Toxicity Assessment of Long-Term Exposure to Non-Thermal Plasma Activated Water in Mice
Abstract
:1. Introduction
2. Results
2.1. Effect of Long-Term PAW Consumption on Vital Teeth: EDX, SEM and AFM Data
2.2. The Effects of Long-Term PAW Consumption on Vital Organs
2.2.1. Weight
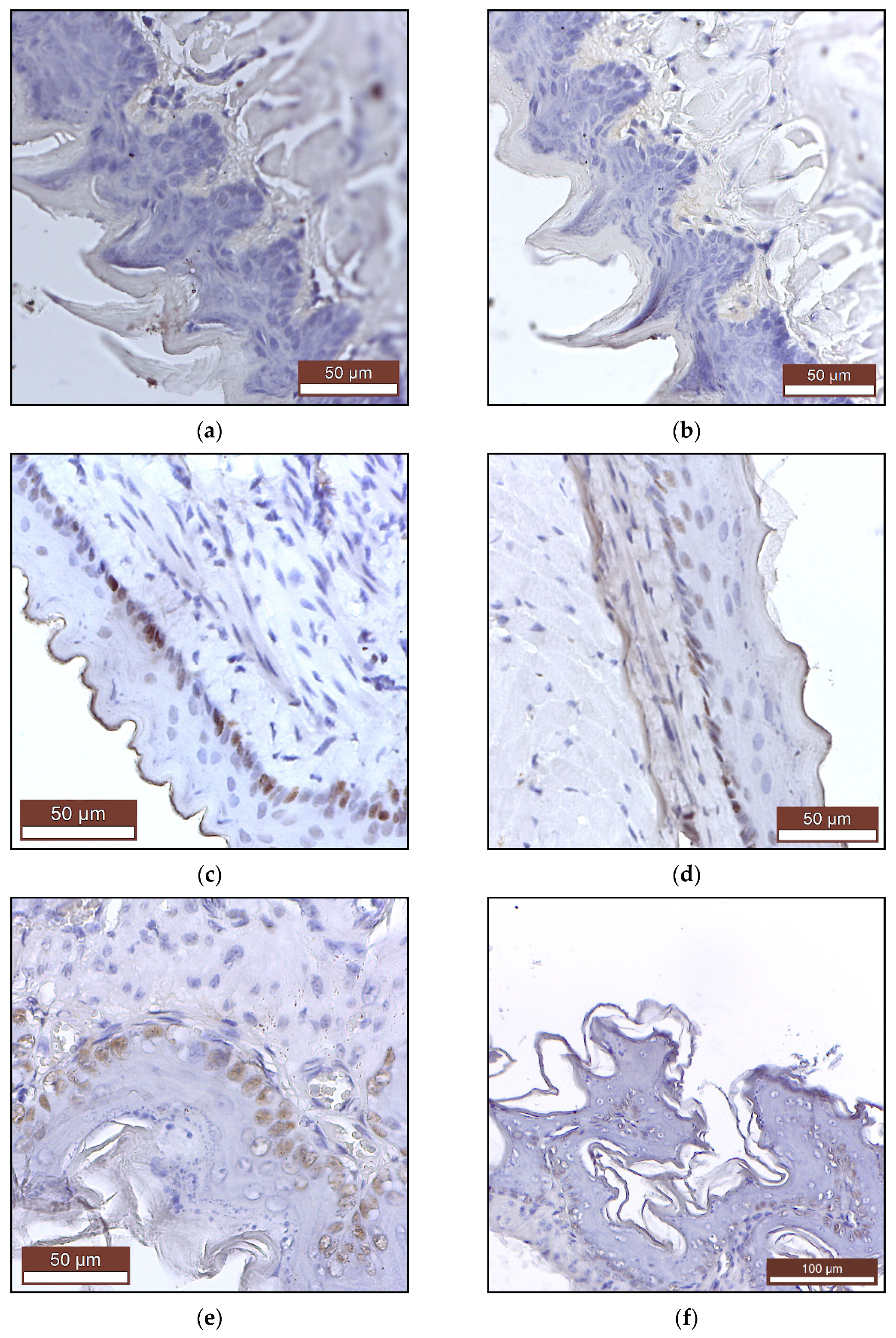
2.2.2. Histopathology and Immunohistochemistry
2.3. The Effects of Long-Term PAW Consumption on Blood Parameters
2.3.1. Hematological Parameters
2.3.2. Biochemical Parameters
2.3.3. Methemoglobin (MetHb) Levels
2.4. Effects of Long-Term PAW Consumption on the Immune Response
3. Discussion
4. Materials and Methods
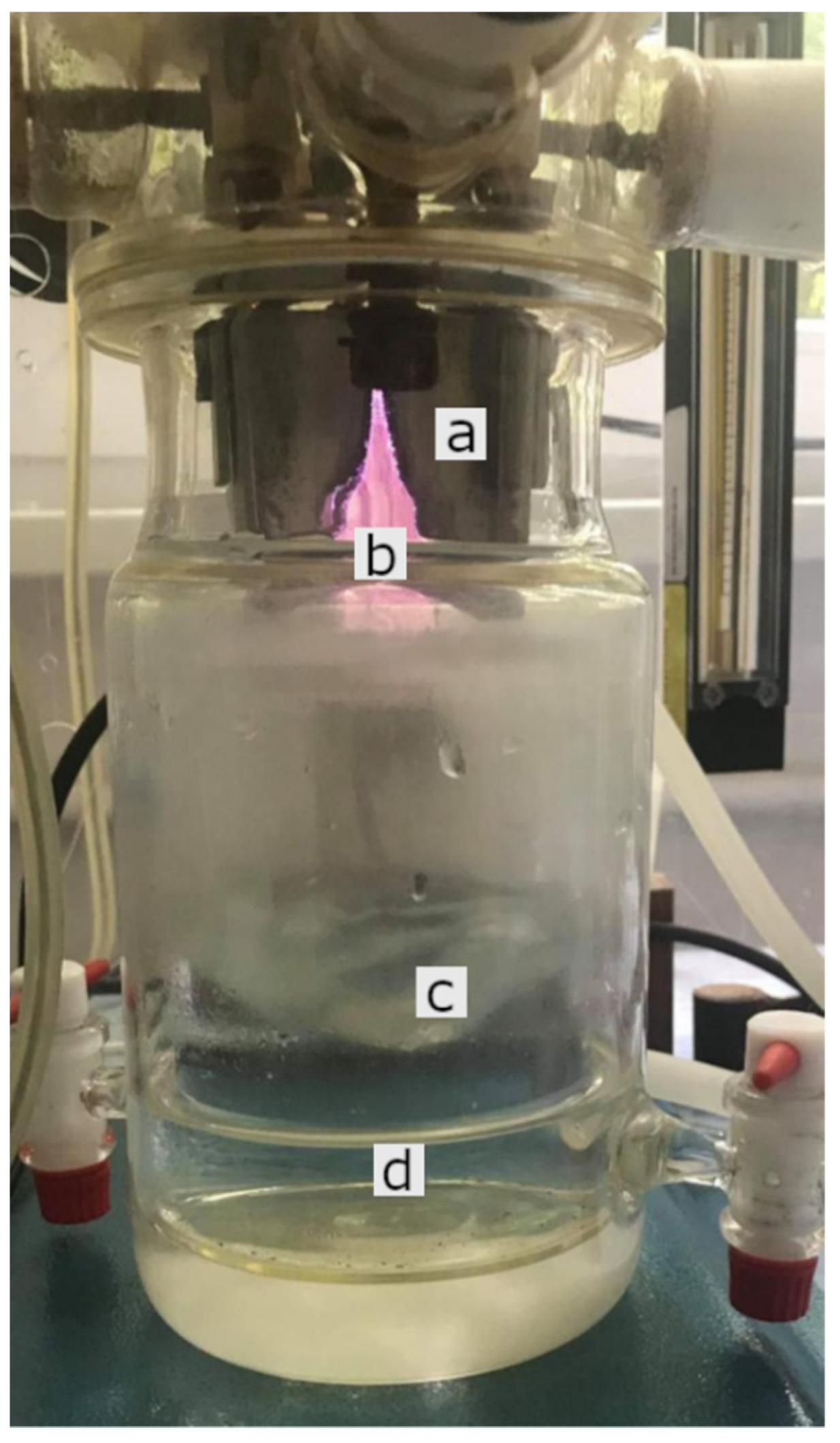
4.1. Plasma Device (GlidingArc Reactor) and Production of PAW
4.2. Animals
4.3. Experimental Design
4.4. Scanning Electronic Microscopy (SEM), Energy Dispersive X-ray Analysis (EDX) and Atomic Force Microscopy (AFM)
4.5. Histopathology and Immunohistochemistry
4.6. Hematology
4.7. Blood Biochemistry
4.8. Immunological Examination
4.9. Determination of Methemoglobin (MetHb)
4.10. Statistical Analysis
4.11. Ethical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Conrads, H.; Schmidt, M. Plasma Generation and Plasma Sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Julák, J.; Hujacová, A.; Scholtz, V.; Khun, J.; Holada, K. Contribution to the Chemistry of Plasma-Activated Water. Plasma Phys. Rep. 2018, 44, 125–136. [Google Scholar] [CrossRef]
- Tarabová, B.; Lukeš, P.; Janda, M.; Hensel, K.; Šikurová, L.; Machala, Z. Specificity of Detection Methods of Nitrites and Ozone in Aqueous Solutions Activated by Air Plasma. Plasma Process. Polym. 2018, 15, 1800030. [Google Scholar] [CrossRef]
- Tarabová, B.; Lukeš, P.; Hammer, M.U.; Jablonowski, H.; von Woedtke, T.; Reuter, S.; Machala, Z. Fluorescence Measurements of Peroxynitrite/Peroxynitrous Acid in Cold Air Plasma Treated Aqueous Solutions. Phys. Chem. Chem. Phys. 2019, 21, 8883–8896. [Google Scholar] [CrossRef] [PubMed]
- Bogle, M.A.; Arndt, K.A.; Dover, J.S. Evaluation of Plasma Skin Regeneration Technology in Low-Energy Full-Facial Rejuvenation. Arch. Dermatol. 2007, 143, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Foster, K.W.; Moy, R.L.; Fincher, E.F. Advances in Plasma Skin Regeneration. J. Cosmet. Dermatol. 2008, 7, 169–179. [Google Scholar] [CrossRef]
- Tümmel, S.; Mertens, N.; Wang, J.; Viöl, W. Low Temperature Plasma Treatment of Living Human Cells. Plasma Process. Polym. 2007, 4, S465–S469. [Google Scholar] [CrossRef]
- Laheij, A.M.G.A.; Kistler, J.O.; Belibasakis, G.N.; Välimaa, H.; de Soet, J.J. Healthcare-Associated Viral and Bacterial Infections in Dentistry. J. Oral Microbiol. 2012, 4, 17659. [Google Scholar] [CrossRef] [Green Version]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of Seed Germination Performance through Cold Plasma Chemistry Technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Fernandez-Gutierrez, S.A.; Pedrow, P.D.; Pitts, M.J.; Powers, J. Cold Atmospheric-Pressure Plasmas Applied to Active Packaging of Apples. IEEE Trans. Plasma Sci. 2010, 38, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Brisset, J.-L.; Pawlat, J. Chemical Effects of Air Plasma Species on Aqueous Solutes in Direct and Delayed Exposure Modes: Discharge, Post-Discharge and Plasma Activated Water. Plasma Chem. Plasma Process. 2016, 36, 355–381. [Google Scholar] [CrossRef]
