analogues: A novel class of potent regulators of cell growth and immune responses. Biochem. Pharmacol. 1991, 42, 1569–1575. [Google Scholar] [CrossRef]
- Bishop, J.E.; Collins, E.D.; Okamura, W.H.; Norman, A.W. Profile of ligand specificity of the vitamin D binding protein for 1α,25-dihydroxyvitamin d3 and its analogs. J. Bone Miner. Res. 2009, 9, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Okamura, W.H.; Norman, A.W. Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995, 16, 200–257. [Google Scholar] [PubMed]
- Evans, T.R.; Colston, K.W.; Lofts, F.J.; Cunningham, D.; Anthoney, D.A.; Gogas, H.; de Bono, J.S.; Hamberg, K.J.; Skov, T.; Mansi, J.L. A phase II trial of the vitamin D analogue Seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br. J. Cancer 2002, 86, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Oettel, K.; Ripple, G.; Staab, M.J.; Horvath, D.; Alberti, D.; Arzoomanian, R.; Marnocha, R.; Bruskewitz, R.; Mazess, R.; et al. Phase I trial of 1alpha-hydroxyvitamin D(2) in patients with hormone refractory prostate cancer. Clin. Cancer Res. 2002, 8, 2820–2827. [Google Scholar] [PubMed]
- Yamada, S.; Shimizu, M.; Yamamoto, K. Structure-function relationships of vitamin D including ligand recognition by the vitamin D receptor. Med. Res. Rev. 2003, 23, 89–115. [Google Scholar] [CrossRef]
- Masuda, S.; Jones, G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol. Cancer Ther. 2006, 5, 797–808. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Nagai, Y.; Sladek, R.; Bastien, Y.; Ho, J.; Petrecca, K.; Sotiropoulou, G.; Diamandis, E.P.; Hudson, T.J.; White, J.H. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol. Endocrinol. 2002, 16, 1243–1256. [Google Scholar] [CrossRef]
- Liu, G.; Wilding, G.; Staab, M.J.; Horvath, D.; Miller, K.; Dresen, A.; Alberti, N.; Arzoomanian, R.; Chappell, R.; Bailey, H.H. Phase II study of 1alpha-hydroxyvitamin D(2) in the treatment of advanced androgen-independent prostate cancer. Clin. Cancer Res. 2003, 9, 4077–4083. [Google Scholar]
- Schwartz, G.G.; Hall, M.C.; Stindt, D.; Patton, S.; Lovato, J.; Torti, F.M. Phase I/II study of 19-nor-1alpha-25-dihydroxyvitamin D2 (paricalcitol) in advanced, androgen-insensitive prostate cancer. Clin. Cancer Res. 2005, 11, 8680–8685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medioni, J.; Deplanque, G.; Ferrero, J.M.; Maurina, T.; Rodier, J.M.; Raymond, E.; Allyon, J.; Maruani, G.; Houillier, P.; Mackenzie, S.; et al. Phase I safety and pharmacodynamic of inecalcitol, a novel VDR agonist with docetaxel in metastatic castration-resistant prostate cancer patients. Clin. Cancer Res. 2014, 20, 4471–4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studzinski, G.P.; Gocek, E.; Coffman, F.; Danilenko, M. Effects of Vitamin D Derivatives on Differentiation, Cell Cycle, and Apoptosis in Hematological Malignancies. Vitamin. D (Forth Edition) 2018, 2, 761–799. [Google Scholar]
