Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery
Abstract
:1. Introduction
2. Results
2.1. Functionalisation of Chitosan and Characterisation of the Hybrid Structure
2.2. In Vitro Degradation of the Hydrogel
2.3. Rheological Properties of Hybrid Hydrogels
2.4. Protein Release from Hybrid Hydrogels
2.5. Assessment of Cell Viability Seeded on/within Hybrid
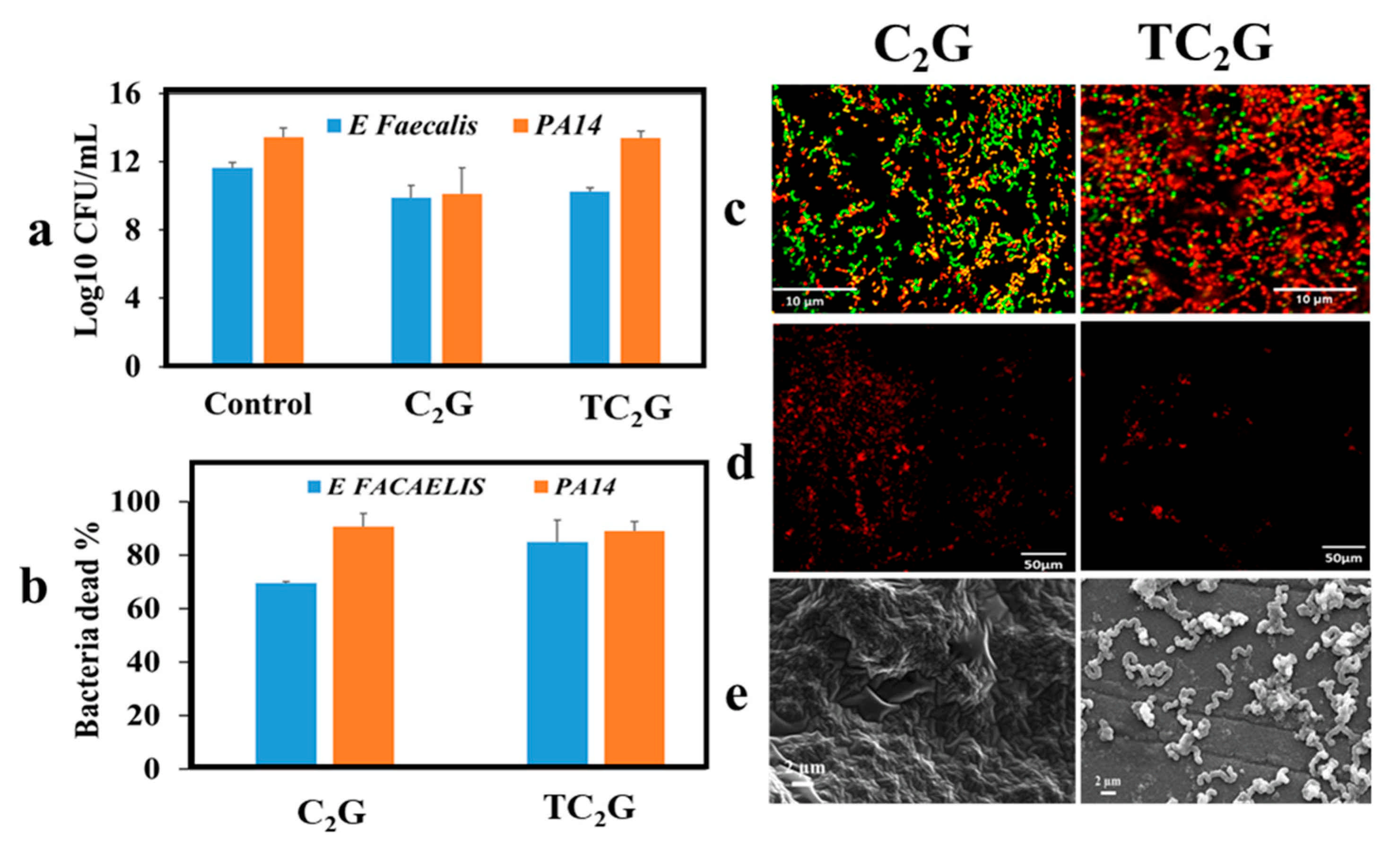
2.6. Assessment of Antibacterial Activities of Hybrid Hydrogels
3. Discussion
3.1. Hybrid Formation
3.2. Degradation Behaviour
3.3. Rheological Properties of Hybrid Hydrogels
3.4. Hybrid Hydrogels As a Drug Delivery System
3.5. Cell-Material Interactions
3.6. Antibacterial Activities of Hybrid Hydrogels
3.7. Limitations of Study and Future Works
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogels
4.2.1. Functionalisation of Chitosan
4.2.2. Synthesis of Glycerol-Modified Silane (GLMS) Precursors
4.2.3. Hydrogel Formation
4.3. Physio-Chemical Characterisation of Hydrogels
4.3.1. Chemical Characterization of Functionalised Polymer and Hydrogels by FTIR and 1H NMR
4.3.2. Rheological Behaviour of the Hydrogels
4.3.3. In Vitro Degradation of the Hydrogel
4.4. Protein Release from Hybrid Hydrogels
4.5. Assessment of Cell-Material Interactions
4.5.1. Culture of Osteoblasts (SaOs-2) with Hydrogels
4.5.2. Indirect Contact Method
4.5.3. Direct Contact Method
4.5.4. Live/Dead Assessment of Cell Encapsulated or Seeded on Hydrogels
4.5.5. Scanning Electron Microscopy (SEM) of Hydrogels Containing Cells
4.6. Antibacterial Effects of Hydrogels
4.6.1. Colony-Forming Units
4.6.2. Adherence Assay
4.6.3. Live/Dead Viability Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silber, J.S.; Anderson, D.G.; Daffner, S.D.; Brislin, B.T.; Leland, J.M.; Hilibrand, A.S.; Vaccaro, A.R.; Albert, T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 2003, 28, 134–139. [Google Scholar] [CrossRef] [PubMed]
- VandeVord, P.J.; Nasser, S.; Wooley, P.H. Immunological responses to bone soluble proteins in recipients of bone allografts. J. Orthop. Res. 2005, 23, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A.; Greenwald, M.A.; Grossi, P. Transmission of Infection with Human Allografts: Essential Considerations in Donor Screening. Clin. Infect. Dis. 2012, 55, 720–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Namnam, N.; Jayash, S.N. Recent advances in bone graft substitute for oral and maxillofacial applications: A review. Int. J. Biosci. 2019, 15, 70–94. [Google Scholar]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Xue, K.; Wang, X.; Yong, P.W.; Young, D.J.; Wu, Y.-L.; Li, Z.; Loh, X.J. Hydrogels as Emerging Materials for Translational Biomedicine. Adv. Ther. 2019, 2, 1800088. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical per-iodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E.; Leijten, J.; Xu, Q.; Khademhosseini, A. The matrix reloaded: The evolution of regenerative hydrogels. Mater. Today 2016, 19, 190–196. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydro-gel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lee, T.-J.; Hsiao, C.-S.; Chen, S.-Y.; Liu, D.-M. Characterization and drug release behavior of chip-like am-phiphilic chitosan–silica hybrid hydrogel for electrically modulated release of ethosuximide: An in vitro study. J. Mater. Chem. 2011, 21, 16077–16085. [Google Scholar] [CrossRef]