- Brisset, J.-L.; Hnatiuc, E. Peroxynitrite: A Re-Examination of the Chemical Properties of Non-Thermal Discharges Burning in Air Over Aqueous Solutions. Plasma Chem. Plasma Process. 2012, 32, 655–674. [Google Scholar] [CrossRef]
- Brisset, J.-L.; Moussa, D.; Doubla, A.; Hnatiuc, E.; Hnatiuc, B.; Kamgang Youbi, G.; Herry, J.-M.; Naïtali, M.; Bellon-Fontaine, M.-N. Chemical Reactivity of Discharges and Temporal Post-Discharges in Plasma Treatment of Aqueous Media: Examples of Gliding Discharge Treated Solutions. Ind. Eng. Chem. Res. 2008, 47, 5761–5781. [Google Scholar] [CrossRef]
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of PH on Bacterial Inactivation in Aqueous Solutions Due to Low-Temperature Atmospheric Pressure Plasma Application. Plasma Process. Polym. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Foster, J.; Sommers, B.S.; Gucker, S.N.; Blankson, I.M.; Adamovsky, G. Perspectives on the Interaction of Plasmas with Liquid Water for Water Purification. IEEE Trans. Plasma Sci. 2012, 40, 1311–1323. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; In Yong, H.; Choe, J.H.; Jeon, H.-J.; Choe, W.; Jo, C. The Use of Atmospheric Pressure Plasma-Treated Water as a Source of Nitrite for Emulsion-Type Sausage. Meat Sci. 2015, 108, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, T.; Ohshima, T.; Usui, E.; Ikawa, S.; Kitano, K.; Maeda, N.; Momoi, Y. Plasma-Treated Water Eliminates Streptococcus Mutans in Infected Dentin Model. Dent. Mater. J. 2017, 36, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia Coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Takai, E.; Kitano, K.; Kuwabara, J.; Shiraki, K. Protein Inactivation by Low-Temperature Atmospheric Pressure Plasma in Aqueous Solution. Plasma Process. Polym. 2012, 9, 77–82. [Google Scholar] [CrossRef]
- Julák, J.; Scholtz, V.; Kotúčová, S.; Janoušková, O. The Persistent Microbicidal Effect in Water Exposed to the Corona Discharge. Phys. Med. 2012, 28, 230–239. [Google Scholar] [CrossRef]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical Modification of Amino Acids by Atmospheric-Pressure Cold Plasma in Aqueous Solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-Phase Chemistry and Bactericidal Effects from an Air Discharge Plasma in Contact with Water: Evidence for the Formation of Peroxynitrite through a Pseudo-Second-Order Post-Discharge Reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwata, N.; Oh, J.-S.; Hahizume, H.; Ohta, T.; Takeda, K.; Ishikawa, K.; Hori, M.; Ito, M. Bactericidal Pathway OfEscherichia Coliin Buffered Saline Treated with Oxygen Radicals. J. Phys. D Appl. Phys. 2017, 50, 155208. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. Aureus and Physicochemical Properties of Plasma Activated Water Stored at Different Temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [Green Version]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Pawłat, J.; Terebun, P.; Kwiatkowski, M.; Tarabová, B.; Kovaľová, Z.; Kučerová, K.; Machala, Z.; Janda, M.; Hensel, K. Evaluation of Oxidative Species in Gaseous and Liquid Phase Generated by Mini-Gliding Arc Discharge. Plasma Chem. Plasma Process. 