Bacteroides fragilis Enterotoxin Upregulates Matrix Metalloproteinase-7 Expression through MAPK and AP-1 Activation in Intestinal Epithelial Cells, Leading to Syndecan-2 Release
Abstract
1. Introduction
2. Results
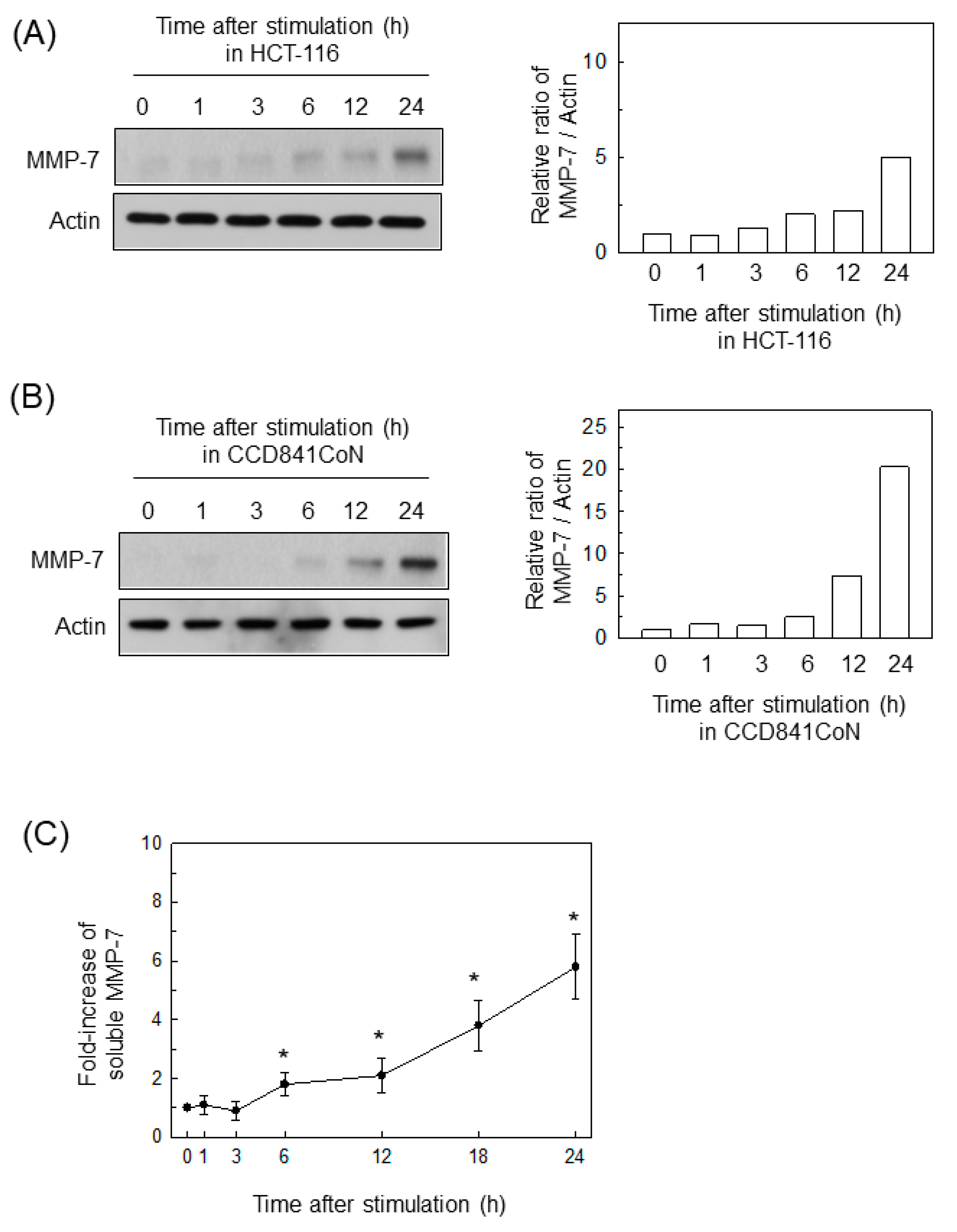
2.1. BFT Upregulates MMP-7 Expression in IECs
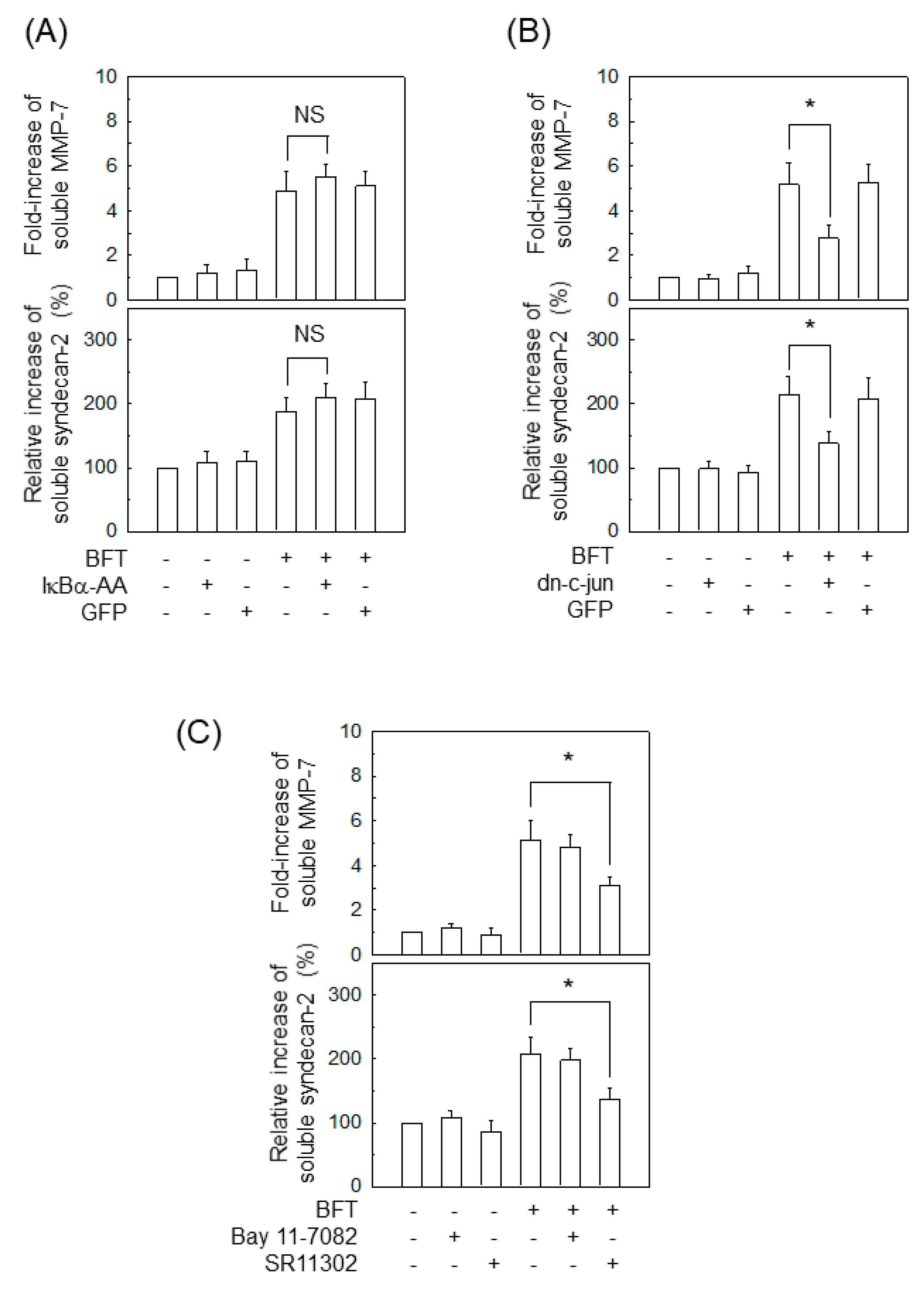
2.2. Activation of NF-κB Is Not Associated with MMP-7 Induction in IECs following BFT Stimulation
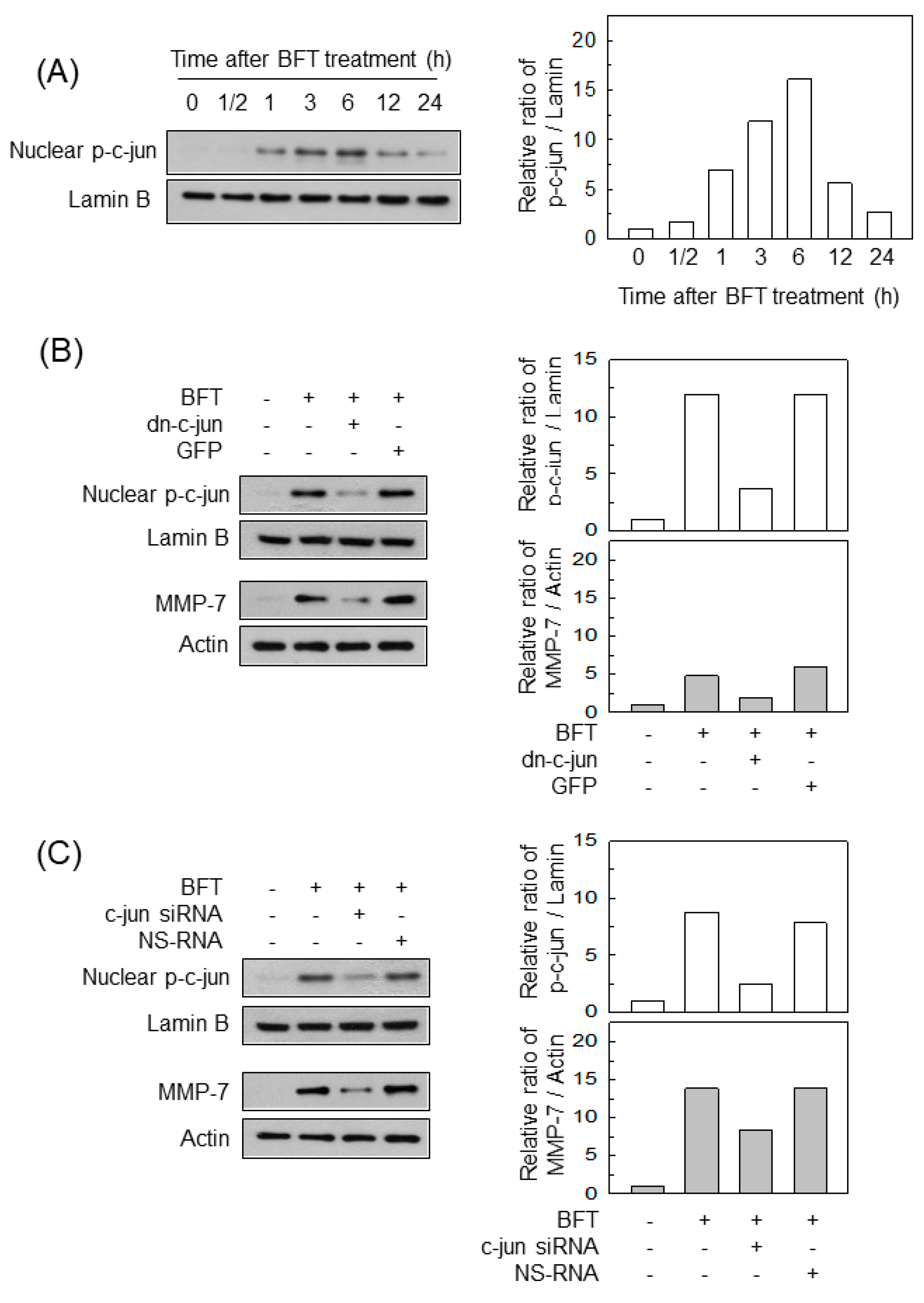
2.3. AP-1 Is Involved in the Upregulation of MMP-7 in BFT-Stimulated IECs
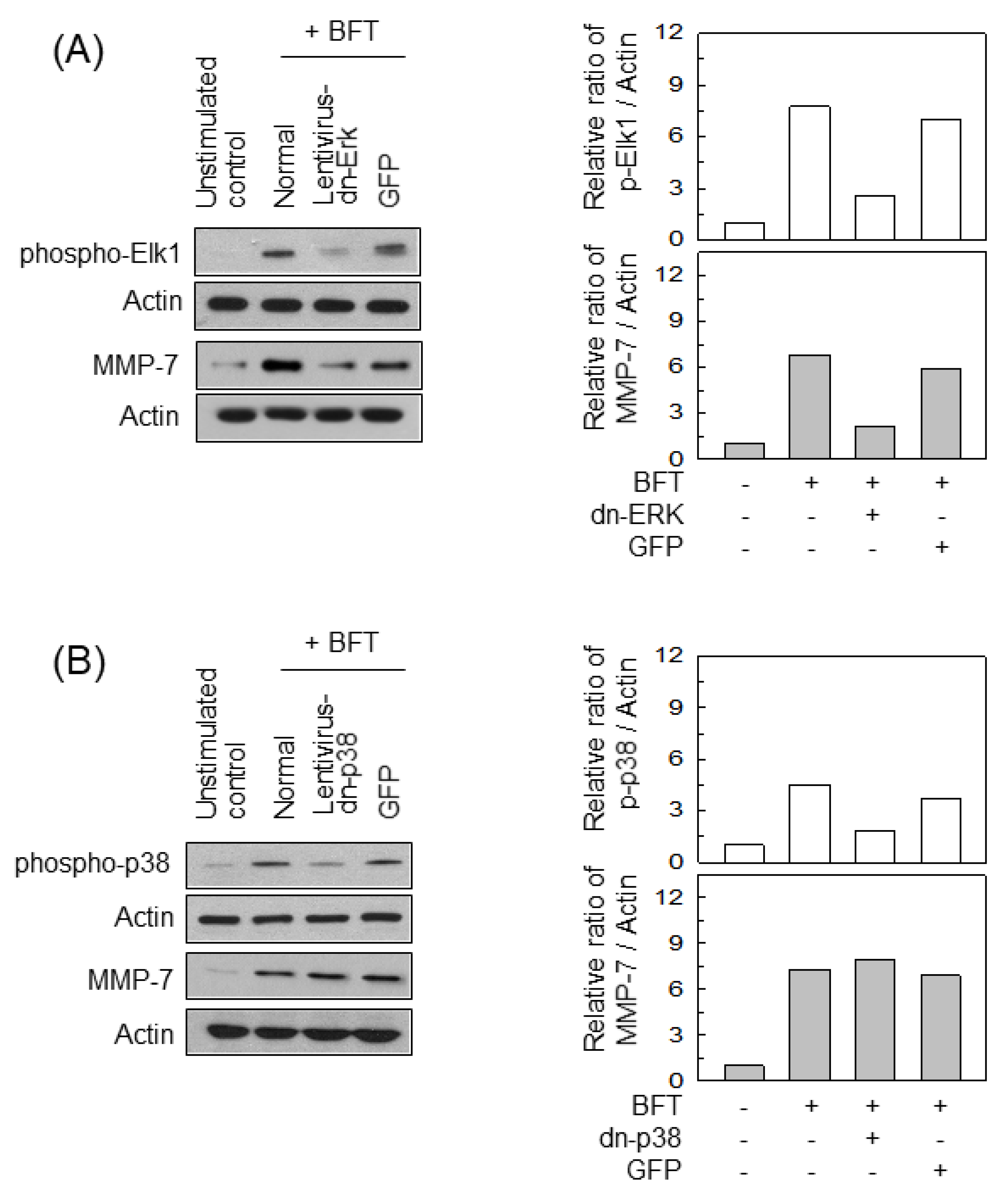
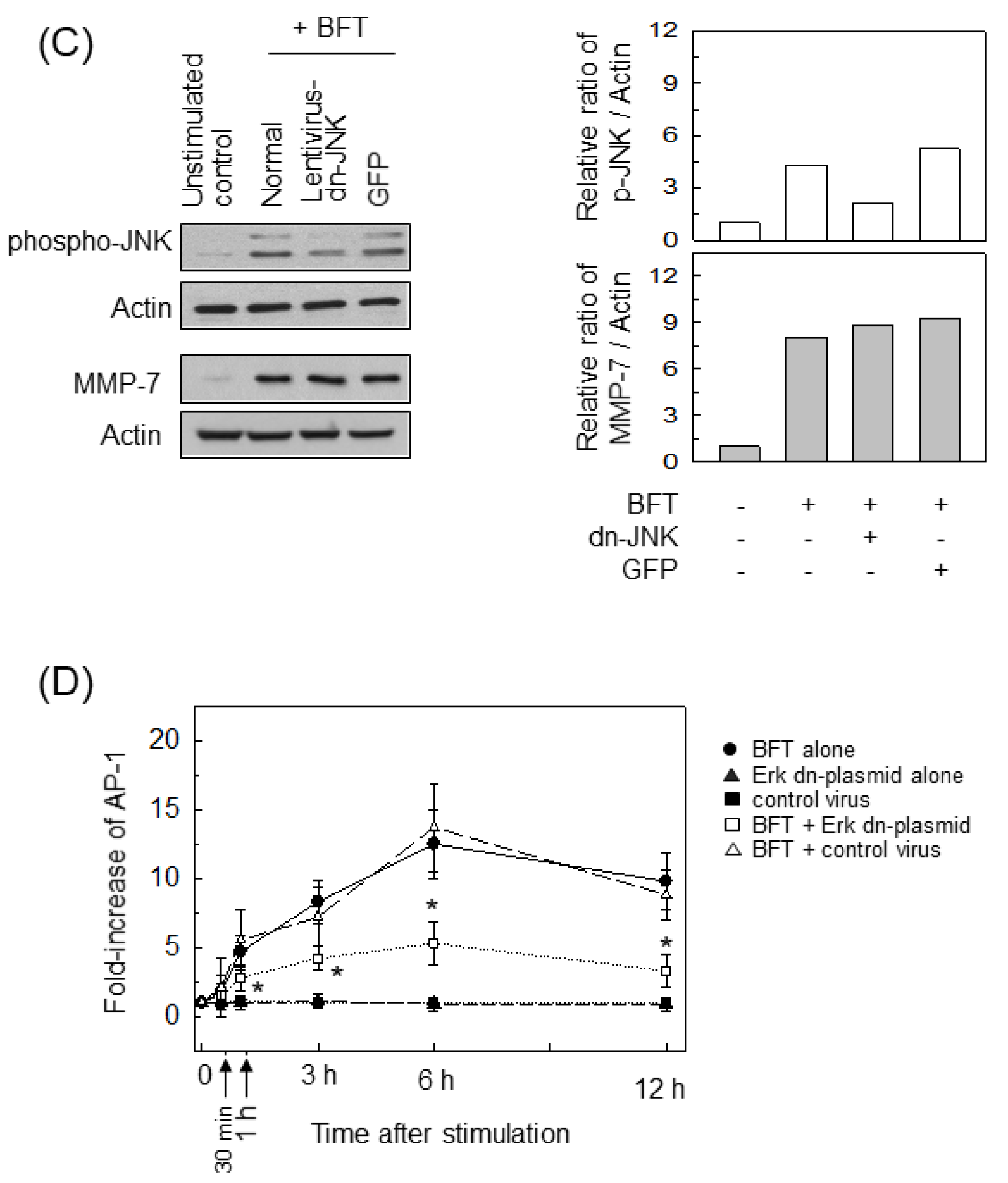
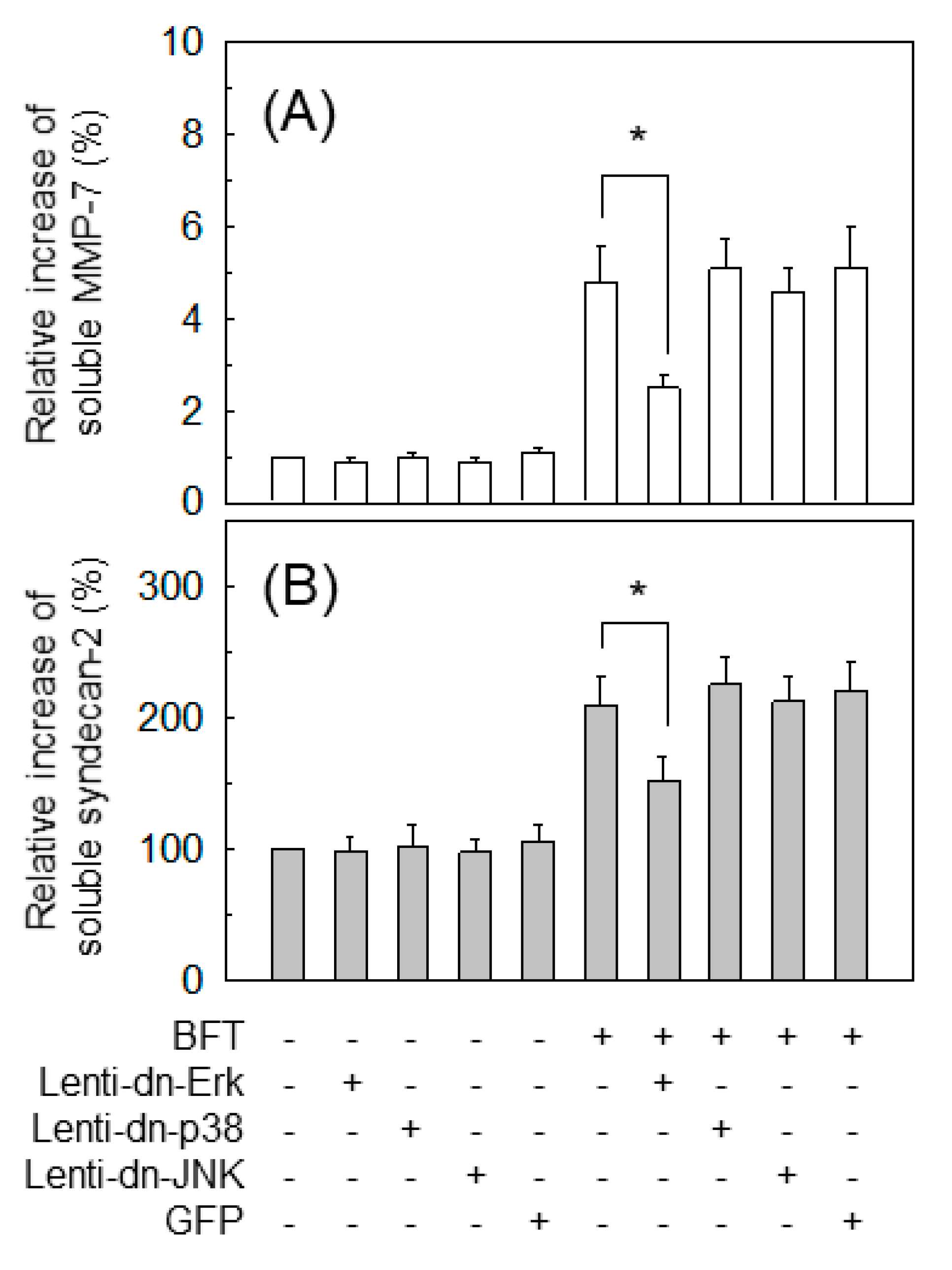
2.4. ERK Is Involved in the Upregulation of MMP-7 in BFT-Stimulated IECs
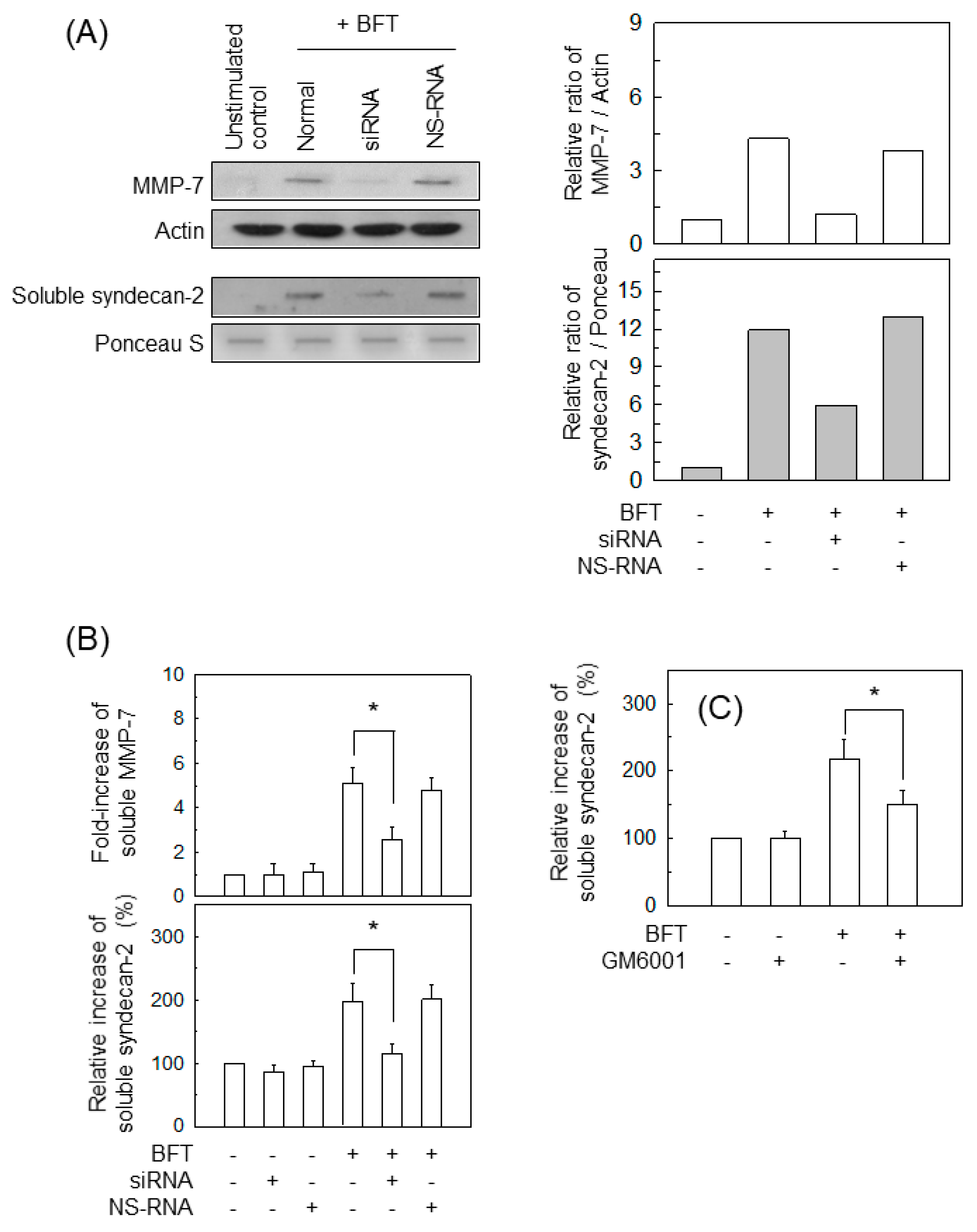
2.5. BFT-Induced MMP-7 Upregulation Is Associated with Syndecan-2 Release in IECs
2.6. AP-1 Signaling Is Involved in Syndecan-2 Release in IECs Stimulated with BFT
2.7. MMP-7-Associated ERK Activation Is Essential for Syndecan-2 Release in BFT-Stimulated IECs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture Conditions and Purification of BFT
4.3. Transfection Assay
4.4. Immunoblots and ELISA
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sears, C.L.; Geis, A.L.; Housseau, F. Bacteroides fragilis subverts mucosal biology: From symbiont to colon carcinogenesis. J. Clin. Investig. 2014, 124, 4166–4172. [Google Scholar] [CrossRef]
