Role of Cytokines in Vitiligo: Pathogenesis and Possible Targets for Old and New Treatments
Abstract
1. Introduction
2. Vitiligo Pathogenesis: Ab Origine Development, from Gene to Cellular Dynamics
2.1. Predisposing Factors
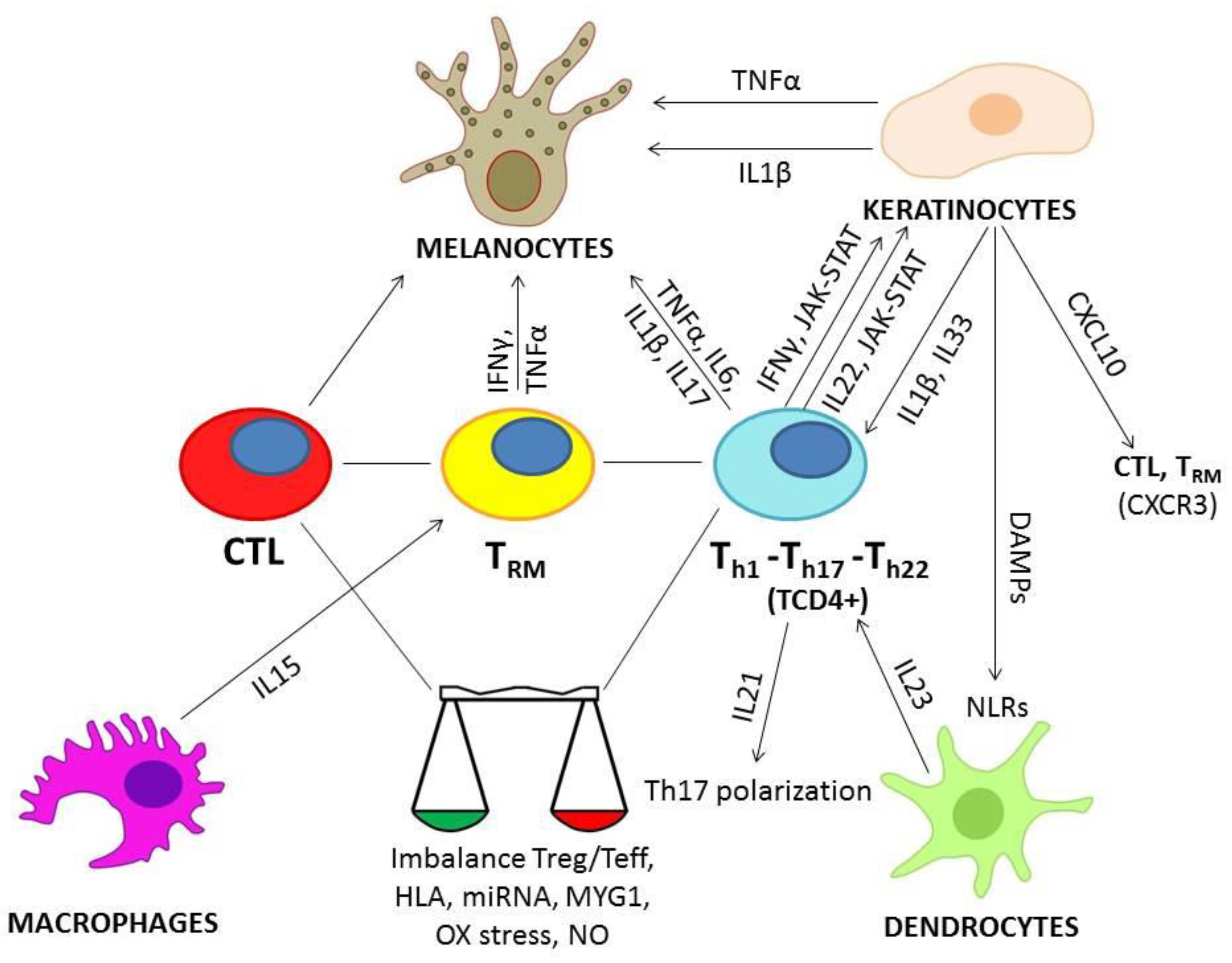
2.2. Role of Cytokines in Vitiligo
2.2.1. INFγ
2.2.2. TNFα
2.2.3. IL-33
2.2.4. IL-1β
2.2.5. IL-6
2.2.6. IL-17
2.2.7. IL-22
2.2.8. IL-21, IL-23, IL-15
3. Vitiligo Treatment Options: Past, Present and Future Therapies
3.1. Systemic Therapeutic Approaches and Biologics for the Treatment of Vitiligo: Rationale, Efficacy and Safety
3.2. Perspectives to Explore
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet 2015, 386, 74–84. [Google Scholar] [CrossRef]
- Van Geel, N.; Bosma, S.; Boone, B.; Speeckaert, R. Classification of segmental vitiligo on the trunk. Br. J. Dermatol. 2014, 170, 322–327. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Odorisio, T.; Sorrenti, S.; Catania, A.; Tartaglia, F.; Carbotta, G.; Pironi, D.; Rendina, R.; D’Armiento, E.; Persechino, S.; et al. Vitiligo and Autoimmune Thyroid Disorders. Front. Endocrinol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Ezzedine, K.; Hamzavi, I.; Pandya, A.; Harris, J.E. New discoveries in the pathogenesis and classification of vitiligo. J. Am. Acad. Dermatol. 2017, 77, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Al-Shobaili, H.A. Update on the genetics characterization of vitiligo. Int. J. Health Sci. 2011, 5, 167–179. [Google Scholar]
- Fain, P.R.; Babu, S.R.; Bennett, D.C.; Spritz, R.A. HLA class II haplotype DRB1*04-DQB1*0301 contributes to risk of familial generalized vitiligo and early disease onset. Pigment. Cell Res. 2006, 19, 51–57. [Google Scholar] [CrossRef]
- Philips, M.-A.; Kingo, K.; Karelson, M.; Rätsep, R.; Aunin, E.; Reimann, E.; Reemann, P.; Porosaar, O.; Vikeså, J.; Nielsen, F.C.; et al. Promoter polymorphism-119C/G in MYG1 (C12orf10) gene is related to vitiligo susceptibility and Arg4Gln affects mitochondrial entrance of Myg1. BMC Med. Genet. 2010, 11, 56. [Google Scholar] [CrossRef]
- Spritz, R.A.; Andersen, G.H. Genetics of Vitiligo. Dermatol. Clin. 2017, 35, 245–255. [Google Scholar] [CrossRef]
- Shang, Z.; Li, H. Altered expression of four miRNA (miR-1238-3p, miR-202-3p, miR-630 and miR-766-3p) and their potential targets in peripheral blood from vitiligo patients. J. Dermatol. 2017, 44, 1138–1144. [Google Scholar] [CrossRef]
- Aguennouz, M.; Guarneri, F.; Oteri, R.; Polito, F.; Giuffrida, R.; Cannavò, S.P. Serum levels of miRNA-21-5p in vitiligo patients and effects of miRNA-21-5p on SOX5, beta-catenin, CDK2 and MITF protein expression in normal human melanocytes. J. Dermatol. Sci. 2020, 101, 22–29. [Google Scholar] [CrossRef]
- Magenta, A.; Dellambra, E.; Ciarapica, R.; Capogrossi, M.C. Oxidative stress, microRNAs and cytosolic calcium homeostasis. Cell Calcium 2016, 60, 207–217. [Google Scholar] [CrossRef]