2019, 39, 627–642. [Google Scholar] [CrossRef] [Green Version]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-Thermal Atmospheric Pressure Discharges. J. Phys. D Appl. Phys. 2005, 38, R1–R24. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal Decontamination of Biological Media by Atmospheric-Pressure Plasmas: Review, Analysis, and Prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-Thermal Plasma Technologies: New Tools for Bio-Decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric-Pressure Plasma Sources for Biomedical Applications. Plasma Sources Sci. Technol. 2012, 21, 043001. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Reuter, S.; Niemi, K.; der Gathen, V.S.; Döbele, H.F. Generation of Atomic Oxygen in the Effluent of an Atmospheric Pressure Plasma Jet. Plasma Sources Sci. Technol. 2008, 18, 015006. [Google Scholar] [CrossRef]
- Ursache, M.; Moraru, R.; Hnatiuc, E.; Nastase, V.; Mares, M. Comparative Assessment of the Relation between Energy Consumption and Bacterial Burden Reduction Using Plasma Activated Water; IEEE: New York, NY, USA, 2014; pp. 1036–1041. ISBN 978-1-4799-5183-3. [Google Scholar]
- Burlica, R.; Kirkpatrick, M.J.; Locke, B.R. Formation of Reactive Species in Gliding Arc Discharges with Liquid Water. J. Electrost. 2006, 64, 35–43. [Google Scholar] [CrossRef]
- Kamgang-Youbi, G.; Herry, J.-M.; Brisset, J.-L.; Bellon-Fontaine, M.-N.; Doubla, A.; Naïtali, M. Impact on Disinfection Efficiency of Cell Load and of Planktonic/Adherent/Detached State: Case of Hafnia Alvei Inactivation by Plasma Activated Water. Appl. Microbiol. Biotechnol. 2008, 81, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined Effects of Long-Living Chemical Species during Microbial Inactivation Using Atmospheric Plasma-Treated Water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [Green Version]
- Kamgang-Youbi, G.; Herry, J.-M.; Meylheuc, T.; Brisset, J.-L.; Bellon-Fontaine, M.-N.; Doubla, A.; Naïtali, M. Microbial Inactivation Using Plasma-Activated Water Obtained by Gliding Electric Discharges. Lett. Appl. Microbiol. 2009, 48, 13–18. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-Term Antibacterial Efficacy of Air Plasma-Activated Water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef] [Green Version]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Lai, T.; Li, B.; Qin, G.; Tian, S. Oxidative Damage Involves in the Inhibitory Effect of Nitric Oxide on Spore Germination of Penicillium Expansum. Curr. Microbiol. 2011, 62, 229–234. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of Plasma Activated Water on Wheat: Germination, Growth Parameters, Photosynthetic Pigments, Soluble Protein Content, and Antioxidant Enzymes Activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma Activated Water: The next Generation Eco-Friendly Stimulant for Enhancing Plant Seed Germination, Vigor and Increased Enzyme Activity, a Study on Black Gram (Vigna Mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea Mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Tian, E.; Liu, D.; Niu, J.; Wang, W.; Qi, Z.; Xia, Y.; Song, Y.; Zhao, Z. Plasma-Activated Water Treatment of Fresh Beef: Bacterial Inactivation and Effects on Quality Attributes. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 113–120. [Google Scholar] [CrossRef]