- Mano, H.; Nishikawa, M.; Yasuda, K.; Ikushiro, S.; Saito, N.; Takano, M.; Kittaka, A.; Sakaki, T. Development of Novel Bioluminescent Sensor to Detect and Discriminate between Vitamin D Receptor Agonists and Antagonists in Living Cells. Bioconjug. Chem. 2015, 26, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Nishikawa, M.; Yasuda, K.; Ikushiro, S.; Saito, N.; Sawada, D.; Honzawa, S.; Takano, M.; Kittaka, A.; Sakaki, T. Novel screening system for high-affinity ligand of heredity vitamin D-resistant rickets-associated vitamin D receptor mutant R274L using bioluminescent sensor. J. Steroid Biochem. Mol. Biol. 2017, 167, 61–66. [Google Scholar] [CrossRef]
- Mano, H.; Ikushiro, S.; Saito, N.; Kittaka, A.; Sakaki, T. Development of a highly sensitive in vitro system to detect and discriminate between vitamin D receptor agonists and antagonists based on split-luciferase technique. J. Steroid Biochem. Mol. Biol. 2018, 178, 55–59. [Google Scholar] [CrossRef]
- Mano, H.; Ikushiro, S.; Sakaki, T. Novel split luciferase-based biosensors for evaluation of vitamin D receptor ligands and their application to estimate CYP27B1 activity in living cells. J. Steroid Biochem. Mol. Biol. 2018, 183, 221–227. [Google Scholar] [CrossRef]
- Mano, H.; Takano, M.; Ikushiro, S.; Kittaka, A.; Sakaki, T. Novel biosensor using split-luciferase for detecting vitamin D receptor ligands based on the interaction between vitamin D receptor and coactivator. Biochem. Biophys. Res. Commun. 2018, 505, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi-Shibata, M.; Sakaki, T.; Ohyama, Y.; Noshiro, M.; Okuda, K.; Yabusaki, Y. Further Oxidation of 24,25-Dihydroxyvitamin D3 by 25-Hydroxyvitamin D3 24- Hydroxylase Itself. Eur. J. Biochem. 1994, 224, 335–343. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Nonaka, Y.; Ohyama, Y.; Inouye, K. Metabolic studies using recombinant Escherichia coli cells producing rat mitochondrial CYP24: CYP24 can convert 1α,25-dihydroxyvitamin D3 to calcitroic acid. JBIC J. Biol. Inorg. Chem. 1999, 262, 43–48. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Komai, K.; Shiozawa, S.; Yamada, S.; Yamamoto, K.; Ohyama, Y.; Inouye, K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000, 267, 6158–6165. [Google Scholar] [CrossRef] [Green Version]
- Sawada, N.; Kusudo, T.; Sakaki, T.; Hatakeyama, S.; Hanada, M.; Abe, D.; Kamao, M.; Okano, T.; Ohta, M.; Inouye, K. Novel metabolism of 1α,25-dihydroxyvitamin D3 with C24–C25 bond cleavage catalyzed by human CYP24A1. Biochemistry 2004, 43, 4530–4537. [Google Scholar] [CrossRef]
- Hamamoto, H.; Kusudo, T.; Urushino, N.; Masuno, H.; Yamamoto, K.; Yamada, S.; Kamakura, M.; Ohta, M.; Inouye, K.; Sakaki, T. Structure-function analysis of vitamin D 24-hydroxylase (CYP24A1) by site-directed mutagenesis: Amino acid resides responsible for species-based difference of CYP24A1 between humans and rats. Mol. Pharmacol. 2006, 70, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yasuda, K.; Takamatsu, M.; Abe, K.; Okamoto, K.; Horibe, K.; Mano, H.; Nakagawa, K.; Tsugawa, N.; Hirota, Y.; et al. Generation of novel genetically modified rats to reveal the molecular mechanisms of vitamin D actions. Sci. Rep. 2020, 10, 5677. [Google Scholar] [CrossRef]