- Sears, C.L. Enterotoxigenic Bacteroides fragilis: A rogue among symbiotes. Clin. Microbiol. Rev. 2009, 22, 349–369. [Google Scholar] [CrossRef]
- Sears, C.L. The toxins of Bacteroides fragilis. Toxicon 2001, 39, 1737–1746. [Google Scholar] [CrossRef]
- Jang, B.; Jung, H.; Chung, H.; Moon, B.I.; Oh, E.S. Syndecan-2 enhances E-cadherin shedding and fibroblast-like morphological changes by inducing MMP-7 expression in colon cancer cells. Biochem. Biophys. Res. Commun. 2016, 12, 47–53. [Google Scholar] [CrossRef]
- Kim, J.M.; Jung, H.Y.; Lee, J.Y.; Youn, J.; Lee, C.H.; Kim, K.H. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur. J. Immunol. 2005, 35, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lim, K.C.; Huang, J.; Saidi, R.F.; Sears, C.L. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 1998, 95, 14979–14984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tian, Y.; Sun, L.; Zhou, L.; Xiao, L.; Tan, R.J.; Tian, J.; Fu, H.; Hou, F.F.; Liu, Y. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J. Am. Soc. Nephrol. 2017, 28, 598–611. [Google Scholar] [CrossRef]
- Brabletz, T.; Jung, A.; Dag, S.; Hlubek, F.; Kirchner, T. β-Catenin Regulates the Expression of the Matrix Metalloproteinase-7 in Human Colorectal Cancer. Am. J. Pathol. 1999, 155, 1033–1038. [Google Scholar] [CrossRef]