- Parlato, M.; Reichert, S.; Barney, N.; Murphy, W.L. Poly (ethylene glycol) hydrogels with adaptable mechanical and deg-radation properties for use in biomedical applications. Macromol. Biosci. 2014, 14, 687–698. [Google Scholar] [CrossRef]
- Metters, A.; Anseth, K.; Bowman, C. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymers 2000, 41, 3993–4004. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (eth-ylene glycol) hydrogels. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Otvos, L., Jr.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical appli-cations. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Tabari, P.M.-; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Wang, D.; Romer, F.; Connell, L.; Walter, C.; Saiz, E.; Yue, S.; Lee, P.D.; McPhail, D.S.; Hanna, J.V.; Jones, J.R. Highly flexible silica/chitosan hybrid scaffolds with oriented pores for tissue regeneration. J. Mater. Chem. B 2015, 3, 7560–7576. [Google Scholar] [CrossRef] [Green Version]
- Mondal, D. Covalently Crosslinked Organic/Inorganic Hybrid Biomaterials for Bone Tissue Engineering Applications. Ph.D. Thesis, Western University, London, ON, Canada, 2018. [Google Scholar]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Murphy, C.; O’Brien, F.; Little, D.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Poologasundarampillai, G.; Yu, B.; Tsigkou, O.; Valliant, E.; Yue, S.; Lee, P.; Hamilton, R.; Stevens, M.; Kasuga, T.; Jones, J. Bioactive silica–poly (γ-glutamic acid) hybrids for bone regeneration: Effect of covalent coupling on dissolution and me-chanical properties and fabrication of porous scaffolds. Soft Matter 2012, 8, 4822–4832. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Yu, B.; Tsigkou, O.; Wang, D.; Romer, F.; Bhakhri, V.; Giuliani, F.; Stevens, M.M.; McPhail, D.S.; Smith, M.E.; et al. Poly(γ-glutamic acid)/Silica Hybrids with Calcium Incorporated in the Silica Network by Use of a Calcium Alkoxide Precursor. Chem.—A Eur. J. 2014, 20, 8149–8160. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Balcioglu, S.; Ulu, A.; Gurses, C. Biomedical applications of hybrid polymer composite materials. In Hybrid Polymer Composite Materials; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 343–408. [Google Scholar]
- Petit, L.; Bouteiller, L.; Brûlet, A.; Lafuma, A.F.; Hourdet, D. Responsive Hybrid Self-Assemblies in Aqueous Media. Langmuir 2007, 23, 147–158. [Google Scholar] [CrossRef]
- Lin, W.-C.; Fan, W.; Marcellan, A.; Hourdet, D.; Creton, C. Large strain and fracture properties of poly (dimethylacryla-mide)/silica hybrid hydrogels. Macromolecules 2010, 43, 2554–2563. [Google Scholar] [CrossRef]
- Kim, K.J.; Joe, Y.A.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Cho, D.-W.; Rhie, J.W. Silica nanoparticles increase human adipose tissue-derived stem cell proliferation through ERK1/2 activation. Int. J. Nanomed. 2015, 10, 2261. [Google Scholar] [CrossRef] [Green Version]