- Yost, A.D.; Joshi, S.G. Atmospheric Nonthermal Plasma-Treated PBS Inactivates Escherichia Coli by Oxidative DNA Damage. PLoS ONE 2015, 10, e0139903. [Google Scholar] [CrossRef]
- Song, H.P.; Kim, B.; Choe, J.H.; Jung, S.; Moon, S.Y.; Choe, W.; Jo, C. Evaluation of Atmospheric Pressure Plasma to Improve the Safety of Sliced Cheese and Ham Inoculated by 3-Strain Cocktail Listeria Monocytogenes. Food Microbiol. 2009, 26, 432–436. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-Thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Tysiąc-Miśta, M.; Dubiel, A.; Brzoza, K.; Burek, M.; Pałkiewicz, K. Air Disinfection Procedures in the Dental Office during the COVID-19 Pandemic. Med. Pr. 2021, 72, 39–48. [Google Scholar] [CrossRef]
- Su, X.; Feng, M.; Rogers, S.; Holsen, T.M.; Thagard, S.M. The Role of High Voltage Electrode Material in the Inactivation of E. Coli by Direct-in-Liquid Electrical Discharge Plasma. Plasma Chem. Plasma Process. 2019, 39, 577–596. [Google Scholar] [CrossRef]
- Kim, H.-J.; Nam, G.-S.; Jang, J.-S.; Won, C.-H.; Kim, H.-W. Cold Plasma Treatment for Efficient Control over Algal Bloom Products in Surface Water. Water 2019, 11, 1513. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.A.; de M.G. Lima, G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Adhikari, M.; Kaushik, N.; Ghimire, B.; Adhikari, B.; Baboota, S.; Al-Khedhairy, A.A.; Wahab, R.; Lee, S.-J.; Kaushik, N.K.; Choi, E.H. Cold Atmospheric Plasma and Silymarin Nanoemulsion Synergistically Inhibits Human Melanoma Tumorigenesis via Targeting HGF/c-MET Downstream Pathway. Cell Commun. Signal. 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [Green Version]
- Daeschlein, G.; Hillmann, A.; Gümbel, D.; Sicher, C.; von Podewils, S.; Stope, M.B.; Jünger, M. Enhanced Anticancer Efficacy by Drug Chemotherapy and Cold Atmospheric Plasma Against Melanoma and Glioblastoma Cell Lines In Vitro. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 153–159. [Google Scholar] [CrossRef]
- Irani, S.; Shahmirani, Z.; Atyabi, S.M.; Mirpoor, S. Induction of Growth Arrest in Colorectal Cancer Cells by Cold Plasma and Gold Nanoparticles. Arch. Med. Sci. 2015, 11, 1286–1295. [Google Scholar] [CrossRef]
- Clemen, R.; Heirman, P.; Lin, A.; Bogaerts, A.; Bekeschus, S. Physical Plasma-Treated Skin Cancer Cells Amplify Tumor Cytotoxicity of Human Natural Killer (NK) Cells. Cancers 2020, 12, 3575. [Google Scholar] [CrossRef]
- Sklias, K.; Santos Sousa, J.; Girard, P.-M. Role of Short- and Long-Lived Reactive Species on the Selectivity and Anti-Cancer Action of Plasma Treatment In Vitro. Cancers 2021, 13, 615. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, S.; Zhang, H.; Kong, X.; Ding, L.; Shen, J.; Lan, Y.; Cheng, C.; Zhu, T.; Xia, W. Selective Effects of Non-Thermal Atmospheric Plasma on Triple-Negative Breast Normal and Carcinoma Cells through Different Cell Signaling Pathways. Sci. Rep. 2017, 7, 7980. [Google Scholar] [CrossRef]
- Girard, P.-M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic Effect of H2O2 and NO2 in Cell Death Induced by Cold Atmospheric He Plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [Green Version]