- Jeon, J.I.; Ko, S.H.; Kim, J.M. Intestinal Epithelial Cells Exposed to Bacteroides fragilis Enterotoxin Regulate NF-κB Activation and Inflammatory Responses through β-Catenin Expression. Infect. Immun. 2019, 87, e00312-19. [Google Scholar] [CrossRef]
- Kelppe, J.; Thorén, H.; Haglund, C.; Sorsa, T.; Hagström, J. MMP-7, -8, -9, E-cadherin, and beta-catenin expression in 34 ameloblastoma cases. Clin. Exp. Dent. Res. 2021, 7, 63–69. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Resp. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Garalla, H.M.; Lertkowit, N.; Tiszlavicz, L.; Reisz, Z.; Holmberg, C.; Beynon, R.; Simpson, D.; Varga, A.; Kumar, J.D.; Dodd, S.; et al. Matrix metalloproteinase (MMP)-7 in Barrett’s esophagus and esophageal adenocarcinoma: Expression, metabolism, and functional significance. Physiol. Rep. 2018, 6, e13683. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Da, C.M.; Liao, B.; Zhang, H.H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Ho, B.Y.; Wu, Y.M.; Chang, K.J.; Pan, T.M. Dimerumic acid inhibits SW620 cell invasion by attenuating H2O2-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int. J. Biol. Sci. 2011, 7, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Hong, E.; Choi, Y.; Kang, D.H.; Oh, E.S. Interleukin-1α promotes extracellular shedding of syndecan-2 via induction of matrix metalloproteinase-7 expression. Biochem. Biophys. Res. Commun. 2014, 446, 487–492. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, K.S.; Sohn, D.H.; Seo, G.S. Polyozellin blocks tumor necrosis factor a-induced interleukin 8 and matrix metalloproteinase 7 production in the human intestinal epithelial cell line HT-29. Arch. Pharm. Res. 2011, 34, 91–97. [Google Scholar] [CrossRef]
