Abstract
Diabetes cardiomyopathy is one of the key factors of mortality among diabetic patients around the globe. One of the prior contributors to the progression of diabetic cardiomyopathy is cardiac mitochondrial dysfunction. The cardiac mitochondrial dysfunction can induce oxidative stress in cardiomyocytes and was found to be the cause of majority of the heart morphological and dynamical changes in diabetic cardiomyopathy. To slow down the occurrence of diabetic cardiomyopathy, it is crucial to discover therapeutic agents that target mitochondrial-induced oxidative stress. Flavonoid is a plentiful phytochemical in plants that shows a wide range of biological actions against human diseases. Flavonoids have been extensively documented for their ability to protect the heart from diabetic cardiomyopathy. Flavonoids’ ability to alleviate diabetic cardiomyopathy is primarily attributed to their antioxidant properties. In this review, we present the mechanisms involved in flavonoid therapies in ameliorating mitochondrial-induced oxidative stress in diabetic cardiomyopathy.
1. Introduction
Diabetes mellitus (DM) is one of the most deadly non-communicable diseases that leads to extensive impairments of organs and body functions. The increasing incidence of DM and its related complications have contributed to the surge of morbidity and mortality rate. DM affects about 463 million people aged between 20 to 79 years in 2019, and this figure is expected to climb up to 700 million by 2045 [1]. Moreover, DM is also one of the root causes for the development of cardiovascular diseases (CVD), which further exacerbate the mortality risk among patients with DM [2]. One of the complications resulting from chronic DM is diabetic cardiomyopathy (DCM). DCM is a cardiac pathological condition in patients with DM characterized by the appearance of aberrant myocardial morphology and cardiac functions in the truancy of other factors, such as coronary artery disease, hypertension, and prominent valvular disease [3]. Due to DCM, patients with DM are more likely to suffer from heart failure compared to their healthy counterparts [3]. This is why DCM is one of the most devastating consequences directly caused by DM.
The heart has a high energy consumption in order to efficiently pump and supply blood throughout the body. Hence, it has a high density of mitochondria population to fuel its activities. However, this dependence exposes the heart to deleterious consequences when mitochondrial malfunction occurs. Mitochondria serve a critical part in oxygen metabolism, hence it is crucial to understand the effects of their dysfunction in patients suffering from metabolic disorders, particularly diabetes [4]. In DCM, the minimal glucose utilization will shift to fatty acid, leading to energetic inefficiency [5]. Since the mitochondria lost its efficiency in energy production, mitochondrial dysfunction will then follow. The role of mitochondrial dysfunction in the progression of DCM has been well established in earlier studies [6,7,8]. As the heart contains a high amount of mitochondria, cardiac mitochondrial dysfunction can lead to cardiac oxidative stress which aggravates the development of DCM. Indeed, the diabetic patients heart mitochondria are typically found to have deteriorated in number and structure, exhibiting increased reactive oxygen species (ROS) emission, and compromised mitochondrial respiratory capacity in the mitochondria [9]. Thus, treatment targeting mitochondrial-induced oxidative stress is very crucial in suppressing DCM.
For more than 40 years, the pathogenesis and mechanisms involved in DCM’s development and progression has been well-studied and documented as well as of its preventive measures and potential therapeutic agents. Despite this, effective remedies for preventing and treating DCM remains unclear [10]. The need of having a treatment for DCM is of utmost importance considering that there is no specific treatment targeting DCM up to the moment [11,12]. Presently, diabetes management is only based on combination of lifestyle modification and therapeutic medications to regulate blood glucose level through glucose-lowering agents or insulin replacement therapy as well as with management of cardiovascular complications [12,13].
Recently, extensive efforts have been invested in studying the use of natural compounds to treat DCM. One of the candidates is flavonoids, which are plant-based polyphenolic compounds found in abundance in some fruits, vegetables, and herbal plants. They have been reported to exert many therapeutic effects against various pathologic conditions, such as cancer, muscle atrophy, inflammation, microbial infection, oxidative stress as well as DM [14,15,16,17,18,19]. These therapeutic effects are mainly mediated through radical scavenging, antioxidant, and anti-inflammatory properties [20,21,22]. Furthermore, flavonoids have gained recognition for their cardioprotective capabilities. Flavonoids have been proven to attenuate the progression of DCM via mitochondrial protection, thereby shielding cardiomyocytes against mitochondrial-induced oxidative stress [23,24,25]. Since flavonoids had showed potentials in alleviating cardiac dysfunction, we sought to review the therapeutic approach of flavonoids in ameliorating diabetic cardiomyopathy by targeting mitochondrial-induced oxidative stress.
2. Diabetic Cardiomyopathy
Rubler et al. [26] was the first to propose the concept of DCM, which has since become widely used in medicine. For decades, there has been an epidemiological relationship found between DM and the pathophysiology of heart failure. The prevalence of DCM is increasing simultaneously with the increased incidence of DM, and it is a main contributing factor to the pathophysiology of heart failure in DM patients [27]. DCM has a long latent phase during which the disease develops silently without observable symptoms. Upon comprehensive clinical investigation, patients may display increased in ventricular mass, substantial myocardial fibrosis, impaired cardiac cell signaling, and diastolic dysfunction which all are features of the early stage of DCM [3,5]. Patients with DCM typically start to exhibit symptoms as systolic function was exacerbated by diabetes-induced uncontrolled cardiac remodeling, which is usually permanent and irreversible, thus progressing towards heart failure [28,29].
Both type 1 (insulin dependent) and type 2 (non-insulin dependent) DM share the same feature, which is hyperglycemia that results from poor insulin action. Chronic hyperglycemia is indeed one of the major components that exacerbate the progression of cardiovascular complications in DM patients. In fact, a study showed that incidence of heart failure among DM patients rise by 8% with every 1% increase in glycated hemoglobin (HbA1c) level [30]. Oxidative stress is a contributing factor for DCM progression [31]. Uncontrolled and persistent hyperglycemia encourage excessive generation of reactive oxygen species (ROS) via several metabolic pathways; elevated glucose uptake through the polyol pathway, enhanced advanced glycation end products (AGEs) production, activation of protein kinase C (PKC) pathway, overactivation of hexosamine pathway and incapacitated antioxidant defense [32].
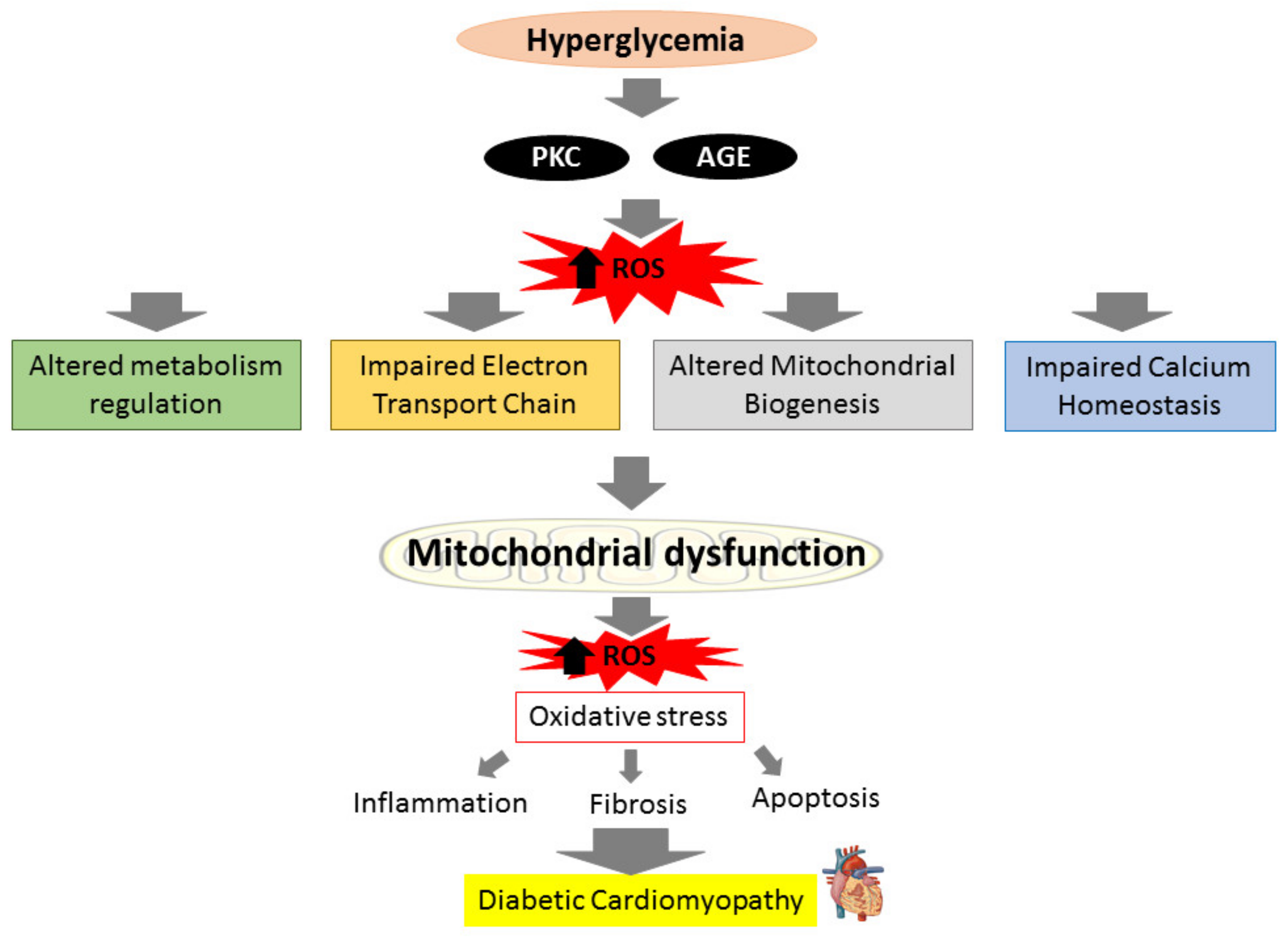
The heart’s energy demands are constantly high due to its need to maintain specialized cellular functions, and cardiomyocytes mitochondria generate more than 95% of their adenine triphosphate (ATP) by oxidative phosphorylation (OXPHOS) [33]. In DM condition, the insulin impairment and inability to utilize glucose in mitochondria will switch from glucose to fatty acid oxidation (FAO) to produce ATP in order to maintain sufficient ATP generation. This process however produces more ROS and causes the OXPHOS process to be disrupted [34]. The cytosolic ROS produced can promote mitochondrial dysfunction by attacking mitochondrial structure further. Upon breach in their structure, fragments of mitochondrial deoxyribonucleic acid (DNA) are released into the cytosol and triggers cardiac inflammation and stimulate the release of pro-inflammatory cytokines which exacerbate the inflammatory process that lead to more mitochondrial damage and loss of function [35,36]. Impaired mitochondria create more ROS, resulting in even worse oxidative damage [37]. ROS accumulation from both mitochondrial dysfunction and hyperglycemia-induced activation of metabolic pathways augments myocardial oxidative stress and aggravates mitochondrial dysfunction, resulting in cardiomyocyte death and the subsequent remodeling of the heart structure in effort to preserve the heart integrity and function. The heart’s ability to contract and relax efficiently is further harmed by uncontrolled and excessive cardiac fibrosis and hypertrophy that lead to irreversible cardiac structural changes, ultimately results in diastolic and systolic dysfunction in DCM [29,38]. The relationship between hyperglycemia and mitochondrial dysfunction towards the progression of DCM is illustrated in brief in Figure 1.