- Colucci, R.; Dragoni, F.; Moretti, S. Oxidative Stress and Immune System in Vitiligo and Thyroid Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 631927. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Li, C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 2019, 25, 1017–1023. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Gibbons, N.C.J.; Zothner, C.; Abou Elloof, M.M.; Wood, J.M. Hydrogen peroxide-mediated oxidative stress disrupts calcium binding on calmodulin: More evidence for oxidative stress in vitiligo. Biochem. Biophys. Res. Commun. 2007, 360, 70–75. [Google Scholar] [CrossRef]
- Tachibana, M. MITF: A stream flowing for pigment cells. Pigment. Cell Res. 2000, 13, 230–240. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Jian, Z.; Li, K.; Song, P.; Zhu, G.; Zhu, L.; Cui, T.; Liu, B.; Tang, L.; Wang, X.; Wang, G.; et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014, 134, 2221–2230. [Google Scholar] [CrossRef]
- Lowenstein, C.J.; Dinerman, J.L.; Snyder, S.H. Nitric oxide: A physiologic messenger. Ann. Intern. Med. 1994, 120, 227–237. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Lerner, L.H.; Lerner, E.A. From bedside to the bench and back. Nitric oxide and cutis. Arch. Dermatol. 1996, 132, 889–893. [Google Scholar] [CrossRef]
- Vaccaro, M.; Irrera, N.; Cutroneo, G.; Rizzo, G.; Vaccaro, F.; Anastasi, G.P.; Borgia, F.; Cannavò, S.P.; Altavilla, D.; Squadrito, F. Differential Expression of Nitric Oxide Synthase Isoforms nNOS and iNOS in Patients with Non-Segmental Generalized Vitiligo. Int. J. Mol. Sci. 2017, 18, 2533. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bagnato, G.; Cristani, M.; Borgia, F.; Spatari, G.; Tigano, V.; Saja, A.; Guarneri, F.; Cannavò, S.P.; Gangemi, S. Oxidation products are increased in patients affected by non-segmental generalized vitiligo. Arch. Dermatol. Res. 2017, 309, 485–490. [Google Scholar] [CrossRef]
- Reichert Faria, A.; Jung, J.E.; Silva de Castro, C.C.; de Noronha, L. Reduced immunohistochemical expression of adhesion molecules in vitiligo skin biopsies. Pathol. Res. Pract. 2017, 213, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, M.; Castellani, C.; Fedrigo, M.; Thiene, G.; Peserico, A.; Alaibac, M.; Angelini, A. Role of alpha5beta1 integrin and MIA (melanoma inhibitory activity) in the pathogenesis of vitiligo. J. Dermatol. Sci. 2013, 71, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yi, H.; He, X.; Luo, L.; Jiang, S.; Shi, Y. miR-9 regulates melanocytes adhesion and migration during vitiligo repigmentation induced by UVB treatment. Exp. Cell Res. 2019, 384, 111615. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.S.; Dwivedi, M.; Begum, R. Decreased suppression of CD8+ and CD4+ T cells by peripheral regulatory T cells in generalized vitiligo due to reduced NFATC1 and FOXP3 proteins. Exp. Dermatol. 2020, 29, 759–775. [Google Scholar] [CrossRef]
- Boniface, K.; Seneschal, J.; Picardo, M.; Taïeb, A. Vitiligo: Focus on Clinical Aspects, Immunopathogenesis, and Therapy. Clin. Rev. Allergy Immunol. 2018, 54, 52–67. [Google Scholar] [CrossRef]