- Shi, M.; Liu, D.; Duan, H.; Han, C.; Wei, B.; Qian, L.; Chen, C.; Guo, L.; Hu, M.; Yu, M.; et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol. Cancer 2010, 9, 269. [Google Scholar] [CrossRef]
- Kim, J.M.; Oh, Y.K.; Kim, Y.J.; Oh, H.B.; Cho, Y.J. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κB plays a major role in the regulation of IL-8 expression. Clin. Exp. Immunol. 2001, 123, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, J.Y.; Youn, Y.M.; Oh, Y.K.; Kang, J.S.; Kim, Y.J.; Kim, K.H. Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-kappaB activation. Eur. J. Immunol. 2006, 2446–2456. [Google Scholar] [CrossRef]
- Ko, S.H.; Jeon, J.I.; Woo, H.A.; Kim, J.M. Bacteroides fragilis enterotoxin upregulates heme oxygenase-1 in dendritic cells via reactive oxygen species-, mitogen-activated protein kinase-, and Nrf2-dependent pathway. World J. Gastroenterol. 2020, 26, 291–306. [Google Scholar] [CrossRef]
- Jeon, J.I.; Choi, J.H.; Lee, K.H.; Kim, J.M. Bacteroides fragilis Enterotoxin Induces Sulfiredoxin-1 Expression in Intestinal Epithelial Cell Lines Through a Mitogen-Activated Protein Kinases- and Nrf2-Dependent Pathway, Leading to the Suppression of Apoptosis. Int. J. Mol. Sci. 2020, 21, 5383. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Rho, D.J.; Jeon, J.I.; Kim, Y.J.; Woo, H.A.; Lee, Y.K.; Kim, J.M. Bacteroides fragilis Enterotoxin Upregulates Heme Oxygenase-1 in Intestinal Epithelial Cells via a Mitogen-Activated Protein Kinase- and NF-kappaB-Dependent Pathway, Leading to Modulation of Apoptosis. Infect. Immun. 2016, 84, 2541–2554. [Google Scholar] [CrossRef][Green Version]
- Roh, H.C.; Yoo, D.Y.; Ko, S.H.; Kim, Y.J.; Kim, J.M. Bacteroides fragilis enterotoxin upregulates intercellular adhesion molecule-1 in endothelial cells via an aldose reductase-, MAPK-, and NF-kappaB-dependent pathway, leading to monocyte adhesion to endothelial cells. J. Immunol. 2011, 187, 1931–1941. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.Y.; Park, J.H.; Lee, S.T.; Han, I.O.; Oh, E.S. The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells. Biochem. Biophys. Res. Commun. 2012, 417, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Grindel, B.J.; Martinez, J.R.; Pennington, C.L.; Muldoon, M.; Stave, J.; Chung, L.W.; Farach-Carson, M.C. Matrilysin/matrix metalloproteinase-7 (MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix. Biol. 2014, 36, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Matsuura, K.; Nakamura, R.; Kojima, A.; Konishi, M.; Akizawa, T. MMP-7 cleaves amyloid beta fragment peptides and copper ion inhibits the degradation. Biometals 2017, 30, 797–807. [Google Scholar] [CrossRef]