- Shie, M.-Y.; Ding, S.-J.; Chang, H.-C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Sreenivas, S.; Pai, K. Thiolated Chitosans: Novel Polymers for Mucoadhesive Drug Delivery—A Review. Trop. J. Pharm. Res. 2008, 7, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Andreas, B.; Hornof, M.; Zoidl, T. Thiolated polymers–thiomers: Modification of chitosan with 2-iminothiolane. Int. J. Pharm 2003, 260, 229–237. [Google Scholar]
- Langoth, N.; Guggi, D.; Pinter, Y.; Andreas, B. Thiolated chitosan: In vitro evaluation of its permeation enhancing properties. J. Control. Rel. 2004, 94, 177. [Google Scholar]
- M Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Poologasundarampillai, G.; Ionescu, C.; Tsigkou, O.; Murugesan, M.; Hill, R.G.; Stevens, M.M.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Synthesis of bioactive class II poly(γ-glutamic acid)/silica hybrids for bone regeneration. J. Mater. Chem. 2010, 20, 8952–8961. [Google Scholar] [CrossRef] [Green Version]
- Poologasundarampillai, G.; Yu, B.; Jones, J.; Kasuga, T. Electrospun silica/PLLA hybrid materials for skeletal regeneration. Soft. Matter. 2011, 7, 10241–10251. [Google Scholar] [CrossRef]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Bounor-Legaré, V.; Ferreira, I.; Verbois, A.; Cassagnau, P.; Michel, A. New transesterification between ester and alkoxysilane groups: Application to ethylene-co-vinyl acetate copolymer crosslinking. Polymers 2002, 43, 6085–6092. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A.O. Encapsulation of Biologicals within Silicate, Siloxane, and Hybrid Sol−Gel Polymers: An Efficient and Generic Approach. J. Am. Chem. Soc. 1998, 120, 8587–8598. [Google Scholar] [CrossRef]
- Da Silva, S.; de Albuquerque, N.; de Almeida, R.; de Abreu, F. Synthesis and charaterization of silica-based aldehyde chitosan hybrid material for biodiesel purification. Materials 2017, 10, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, T.; Moreira, C.D.; Costa-Júnior, E.S.; Pereira, M.M. In vitro degradation of chitosan composite foams for biomedical applications and effect of bioactive glass as a crosslinker. Biomed. Glasses 2018, 4, 45–56. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.; Rabea, E.I.; Taktak, N.E. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydr. Polym. 2014, 111, 670–682. [Google Scholar] [CrossRef]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mater. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef]
- Connell, L.S.; Romer, F.; Suárez, M.; Valliant, E.M.; Zhang, Z.; Lee, P.D.; Smith, M.E.; Hanna, J.V.; Jones, J.R. Chemical characterisation and fabrication of chitosan–silica hybrid scaffolds with 3-glycidoxypropyl trimethoxysilane. J. Mater. Chem. B 2014, 2, 668–680. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Razavi, S.M.A.; Mikkonen, K.S. Novel nanobiocomposite hydrogels based on sage seed gum-laponite: Physico-chemical and rheological characterization. Carbohydr. Polym. 2018, 192, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kadib, A.; Bousmina, M. Chitosan bio-based organic–inorganic hybrid aerogel microspheres. Chem.–A Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Organic–inorganic hybrid of chitosan/organoclay bionanocomposites for hexavalent chromium uptake. J. Colloid Interface Sci. 2011, 361, 509–520. [Google Scholar] [CrossRef]