- Boniface, K.; Passeron, T.; Seneschal, J.; Tulic, M.K. Targeting Innate Immunity to Combat Cutaneous Stress: The Vitiligo Perspective. Front. Immunol. 2021, 12, 613056. [Google Scholar] [CrossRef]
- Li, S.; Kang, P.; Zhang, W.; Jian, Z.; Zhang, Q.; Yi, X.; Guo, S.; Guo, W.; Shi, Q.; Li, B.; et al. Activated NLR family pyrin domain containing 3 (NLRP3) inflammasome in keratinocytes promotes cutaneous T-cell response in patients with vitiligo. J. Allergy Clin. Immunol. 2020, 145, 632–645. [Google Scholar] [CrossRef]
- Levandowski, C.B.; Mailloux, C.M.; Ferrara, T.M.; Gowan, K.; Ben, S.; Jin, Y.; McFann, K.K.; Holland, P.J.; Fain, P.R.; Dinarello, C.A.; et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1β processing via the NLRP1 inflammasome. Proc. Natl. Acad. Sci. USA 2013, 110, 2952–2956. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Wańkowicz-Kalińska, A.; van den Wijngaard, R.M.; Tigges, B.J.; Westerhof, W.; Ogg, G.S.; Cerundolo, V.; Storkus, W.J.; Das, P.K. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab. Investig. 2003, 83, 683–695. [Google Scholar] [CrossRef]
- Chatterjee, S.; Eby, J.M.; Al-Khami, A.A.; Soloshchenko, M.; Kang, H.K.; Kaur, N.; Naga, O.S.; Murali, A.; Nishimura, M.I.; Caroline Le Poole, I.; et al. A quantitative increase in regulatory T cells controls development of vitiligo. J. Investig. Dermatol. 2014, 134, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Erf, G.F. IFN-γ, IL-21, and IL-10 co-expression in evolving autoimmune vitiligo lesions of Smyth line chickens. J. Investig. Dermatol. 2012, 132 Pt 1, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wei, Y.; Sun, Y.; Shi, W.; Yang, J.; Zhu, L.; Li, M. Interferon-gamma Inhibits Melanogenesis and Induces Apoptosis in Melanocytes: A Pivotal Role of CD8+ Cytotoxic T Lymphocytes in Vitiligo. Acta Derm. Venereol. 2015, 95, 664–670. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, M.; Lin, F.; Liu, D.; Hong, W.; Lu, L.; Zhu, Y.; Xu, A. Interferon-γ induces senescence in normal human melanocytes. PLoS ONE 2014, 9, e93232. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Abdel Motaleb, A.A.; Tawfik, Y.M.; El-Mokhtar, M.A.; Elkady, S.; El-Gazzar, A.F.; ElSayed, S.K.; Awad, S.M.; Cutaneous, J.A.K. Expression in Vitiligo. J. Cutan. Med. Surg. 2021, 25, 157–162. [Google Scholar] [CrossRef]
- Caretto, D.; Katzman, S.D.; Villarino, A.V.; Gallo, E.; Abbas, A.K. Cutting edge: The Th1 response inhibits the generation of peripheral regulatory T cells. J. Immunol. 2010, 184, 30–34. [Google Scholar] [CrossRef]
- Samaka, R.M.; Basha, M.A.; Menesy, D. Role of Janus kinase 1 and signal transducer and activator of transcription 3 in vitiligo. Clin. Cosmet. Investig. Dermatol. 2019, 12, 469–480. [Google Scholar] [CrossRef]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef]