- Elsaadany, M.; Subramanian, G.; Ayan, H.; Yildirim-Ayan, E. Exogenous Nitric Oxide (NO) Generated by NO-Plasma Treatment Modulates Osteoprogenitor Cells Early Differentiation. J. Phys. D Appl. Phys. 2015, 48, 345401. [Google Scholar] [CrossRef]
- Balzer, J.; Heuer, K.; Demir, E.; Hoffmanns, M.A.; Baldus, S.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. Non-Thermal Dielectric Barrier Discharge (DBD) Effects on Proliferation and Differentiation of Human Fibroblasts Are Primary Mediated by Hydrogen Peroxide. PLoS ONE 2015, 10, e0144968. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Fridman, A.; Friedman, G.; Clyne, A.M. Non-Thermal Dielectric Barrier Discharge Plasma Treatment of Endothelial Cells. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 3578–3581. [Google Scholar] [CrossRef]
- Baik, K.Y.; Kim, Y.H.; Hur, E.-H. Selective Toxicity on Canine Blood Cells by Using Atmospheric-Pressure Plasma Jets. J. Korean Phys. Soc. 2012, 60, 965–969. [Google Scholar] [CrossRef]
- Ye, F.; Kaneko, H.; Nagasaka, Y.; Ijima, R.; Nakamura, K.; Nagaya, M.; Takayama, K.; Kajiyama, H.; Senga, T.; Tanaka, H.; et al. Plasma-Activated Medium Suppresses Choroidal Neovascularization in Mice: A New Therapeutic Concept for Age-Related Macular Degeneration. Sci. Rep. 2015, 5, 7705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, S.; Zhang, J.; Wang, Z.; Liu, D.; Guo, L.; Cheng, C.; Cheng, Y.; Xu, D.; Kong, M.G.; et al. Plasma-Activated Thermosensitive Biogel as an Exogenous ROS Carrier for Post-Surgical Treatment of Cancer. Biomaterials 2021, 276, 121057. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, S.; Li, B.; Wang, S.; Zhang, H.; Li, Q.; Liu, Z.; Guo, B.; Liu, D.; Xu, D. Systematic Safety Evaluation of Cold Plasma-Activated Liquid in Rabbits. Front. Phys. 2021, 9, 481. [Google Scholar] [CrossRef]
- Xu, D.; Cui, Q.; Xu, Y.; Wang, B.; Tian, M.; Li, Q.; Liu, Z.; LIU, D.; Chen, H.; Kong, M.G. Systemic Study on the Safety of Immuno-Deficient Nude Mice Treated by Atmospheric Plasma-Activated Water. Plasma Sci. Technol. 2018, 20, 044003. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Privat-Maldonado, A.; Gorbanev, Y.; O’Connell, D.; Vann, R.; Chechik, V.; van der Woude, M.W. Nontarget Biomolecules Alter Macromolecular Changes Induced by Bactericidal Low–Temperature Plasma. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 121–128. [Google Scholar] [CrossRef]
- İbiş, F.; Ercan, U.K. Inactivation of Biofilms in Endotracheal Tube by Cold Atmospheric Plasma Treatment for Control and Prevention of Ventilator-Associated Pneumonia. Plasma Process. Polym. 2020, 17, 2000065. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and Biological Mechanisms of Direct Plasma Interaction with Living Tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Ye, G.; Zhang, Q.; Wang, J.; Zhang, J.; Fang, J. In Vitro Studies of the Antimicrobial Effect of Non-Thermal Plasma-Activated Water as a Novel Mouthwash. Eur. J. Oral Sci. 2017, 125, 463–470. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and Medical Applications of Plasma-Activated Media, Water and Solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Han, S.H.; Suh, H.J.; Hong, K.B.; Kim, S.Y.; Min, S.C. Oral Toxicity of Cold Plasma-Treated Edible Films for Food Coating. J. Food Sci. 2016, 81, T3052–T3057. [Google Scholar] [CrossRef]