- Ebisike, K.; Okoronkwo, A.E.; Alaneme, K.K. Synthesis and characterization of Chitosan–silica hybrid aerogel using sol-gel method. J. King Saud Univ.—Sci. 2020, 32, 550–554. [Google Scholar] [CrossRef]
- Park, J.-U.; Jeong, S.-H.; Song, E.-H.; Song, J.; Kim, H.-E.; Kim, S. Acceleration of the healing process of full-thickness wounds using hydrophilic chitosan–silica hybrid sponge in a porcine model. J. Biomater. Appl. 2018, 32, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Ma, I.W.; Ammar, S.; Bashir, S.; Selvaraj, M.; Assiri, M.A.; Ramesh, K.; Ramesh, S. Preparation of Hybrid Chitosan/Silica Composites via Ionotropic Gelation and Its Electrochemical Impedance Studies. Prog. Org. Coat. 2020, 145, 105679. [Google Scholar] [CrossRef]
- Anitha, A.; Deepa, N.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohydr. Polym. 2011, 83, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-L.; Su, Y.-H.; Lai, J.-Y. In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using γ-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymers 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Chao, A.-C. Preparation of porous chitosan/GPTMS hybrid membrane and its application in affinity sorption for tyrosinase purification with Agaricus bisporus. J. Membr. Sci. 2008, 311, 306–318. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Costa, M.A.; Fernandes, M.H. Physical, chemical and in vitro biological profile of chitosan hybrid membrane as a function of organosiloxane concentration. Acta Biomater. 2009, 5, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.G.; Karuppannan, R.S.; Kariduraganavar, M.Y. Development of Hybrid Membranes Using Chitosan and Silica Precursors for Pervaporation Separation of Water + Isopropanol Mixtures. J. Chem. Eng. Data 2010, 55, 2084–2092. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef]
- Depan, D.; Kumar, B.; Singh, R.P. Preparation and characterization of novel hybrid of chitosan-g-PDMS and sodium montmorrilonite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 184–190. [Google Scholar] [CrossRef]
- Toskas, G.; Cherif, C.; Hund, R.-D.; Laourine, E.; Mahltig, B.; Fahmi, A.; Heinemann, C.; Hanke, T. Chitosan(PEO)/silica hybrid nanofibers as a potential biomaterial for bone regeneration. Carbohydr. Polym. 2013, 94, 713–722. [Google Scholar] [CrossRef]
- Gabrielli, L.; Connell, L.; Russo, L.; Jiménez-Barbero, J.; Nicotra, F.; Cipolla, L.; Jones, J.R. Exploring GPTMS reactivity against simple nucleophiles: Chemistry beyond hybrid materials fabrication. RSC Adv. 2014, 4, 1841–1848. [Google Scholar] [CrossRef]
- Gabrielli, L.; Russo, L.; Poveda, A.; Jones, J.R.; Nicotra, F.; Jiménez-Barbero, J.; Cipolla, L. Epoxide Opening versus Silica Condensation during Sol-Gel Hybrid Biomaterial Synthesis. Chem.—A Eur. J. 2013, 19, 7856–7864. [Google Scholar] [CrossRef]