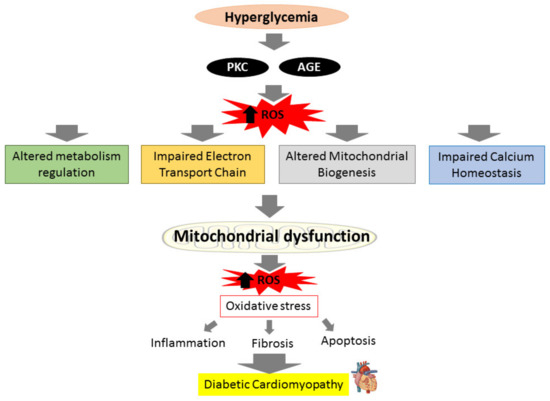
Figure 1.
Prolonged hyperglycemia can produce reactive oxygen species (ROS) via activation of protein kinase C (PKC) pathways and advanced glycation end products (AGEs) production, leading to altered metabolism regulation, altered mitochondrial biogenesis, impaired mitochondrial calcium handling, and impaired electron transport chain. These actions will cause mitochondrial to deteriorate and generate more ROS. The ROS produced results in oxidative stress, which can initiate inflammation, fibrosis, and apoptosis, causing diabetic cardiomyopathy (DCM).
3. Mitochondrial-Induced Oxidative Stress in Diabetic Cardiomyopathy
Mitochondrial dysfunction is the destruction of mitochondrial morphology, respiratory chain disruption, biogenic dysfunction, gene alterations, mitochondrial population deprivation, and alteration in the oxidative protein’s activity in cells [39]. Although ROS is the main by-product of oxygen metabolism by the mitochondria, hyperglycemic condition may also induce its excessive production. This results in the accumulation of the ROS, leading to mitochondrial oxidative damage that attack its protein, DNA, and lipid structures [40]. As mitochondrial DNA are lacking histone protection, it is very susceptible to oxidative damage which disrupts its respiratory chains and biogenesis [41]. Accumulation of ROS not only will disrupt the mitochondrial normal functions, but also induces the development of mitochondrial permeability transition pores (mPTP) that leads to the depolarization of the mitochondrial membrane and release of factors of cell death into the cytosol [42].
3.1. Altered Metabolic Regulation
Cardiomyocytes are high-energy-consuming cells with mitochondria as their primary source of energy supply. Mitochondria is a major organelle for glucose and fatty acid metabolism. Impaired mitochondrial activity can impede insulin signaling by meddling with acyl-CoA oxidation from fatty acid, diacylglycerol accumulation, PKC stimulation, AGEs production and ROS formation [43]. Under normal circumstances, β-oxidation accounts for around 70% of the energy supply in the heart, with the rest coming from the oxidation of other substances such as glucose, ketone bodies, lactate, and amino acids. [44]. It is worth bringing up that fatty acids, as energy metabolic sources, require around 12% more oxygen to generate the same proportion of ATP as glucose. Nevertheless, FAO increases while glucose oxidation decreases. In DCM patients, FAO is the primary source of ATP generation, which can result in increased oxygen demand and respiratory dysfunction in mitochondria. [45].
In DCM condition, the surge in serum fatty acid promotes an increase in fatty acid utilization and FAO. Hyperglycemic condition downregulate activation of 5′ adenosine monophosphate-activated protein kinase (AMPK) and causes reduction in peroxisome proliferator-activated receptor-gamma coactivator 1 (PGC-1) regulation. The FAO rate increases in conjunction with the decreased peroxisome proliferator-activated receptors (PPARs) activity, including PPARα activity where its activation is triggered by PGC-1. However, acyl-CoA overload generated from excess fatty acid can lead to excessive mitochondrial ROS generation [46]. Particularly, the production of byproduct from β-oxidation such as nicotinamide adenine dinucleotide (NAD) + hydrogen (H) (NADH) and flavin adenine dinucleotide (FADH2) are both increased in excess, leading to generation of ROS in the electron transport chain (ETC) [47]. Downregulation of the cardiac-specific manganese superoxide dismutase (MnSOD) or AMPK activity further elevates ROS production in the mitochondria, which allows mitochondrial disruptions and FAO [8,48].
Intriguingly, elevated free fatty acid uptake has been linked with the surge of uncoupling protein 3 (UCP3) in cardiac muscle, whose function is to facilitate anion transfer from inner to outer membrane of the mitochondria [49,50]. In cardiomyocytes, UCP3 is upregulated by increase circulating free fatty acids via activation of PPARα activation [51]. Even though UCP3 appears to specifically involve in encouraging fatty acid oxidation, it is indirectly influencing glucose homeostasis [52]. Subsequently, UCP3 reduced mitochondrial electrochemical gradient which further deprived ATP generation [53]. In addition, proton leak from the OXPHOS that triggered by increase of FAO also enhance the regulation of UCP3 as proton leakage is precisely regulated and be catalyzed or suppressed by UCP3 [54].
3.2. Impaired Electron Transport Chain (ETC)
There is tremendous data that mitochondrial ROS generation triggers the development of DCM. Indeed, patients with DM possess defective cardiac mitochondria, with increased hydrogen peroxide outflow, reduced respiratory capability, and elevated levels of oxidized proteins [9]. One of the primary machineries that produce excessive ROS under hyperglycemic condition is the ETC itself. ETC is the primary site of mitochondrial ATP generation within all cells particularly cardiomyocytes. The ETC is made up of protein complexes I, II, III, and IV as well as the electron transfer carriers, ubiquinone (Co-enzyme) and cytochrome C, and is where ATPs are being produced during OXPHOS. At the inner mitochondrial membrane (IMM), electrons from NADH and FADH2, the byproduct of β-oxidation, are transferred through the respiratory chain to oxygen, which is then reduced to water at complex IV [55]. The flow of protons into the intermembrane gap is fueled by this mechanism, which creates a proton gradient which generate mitochondrial membrane potential (ΔΨm) that promotes ATP production by the ATP synthase [56]. Due to the incomplete reduction of oxygen, some electrons (approximately 0.1%) that escape from ETC can induce superoxide (ROS) generation [57].
Past studies suggested superoxide generation from ETC as the primary event in hyperglycemia-induced mitochondrial dysfunction [58,59]. High glucose levels in the cell and glucose-depleted pyruvate boost respiration by raising the ETC’s capacity, leading to mitochondrial membrane hyperpolarization and superoxide generation [60]. Superoxide formation can occur when the electron flow is reduced, especially at the first three complexes, where flavins or quinones might operate as single electron donors [55]. The generation of ROS can also be triggered by the reverse electron flow via complex I [61]. Interestingly, the protection exerted when complex I or Il were inhibited implies that ETC superoxide generation occurs via reverse electron transfer during high glucose exposure. Furthermore, many treatments, such as inhibiting ETC complex II activity, uncoupling OXPHOS, upregulation of uncoupling protein-1, or MnSOD, can reduce hyperglycemia-induced ROS production [59].
Abnormalities in hyperglycemic condition caused by oxidation can escalate methylglyoxal adduct production and elevate O-linked β-N-acetylglucosaminylation (O-GlcNAcylation). These are examples of post-translational changes that lead to mitochondrial and systolic function deterioration [62]. Hyperglycemia disrupts the activity of the respiratory mechanisms in cardiac mitochondria and causes O-GlcNAcylation dysregulation [63]. In normal circumstances, the O-GlcNAc transferase (OGT) is found in the IMM and interacts with complex IV of ETC. This enzyme is poorly localized to the mitochondrial matrix in hyperglycemic condition, and thus the OGT-complex IV connection is disrupted, resulting in lowered complex IV activity and reduced ΔΨm [63]. O-GlcNAcylation of mitochondrial dynamics proteins, including mitochondrial dynamin-related protein 1 and optic atrophy gene 1 leads to mitochondrial rupture, perpetuating mitochondrial failure [64,65].
In DCM, ATP synthase activity is typically found to be impaired, which compromises mitochondrial function. Persistent hyperglycemia induces overexpression of mitochondrial calpain-1, a calcium-activated intracellular proteinase [66]. Calpain-1 was found to cleave ATP synthase, leading to reduced ATP synthase function which triggers excessive mitochondrial superoxide formation [67]. Its activation is thought to be mediated by nicotinamide adenine dinucleotide phosphate oxidase (NOX) subunit, gp91phox [68]. These findings highlight the relevance of ETC in mitochondria as a significant source in generating superoxide in DCM.
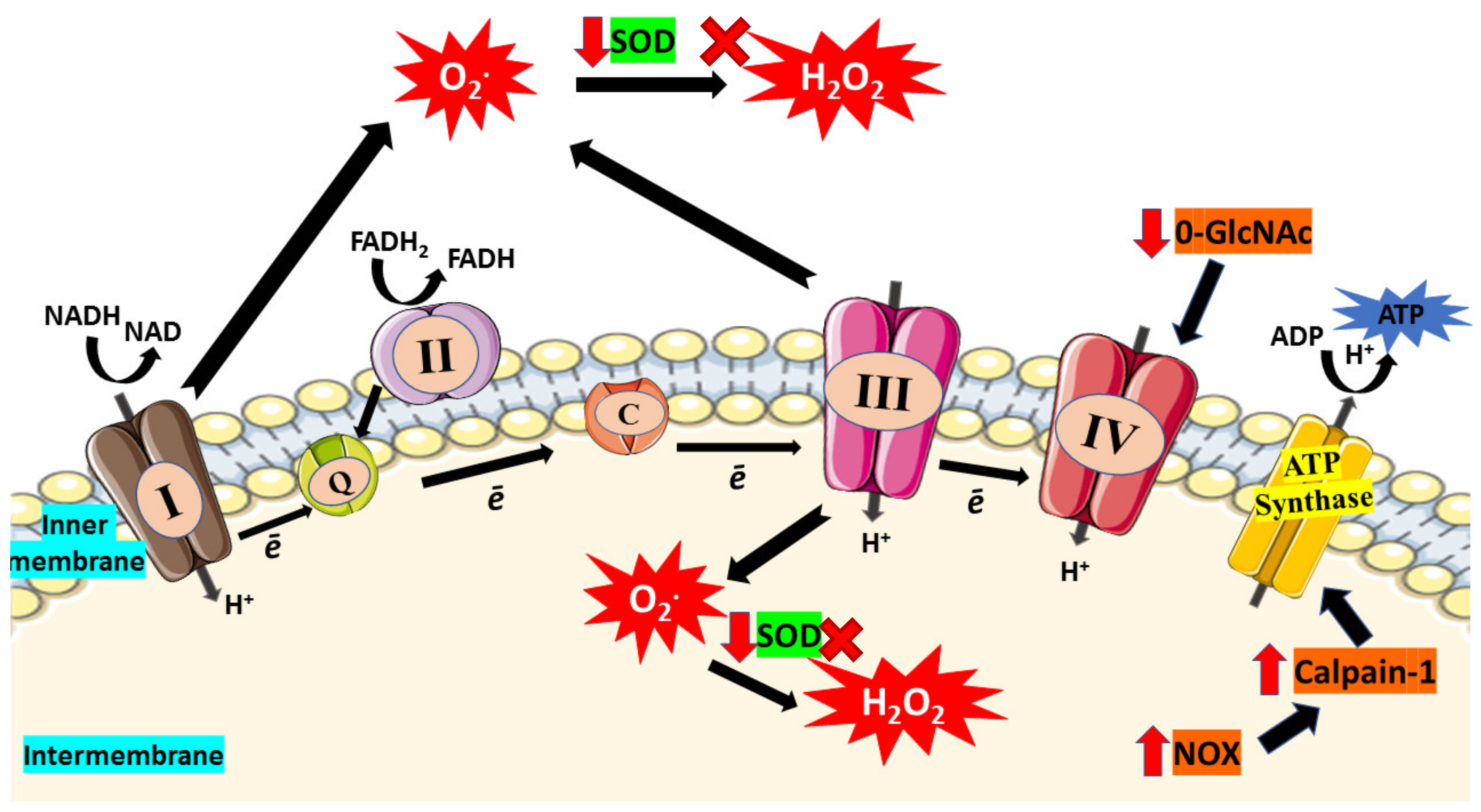
Apart from that, cardiolipin, a phospholipid in IMM, has been suggested to play a significant role in controlling energy generation by optimizing the IMM proteins and complexes activities involved in OXPHOS [69,70,71]. Hence, deterioration of cardiolipin structure can affect the ATP production in ETC. According to a study, streptozotocin-induced diabetic rodents displayed prominent changes in the interfibrillar of mitochondrial population, including depleted cardiolipin concentration and electron flow capacity [72]. This finding shows that depletion of cardiolipin leads to diminished electron flow capacity and consequently enhanced superoxide production from ETC. Besides, increased level of ROS can alter mitochondrial cardiolipin and leads to mitochondrial architecture disruption, including mitochondrial disintegration, cristae disruption, and swelling which have been observed in cardiomyocytes from diabetic hearts [73,74]. Figure 2 illustrated impaired ETC which then enhance oxidative stress in mitochondria.