- Umbreit, J. Methemoglobin—It’s Not Just Blue: A Concise Review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef]
- Naoum, P.C.; Radispiel, J.; da S. Moraes, M. Dosagem Espectrométrica de Metaemoglobina Sem Interferentes Químicos Ou Enzimáticos. Rev. Bras. Hematol. Hemoter. 2004, 26, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, Pharmacology, and Clinical Management. Ann. Emerg. Med. 1999, 34, 646–656. [Google Scholar] [CrossRef]
- Bradberry, S. Methaemoglobinaemia. Medicine 2016, 44, 91–92. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold Atmospheric Plasma Activated Water as a Prospective Disinfectant: The Crucial Role of Peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Bostanaru, A.-C.; Hnatiuc, E.; Rosca, I.; Vasiliu, A.L.; Doroftei, M.; Ursu, L.; Ailincai, L.I.; Nastasa, V.; Mares, M. Long thermal interactions of PAW with normal tooth structure and different dental biomaterials. In Advanced Topics in Optoelectronics, Microelectronics, and Nanotechnologies Viii; Vladescu, M., Tamas, R., Cristea, I., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2016; Volume 10010, p. UNSP 100103F. ISBN 978-1-5106-0424-7. [Google Scholar]
- Cheng, Y.-C.; Wu, C.-H.; Liu, C.-T.; Lin, C.-Y.; Chiang, H.-P.; Chen, T.-W.; Chen, C.-Y.; Wu, J.-S. Tooth Bleaching by Using a Helium-Based Low-Temperature Atmospheric Pressure Plasma Jet with Saline Solution. Plasma Process. Polym. 2017, 14, 1600235. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.L.; Liu, C.M.; Tian, Y.; Yu, S.; Wang, K.L.; Zhang, J.; Fang, J. Investigation of Cold Atmospheric Plasma-Activated Water for the Dental Unit Waterline System Contamination and Safety Evaluation in Vitro. Plasma Chem. Plasma Process. 2017, 37, 1091–1103. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Stoffels, E.; Walraven, R.; Tielbeek, P.J.A.; Koolhoven, R.A. Plasma Treatment of Dental Cavities: A Feasibility Study. IEEE Trans. Plasma Sci. 2004, 32, 1540–1543. [Google Scholar] [CrossRef]
- El-Wassefy, N.A. The Effect of Plasma Treatment and Bioglass Paste on Enamel White Spot Lesions. Saudi J. Dent. Res. 2017, 8, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Scaramucci, T.; Carvalho, J.C.; Hara, A.T.; Zero, D.T. Causes of Dental Erosion: Intrinsic Factors. In Dental Erosion and Its Clinical Management; Amaechi, B.T., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 35–67. ISBN 978-3-319-13993-7. [Google Scholar]
- Hara, A.T.; Carvalho, J.C.; Zero, D.T. Causes of Dental Erosion: Extrinsic Factors. In Dental Erosion and Its Clinical Management; Amaechi, B.T., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 69–96. ISBN 978-3-319-13993-7. [Google Scholar]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–Remineralization Dynamics in Teeth and Bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. 6. Calcium Phosphates in Enamel, Dentin and Bone. Calcium Phosphates Oral Biol. Med. 1991, 15, 108–129. [Google Scholar] [CrossRef]
- Hnatiuc, E.; Hnatiuc, B. Sistem Multielectrod Pentru Realizarea Reactoarelor Electrochimice Cu Plasma Rece Si Circuit Pentru Comanda Si Reglajul Functionarii Acestora, OSIM Romania, BOPI nr. 6/1997. p. 75. Available online: https://osim.ro/wp-content/uploads/Publicatii-OSIM/BOPI-Inventii/1997/bopi0697.pdf (accessed on 21 October 2021).