- Wang, D.; Liu, W.; Feng, Q.; Dong, C.; Liu, Q.; Duan, L.; Huang, J.; Zhu, W.; Li, Z.; Xiong, J.; et al. Effect of inorganic/organic ratio and chemical coupling on the performance of porous silica/chitosan hybrid scaffolds. Mater. Sci. Eng. C 2017, 70, 969–975. [Google Scholar] [CrossRef]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.; Smidsrød, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-induced degradation of chitosan: The characterisation of degraded chi-tosan scaffolds. J. Tissue Repair Regen. 2017, 1, 12. [Google Scholar]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Shirosaki, Y.; Hirai, M.; Hayakawa, S.; Fujii, E.; Lopes, M.A.; Santos, J.D.; Osaka, A. Preparation and in vitro cytocompatibility of chitosan—Siloxane hybrid hydrogels. J. Biomed. Mater. Res. Part A 2015, 103, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, Y.; Wang, L.; Ge, X.; Fu, M.; Wang, P.; Wang, Q. High-Strength Nanocomposite Hydrogels with Swelling-Resistant and Anti-Dehydration Properties. Polymers 2018, 10, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanov, I.; Hinojosa-Caballero, D.; Maspoch, S.; Hoyo, J.; Tzanov, T. Enzymatic synthesis of a thiolated chitosan-based wound dressing crosslinked with chicoric acid. J. Mater. Chem. B 2018, 6, 7943–7953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, L.; Chen, X.; Chen, W. Rheological Characterization of in Situ Crosslinkable Hydrogels Formulated from Oxidized Dextran and N-Carboxyethyl Chitosan. Biomacromolecules 2007, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Hou, Q.; Paul, A.; Shakesheff, K.M. Injectable scaffolds for tissue regeneration. J. Mater. Chem. 2004, 14, 1915–1923. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.L.; Gil, M.H. Rheological Study of Genipin Cross-Linked Chitosan Hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [Green Version]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.; Hoemann, C.; Leroux, J.; Atkinson, B.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Alghooneh, A.; Razavi, S.M.A.; Behrouzian, F. Rheological characterization of hydrocolloids interaction: A case study on sage seed gum-xanthan blends. Food Hydrocoll. 2017, 66, 206–215. [Google Scholar] [CrossRef]
- Vigilato, M.Á.; Horn, M.; Martins, V.C.A.; Plepis, A.M.D.G. Rheological study of gels based on chitosan and carbon nanotubes. Braz. J. Therm. Anal. 2015, 4, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.Y.; Chalisserry, E.P.; Kim, M.H.; Kang, H.W.; Choi, I.-W.; Nam, S.Y. The Influence of Astaxanthin on the Proliferation of Adipose-derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels. Materials 2019, 12, 2416. [Google Scholar] [CrossRef] [Green Version]
- Karvinen, J.; Ihalainen, T.O.; Calejo, M.T.; Jönkkäri, I.; Kellomäki, M. Characterization of the microstructure of hydrazone crosslinked polysaccharide-based hydrogels through rheological and diffusion studies. Mater. Sci. Eng. C 2019, 94, 1056–1066. [Google Scholar] [CrossRef]
- Prasanth Koppolu, B.; Smith, S.G.; Ravindranathan, S.; Jayanthi, S.; Kumar, T.K.S.; Zaharoff, D.A. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 2014, 35, 4382–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-based nanoparticles for protein delivery: Design, strategies and applications. J. Mater. Chem. B 2016, 4, 4060–4071. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Rafailovich, M.; Sokolov, J.; Xu, D.; Yang, N.L.; McLeod, K. Fibronectin fibrillogenesis on sulfonated poly-styrene surfaces. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 64, 684–692. [Google Scholar]