- Yasuda, K.; Nishikawa, M.; Okamoto, K.; Horibe, K.; Mano, H.; Yamaguchi, M.; Okon, R.; Nakagawa, K.; Tsugawa Okano, T.; Kawagoe, F.; et al. Elucidation of metabolic pathways of 25-hydroxyvitamin D3 mediated by CYP24A1 and Cyp3A using Cyp24a1 knockout rats generated by CRISPR/Cas9 System. J. Biol. Chem. 2021, 296, 100668. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, M.; Tsukahara, Y.; Iwasaki-Miyamoto, Y.; MihoriShimazaki, M.; Yamada, S.; Inaba, S.; Oda, M.; Shimizu, M.; Makishima, M.; Tokiwa, H.; et al. Crystal structures of hereditary vitamin D-resistant rickets-associated vitamin D receptor mutants R270L and W282R bound to 1,25-dihydroxyvitamin D3 and synthetic ligands. J. Med. Chem. 2013, 56, 6745–6760. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Ishii, C.; Ryoji, M. Role of distal upstream sequence in vitamin D-induced expression of human CYP24 gene. Biochem. Biophys. Res. Commun. 2007, 358, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Masuno, H.; Choi, M.; Nakashima, K.; Taga, T.; Ooizumi, H.; Umesono, K.; Sicinska, W.; VanHooke, J.; DeLuca, H.F.; et al. Three-dimensional modeling of and ligand docking to vitamin D receptor ligand binding domain. Proc. Natl. Acad. Sci. USA 2000, 97, 1467–1472. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Cho, E.J.; Jo, S.m.; Sung, B.R.; Lee, S.; Yun, S.H. A split luciferase complementation assay for studying in vivo protein-protein interactions in filamentous ascomycetes. Curr. Genet. 2012, 58, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Tashakor, A.; Hosseinkhani, S. Split-luciferase complementary assay: Applications, recent developments, and future perspectives. Anal. Bioanal. Chem. 2014, 406, 5541–5560. [Google Scholar] [CrossRef]
- Shavkunov, A.S.; Ali, S.R.; Panova-Elektronova, N.I.; Laezza, F. Split-luciferase complementation assay to detect channel-protein interactions in live cells. Methods Mol. Biol. 2015, 1278, 497–514. [Google Scholar] [PubMed]
- Varnum, M.M.; Clayton, K.A.; Yoshii-Kitahara, A.; Yonemoto, G.; Koro, L.; Ikezu, S.; Ikezu, T. A split-luciferase complementation, real-time reporting assay enables monitoring of the disease-associated transmembrane protein TREM2 in live cells. J. Biol. Chem. 2017, 292, 10651–10663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustaqil, M.; Bhumkar, A.; Gonzalez, L.; Raoul, L.; Hunter, D.J.B.; Carrive, P.; Sierecki, E.; Gambin, Y. A Split-Luciferase Reporter Recognizing GFP and mCherry Tags to Facilitate Studies of Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2681. [Google Scholar] [CrossRef] [Green Version]
- Forster, L.; Grätz, L.; Mönnich, D.; Bernhardt, G.; Pockes, S. A Split Luciferase Complementation Assay for the Quantification of beta-Arrestin2 Recruitment to Dopamine D(2)-Like Receptors. Int. J. Mol. Sci. 2020, 21, 6103. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, M.; Thurner, L.; Romantini, N.; Zimmermann, M.; Meger, B.; Behe, M.; Waldhoer, M.; Schertler, G.F.X.; Berger, P. New Insights into Arrestin Recruitment to GPCRs. Int. J. Mol. Sci. 2020, 21, 4949. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Lytle, N.K.; Gammon, S.T.; Wang, L.; Hayes, T.K.; Sutton, M.N.; Bast, R.C., Jr.; Der, C.J.; Piwnica-Worms, D.; McCormick, F.; et al. Analysis of RAS protein interactions in living cells reveals a mechanism for pan-RAS depletion by membrane-targeted RAS binders. Proc. Natl. Acad. Sci. USA 2020, 117, 12121–12130. [Google Scholar] [CrossRef] [PubMed]