- Pasca, S.; Nastasa, V.; Bostanaru, A.; Grecu, M.; Mares, M.; Hnatiuc, E. Toxic Amyloid Formation in Mice: A Possible Link between Bisphenol A Exposure and PAW Administration Using Polycarbonate Bottles; IEEE: New York, NY, USA, 2017; pp. 1075–1080. ISBN 978-1-5090-4489-4. [Google Scholar]
- OECD Test No.408: Repeated Dose 90-Day Oral Toxicity Study in Rodents; OECD Publishing: Paris, France, 2018.
- Marxfeld, H.A.; Küttler, K.; Dammann, M.; Gröters, S.; van Ravenzwaay, B. Body and Organ Weight Data in 28-Day Toxicological Studies in Two Mouse Strains. Data Brief 2019, 27, 104632. [Google Scholar] [CrossRef]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting Proliferating Cell Nuclear Antigen (PCNA) as an Effective Strategy to Inhibit Tumor Cell Proliferation. Curr. Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef]
- Lu, E.M.-C.; Ratnayake, J.; Rich, A.M. Assessment of Proliferating Cell Nuclear Antigen (PCNA) Expression at the Invading Front of Oral Squamous Cell Carcinoma. BMC Oral Health 2019, 19, 233. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ichikawa, J.; Ochiai, T.; Yoshida, Y.; Haro, H.; Suzuki-Karasaki, Y. Combined Anticancer Effect of Plasma-Activated Infusion and Salinomycin by Targeting Autophagy and Mitochondrial Morphology. Front. Oncol. 2021, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
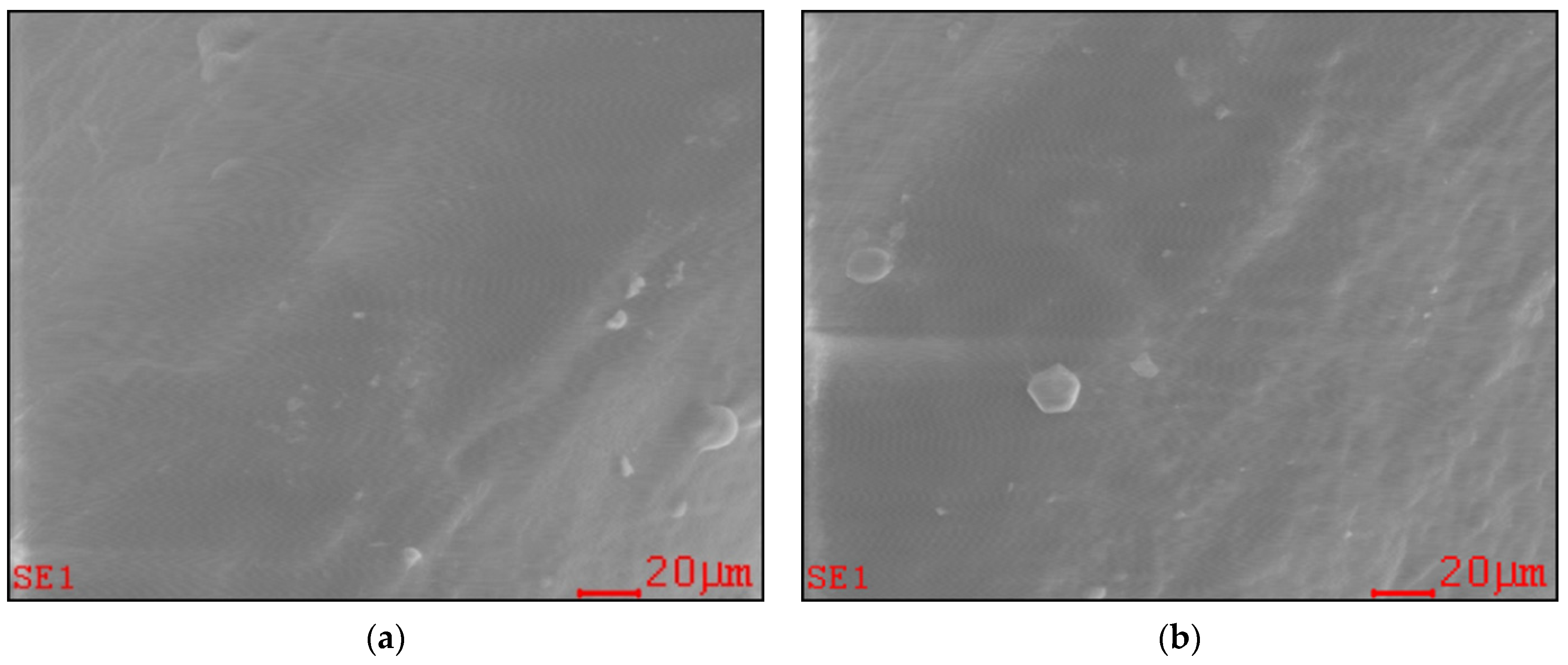



| Minerals | Control(Mean ± SD) | PAW(Mean ± SD) |
|---|---|---|
| Carbon (C) | 66.19 ± 6.04 | 58.39 ± 7.85 |
| Nitrogen (N) | 10.28 ± 1.69 | 8.37 ± 1.41 |
| Oxygen (O) | 19.70 ± 4.34 | 24.13 ± 5.10 |
| Fluorine (F) | 0.14 ± 0.06 | 0.20 ± 0.16 |
| Sodium (Na) | 0.11 ± 0.07 | 0.18 ± 0.06 |
| Magnesium (Mg) | 0.16 ± 0.11 | 0.29 ± 0.15 |
| Aluminum (Al) | 0.05 ± 0.03 | 0.12 ± 0.07 |
| Silicon (Si) | 0.05 ± 0.02 | 0.05 ± 0.01 |
| Phosphorus (P) | 1.52 ± 1.18 | 3.39 ± 1.59 |
| Chlorine (Cl) | 0.00 ± 0.00 | 0.01 ± 0.01 |
| * Potassium (K) | 0.02 ± 0.01 | 0.06 ± 0.02 |
| Calcium (Ca) | 2.07 ± 1.57 | 4.75 ± 2.19 |
| ORGAN | Control (Mean ± SD) | PAW (Mean ± SD) | ||
|---|---|---|---|---|
| Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | |
| Heart | 0.18 ± 0.02 | 0.49 ± 0.04 | 0.17 ± 0.03 | 0.49 ± 0.08 |
| * Spleen | 0.16 ± 0.03 | 0.43 ± 0.04 | 0.13 ± 0.02 | 0.36 ± 0.03 |
| Liver | 1.45 ± 0.01 | 3.96 ± 0.37 | 1.33 ± 0.14 | 3.87 ± 0.31 |
| Kidneys | 0.45 ± 0.08 | 1.22 ± 0.12 | 0.39 ± 0.05 | 1.14 ± 0.10 |
| Brain | 0.49 ± 0.01 | 1.34 ± 0.15 | 0.50 ± 0.04 | 1.43 ± 0.11 |
| Bodyweight | 36.81 ± 4.95 | 34.43 ± 3.15 | ||
| Parameter | Control | PAW |
|---|---|---|
| RBC (1012/L) | 9.39 ± 0.55 | 9.22 ± 1.20 |
| HGB (g/dL) | 14.12 ± 0.93 | 13.05 ± 2.00 |
| HCT (%) | 45.48 ± 3.66 | 44.19 ± 7.26 |
| MCV (fl) | 48.00 ± 3.33 | 47.80 ± 4.96 |
| MCH (pg) | 14.64 ± 0.83 | 14.28 ± 0.91 |
| MCHC (g/dL) | 31.84 ± 2.62 | 31.64 ± 2.40 |
| RDWc (%) | 18.90 ± 1.55 | 18.81 ± 1.19 |
| RDWs (%) | 35.56 ± 6.73 | 33.83 ± 7.48 |
| PLT (109/L) | 367.10 ± 147.10 | 368.70 ± 118.10 |
| PCT (%) | 0.35 ± 0.05 | 0.32 ± 0.93 |
| MPV (fl) | 6.47 ± 0.26 | 6.66 ± 0.42 |
| PDWc (%) | 28.30 ± 1.45 | 29.10 ± 2.11 |
| PDWs (fl) | 7.84 ± 0.79 | 7.84 ± 0.87 |
| WBC (109/L) | 3.59 ± 0.82 | 3.77 ± 1.37 |
| NEU (109/L) | 0.36 ± 0.07 | 0.34 ± 0.10 |
| LIMF (109/L) | 2.94 ± 0.76 | 3.32 ± 1.22 |
| MONO (109/L) | 0.11 ± 0.02 | 0.12 ± 0.05 |