- Richmond, J.M.; Bangari, D.S.; Essien, K.I.; Currimbhoy, S.D.; Groom, J.R.; Pandya, A.G.; Youd, M.E.; Luster, A.D.; Harris, J.E. Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis during Vitiligo and May Serve as Biomarkers of Disease. J. Investig. Dermatol. 2017, 137, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Salas-Alanis, J.C. The role of tumor necrosis factor-α in the pathogenesis of vitiligo. Am. J. Clin. Dermatol. 2013, 14, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mansuri, M.S.; Kadam, A.; Palit, S.P.; Dwivedi, M.; Laddha, N.C.; Begum, R. Tumor Necrosis Factor-alpha affects melanocyte survival and melanin synthesis via multiple pathways in vitiligo. Cytokine 2021, 140, 155432. [Google Scholar] [CrossRef]
- Biton, J.; Boissier, M.C.; Bessis, N. TNFα: Activator or inhibitor of regulatory T cells? Joint Bone Spine 2012, 79, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, H.; Han, D.; Mou, K. Interleukin-33 affects cytokine production by keratinocytes in vitiligo. Clin. Exp. Dermatol. 2015, 40, 163–170. [Google Scholar] [CrossRef]
- Vaccaro, M.; Cicero, F.; Mannucci, C.; Calapai, G.; Spatari, G.; Barbuzza, O.; Cannavò, S.P.; Gangemi, S. IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch. Dermatol. Res. 2016, 308, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Marie, J.; Kovacs, D.; Pain, C.; Jouary, T.; Cota, C.; Vergier, B.; Picardo, M.; Taieb, A.; Ezzedine, K.; Cario-André, M. Inflammasome activation and vitiligo/nonsegmental vitiligo progression. Br. J. Dermatol. 2014, 170, 816–823. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Rani, S.; Srivastava, N.; Kumar, R.; Parsad, D. Increased systemic and epidermal levels of IL-17A and IL-1β promotes progression of non-segmental vitiligo. Cytokine 2017, 91, 153–161. [Google Scholar] [CrossRef]
- Laddha, N.C.; Dwivedi, M.; Mansuri, M.S.; Singh, M.; Patel, H.H.; Agarwal, N.; Shah, A.M.; Begum, R. Association of neuropeptide Y (NPY), interleukin-1B (IL1B) genetic variants and correlation of IL1B transcript levels with vitiligo susceptibility. PLoS ONE 2014, 9, e107020. [Google Scholar]
- Kholmanskikh, O.; van Baren, N.; Brasseur, F.; Ottaviani, S.; Vanacker, J.; Arts, N.; van der Bruggen, P.; Coulie, P.; De Plaen, E. Interleukins 1alpha and 1beta secreted by some melanoma cell lines strongly reduce expression of MITF-M and melanocyte differentiation antigens. Int. J. Cancer 2010, 127, 1625–1636. [Google Scholar] [CrossRef]
- Sushama, S.; Dixit, N.; Gautam, R.K.; Arora, P.; Khurana, A.; Anubhuti, A. Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF-α) in vitiligo-New insight into pathogenesis of disease. J. Cosmet. Dermatol. 2019, 18, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Karagün, E.; Baysak, S. Levels of TNF-α, IL-6, IL-17, IL-37 cytokines in patients with active vitiligo. Aging Male 2020, 23, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Hu, D.N.; Chen, M.; Li, S.S. Subtoxic levels hydrogen peroxide-induced expression of interleukin-6 by epidermal melanocytes. Arch. Dermatol. Res. 2012, 304, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Ortonne, J.P. Activation of the unfolded protein response in vitiligo: The missing link? J. Investig. Dermatol. 2012, 132, 2502–2504. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Jadeja, S.D.; Vaishnav, J.; Mansuri, M.S.; Shah, C.; Mayatra, J.M.; Shah, A.; Begum, R. Investigation of the Role of Interleukin 6 in Vitiligo Pathogenesis. Immunol. Investig. 2020, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, D.A.; Shaker, O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin. Exp. Dermatol. 2011, 36, 292–297. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Y.; Chen, S.; Wang, L.; Jiang, M.; Xiang, L. Circulating CCL20: A potential biomarker for active vitiligo together with the number of Th1/17 cells. J. Dermatol. Sci. 2019, 93, 92–100. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, Y.L.; Li, K.; Hamzavi, I.; Gao, T.W.; Huggins, R.H.; Lim, H.W.; Mi, Q.S. Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development. Pigment. Cell Melanoma Res. 2015, 28, 324–329. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Elela, M.A.; Hegazy, R.A.; Fawzy, M.M.; Rashed, L.A.; Rasheed, H. Interleukin 17, interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: A case-controlled study on eighty-four patients. Eur. J. Dermatol. 2013, 23, 350–355. [Google Scholar] [CrossRef]
- Dong, J.; An, X.; Zhong, H.; Wang, Y.; Shang, J.; Zhou, J. Interleukin-22 participates in the inflammatory process of vitiligo. Oncotarget 2017, 8, 109161–109174. [Google Scholar] [CrossRef][Green Version]
- Vaccaro, M.; Cannavò, S.P.; Imbesi, S.; Cristani, M.; Barbuzza, O.; Tigano, V.; Gangemi, S. Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int. J. Dermatol. 2015, 54, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Mukhtar, M.M.; Bakheit, K.H.; Hamdan, H.Z. Plasma Levels of Interleukin-17, Interleukin-23, and Transforming Growth Factor-β in Sudanese Patients with Vitiligo: A Case-Control Study. Indian J. Dermatol. 2015, 60, 635. [Google Scholar] [CrossRef] [PubMed]
- Atwa, M.A.; Ali, S.M.M.; Youssef, N.; Marie, R.E.M. Elevated serum level of interleukin-15 in vitiligo patients and its correlation with disease severity but not activity. J. Cosmet. Dermatol. 2020, 20, 2640–2644. [Google Scholar] [CrossRef]
- Tokura, Y.; Phadungsaksawasdi, P.; Kurihara, K.; Fujiyama, T.; Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol. 2021, 11, 618897. [Google Scholar] [CrossRef]
- Seneschal, J.; Boniface, K.; D’Arino, A.; Picardo, M. An update on Vitiligo pathogenesis. Pigment. Cell Melanoma Res. 2021, 34, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, F.C. Therapeutic monoclonal antibodies. Lancet 2000, 355, 735–740. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Gottlieb, A.B. Ustekinumab for psoriasis and psoriatic arthritis. J. Rheumatol. Suppl. 2012, 89, 86–89. [Google Scholar] [PubMed]
- Elkady, A.; Bonomo, L.; Amir, Y.; Vekaria, A.S.; Guttman-Yassky, E. Effective use of ustekinumab in a patient with concomitant psoriasis, vitiligo, and alopecia areata. JAAD Case Rep. 2017, 3, 477–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Méry-Bossard, L.; Bagny, K.; Chaby, G.; Khemis, A.; Maccari, F.; Marotte, H.; Perrot, J.L.; Reguiai, Z.; Sigal, M.L.; Avenel-Audran, M.; et al. New-onset vitiligo and progression of pre-existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 181–186. [Google Scholar] [CrossRef]