- Bebb, J.R.; Letley, D.P.; Thomas, R.J.; Aviles, F.; Collins, H.M.; Watson, S.A.; Hand, N.M.; Zaitoun, A.; Atherton, J.C. Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut 2003, 52, 1408–1413. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, C.A.; Chen, P.J.; Chiang, Y.C.; Chen, Y.L.; Mao, T.L.; Lin, H.W.; Lin Chiang, W.H.; Cheng, W.F. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem. J. 2012, 442, 293–302. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, F.; Zheng, Z.; Xiao, Z.; Yang, Q.; Gong, N.; Zhou, J.; Zuo, D.; Ai, J. MMP-7 affects peritoneal ultrafiltration associated with elevated aquaporin-1 expression via MAPK/ERK pathway in peritoneal mesothelial cells. J. Cell Mol. Med. 2021, 25, 6887–6898. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, C.A.; Drake, K.L.; Siddavatam, P.; Lawhon, S.D.; Nunes, J.E.; Gull, T.; Khare, S.; Everts, R.E.; Lewin, H.A.; Adams, L.G. Systems biology analysis of Brucella infected Peyer’s patch reveals rapid invasion with modest transient perturbations of the host transcriptome. PLoS ONE 2013, 8, e81719. [Google Scholar] [CrossRef]
- Haynes, A.; Ruda, F.; Oliver, J.; Hamood, A.N.; Griswold, J.A.; Park, P.W.; Rumbaugh, K.P. Syndecan 1 shedding contributes to Pseudomonas aeruginosa sepsis. Infect. Immun. 2005, 73, 7914–7921. [Google Scholar] [CrossRef] [PubMed]