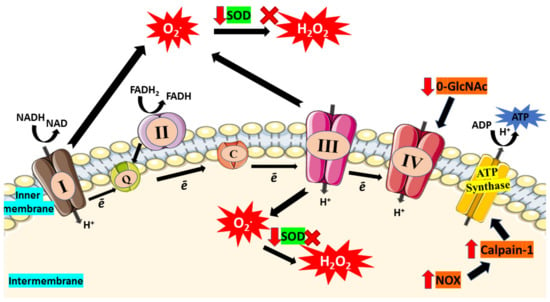
Figure 2.
In diabetic condition, hyperglycemia causes impairment of electron transport chain (ETC). Due to incomplete reduction of oxygen, electron (ē) will escape from the ETC and lead to superoxide (O2*) production. Furthermore, glucose-depletion in the mitochondria boost respiration and enhance ETC capacity which then enhances production of superoxide that leads to increased consumption of SOD. Reduction in SOD activity results in the accumulation of superoxides and reduced their conversion to hydrogen peroxide (H2O2). Moreover, elevated O-GlcNAc was found in hyperglycemic condition which then reduces the activity of complex IV. The activity of ATP synthase also declines as high glucose triggers NOX expression and further enhances expression of calpain-1, leading to ATP synthase cleavage and thus reduces the production of ATP. Red arrow indicates increase/decrease of level/activity; black arrow indicates flow of mechanisms in ETC; ‘x’ symbol indicates inhibition of H2O2 production.
3.3. Altered Mitochondrial Biogenesis
Mitochondrial biogenesis is a process by which the mitochondrial population in a cell multiplies. One of the factors that stimulate to the alterations in mitochondrial biogenesis, respiratory function, and/or lowered ATP production is diabetes. Hence, diabetes-induced impaired mitochondrial biogenesis will diminish mitochondrial function. In normal physiology, mitochondrial DNA transcription is triggered by AMPK and activated by the family PGC-1 proteins, where it is considered as the master regulator in mitochondrial biogenesis [75]. In contrast, insulin resistant uncoupling protein-diphtheria toxin a (UCP-DTA) transgenic mice showed elevation in PGC-1 expression consistent with the promotion of PPARα in the heart, whose function is known to activate metabolic genes in the heart [76]. This further confirms that PGC-1 plays a key role in mitochondrial biogenesis and that its reduced activity in diabetes condition suppresses mitochondrial biogenesis. In DCM, preliminary studies exhibited that hypoadiponectinemia impaired AMPK-PGC-1α signaling [77], more recently, in a model for type 2 DM with high fat diet, adiponectin was found to partial rescue mitochondrial biogenesis in cardiac cells, via PGC-1α-mediated signaling [78]. When mitochondrial biogenesis is disrupted, mitochondrial biogenesis is inhibited, hence ATP generation is hampered. As a result, mitochondrial ATP synthesis will rise, increasing the burden in the mitochondria, resulting in the production of ROS and oxidative damage.
3.4. Impaired Mitochondrial Calcium Homeostasis
One of the primary drivers for mitochondrial-induced oxidative stress has been identified to be impairment in mitochondrial calcium handling. Calcium homeostasis is important in the regulation of cellular metabolism, muscle contraction, and signal transduction [3]. Furthermore, mitochondria is an important organelle for calcium regulation and storage [79,80]. For ATP generation in mitochondria, a transmembrane potential gradient, also known as a proton (Ca2+) gradient, is required. A uniporter transports calcium down the concentration gradient, while a Na+/Ca2+ exchanger removes the accumulated calcium. Calcium uptake into a mitochondrion is necessary for Krebs cycle activation and ATP generation. Furthermore, calcium transport from the cytosol to the mitochondria is also responsible for regulating ATP supply and demand for cardiac function [81]. Calcium uptake in mitochondria may also function as a buffering system, eliminating local calcium and adjusting accumulated cytosolic calcium level, hence controlling the activity of calcium-dependent mitochondrial enzyme activities [82].
In hyperglycemic condition, excessive calcium loading in mitochondrial matrix can result in the opening of the mPTP complex with large amounts of Ca2+ released into the cytoplasm, ultimately leading to activation of apoptotic factors in cardiomyocytes [83,84]. mPTP is a known cause for mitochondrial swelling, equilibration of ionic gradient, and depletion of ΔΨm, and thus leading to the impairment of ATP production in ETC. Apart from that, cardiolipin in IMM is also vulnerable to free radical and has been demonstrated to play a key role in calcium maneuver and apoptosis [85]. In addition, PGC-1 is known to be involved in mitochondrial production and respiratory activity regulated by calcium-dependent mechanisms [86]. It has been shown to have a role in calcium signaling and calcium-mediated oxidative damage [87]. Therefore, it is plausible that impaired mitochondrial calcium handling adds to oxidative stress and disrupt energy homeostasis in DCM. The overall mechanisms of mitochondrial-induced oxidative stress in DCM were illustrated in Figure 3.
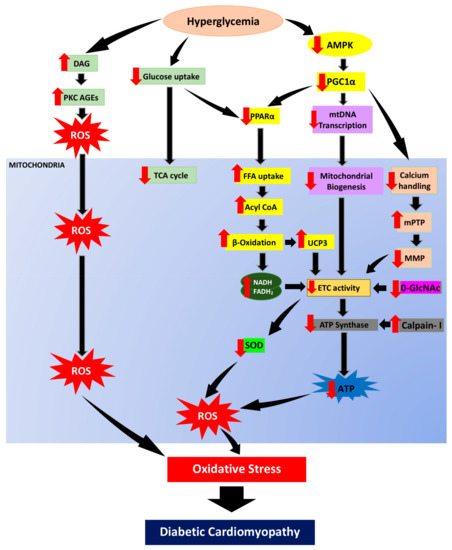
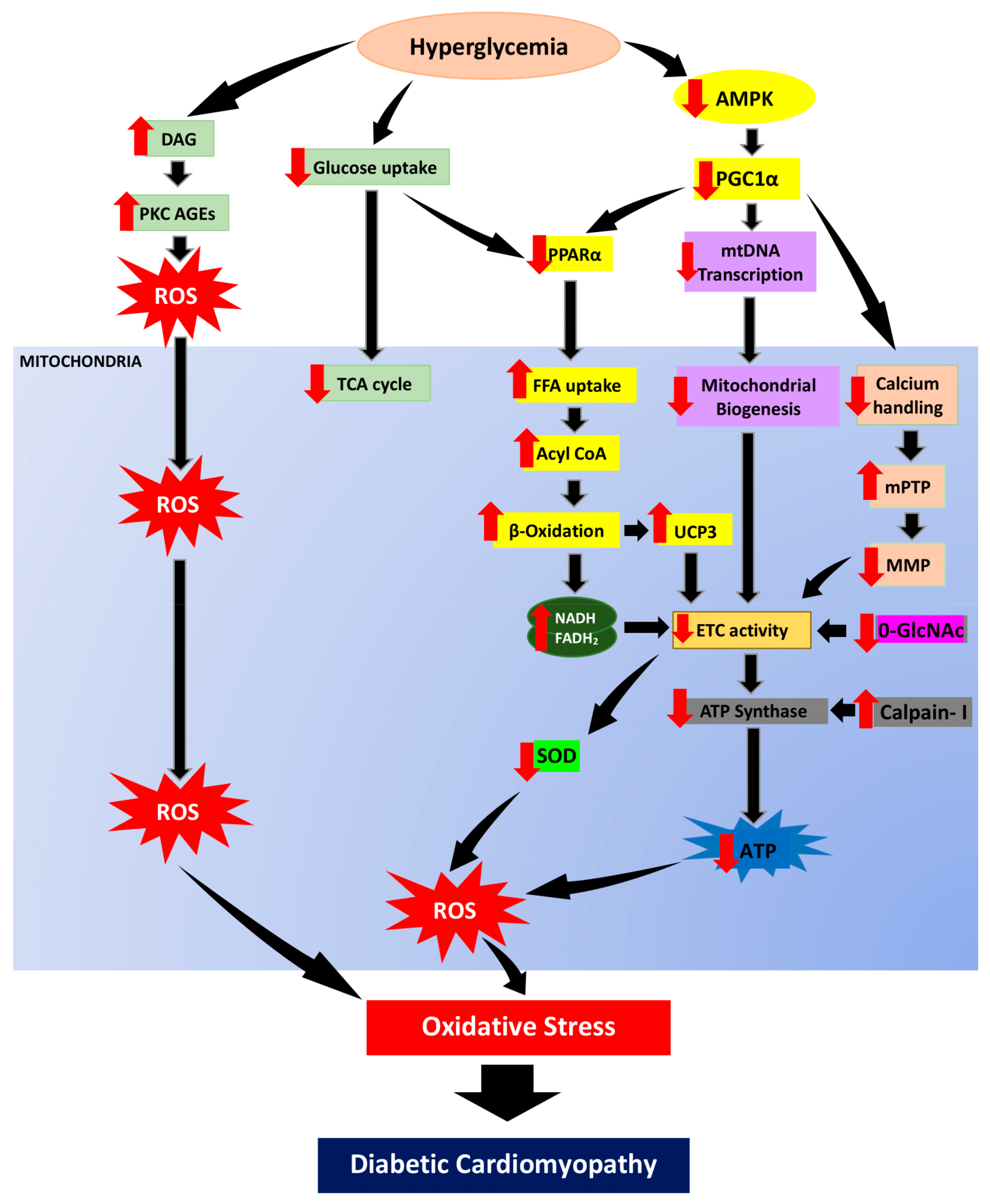
Figure 3.