- Malloy, P.J.; Pike, J.W.; Feldman, D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr. Rev. 1999, 20, 156–188. [Google Scholar] [PubMed] [Green Version]
- Ohyama, Y.; Noshiro, M.; Okuda, K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 1991, 278, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, Y.; Ozono, K.; Uchida, M.; Shinki, T.; Kato, S.; Suda, T.; Yamamoto, O.; Noshiro, M.; Kato, Y. Identification of a vitamin D-responsive element in the 5’-flanking region of the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J. Biol. Chem. 1994, 269, 10545–10550. [Google Scholar] [CrossRef]
- Zierold, C.; Mings, J.A.; DeLuca, H.F. Parathyroid hormone regulates 25-hydroxyvitamin D3-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA 2001, 98, 13572–13576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhawan, P.; Peng, X.; Sutton, A.L.; MacDonald, P.N.; Croniger, C.M.; Trautwein, C.; Centrella, M.; McCarthy, T.L.; Christakos, S. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol. Cell Biol. 2005, 25, 472–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1α,25-dihydroxyvitamin D3. J. Biol. Chem. 2010, 285, 15599–15610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.B.; Lee, S.M.; Carlson, A.H.; Benkusky, N.A.; Kaufmann, M.; Jones, G.; Pike, J.W. A chromatin-based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non-renal tissues. J. Biol. Chem. 2019, 294, 14467–14481. [Google Scholar] [CrossRef] [Green Version]
- Sakaki, T.; Sawada, N.; Abe, D.; Komai, K.; Shiozawa, S.; Nonaka, Y.; Nakagawa, K.; Okano, T.; Ohta, M.; Inouye, K. Metabolism of 26,26,26,27,27,27-F6-1α,25-dihydroxyvitamin D3 by CYP24: Species-based difference between humans and rats. Biochem. Pharmacol. 2003, 65, 1957–1965. [Google Scholar] [CrossRef]
- Kusudo, T.; Sakaki, T.; Abe, D.; Fujishima, T.; Kittaka, A.; Takayama, H.; Ohta, M.; Inouye, K. Metabolism of 20-epimer of 1α,25-dihydroxyvitamin D3 by CYP24: Species-based difference between humans and rats. Biochem. Biophys. Res. Commun. 2003, 309, 885–892. [Google Scholar] [CrossRef]
- Kusudo, T.; Sakaki, T.; Abe, D.; Fujishima, T.; Kittaka, A.; Takayama, H.; Hatakeyama, S.; Ohta, M.; Inouye, K. Metabolism of A-ring diastereomers of 1α,25-dihydroxyvitamin D3 by CYP24A1. Biochem. Biophys. Res. Commun. 2004, 321, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Abe, D.; Sakaki, T.; Kusudo, T.; Kittaka, A.; Saito, N.; Suhara, Y.; Fujishima, T.; Takayama, H.; Hamamoto, H.; Kamakura, M.; et al. Metabolism of 2α-propoxy-1α,25-dihydroxyvitamin D3 and 2α-(3-hydroxypropoxy)- 1α,25-dihydroxyvitamin D3 by human CYP27A1 and CYP24A1. Drug Metab. Dispos. 2005, 33, 778–784. [Google Scholar] [CrossRef]
- Urushino, N.; Nakabayashi, S.; Arai, M.A.; Kittaka, A.; Chen, T.C.; Yamamoto, K.; Hayashi, K.; Kato, S.; Ohta, M.; Kamakura, M.; et al. Kinetic Studies of 25-Hydroxy-19-Nor-Vitamin D3 and 1α,25-Dihydroxy-19-Nor-Vitamin D3 Hydroxylation by CYP27B1 and CYP24A1. Drug Metab. Dispos. 2007, 35, 1482–1488. [Google Scholar] [CrossRef]