- Henry-Stanley, M.J.; Hess, D.J.; Erlandsen, S.L.; Wells, C.L. Ability of the heparan sulfate proteoglycan syndecan-1 to participate in bacterial translocation across the intestinal epithelial barrier. Shock 2005, 24, 571–576. [Google Scholar] [CrossRef]
- Freissler, E.; Meyer auf der Heyde, A.; David, G.; Meyer, T.F.; Dehio, C. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell Microbiol. 2000, 2, 69–82. [Google Scholar] [CrossRef]
- Bobardt, M.D.; Saphire, A.C.; Hung, H.C.; Yu, X.; Van der Schueren, B.; Zhang, Z.; David, G.; Gallay, P.A. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 2003, 18, 27–39. [Google Scholar] [CrossRef]
- Han, J.; Shi, Y.; Willis, G.; Imani, J.; Kwon, M.Y.; Li, G.; Ayaub, E.; Ghanta, S.; Ng, J.; Hwang, N.; et al. Mesenchymal stromal cell-derived syndecan-2 regulates the immune response during sepsis to foster bacterial clearance and resolution of inflammation. FEBS J. 2021. [Google Scholar] [CrossRef]
- Jang, B.; Yun, J.H.; Choi, S.; Park, J.; Shin, D.H.; Lee, S.T.; Lee, W.; Oh, E.S. Tyrosine 51 residue of the syndecan-2 extracellular domain is involved in the interaction with and activation of pro-matrix metalloproteinase-7. Sci. Rep. 2019, 9, 10625. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Jeon, J.I.; Myung, H.S.; Kim, Y.J.; Kim, J.M. Bacteroides fragilis Enterotoxin Induces Formation of Autophagosomes in Endothelial Cells but Interferes with Fusion with Lysosomes for Complete Autophagic Flux through a Mitogen-Activated Protein Kinase-, AP-1-, and C/EBP Homologous Protein-Dependent Pathway. Infect. Immun. 