In hyperglycemic condition, reduction of glucose uptake will suppress glucose oxidation. Hence, the energy metabolism will shift from glucose to fatty acid utilization. AMPK, regulator of energy homeostasis, will be downregulated hence suppressing PGC1α expression. PGC1α suppression downregulates PPARα and enhances free fatty acid uptake, acyl CoA as well as escalating β-oxidation. In parallel with that, the TCA cycle is also deprived. The increase of DAG resulting from persistent hyperglycemia enhances PKC activation and AGEs formation which then promote ROS generation. In addition, the surge of β-oxidation produce byproduct, NADH and FADH2 as well as enhanced UCP3 expression further cause reduction in electron transport chain activity. Furthermore, PGC1α suppression also leads to mitochondrial biogenesis impairment via reduction of mitochondrial transcription. PGC1α suppression will also cause poor calcium handling which then enhance mitochondrial permeability transition pore (mPTP) opening and diminishes mitochondrial membrane polarization. Enhanced β-oxidation, impaired of mitochondrial biogenesis and poor calcium handling will then cause reduction of ETC hence cause overproduction of superoxide and hydrogen peroxide as well as downregulation of O-GlcNAc. Calpain-1 activity enhancement cleaves and diminish ATP synthase activity which will cause reduction in ATP production. These mechanisms of mitochondrial dysfunction are the root to oxidative stress and consequently lead to diabetic cardiomyopathy development. Black arrow indicates flow of mechanisms involved; red arrow indicates increase/decrease of level/activity.
4. Therapeutic Role of Flavonoid in Alleviating Mitochondrial Dysfunction-Induced Oxidative Stress in Diabetic Cardiomyopathy
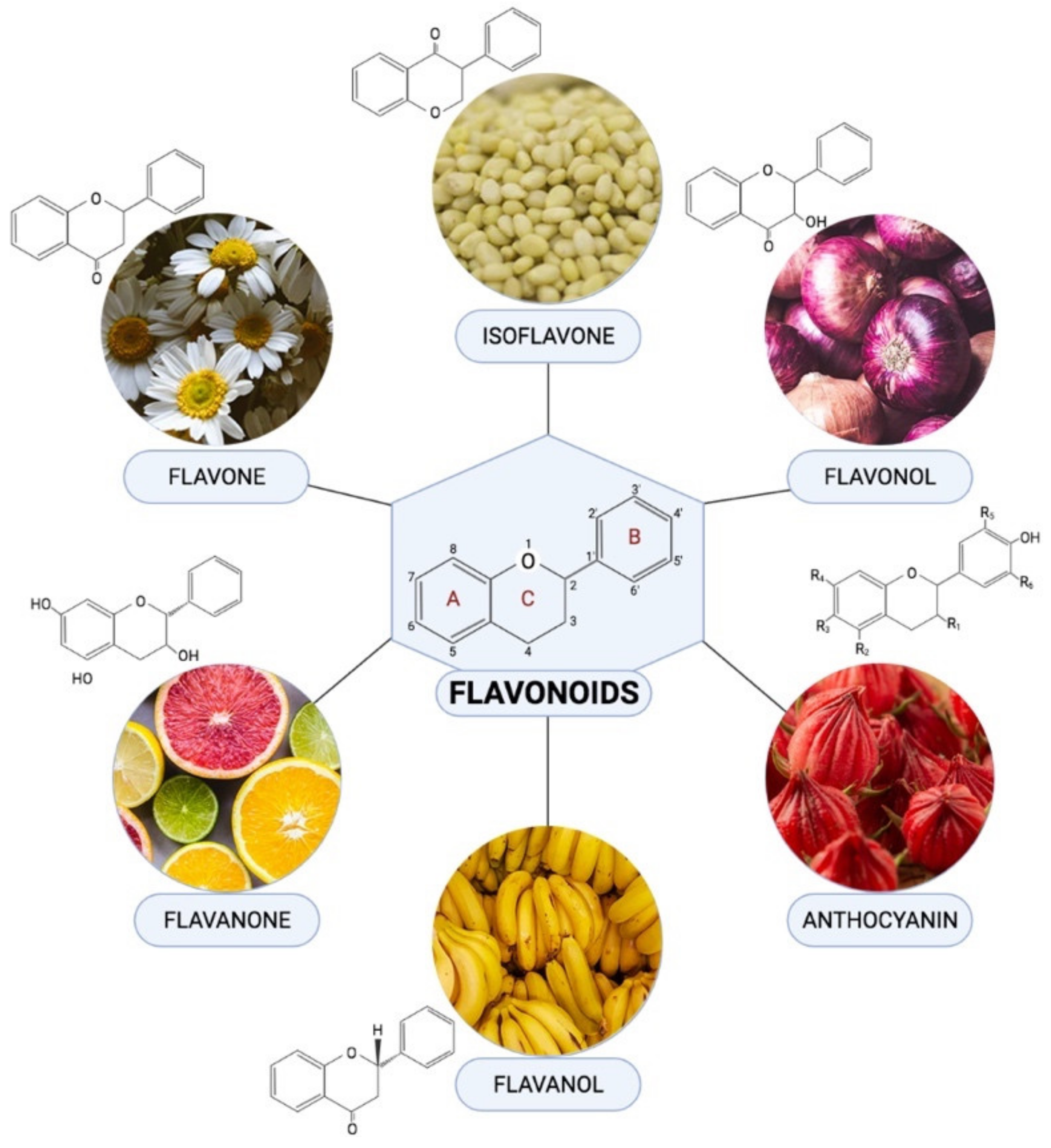
Flavonoids are one of the most diverse families of bioactive phytochemicals, with over 9000 different compounds. According to IUPAC Recommendations (2017), the term “flavonoid” refers to compounds that have the basic structure of phenyl-substituted propylbenzene derivatives with C15 skeleton, C16 skeleton, and flavonolignans with C6–C3 lignan precursors [88]. Flavonoids are divided into six subclasses; isoflavones, flavones, flavanols, flavonols, flavanones, and anthocyanins, are abundant in plants and their metabolic routes have been thoroughly explored using biochemical and molecular approaches [89,90]. Many plants, including pomelos, blueberries, roselle, oranges, grapefruit, lemons, and limes, are all rich in flavonoids [91].
Flavonoids have been demonstrated to alleviate pathological disorders, such as diabetes, cancer, obesity, and cardiovascular diseases. Flavonoids are abundant plant-based natural compounds with a good potential for medicinal and biological actions. These compounds showed the ability to exert anti-oxidative, anti-inflammatory, anti-fibrotic, and anti-apoptotic activities as reported previously [92,93,94]. Given the role of mitochondrial-induced oxidative stress in the progression of DCM, this review aims to summarize mechanisms of action of flavonoids in alleviating DCM by targeting mitochondrial-induced oxidative stress. Figure 4 demonstrated the chemical structure of flavonoid subclasses.
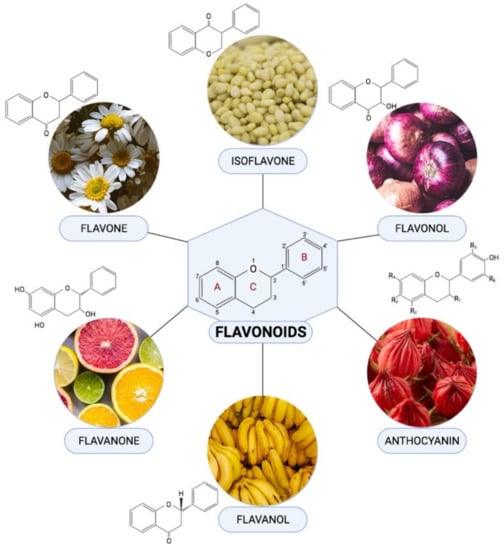
Figure 4.
Chemical structures and example of sources where they are found abundant in for each flavonoid subclasses.
4.1. Flavones
Flavone is one of the significant flavonoid subclasses. They are found as glycosides in celery, parsley, red peppers, mint, and ginkgo biloba. This group of flavonoids includes luteolin, apigenin, and tangeritin [95]. They have a double bond between positions 2 and 3 of the main C ring, as well as a ketone in position 4. The hydroxyl group at position 5 of the A ring is found in the majority of flavones from vegetables and fruits, but hydroxylation in other parts, most commonly in position 7 of the A ring or 3′ and 4′ of the B ring, varies depending on type of the vegetable or fruit [96].
Luteolin is one of the most prevalent flavones that can be found in variety of vegetables, fruits and herbs such as apple, cabbage, carrot, tea, and celery. A previous study by Yang and colleagues [97] which utilizes streptozotocin-induced diabetic rodents ischemia reperfusion model showed that luteolin treatment, at 100 mg/kg dose, was able to increase cardiac MnSOD and endothelium nitric oxide synthase (eNOS) expression as well as decrease Ca2+ induced mPTP opening and ΔΨm.
In addition, myricitrin, a flavone that can be found in abundance in berries and teas, has been proven to suppress high glucose-induced superoxide production in mitochondria, depolarization of mitochondrial membrane potential and restored mPTP formation in diabetic cardiomyopathy via in vitro study [98]. Another study has reported that flavonoid from Abroma augusta L. (Malvaceae) leaf extract containing predominantly rutin improved co-enzyme Q9 and Q10 levels in the mitochondria by acting as antioxidants through scavenging ROS and thereby inhibit lipid peroxidation [99].
4.2. Isoflavones
Isoflavones are phytocompounds with a chemical composition based on the 3-phenyl chromen-4-one backbone. Isoflavones are secondary plant metabolites extensively studied for its wide range of therapeutic effects, including antioxidant, chemopreventive, anti-inflammatory, anti-allergic, antibacterial, and cardioprotective effects [100,101]. The highest content of isoflavones is identified to be in roots and seeds. Other medicinal plants with high isoflavones content include red clover, dyer’s broom, lucerne, and sohphlang flax. Beside soy, other legumes such as lupin beans, kudzu, barley, and fava beans are rich in isoflavones [102,103]. The most important types of isoflavones are genistein, daidzein, glycitein, formononetin, biochanin A, and equol [104].
Isoflavone has been reported to alleviate mitochondrial-induced oxidative stress on DCM. Recently, Upadhayay et al. [24] reported that isoflavone was able to reduce mitochondrial-induced oxidative damage by reducing ROS generation in mitochondria and depolarization of mitochondrial membrane through silent information regulator 1 (SIRT-1) pathway or PPAR-α, which further attenuated mitochondrial dysfunction, thus conserving cardiomyocytes health. Besides, recent study conducted by Laddha and colleagues has confirmed that streptozotocin-induced type 1 diabetic rats were shown to maintain AMPK and SIRT-1 levels to normal levels whereby both activities are important in controlling free fatty acid uptake as well biogenesis in cardiac mitochondria [105].