| EOS (109/L) | <min | <min |
| BASO (109/L) | <min | <min |
| NE (%) | 11.94 ± 2.84 | 10.80 ± 1.88 |
| LIMF (%) | 84.61 ± 3.76 | 85.12 ± 2.87 |
| MONO (%) | 3.60 ± 0.92 | 3.27 ± 0.99 |
| EOS (%) | - | - |
| BASO (%) | - | - |
| Parameter | Control | PAW |
|---|---|---|
| BUN (mg/dL) | 27.20 ± 5.59 | 29.90 ± 6.36 |
| GLU (mg/dL) | 137.50 ± 30.28 | 142.90 ± 11.97 |
| ALP/ALK (U/L) | 126.60 ± 20.44 | 135.10 ± 15.41 |
| GPT/ALT (U/L) | 21.10 ± 8.27 | 20.40 ± 4.97 |
| T-PRO (g/dL) | 5.25 ± 0.46 | 5.15 ± 0.69 |
| CREAT (mg/dL) | 0.96 ± 0.18 | 0.94 ± 0.20 |
| Cytokine | Control | PAW |
|---|---|---|
| IL-1a | 4.17 ± 0.71 | 3.54 ± 0.37 |
| IL-1b | <min | <min |
| IL-2 | 3.20 ± 0.01 | 2.67 ± 5.98 |
| IL-3 | 3.04 ± 0.61 | 2.17 ± 0.46 |
| IL-4 | 0.78 ± 0.49 | <min |
| IL-5 | 3.68 ± 0.76 | 1.92 ± 1.19 |
| IL-6 | 2.94 ± 0.26 | 2.30 ± 0.39 |
| IL-9 | 13.45 ± 2.5 | 6.89 ± 6.33 |
| IL-10 | 44.40 ± 27.65 | 15.25 ± 4.91 |
| IL-12 (p40) | 274.06 ± 46.64 | 214.86 ± 18.95 |
| IL-12 (p70) | 82.91 ± 13.87 | 60.61 ± 7.65 |
| IL-13 | 35.62 ± 10.56 | 60.61 ± 30.20 |
| IL-17A | 51.32 ± 16.76 | 12.78 ± 16.19 |
| IL-17F | 0.36 ± 0.32 | 0.29 ± 0.32 |
| IL-21 | <min | <min |
| IL-22 | 1.29 ± 0.64 | 1.71 ± 0.52 |
| IL-23 (p19) | 4.10 ± 4.30 | 2.55 ± 2.70 |
| IL-25 | 2.59 ± 1.53 | 0.53 ± 0.26. |
| IL-27 | 1.48 ± 1.46 | 0.34 ± 0.19 |
| IL-31 | 3.47 ± 4.75 | 3.43 ± 4.69 |
| IL-33 | <min | <min |
| Eotaxin | 249.84 ± 69.02 | 211.09 ± 73.41 |
| G-CSF | 44.21 ± 7.69 | 38.29 ± 13.06 |
| GM-CSF | 24.10 ± 2.72 | 19.91 ± 4.35 |
| IFN-γ | 11.03 ± 1.88 | 5.72 ± 2.02 |
| KC | 14.15 ± 1.53 | 21.39 ± 23.43 |
| CD40L | <min | <min |
| MIP-3α | 0.60 ± 0.40 | 0.87 ± 1.02 |
| MCP-1 | 85.80 ± 15.36 | 52.14 ± 13.91 |
| MIP-1α | 2.47 ± 0.50 | 1.10 ± 0.70 |
| MIP-1β | 6.42 ± 4.25 | 6.72 ± 5.09 |
| RANTES | 26.08 ± 5.76 | 17.64 ± 2.07 |
| TNF-α | 45.54 ± 10.67 | 43.07 ± 14.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastasa, V.; Pasca, A.-S.; Malancus, R.-N.; Bostanaru, A.-C.; Ailincai, L.-I.; Ursu, E.-L.; Vasiliu, A.-L.; Minea, B.; Hnatiuc, E.; Mares, M. Toxicity Assessment of Long-Term Exposure to Non-Thermal Plasma Activated Water in Mice. Int. J. Mol. Sci. 2021, 22, 11534. https://doi.org/10.3390/ijms222111534
Nastasa V, Pasca A-S, Malancus R-N, Bostanaru A-C, Ailincai L-I, Ursu E-L, Vasiliu A-L, Minea B, Hnatiuc E, Mares M. Toxicity Assessment of Long-Term Exposure to Non-Thermal Plasma Activated Water in Mice. International Journal of Molecular Sciences. 2021; 22(21):11534. https://doi.org/10.3390/ijms222111534
Chicago/Turabian StyleNastasa, Valentin, Aurelian-Sorin Pasca, Razvan-Nicolae Malancus, Andra-Cristina Bostanaru, Luminita-Iuliana Ailincai, Elena-Laura Ursu, Ana-Lavinia Vasiliu, Bogdan Minea, Eugen Hnatiuc, and Mihai Mares. 2021. "Toxicity Assessment of Long-Term Exposure to Non-Thermal Plasma Activated Water in Mice" International Journal of Molecular Sciences 22, no. 21: 11534. https://doi.org/10.3390/ijms222111534
APA StyleNastasa, V., Pasca, A.-S., Malancus, R.-N., Bostanaru, A.-C., Ailincai, L.-I., Ursu, E.-L., Vasiliu, A.-L., Minea, B., Hnatiuc, E., & Mares, M. (2021). Toxicity Assessment of Long-Term Exposure to Non-Thermal Plasma Activated Water in Mice. International Journal of Molecular Sciences, 22(21), 11534. https://doi.org/10.3390/ijms222111534