- Saito, N.; Suhara, Y.; Abe, D.; Kusudo, T.; Ohta, M.; Yasuda, K.; Sakaki, T.; Honzawa, S.; Fujishima, T.; Kittaka, A. Synthesis of 2α-propoxy-1α,25-dihydroxyvitamin D3 and comparison of its metabolism by human CYP24A1 and rat CYP24A1. Bioorg. Med. Chem. 2009, 17, 4296–4301. [Google Scholar] [CrossRef]
- Sawada, D.; Tsukuda, Y.; Yasuda, K.; Sakaki, T.; Saito, H.; Takagi, K.-I.; Takenouchi, K.; Chen, T.; Reddy, G.S.; Kittaka, A. Synthesis and Biological Activities of 1α,4α,25- and 1α,4β,25-Trihydroxyvitamin D3 and Their Metabolism by Human CYP24A1 and UDP-Glucuronosyltransferase. Chem. Pharm. Bull. 2012, 60, 1343–1346. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Ikushiro, S.; Kamakura, M.; Takano, M.; Saito, N.; Kittaka, A.; Chen, T.; Ohta, M.; Sakaki, T. Human cytochrome P450-dependent differential metabolism among three 2α-substituted-1α,25-dihydroxyvitamin D3 analogs. J. Steroid Biochem. Mol. Biol. 2013, 133, 84–92. [Google Scholar] [CrossRef]
- Yasuda, K.; Iwanaga, Y.; Ogawa, K.; Mano, H.; Ueno, S.; Kimoto, S.; Ohta, M.; Kamakura, M.; Ikushiro, S.; Sakaki, T. Human hepatic metabolism of the anti-osteoporosis drug eldecalcitol (ED-71) involves sterol C4-methyl oxidase. Pharmacol. Res. Prospect. 2015, 3, e00120. [Google Scholar]
- Takano, M.; Yasuda, K.; Higuchi, E.; Tohyama, E.; Takeuchi, A.; Sakaki, T.; Kittaka, A. Synthesis, metabolism, and biological activity of 2-[3-(tetrazolyl) propyl]-1α,25-dihydroxy-19-norvitamin D3. J. Steroid Biochem. Mol. Biol. 2016, 164, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, F.; Sugiyama, T.; Yasuda, K.; Uesugi, M.; Sakaki, T.; Kittaka, A. Concise synthesis of 23-hydroxylated vitamin D3 metabolites. J. Steroid Biochem. Mol. Biol. 2018, 186, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, F.; Yasuda, K.; Mototani, S.; Sugiyama, T.; Uesugi, M.; Sakaki, T.; Kittaka, A. Synthesis and CYP24A1-Dependent Metabolism of 23-Fluorinated Vitamin D3 Analogues. ACS Omega 2019, 4, 11332–11337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawagoe, F.; Mototani, S.; Yasuda, K.; Nagasawa, K.; Uesugi, M.; Sakaki, T.; Kittaka, A. Introduction of Fluorine Atoms to Vitamin D3 Side-chain and Synthesis of 24,24- Difluoro-25-hydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2019, 195, 105477. [Google Scholar] [CrossRef]
- Milczarek, M.; Chodyński, M.; Pietraszek, A.; Stachowicz-Suhs, M.; Yasuda, K.; Sakaki, T.; Wietrzyk, J.; Kutner, A. Synthesis, CYP24A1-Dependent Metabolism and Antiproliferative Potential against Colorectal Cancer Cells of 1,25-Dihydroxyvitamin D2 Derivatives Modified at the Side Chain and the A-Ring. Int. J. Mol. Sci. 2020, 21, 642. [Google Scholar] [CrossRef] [Green Version]
- Prosser, D.E.; Kaufmann, M.; O’Leary, B.; Byford, V.; Jones, G. Single A326G mutation converts human CYP24A1 from 25-OH-D3-24-hydroxylase into -23-hydroxylase, generating 1,25-(OH)2D3-26,23-lactone. Proc. Natl. Acad. Sci. USA 2007, 104, 12673–12678. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, Y.; Ozono, K.; Uchida, M.; Yoshimura, M.; Shinki, T.; Suda, T.; Yamamoto, O. Functional assessment of two vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J. Biol. Chem. 1996, 271, 30381–30385. [Google Scholar] [CrossRef] [Green Version]