However, studies made on the effects of isoflavones on cardiac diabetes model are rather meagre in number. Yet, we can still refer to its therapeutic effect on other cardiac pathology models as well. There are several studies that reported favorable effects of isoflavones on cardiac mitochondria. Recently, isoflavones was shown to give positive effects on mitochondria by alleviating the excessive mitochondria Ca2+ uptake in isolated heart [106]. Apart from that, isoflavones was also capable in improving disturbance in ΔΨm as well as reduction of intracellular ROS release, thus proving that isoflavone was able limit oxidative stress induced by mitochondria [107,108]. In addition, isoflavones also alleviate ΔΨm loss as well as curbing mPTP opening which exhibiting cardiac protective effect [108].
4.3. Flavonol
Flavonoids with a ketone group are known as flavonols. Flavonols can be found plentiful fruits and vegetables. Kaempferol, quercetin, myricetin, and fisetin are among the most widely studied flavonols and they can be found in abundant in common daily diet including in onions, kale, lettuce, apples, and berries. Flavonol consumption has been proved to a variety of health advantages, including antioxidant potential and a lower risk of cardiovascular disease. Flavonols have a hydroxyl group in position 3 of the C ring, which can be glycosylated, unlike flavones. Flavonols have a vast spectrum of methylation and hydroxylation forms, and they are the most prevalent and largest subclass of flavonoids in fruits and vegetables based on their many glycosylation patterns [109].
Flavonol has a vast potential in protecting heart mitochondria. Earlier study has revealed that flavonol was able to enhance mitochondrial biogenesis by increasing mitochondrial DNA content via upregulation of nuclear factor erythroid 2-related factor, Nrf-1, Nrf-2, and mitochondrial transcription factor A (TFAM) expression [110]. Furthermore, flavonol also was capable to improve complexes I, III, and IV activities as well as upregulate expression of UCP-2 and UCP-3 [110]. These findings show that flavonols have promising capability in protecting against cardiovascular disease development.
Indeed, previous study reported that flavanols found in Abroma augusta L. family of Malvaceae including rutin, myricetin, and quercetin have been proven to revive the ubiquinones (co-enzyme Q) function, which is important in electron carriers’ distribution within cell organelles chiefly and thus reduce ROS production in myocardial mitochondrial of T2DM [99]. In another cardiac study model, quercetin also was found to control free FAO by modulating phosphorylation of AMPK via upregulation of AMPKα2, PPARα, and PCG-1α genes where these genes are crucial in altered energy metabolism mechanisms [111,112]. The derivative of myricetin, dihydromyricetin, could boost mitochondrial performance in streptozotocin-induced diabetic rodents, thus reducing oxidative stress. In this study, the ATP levels and complexes Ι/ΙΙ/ΙΙΙ/ΙV maneuver in ETC as well as ΔΨm were enhanced when treated with dihydromyricetin in the cardiomyocytes [113].
Recently, Ni and colleagues [23] have demonstrated that flavonol icariin could upregulate Apelin, the gene in myocardium and the mitochondrial matrix gene Sirt3, hence elevates the ΔΨm and reduces mitochondria ROS production. Another study has shown that flavonol from quercetin could induce peroxiredoxin-3 (Prx-3) expression, a mitochondrial antioxidant, causing a significant decrease in myocardial biomarkers for mitochondrial uncoupling and redox stress, UCP3 protein expression, and reduction of cardiac thioredoxin-2 (Trx-2) expression as well as thioredoxin reductase-2 (TrxR2) activity. Therefore, it could upregulate the expression of Nrf2/Nrf1 and consequently elevate Prx-3 expression [114]. An in vivo study conducted by using Murraya koenigii (curry) and Moringa oleifera leaf extract that contain quercetin and kaempferol has reported that these flavonols were able to enhance the expression SOD1 gene, PGC 1α gene, and ATPase and improve mitochondrial function in the diabetic heart [115].
The disruption of mitochondrial transmembrane potential is one of the causes that lead to mitochondrial induce oxidative stress. Taxifolin (dihydroquercetin), a subclass of flavonol could restore mitochondrial transmembrane potential in H9c2 cell lines (Sun et al. 2014). Wu and the team [113] have reported that the dihydromyricetin could enhance the ATP content levels, citrate synthase activity and complex Ι/ΙΙ/ΙΙΙ/ΙV and ATP synthase activities as well as increase in ΔΨm.
4.4. Flavanol
Flavanols are the 3-hydroxy derivatives of flavanones commonly known as dihydroflavonols or catechins. They are a multi-substituted and highly diverse subclass of flavonoids. [96]. Due to the hydroxyl group attached to position 3 of the C rings, flavanols are also known as flavan-3-ols. There is no double bond between positions 2 and 3, unlike many flavonoids. Fruits such as bananas, pears, apples, blueberries, and peaches are rich in flavanols.
In earlier study, flavanol has appear to protect heart mitochondria via various mechanisms. This includes protection effect of flavanol via meddling with ETC complexes activities through deprivation of complex I activity, consequently mitochondrial membrane depolarization which then of ROS production (NO and H2O2) [116]. This has been corroborated by previous study on T2DM model where epigallocatechin-3-gallate (EGCG), a flavanol, attenuated myocardial deterioration and showed beneficial effects on myocardial mitochondrial components. EGCG has been demonstrated to revive complex I, III, and IV, as well as voltage-dependent anion-selective channel 1 (VDAC1) activities that produce major ROS. Mitochondrial DNA (mtDNA) copies and the mitochondrial dehydrogenase activity were significantly revived in treatment model [117]. This evidence indicated that EGCG could be an effective substances to protect mitochondria-induced oxidative stress in cardiomyocytes of T2DM.
Moreover, epicatechin is one of the flavanols reported to attenuate DCM through modulation of mitochondrial-induced oxidative stress. Ramírez-Sánchez and colleagues [118] have demonstrated that epicatechin could block the suppressive effect of high glucose on heart mitochondrial biogenesis involving mitofilin, SIRT1, PGC-1α, and TFAM levels. Treatment with epicatechin also has reversed the high level of eNOS-O-GlcNAc in the diabetic heart.
4.5. Anthocyanins
In terrestrial plants, anthocyanins are one of the most common and abundantly distributed secondary metabolites. Anthocyanins are responsible for red, purple, and blue colors in the flowers, seeds, and fruits of numerous plant species. [119,120]. Anthocyanins are natural antioxidants because they are electron deficient, making them highly reactive to ROS [121]. More than 600 anthocyanins have been extracted from a variety of plant species. They are based on the flavylium ion, which has a single fundamental core structure. As a result, the C15 skeleton is formed with a chromane ring with a second aromatic ring B in position 2 (C6-C3-C6) containing single or more sugar molecules attached at various hydroxylated sites on the basic structure. The C3 hydroxyl in the C ring commonly conjugates sugar molecules to the anthocyanidin structure [121]. Anthocyanins and anthocyanin-rich foods have been found to exhibit a variety of biological functions mostly as an antioxidant that may benefit for human wellbeing [122]. The role of anthocyanins has been proven to improve DCM through modulation of mitochondrial-induced oxidative stress. Anthocyanins mainly can be found in plant such as roselle, blackberries and blackcurrants [123]. However, the study that was conducted by using anthocyanins in targeting mitochondrial-induced oxidative stress in DCM is still very limited.
Mitochondria damage is a key factor leading to cardiomyocytes impairment and cell death as well as other cardiac diseases and making mitochondria an attractive target for pharmacological interventions. As a matter of fact, protocatechuic acid (PCA), a primary metabolite of anthocyanins that found in roselle, has been shown to possess as an antioxidant. In an in vitro study by Semaming et al. [122], PCA significantly reduced mitochondrial ROS level and attenuated mitochondrial membrane depolarization. They also found that PCA treatment attenuated mitochondrial swelling as the ROS level decrease. Not only that, PCA treatment alone was able to reduce blood glucose level via enhancing GLUT4 translocation and adiponectin secretion caused by elevated PPARɣ activity in adipocytes. This shows that mediating this mechanism is crucial in alleviating increase of FAO in the mitochondria [124].
Although the use of anthocyanins on diabetic cardiomyopathy research has not yet been extensively investigated, we can presume the result of its interventions by looking at findings of its impact on different cardiac disease models. Previously, anthocyanins has proven to attenuate oxidative stress by scavenge ROS via various mechanisms including direct scavenge ROS, induction of enzymes (superoxide dismutase, catalase) responsible for ROS removal or modulation of ROS forming enzymes (NADPH oxidase) in mitochondria [125,126]. This has been confirmed by another study reporting that anthocyanins was able to quench ROS and thus preserve mitochondrial complex activities in heart [127].
4.6. Flavanones
Flavanones are another important compound found in citrus fruits, including oranges, lemons, and grapes. This group of flavonoids includes hesperidin, naringenin, and eriodyctiol. Because of their free radical-scavenging characteristics, flavanones have been linked to various health advantages [96]. Citrus fruit juice and peel contain these substances, which give them a bitter taste. Citrus flavonoids have pharmacological actions that include antioxidant, anti-inflammatory, anti-hyperglycemia, and anti-hypercholestrolemia. The C ring is saturated in flavanones (saturated double bond between positions 2 and 3), giving them the alternative name of dihydroflavonols, and distinguishes them from flavones [109].
Naringin, a major flavanone glycoside found mostly in citrus fruits, has been reported to alleviate mitochondrial-induce oxidative stress by preventing the high glucose-induced loss in mitochondrial membrane potential [128]. In another study conducted by You and colleagues [129], naringin also reduced the downregulation of mitochondrial ATP-sensitive potassium channels, which is important in sensing the metabolic changes in pancreatic beta cells and thus protecting the cardiomyocytes against the hyperglycemic condition.