2017, 85, e00420-17. [Google Scholar] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, J.I.; Lee, K.H.; Kim, J.M. Bacteroides fragilis Enterotoxin Upregulates Matrix Metalloproteinase-7 Expression through MAPK and AP-1 Activation in Intestinal Epithelial Cells, Leading to Syndecan-2 Release. Int. J. Mol. Sci. 2021, 22, 11817. https://doi.org/10.3390/ijms222111817
Jeon JI, Lee KH, Kim JM. Bacteroides fragilis Enterotoxin Upregulates Matrix Metalloproteinase-7 Expression through MAPK and AP-1 Activation in Intestinal Epithelial Cells, Leading to Syndecan-2 Release. International Journal of Molecular Sciences. 2021; 22(21):11817. https://doi.org/10.3390/ijms222111817
Chicago/Turabian StyleJeon, Jong Ik, Keun Hwa Lee, and Jung Mogg Kim. 2021. "Bacteroides fragilis Enterotoxin Upregulates Matrix Metalloproteinase-7 Expression through MAPK and AP-1 Activation in Intestinal Epithelial Cells, Leading to Syndecan-2 Release" International Journal of Molecular Sciences 22, no. 21: 11817. https://doi.org/10.3390/ijms222111817
APA StyleJeon, J. I., Lee, K. H., & Kim, J. M. (2021). Bacteroides fragilis Enterotoxin Upregulates Matrix Metalloproteinase-7 Expression through MAPK and AP-1 Activation in Intestinal Epithelial Cells, Leading to Syndecan-2 Release. International Journal of Molecular Sciences, 22(21), 11817. https://doi.org/10.3390/ijms222111817