- Shankar, V.N.; Dilworth, F.J.; Makin, H.L.; Schroeder, N.J.; Trafford, D.J.; Kissmeyer, A.M.; Calverley, M.J.; Binderup, E.; Jones, G. Metabolism of the vitamin D analog EB1089 by cultured human cells: Redirection of hydroxylation site to distal carbons of the side-chain. Biochem. Pharmacol. 1997, 53, 783–793. [Google Scholar] [CrossRef]
- Lechner, D.; Manhardt, T.; Bajna, E.; Posner, G.H.; Cross, H.S. A 24-phenylsulfone analog of vitamin D inhibits 1alpha,25-dihydroxyvitamin D(3) degradation in vitamin D metabolism-competent cells. J. Pharmacol. Exp. Ther. 2007, 320, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.; Egger, H.; Astecker, N.; Herzig, G.; Schüssler, M.; Vorisek, G. Selective inhibitors of CYP24: Mechanistic tools to explore vitamin D metabolism in human keratinocytes. Steroids 2001, 66, 451–462. [Google Scholar] [CrossRef]
- Kittaka, A.; Suhara, Y.; Takayanagi, H.; Fujishima, T.; Kurihara, M.; Takayama, H. A concise and efficient route to 2alpha-(omega-hydroxyalkoxy)-1alpha,25-dihydroxy vitamin D3: Remarkably high affinity to vitamin D receptor. Org. Lett. 2000, 2, 2619–2622. [Google Scholar] [CrossRef] [PubMed]
- Suhara, Y.; Nihei, K.I.; Tanigawa, H.; Fujishima, T.; Konno, K.; Nakagawa, K.; Okano, T.; Takayama, H. Syntheses and biological evaluation of novel 2alpha-substituted 1alpha,25-dihydroxyvitamin D3 analogues. Bioorg. Med. Chem. Lett. 2000, 10, 1129–1132. [Google Scholar] [CrossRef]
- Suhara, Y.; Nihei, K.I.; Kurihara, M.; Kittaka, A.; Yamaguchi, K.; Fujishima, T.; Konno, K.; Miyata, N.; Takayama, H. Efficient and versatile synthesis of novel 2alpha-substituted 1alpha,25-dihydroxyvitamin D(3) analogues and their docking to vitamin D receptors. J. Org. Chem. 2001, 66, 8760–8771. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Suhara, Y.; Kurihara, M.; Fujishima, T.; Honzawa, S.; Takayanagi, H.; Kozono, T.; Matsumoto, M.; Ohmori, M.; Miyata, N.; et al. Design and efficient synthesis of 2 alpha-(omega-hydroxyalkoxy)-1 alpha,25-dihydroxyvitamin D3 Analogues, including 2-epi-ED-71 and their 20-epimers with HL-60 cell differentiation activity. J. Org. Chem. 2004, 69, 7463–7471. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Nakagawa, K.; Suhara, Y.; Kittaka, A.; Nihei, K.; Konno, K.; Takayama, H.; Ozono, K.; Okano, T. Biological activities of 2alpha-substituted analogues of 1alpha,25-dihydroxyvitamin D3 in transcriptional regulation and human promyelocytic leukemia (HL-60) cell proliferation and differentiation. Biol. Pharm. Bull. 2006, 29, 2246–2250. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Yoshida, A.; Saito, N.; Fujishima, T.; Honzawa, S.; Suhara, Y.; Kishimoto, S.; Sugiura, T.; Waku, K.; Takayama, H.; et al. Efficient synthesis of 2-modified 1alpha,25-dihydroxy-19-norvitamin D3 with Julia olefination: High potency in induction of differentiation on HL-60 cells. J. Org. Chem. 2003, 68, 7407–7415. [Google Scholar] [CrossRef]
- Arai, M.A.; Takeyama, K.; Ito, S.; Kato, S.; Chen, T.C.; Kittaka, A. High-throughput system for analyzing ligand-induced cofactor recruitment by vitamin D receptor. Bioconjug. Chem. 2007, 18, 614–620. [Google Scholar] [CrossRef]