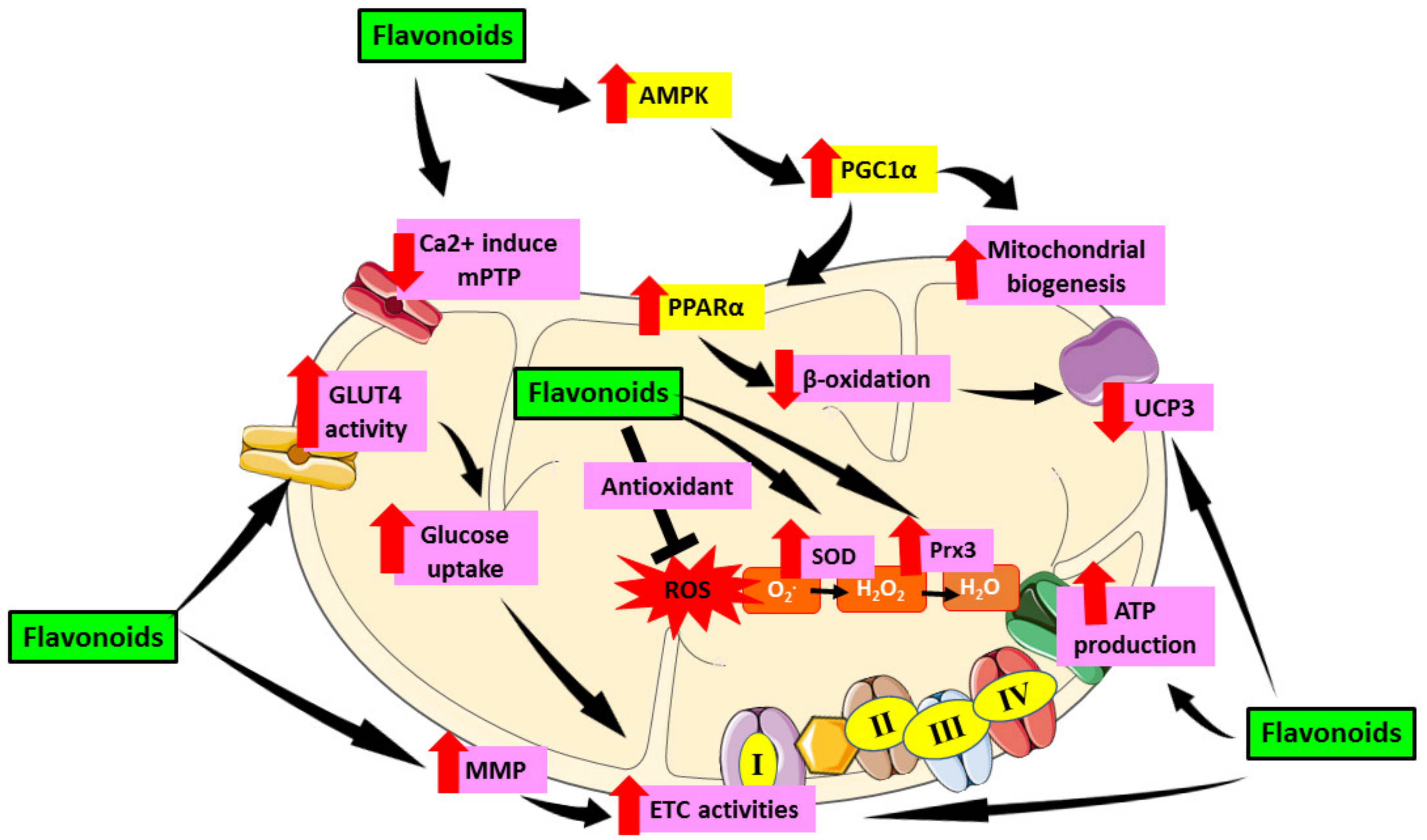
Similar to anthocyanins, the extensive study on the effect of flavanone in mitochondrial-induced oxidative stress in DCM is still limited. However, in other cardiac models, flavanone has been demonstrated to modulate mitochondrial function in cardiomyocytes. Previously, flavanone was found to ameliorate mitochondrial disruption in cardiomyocytes by reducing impaired mitochondrial membrane potential and suppressing mitochondrial ROS levels and increase mitochondrial antioxidant via regulation of AMPK-mTOR signaling pathways [130,131]. Moreover, flavanone was able to alleviate mitochondrial membrane potential collapse and preserve mitochondrial complex II activity on isolated heart mitochondria [131]. Aside from that, Ca2+ overload was reduced significantly with the treatment of flavanone and hence reviving mitochondrial function in the heart [132]. Table 1 shows an overview of the role of flavonoids in alleviating mitochondrial-induced oxidative stress in DCM. Figure 5 demonstrated the role of flavonoids in targeting mitochondrial induce oxidative stress in DCM.

Table 1.
Summary of flavonoid and its subclasses in targeting mitochondrial-induce oxidative stress in DCM.
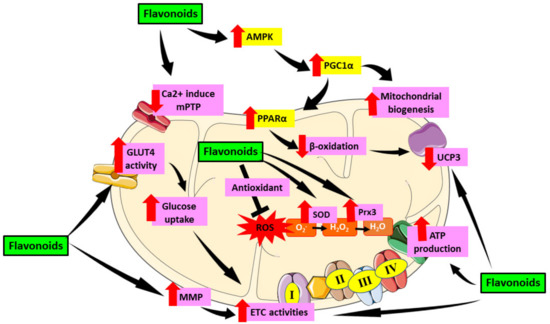
Figure 5.
Role of flavonoids in targeting mitochondrial induce oxidative stress in DCM. Flavonoids have been proven to alleviate mitochondrial dysfunction by targeting mechanisms involving oxidative stress in mitochondria including activation of AMPK which then activate PGC1α. PGC1α enhances mitochondrial biogenesis as well as PPARα expression and cause reduction of β-oxidation in mitochondria which then downregulate UCP3. Flavonoids also reduce the formation of Ca2+ induced mPTP which preserve mitochondrial number and prevent apoptosis. Furthermore, flavonoids were proven to enhance GLUT4 activity which then led to increase glucose uptake as well as enhance MMP that cause increase in ETC activities which further elevate ATP production. Moreover, flavonoids can act as antioxidant and scavenge ROS as well as increase SOD and Prx3 enzyme which later attenuate oxidative stress. This figure is illustrated based on the review of the previous research. Black arrow indicates flow of mechanisms; red arrow indicates increase/decrease of level/activity.
5. Future Prospects of Flavonoids Aiming at Reducing Mitochondrial-Induced Oxidative Stress in Diabetic Cardiomyopathy
Mitochondrial dysfunction is the hallmark of cardiac degeneration in DCM. Therefore, it is of utmost importance to curb and alleviate mitochondrial dysfunction in patients with DM. Given enormous evidence linking that mitochondrial-induced oxidative damage can lead to progression of DCM, it is reasonable to assume that lessening oxidative damage would protect the cardiac against detrimental adjustment by diabetes. To the best of our knowledge, there is no clinical study using flavonoids as an antioxidant targeting mitochondrial-induced oxidative stress in DCM. In addition, the preclinical study in this field also is limited. To sustain the improvement of a specific and successful therapy, future studies should investigate the use of flavonoid-rich source targeting specifically in mitochondrial ROS production pathways. Nevertheless, the inhibition of mitochondrial ROS supply might be a useful strategy to prevent the alteration caused by oxidative stress on myocardial structure and function.
We postulate that the lack of clinical trial on this topic could be due to several reasons. This could be a consequence of difficulties in getting human cardiac mitochondrial samples as it is invasive or a lack of proven biomarkers indicative of mitochondrial ROS production, specifically from DCM. Taking into consideration of previous findings obtained in DCM experimental models with the treatment of flavonoids, it is worth assessing whether the flavonoid compound could be used for the treatment of patients with DCM caused by mitochondrial-induced oxidative stress. Flavonoids can be considered as a tool to prevent DCM in patients with DM or to be use in the conjunction with existing treatment to lower the morbidity and mortality rate due to DCM.
6. Conclusions
In a nutshell, it is clear that prolonged hyperglycemia can cause mitochondrial-induced oxidative stress and lead to development of DCM. There are scientific evidence showing that flavonoids can protect cardiomyocytes against mitochondrial-induced oxidative stress caused by DM against perturbations, such as altered energy metabolism, impaired calcium handling, altered mitochondrial biogenesis, and altered ETC. Therefore, flavonoids show promising potential in alleviating DCM by protection against mitochondrial-induced oxidative stress. However, studies exploring this potential are rather scarce especially by using isoflavones, anthocyanins, and flavanones. Several mechanisms were also poorly investigated by previous studies even though it is very important in alleviating mitochondrial-induced oxidative stress and intervene by flavonoid. To prove that flavonoids could suppress mitochondrial-induced oxidative stress that causes DCM, in-depth studies are very much needed in the future especially by looking into its effects on altered ETC, mitochondrial biogenesis and calcium handling. Currently, most studies of the effects of flavonoids on cardiac mitochondrial-induced oxidative stress are focusing on animals and cell culture studies rather than clinical study. Hence, more clinical studies examining the beneficial effect of flavonoids on mitochondrial-induced oxidative stress should be conducted as flavonoid has huge potential in ameliorate DCM which then reduce heart failure risk in diabetic patients.
Author Contributions
Conceptualization, S.S. and S.B.B.; resources, S.S., F.F.J. and N.A.M.N.; writing—original draft preparation, S.S.; writing—review and editing, S.B.B.; K.-Y.C., H.K., I.S.T. and J.L.; supervision, S.B.B. and K.-Y.C. The authors have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Utas Maju Sdn. Bhd. (NN-2021-003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| DM | Diabetes mellitus |
| CVD | Cardiovascular disease |
| DCM | Diabetic cardiomyopathy |
| ROS | Reactive oxygen species |
| AGE | Advanced glycation end product |
| PKC | Protein kinase C |
| ATP | Adenine triphosphate |
| OXPHOS | Oxidative phosphorylation |
| DNA | Deoxyribonucleic acid |
| mPTP | Mitochondrial permeability transition pore |
| FAO | Fatty acid oxidation |
| PPAR | Peroxisome proliferator-activated receptor |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| PGC-1 | Peroxisome proliferator-activated receptor-gamma coactivator 1 |
| NADH | Nicotinamide adenine dinucleotide (NAD) + hydrogen (H) |
| FADH2 | Flavin adenine dinucleotide |
| ETC | Electron transport chain |
| MnSOD | Manganese superoxide dismutase |
| UCP | Uncoupling protein |
| ΔΨm | Inner mitochondrial membrane |
| MMP | Mitochondrial membrane polarization |
| O-GlcNAc | O-linked β-N-acetylglucosamine |
| OGT | O-GlcNAc transferase |
| NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| UCP-DTA | Uncoupling Protein-diphtheria Toxin A |
| eNOS | Endothelial nitric oxide synthase |
| SIRT1 | Silent information regulator 1 |
| Nrf | Nuclear factor erythroid 2–related factor 2 |
| TFAM | Mitochondrial transcription factor A |
| Prx3 | Peroxiredoxin-3 |
| Trx2 | Thioredoxin-2 |
| VDAC | Voltage-dependent anion channel 1 |
| EGCG | Epigallocatechin-3-gallate |
| PCA | Protocatechuic acid |
| GLUT4 | Glucose transporter type 4 |
References
- International Diabetes Federation (IDF). Diabetes Facts & Figures. 2019. Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 30 June 2021).