- Jeworrek, C.; Hollmann, O.; Steitz, R.; Winter, R.; Czeslik, C. Interaction of IAPP and Insulin with Model Interfaces Studied Using Neutron Reflectometry. Biophys. J. 2009, 96, 1115–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayash, S.N.; Hashim, N.M.; Misran, M.; Baharuddin, N. Formulation and in vitro and in vivo evaluation of a new osteo-protegerin–chitosan gel for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 398–407. [Google Scholar] [CrossRef]
- Jahromi, M.Z.; Ranjbarian, P.; Shiravi, S. Cytotoxicity Evaluation of Iranian Propolis and Calcium Hydroxide on Dental Pulp Fibroblasts. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 130–133. [Google Scholar] [CrossRef]
- Cai, Y.; Che, J.; Yuan, M.; Shi, X.; Chen, W.; Yuan, W.-E. Effect of glycerol on sustained insulin release from PVA hydrogels and its application in diabetes therapy. Exp. Ther. Med. 2016, 12, 2039–2044. [Google Scholar] [CrossRef] [Green Version]
- Puri, S.; Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Drug incorporation and release of water soluble drugs from novel functionalised poly(glycerol adipate) nanoparticles. J. Control. Release 2008, 125, 59–67. [Google Scholar] [CrossRef]
- McKinnon, D.D.; Domaille, D.W.; Cha, J.N.; Anseth, K.S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 2014, 26, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Palma, P.; Matos, S.; Ramos, J.; Guerra, F.; Figueiredo, M.; Kauser, J. New formulations for space provision and bone re-generation. Biodental Eng. 2010, I, 71–76. [Google Scholar]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines 2019, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Borgwardt, L.; Høiby, N.; Wu, H.; Sørensen, T.S.; Borgwardt, A. Prosthesis infections after orthopedic joint re-placement: The possible role of bacterial biofilms. Orthop. Rev. 2013, 5, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Sun, J.; Lu, Y.; Wu, T.; Pang, J.; Hu, Y. In situ self-assembly chitosan/ε-polylysine bionanocomposite film with enhanced antimicrobial properties for food packaging. Int. J. Biol. Macromol. 2019, 132, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Sousa, D.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.A.; Chen, Y.; Guo, K.; Zhang, Z.; Brennan, J.D. Sugar-modified silanes: Precursors for silica monoliths. J. Mater. Chem. 2004, 14, 1469–1479. [Google Scholar] [CrossRef]
- Brook, M.A.; Chen, Y.; Guo, K.; Zhang, Z.; Jin, W.; Deisingh, A.; Cruz-Aguado, J.; Brennan, J.D. Proteins Entrapped in Silica Monoliths Prepared from Glyceroxysilanes. J. Sol-Gel Sci. Technol. 2004, 31, 343–348. [Google Scholar] [CrossRef]
- Hartmann, S.; Brandhuber, D.; Hüsing, N. Glycol-Modified Silanes: Novel Possibilities for the Synthesis of Hierarchically Organized (Hybrid) Porous Materials. Acc. Chem. Res. 2007, 40, 885–894. [Google Scholar] [CrossRef]