- Chen, T.; Persons, K.; Zheng, S.; Mathieu, J.; Holick, M.; Lee, Y.; Bao, B.; Arai, M.; Kittaka, A. Evaluation of C-2-substituted 19-nor-1α,25-dihydroxyvitamin D3 analogs as therapeutic agents for prostate cancer. J. Steroid Biochem. Mol. Biol. 2007, 103, 717–720. [Google Scholar] [CrossRef]
- Flanagan, J.N.; Zheng, S.; Chiang, K.C.; Kittaka, A.; Sakaki, T.; Nakabayashi, S.; Zhao, X.; Spanjaard, R.A.; Persons, K.S.; Mathieu, J.S.; et al. Evaluation of 19-nor-2alpha-(3-hydroxypropyl)-1alpha,25- dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Res. 2009, 29, 3547–3553. [Google Scholar]
- Chen, T.C.; Kittaka, A. Novel vitamin d analogs for prostate cancer therapy. Int. Sch. Res. Not. 2011, 2011, 301490. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.C.; Yeh, C.N.; Chen, H.Y.; Lee, J.M.; Juang, H.H.; Chen, M.F.; Takano, M.; Kittaka, A.; Chen, T.C. 19-Nor-2α-(3-hydroxypropyl)-1α,25- dihydroxyvitamin D3 (MART-10) is a potent cell growth regulator with enhanced chemotherapeutic potency in liver cancer cells. Steroids 2011, 76, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Nakagawa, K.; Mimatsu, S.; Sawada, N.; Sakaki, T.; Kubodera, N.; Kamao, M.; Tsugawa, N.; Suhara, Y.; Okano, T. Nongenomic effects of 1α,25-dihydroxyvitamin D3 on cartilage formation deduced from comparisons between Cyp27b1 and Vdr knockout mice. Biochem. Biophys. Res. Commun. 2017, 483, 359–365. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kaori Yasuda, K.; Takamatsu, M.; Abe, K.; Nakagawa, K.; Tsugawa, N.; Hirota, Y.; Tanaka, K.; Yamashita, S.; Ikushiro, S.; et al. Generation of 1,25-dihydroxyvitamin D3 in Cyp27b1 knockout mice by treatment with 25-hydroxyvitamin D3 rescued their rachitic phenotypes. J. Steroid Biochem. Mol. Biol. 2019, 185, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, S.; Lu, R.; Zhou, D.; Zhou, J.; Carmeliet, G.; Petrof, E.; Claud, E.C.; Sun, J. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci. Rep. 2015, 5, 10642. [Google Scholar] [CrossRef] [Green Version]
- Norman, A.W.; Bishop, J.E.; Collins, E.D.; Seo, E.G.; Satchell, D.P.; Dormanen, M.C.; Zanello, S.B.; Farach-Carson, M.C.; Bouillon, R.; Okamura, W.H. Differing shapes of 1 alpha,25-dihydroxyvitamin D3 function as ligands for the D-binding protein, nuclear receptor and membrane receptor: A status report. J. Steroid Biochem. Mol. Biol. 1996, 56, 13–22. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Norman, A.W. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci. Signal. 2009, 2, re4. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorija, K.; Cox, M.; Sisk, J.M.; Dowd, D.R.; MacDonald, P.N.; Thompson, C.C.; Demay, M.B. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol. Endocrinol. 2005, 19, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munetsuna, E.; Kawanami, R.; Nishikawa, M.; Ikeda, S.; Nakabayashi, S.; Ohta, M.; Kamakura, M.; Ikushiro, S.; Sakaki, T. Anti-proliferative activity of 25-hydroxyvitamin D3 in human prostate cells. Mol. Cell Endocrinol. 2014, 382, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).