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Cieluch, A.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Can we prevent mitochondrial dysfunction and diabetic cardiomyopathy in Type 1 diabetes mellitus? Pathophysiology and treatment options. Int. J. Mol. Sci. 2020, 21, 2852. [Google Scholar] [CrossRef]
- Maack, C.; Lehrke, M.; Backs, J.; Heinzel, F.R.; Hulot, J.-S.; Marx, N.; Paulus, W.J.; Rossignol, P.; Taegtmeyer, H.; Bauersachs, J. Heart failure and diabetes: Metabolic alterations and therapeutic interventions: A state-of-the-art review from the Translational Research Committee of the Heart Failure Association–European Society of Cardiology. Eur. Heart J. 2018, 39, 4243–4254. [Google Scholar] [CrossRef] [Green Version]
- Bingchao, Q.; Linjie, H.; Zhao, Y.; Zhang, L.; Yuanfang, H.; Li, J.; Congye, L.; Zhang, B.; Qichao, H.; Jinliang, X. Akap1 deficiency exacerbates diabetic cardiomyopathy in mice by NDUFS1-mediated mitochondrial dysfunction and apoptosis. Diabetologia 2020, 63, 1072–1087. [Google Scholar]
- Dia, M.; Gomez, L.; Thibault, H.; Tessier, N.; Leon, C.; Chouabe, C.; Ducreux, S.; Gallo-Bona, N.; Tubbs, E.; Bendridi, N. Reduced reticulum–mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res. Cardiol. 2020, 115, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.-H. Hyperglycemia-driven inhibition of AMP-activated protein kinase α2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation 2019, 139, 1913–1936. [Google Scholar] [CrossRef] [PubMed]
- Katunga, L.A.; Gudimella, P.; Efird, J.T.; Abernathy, S.; Mattox, T.A.; Beatty, C.; Darden, T.M.; Thayne, K.A.; Alwair, H.; Kypson, A.P. Obesity in a model of gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol. Metab. 2015, 4, 493–506. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Mariappan, V.; Budin, S.B. Mitochondrial dysfunction in diabetic cardiomyopathy: The possible therapeutic roles of phenolic acids. Int. J. Mol. Sci. 2020, 21, 6043. [Google Scholar] [CrossRef]
- Tay, Y.; Bakar, M.; Azmi, M.; Saad, N.; Awang, K.; Litaudon, M.; Kassim, M. (Eds.) Inhibition of Carbohydrate Hydrolysing Enzymes, Antioxidant Activity and Polyphenolic Content of Beilschmiedia Species Extracts. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 716, p. 012007. [Google Scholar]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic cardiomyopathy: Current and future therapies. Beyond glycemic control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-F.; Wang, S.-W.; Wang, X.-X.; Weng, Y.-Y.; Fan, X.-Y.; Sheng, H.; Zhu, X.-T.; Lou, L.-J.; Zhang, F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bedir, F.; Kocatürk, H.; Yapanoğlu, T.; Gürsul, C.; Arslan, R.; Mammadov, R.; Çoban, A.; Altuner, D.; Suleyman, H. Protective effect of taxifolin against prooxidant and proinflammatory kidney damage associated with acrylamide in rats. Biomed. Pharmacother. 2021, 139, 111660. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, X.; Yin, K.; Qi, X.; Zhang, Y.; Zhang, J.; Li, S.; Lin, H. Dibutyl phthalate-induced oxidative stress, inflammation and apoptosis in grass carp hepatocytes and the therapeutic use of taxifolin. Sci. Total Environ. 2021, 764, 142880. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A Flavonoid as an Effective Sensitizer for Anticancer Therapy; Insights into Multifaceted Mechanisms and Applicability for Combination Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, G.; Qu, H.; Wang, K.; Jing, S.; Guan, S.; Su, L.; Li, Q.; Wang, D. Taxifolin, an Inhibitor of Sortase A, Interferes with the Adhesion of Methicillin-Resistant Staphylococcal aureus. Front. Microbiol. 2021, 12, 1876. [Google Scholar]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Xiao, C.; Xia, M.-L.; Wang, J.; Zhou, X.-R.; Lou, Y.-Y.; Tang, L.-H.; Zhang, F.-J.; Yang, J.-T.; Qian, L.-B. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxidative Med. Cell. Longev. 2019, 2019, 2719252. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Liu, H.; Li, P.; Zhang, H.; Cheng, G. Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem. Biophys. Res. Commun. 2017, 490, 552–559. [Google Scholar] [CrossRef]
- Ni, T.; Lin, N.; Huang, X.; Lu, W.; Sun, Z.; Zhang, J.; Lin, H.; Chi, J.; Guo, H. Icariin ameliorates diabetic cardiomyopathy through Apelin/Sirt3 Signalling to improve mitochondrial dysfunction. Front. Pharmacol. 2020, 11, 256. [Google Scholar] [CrossRef]
- Upadhyay, S.; Mantha, A.K.; Dhiman, M. Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes. J. Ethnopharmacol. 2020, 258, 112690. [Google Scholar] [CrossRef]
- Xue, W.; Wang, X.; Tang, H.; Sun, F.; Zhu, H.; Huang, D.; Dong, L. Vitexin attenuates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction induced by mitochondrial dynamics imbalance. Biomed. Pharmacother. 2020, 124, 109849. [Google Scholar] [CrossRef] [PubMed]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Shah, V.N.; Bailey, R.; Wu, M.; Foster, N.C.; Pop-Busui, R.; Katz, M.; Crandall, J.; Bacha, F.; Nadeau, K.; Libman, I. Risk factors for cardiovascular disease (CVD) in adults with type 1 diabetes: Findings from prospective real-life T1D exchange registry. J. Clin. Endocrinol. Metab. 2020, 105, e2032–e2038. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M. Diabetic cardiomyopathy: Definition, diagnosis, and therapeutic implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Bounias, I.; Olsson, M.; Gudbjörnsdottir, S.; Svensson, A.-M.; Rosengren, A. Glycaemic control and incidence of heart failure in 20 985 patients with type 1 diabetes: An observational study. Lancet 2011, 378, 140–146. [Google Scholar] [CrossRef]
- Mohammed Yusof, N.L.; Zainalabidin, S.; Mohd Fauzi, N.; Budin, S.B. Hibiscus sabdariffa (roselle) polyphenol-rich extract averts cardiac functional and structural abnormalities in type 1 diabetic rats. Appl. Physiol. Nutr. Metab. 2018, 43, 1224–1232. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S. Collateral Glucose-Utlizing Pathwaya in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2021, 22, 94. [Google Scholar]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Habibi, J.; DeMarco, V.G.; Martinez-Lemus, L.A.; Ma, L.; Whaley-Connell, A.T.; Aroor, A.R.; Domeier, T.L.; Zhu, Y.; Meininger, G.A. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 2015, 66, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Collins, L.V.; Hajizadeh, S.; Holme, E.; Jonsson, I.-M.; Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004, 75, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003. [Google Scholar] [PubMed]
- Yusof, N.L.M.; Zainalabidin, S.; Fauzi, N.M.; Budin, S.B. Attenuation of Cardiac Functional and Structural Abnormalities by Hibiscus Sabdariffa Polyphenol-Rich Extract in Type-1-Induced Diabetic Rats. Int. J. Cardiol. 2018, 273, 19. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wu, R.; Bai, J.; Hou, Y.; Zeng, Y.; Zhang, Y.; Wang, X.; Wang, Z.; Meng, X. Mitochondrial MPTP: A novel target of ethnomedicine for stroke treatment by apoptosis inhibition. Front. Pharmacol. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, J.D.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Picard, M.; Wright, K.J.; Ritchie, D.; Thomas, M.M.; Hepple, R.T. Mitochondrial function in permeabilized cardiomyocytes is largely preserved in the senescent rat myocardium. PLoS ONE 2012, 7, e43003. [Google Scholar] [CrossRef] [Green Version]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Bai, J.; Liu, C.; Zhu, P.; Li, Y. Novel Insights into Molecular Mechanism of Mitochondria in Diabetic Cardiomyopathy. Front. Physiol. 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Corvera, S. The role of mitochondria in the pathogenesis of Type 2 Diabetes. Endocr. Rev. 2010, 31, 364–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, N.J.; Rajasekaran, N.S.; Abel, E.D.; Bugger, H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic. Biol. Med. 2021, 169, 317–342. [Google Scholar] [CrossRef] [PubMed]
- Viglino, C.; Foglia, B.; Montessuit, C. Chronic AICAR treatment prevents metabolic changes in cardiomyocytes exposed to free fatty acids. Pflüg. Arch. Eur. J. Physiol. 2019, 471, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P. Skeletal muscle uncoupling protein 3 (UCP3): Mitochondrial uncoupling protein in search of a function. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 265–270. [Google Scholar] [CrossRef]
- Stavinoha, M.A.; RaySpellicy, J.W.; Essop, M.F.; Graveleau, C.; Abel, E.D.; Hart-Sailors, M.L.; Mersmann, H.J.; Bray, M.S.; Young, M.E. Evidence for mitochondrial thioesterase 1 as a peroxisome proliferator-activated receptor-α-regulated gene in cardiac and skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E888–E895. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Panagia, M.; Hauton, D.; Gibbons, G.F.; Clarke, K. Plasma free fatty acids and peroxisome proliferator–activated receptor α in the control of myocardial uncoupling protein levels. Diabetes 2005, 54, 3496–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giralt, M.; Villarroya, F. Mitochondrial uncoupling and the regulation of glucose homeostasis. Curr. Diabetes Rev. 2017, 13, 386–394. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Tiano, L.; Louw, J.; Jastroch, M.; Mazibuko-Mbeje, S.E. Uncoupling proteins as a therapeutic target to protect the diabetic heart. Pharmacol. Res. 2018, 137, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, R.R. Electron carriers and energy conservation in mitochondrial respiration. ChemTexts 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta 2018, 1859, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Du, X.-L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Teshima, Y.; Takahashi, N.; Nishio, S.; Saito, S.; Kondo, H.; Fukui, A.; Aoki, K.; Yufu, K.; Nakagawa, M.; Saikawa, T. Production of Reactive Oxygen Species in the Diabetic Heart–Roles of Mitochondria and NADPH Oxidase. Circ. J. 2013, CJ-13-1187. [Google Scholar] [CrossRef] [Green Version]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Baseler, W.A.; Dabkowski, E.R.; Williamson, C.L.; Croston, T.L.; Thapa, D.; Powell, M.J.; Razunguzwa, T.T.; Hollander, J.M. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: Contribution of protein import dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R186–R200. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.S.; Ma, J.; Hart, G.W. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, 6050–6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawlowski, T.; Suarez, J.; Scott, B.; Torres-Gonzalez, M.; Wang, H.; Schwappacher, R.; Han, X.; Yates, J.R.; Hoshijima, M.; Dillmann, W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J. Biol. Chem. 2012, 287, 30024–30034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, A.; Suarez, J.; Gawlowski, T.; Han, W.; Wang, H.; Scott, B.T.; Dillmann, W.H. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1296–R1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, L.; Ni, R.; Cao, T.; Zheng, D.; Xiong, S.; Greer, P.A.; Fan, G.-C.; Peng, T. Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim. Biophys. Acta 2016, 1862, 2023–2033. [Google Scholar] [CrossRef]