- Pipattanawarothai, A.; Suksai, C.; Srisook, K.; Trakulsujaritchok, T. Non-cytotoxic hybrid bioscaffolds of chitosan-silica: Sol-gel synthesis, characterization and proposed application. Carbohydr. Polym. 2017, 178, 190–199. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. In situ gel loaded with chitosan-coated simvastatin nanoparticles: Promising delivery for ef-fective anti-proliferative activity against tongue carcinoma. Marine Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Kurakula, M.; Naveen, N.R.; Patel, B.; Manne, R.; Patel, D.B. Preparation, optimization and evaluation of chitosan-based avanafil nanocomplex utilizing antioxidants for enhanced neuroprotective effect on PC12 cells. Gels 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Jayash, S.N.; Hashim, N.M.; Misran, M.; Baharuddin, N. In vitro evaluation of osteoprotegerin in chitosan for potential bone defect applications. PeerJ 2016, 4, e2229. [Google Scholar] [CrossRef] [PubMed]
- Jayash, S.N.; Hashim, N.M.; Misran, M.; Ibrahim, N.; AL-Namnam, N.M.; Baharuddin, N. Analysis on efficacy of chitosan-based gel on bone quality and quantity. Front. Mater. 2021, 8, 46. [Google Scholar] [CrossRef]
- ISO 10993–12. 2012 Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Trujillo, S.; Pérez-Román, E.; Kyritsis, A.; Gómez Ribelles, J.L.; Pandis, C. Organic–inorganic bonding in chitosan–silica hybrid networks: Physical properties. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1391–1400. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with algi-nate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Naveen, N.R.; Kurakula, M.; Gowthami, B. Process optimization by response surface methodology for preparation and evaluation of methotrexate loaded chitosan nanoparticles. Mater. Today Proc. 2020, 33, 2716–2724. [Google Scholar] [CrossRef]
- Koch, M.; Włodarczyk-Biegun, M.K. Faithful scanning electron microscopic (SEM) visualization of 3D printed alginate-based scaffolds. Bioprinting 2020, 20, e00098. [Google Scholar] [CrossRef]







| Hydrogel | Chitosan/Thiolated Chitosan (mg) | GPTMS (mg) | GLMS (mg) |
|---|---|---|---|
| TC1G/C1G | 17 | 6.25 | 100 |
| TC2G/C2G | 17 | 6.25 | 50 |
| TC10G/C10G | 17 | 6.25 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayash, S.N.; Cooper, P.R.; Shelton, R.M.; Kuehne, S.A.; Poologasundarampillai, G. Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery. Int. J. Mol. Sci. 2021, 22, 12267. https://doi.org/10.3390/ijms222212267
Jayash SN, Cooper PR, Shelton RM, Kuehne SA, Poologasundarampillai G. Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery. International Journal of Molecular Sciences. 2021; 22(22):12267. https://doi.org/10.3390/ijms222212267
Chicago/Turabian StyleJayash, Soher N., Paul R. Cooper, Richard M. Shelton, Sarah A. Kuehne, and Gowsihan Poologasundarampillai. 2021. "Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery" International Journal of Molecular Sciences 22, no. 22: 12267. https://doi.org/10.3390/ijms222212267
APA StyleJayash, S. N., Cooper, P. R., Shelton, R. M., Kuehne, S. A., & Poologasundarampillai, G. (2021). Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery. International Journal of Molecular Sciences, 22(22), 12267. https://doi.org/10.3390/ijms222212267