- Ni, R.; Zheng, D.; Xiong, S.; Hill, D.J.; Sun, T.; Gardiner, R.B.; Fan, G.-C.; Lu, Y.; Abel, E.D.; Greer, P.A. Mitochondrial calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: A novel mechanism contributing to diabetic cardiomyopathy. Diabetes 2016, 65, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, Q.; Arnold, M.; Peng, T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc. Res. 2009, 84, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J.; Hartmann, M.; Rehling, P. The role of mitochondrial cardiolipin in heart function and its implication in cardiac disease. Biochim. Biophys. Acta 2019, 1865, 810–821. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: Implications for pharmacological cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1341–H1352. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: Molecular and pharmacological aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabkowski, E.R.; Williamson, C.L.; Bukowski, V.C.; Chapman, R.S.; Leonard, S.S.; Peer, C.J.; Callery, P.S.; Hollander, J.M. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H359–H369. [Google Scholar] [CrossRef] [Green Version]
- Galloway, C.A.; Yoon, Y. Mitochondrial dynamics in diabetic cardiomyopathy. Antioxid. Redox Signal. 2015, 22, 1545–1562. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, J.; Ghosh, S.; Delbridge, L.M.; Petzer, A.; Hickey, A.J.; Crampin, E.J.; Hanssen, E.; Rajagopal, V. Changes in mitochondrial morphology and organization can enhance energy supply from mitochondrial oxidative phosphorylation in diabetic cardiomyopathy. Am. J. Physiol. Cell Physiol. 2017, 312, C190–C197. [Google Scholar] [CrossRef] [PubMed]
- Buccoliero, C.; Dicarlo, M.; Pignataro, P.; Gaccione, F.; Colucci, S.; Colaianni, G.; Grano, M. The Novel Role of PGC1α in Bone Metabolism. Int. J. Mol. Sci. 2021, 22, 4670. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Nogee, D.P.; Zechner, J.F.; Yea, K.; Gierasch, C.M.; Kovacs, A.; Medeiros, D.M.; Kelly, D.P.; Duncan, J.G. The transcriptional coactivators, PGC-1α and β, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J. Mol. Cell. Cardiol. 2012, 52, 701–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Zhang, H.; Liu, P.; Wang, H.; Liu, J.; Gao, C.; Liu, Y.; Lian, K.; Yang, L.; Sun, L. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res. Cardiol. 2013, 108, 329. [Google Scholar] [CrossRef]
- Wang, H.; Yan, W.; Zhang, J.; Zhang, F.; Gao, C.; Wang, Y.; Bondlaw, W.; Tao, L. Adiponectin partially rescues high glucose/high fat-induced impairment of mitochondrial biogenesis and function in a PGC-1α dependent manner. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 590–599. [Google Scholar]
- Drago, I.; Davis, R.L. Inhibiting the mitochondrial calcium uniporter during development impairs memory in adult Drosophila. Cell Rep. 2016, 16, 2763–2776. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Fang, D.; Swerdlow, R.H.; Yu, H.; Chen, J.X.; Yan, S.S. Antioxidants rescue mitochondrial transport in differentiated Alzheimer’s disease trans-mitochondrial cybrid cells. J. Alzheimer’s Dis. 2016, 54, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Prado-Garcia, H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer. Int. J. Oncol. 2019, 54, 1155–1167. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, P.; Di Lisa, F.; Fogolari, F.; Lippe, G. From ATP to PTP and back: A dual function for the mitochondrial ATP synthase. Circ. Res. 2015, 116, 1850–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, C.; Bonora, M.; Sbano, L.; Morciano, G.; Aquila, G.; Campo, G.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. The mitochondrial permeability transition pore. In Mitochondrial Biology and Experimental Therapeutics; Springer: Berlin, Germany, 2018; pp. 47–73. [Google Scholar]
- Moltedo, O.; Remondelli, P.; Amodio, G. The mitochondria–endoplasmic reticulum contacts and their critical role in aging and age-associated diseases. Front. Cell Dev. Biol. 2019, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, inflammation, and oxidative stress: An integrative view in metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, K.; Vandecasteele, G.; Carli, C.; Romagnoli, A.; Szabadkai, G.; Rizzuto, R. Regulation of Ca2+ signalling and Ca2+-mediated cell death by the transcriptional coactivator PGC-1 α. Cell Death Differ. 2006, 13, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Rauter, A.P.; Ennis, M.; Hellwich, K.-H.; Herold, B.J.; Horton, D.; Moss, G.P.; Schomburg, I. Nomenclature of flavonoids (IUPAC recommendations 2017). Pure Appl. Chem. 2018, 90, 1429–1486. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26, 17A–21A. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qiao, L.; Shen, Y.; Jiang, P.; Chen, J.; Ye, X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J. Food Sci. 2013, 78, C37–C42. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Wang, Y.; Liu, X.; Ma, S.; Yang, R. Effects of genistein on Nrf2/HO-1 pathway in myocardial tissues of diabetic rats. J. Cent. South Univ. Med. Sci. 2019, 44, 850–856. [Google Scholar]
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev. Res. 2019, 80, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-T.; Qian, L.-B.; Zhang, F.-J.; Wang, J.; Ai, H.; Tang, L.-H.; Wang, H.-P. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J. Cardiovasc. Pharmacol. 2015, 65, 349–356. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Shen, Q.; Liu, G.; Ye, J.; Sun, G.; Sun, X. Myricitrin attenuates high glucose-induced apoptosis through activating Akt-Nrf2 signaling in H9c2 cardiomyocytes. Molecules 2016, 21, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J. Transl. Med. 2015, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirumbolo, S. Dietary assumption of plant polyphenols and prevention of allergy. Curr. Pharm. Des. 2014, 20, 811–839. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- Andor, B.; Danciu, C.; Alexa, E.; Zupko, I.; Hogea, E.; Cioca, A.; Coricovac, D.; Pinzaru, I.; Pătrașcu, J.M.; Mioc, M. Germinated and ungerminated seeds extract from two lupinus species: Biological compounds characterization and in vitro and in vivo evaluations. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.-P. Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef] [Green Version]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.-A.; Soica, C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021, 284, 119664. [Google Scholar] [CrossRef] [PubMed]
- Colareda, G.A.; Matera, S.I.; Bayley, M.; Ragone, M.I.; Flores, M.L.; Córdoba, O.L.; Consolini, A.E. Lepidium meyenii (maca) and soy isoflavones reduce cardiac stunning of ischemia-reperfusion in rats by mitochondrial mechanisms. J. Tradit. Complement. Med. 2021, 3, 4. [Google Scholar]
- Farruggio, S.; Raina, G.; Cocomazzi, G.; Librasi, C.; Mary, D.; Gentilli, S.; Grossini, E. Genistein improves viability, proliferation and mitochondrial function of cardiomyoblasts cultured in physiologic and peroxidative conditions. Int. J. Mol. Med. 2019, 44, 2298–2310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, R.; Li, L.; Li, S.; Fan, G.; Zhao, X.; Wang, Y. Tongmai formula improves cardiac function via regulating mitochondrial quality control in the myocardium with ischemia/reperfusion injury. Biomed. Pharmacother. 2020, 132, 110897. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. Flavonoid properties of five families newly incorporated into the order Caryophyllales. Bull. Natl. Mus. Nat. Sci. 2013, 39, 25–51. [Google Scholar]
- Rajagopalan, G.; Chandrasekaran, S.P.; Carani Venkatraman, A. Troxerutin attenuates diet-induced oxidative stress, impairment of mitochondrial biogenesis and respiratory chain complexes in mice heart. Clin. Exp. Pharmacol. Physiol. 2017, 44, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, M.; Mazibuko, S.E.; Joubert, E.; De Beer, D.; Johnson, R.; Pheiffer, C.; Louw, J.; Muller, C.J. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine 2014, 21, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Khalil, S.R.; Awad, A.; Khairy, G.M. Quercetin Reverses Altered Energy Metabolism in the Heart of Rats Receiving Adriamycin Chemotherapy. Cardiovasc. Toxicol. 2018, 18, 109–119. [Google Scholar] [CrossRef]
- Wu, B.; Lin, J.; Luo, J.; Han, D.; Fan, M.; Guo, T.; Tao, L.; Yuan, M.; Yi, F. Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. BioMed Res. Int. 2017, 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Arkat, S.; Umbarkar, P.; Singh, S.; Sitasawad, S.L. Mitochondrial peroxiredoxin-3 protects against hyperglycemia induced myocardial damage in diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 97, 489–500. [Google Scholar] [CrossRef]
- Ahmed, E.; Ebrahim, H.S.E.D. Biochemical and Molecular Study on the Effect of Murraya Koenigii (Curry) and Moringa Oleifera on Cardiac Mitochondrial Dysfunction in Diabetic Rats. World J. Pharm. Pharm. Sci. 2018, 7, 1062–1076. [Google Scholar]
- Iglesias, D.E.; Bombicino, S.S.; Boveris, A.; Valdez, L.B. (+)-Catechin inhibits heart mitochondrial complex I and nitric oxide synthase: Functional consequences on membrane potential and hydrogen peroxide production. Food Funct. 2019, 10, 2528–2537. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Feng, Z.; Long, J. (–)-Epigallocatechin-3-gallate attenuated myocardial mitochondrial dysfunction and autophagy in diabetic Goto–Kakizaki rats. Free. Radic. Res. 2014, 48, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, I.; Rodríguez, A.; Ulloa, A.M.; Ceballos, G.; Villarreal, F. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes Vasc. Dis. Res. 2016, 13, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Semaming, Y.; Kumfu, S.; Pannangpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol. 2014, 223, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.-C.; Budin, S.B.; Othman, F.; Latip, J.; Zainalabidin, S. Roselle polyphenols exert potent negative inotropic effects via modulation of intracellular calcium regulatory channels in isolated rat heart. Cardiovasc. Toxicol. 2017, 17, 251–259. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Jayasingha, J.A.C.C.; Kim, G.-Y. Anthocyanins from Hibiscus syriacus L. Inhibit NLRP3 Inflammasome in BV2 Microglia Cells by Alleviating NF-κB-and ER Stress-Induced Ca2+ Accumulation and Mitochondrial ROS Production. Oxidative Med. Cell. Longev. 2021, 2021, 17. [Google Scholar] [CrossRef]
- Skemiene, K.; Liobikas, J.; Borutaite, V. Anthocyanins as substrates for mitochondrial complex I–protective effect against heart ischemic injury. FEBS J. 2015, 282, 963–971. [Google Scholar] [CrossRef]
- Li, F.; Lang, F.; Wang, Y.; Zhai, C.; Zhang, C.; Zhang, L.; Hao, E. Cyanidin ameliorates endotoxin-induced myocardial toxicity by modulating inflammation and oxidative stress through mitochondria and other factors. Food Chem. Toxicol. 2018, 120, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, K.; You, Q.; Huang, R.; Li, S. Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int. J. Mol. Med. 2013, 32, 396–402. [Google Scholar] [CrossRef] [Green Version]
- You, Q.; Wu, Z.; Wu, B.; Liu, C.; Huang, R.; Yang, L.; Guo, R.; Wu, K.; Chen, J. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016, 230, 197–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.-W.; Lee, J.H. Amelioration of high fructose-induced cardiac hypertrophy by naringin. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Salehcheh, M.; Alboghobeish, S.; Dehghani, M.A.; Zeidooni, L. Multi-walled carbon nanotubes induce oxidative stress, apoptosis, and dysfunction in isolated rat heart mitochondria: Protective effect of naringin. Environ. Sci. Pollut. Res. 2020, 27, 13447–13456. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Sun, X.; Chen, R.-C.; Yang, Z.-H.; Sun, G.-B.; Wang, M.; Ma, X.-J.; Yang, L.-J.; Sun, X.-B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).