- Anthony, N.; Bourneau-Martin, D.; Ghamrawi, S.; Lagarce, L.; Babin, M.; Briet, M. Drug-induced vitiligo: A case/non-case study in Vigibase®, the WHO pharmacovigilance database. Fundam. Clin. Pharmacol. 2020, 34, 736–742. [Google Scholar] [CrossRef]
- Gedikli, O.K.; Kilic, G. New-onset vitiligo as an unusual cutaneous reaction under ustekinumab therapy in patients with psoriatic arthritis. Acta Reumatol. Port. 2020, 45, 301–303. [Google Scholar] [PubMed]
- Palazzo, G. Resolution of post-adalimumab vitiligo with secukinumab in a patient with psoriasis vulgaris. Oxf. Med. Case Rep. 2020, 2020, omz134. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Benito, L.M.; Baniandrés-Rodríguez, O. New-Onset Vitiligo during Treatment with Secukinumab: Report of Two Cases and Review of the Literature. Clin. Drug Investig. 2020, 40, 1089–1091. [Google Scholar] [CrossRef]
- Speeckaert, R.; Mylle, S.; van Geel, N. IL-17A is not a treatment target in progressive vitiligo. Pigment. Cell Melanoma Res. 2019, 32, 842–847. [Google Scholar] [CrossRef]
- Raimondo, A.; Guglielmi, G.; Marino, C.; Ligrone, L.; Lembo, S. Hair whitening in a patient with psoriasis on adalimumab reversed after switching to ixekizumab. JAAD Case Rep. 2021, 11, 51–53. [Google Scholar] [CrossRef]
- Tirado-Sánchez, A.; Bonifaz, A. Simultaneous Bullous Pemphigoid and Vitiligo Associated with Adalimumab Therapy in a Patient with Psoriasis Vulgaris. Indian Dermatol. Online J. 2020, 11, 229–231. [Google Scholar] [PubMed]
- Phan, K.; Charlton, O.; Smith, S.D. New onset vitiligo in a patient with hidradenitis suppurativa treated with adalimumab. Dermatol. Ther. 2020, 33, e13347. [Google Scholar] [CrossRef]
- Posada, C.; Flórez, A.; Batalla, A.; Alcázar, J.J.; Carpio, D. Vitiligo during Treatment of Crohn’s Disease with Adalimumab: Adverse Effect or Co-Occurrence? Case Rep. Dermatol. 2011, 3, 28–31. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, Y.J.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C. Development of Vitiligo during Treatment with Adalimumab: A Plausible or Paradoxical Response? Ann. Dermatol. 2015, 27, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gao, Y.; Ding, Y. Vitiligo in a patient receiving infliximab for chronic plaque psoriasis. Dermatol. Ther. 2019, 32, e12917. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.H.; Lee, D.W.; Choi, J.E.; Ahn, H.H.; Kye, Y.C.; Seo, S.H. A Type II Segmental Vitiligo Developed under Infliximab Treatment for Ulcerative Colitis. Ann. Dermatol. 2017, 29, 826–827. [Google Scholar] [CrossRef]
- Luber, R.P.; Chamberlain, A.J.; Sparrow, M.P. New onset vitiligo following commencement of infliximab in Crohn disease. Intern. Med. J. 2017, 47, 972–973. [Google Scholar] [CrossRef]
- Carvalho, C.L.; Ortigosa, L.C. Segmental vitiligo after infliximab use for rheumatoid arthritis--a case report. An. Bras. Dermatol. 2014, 89, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Mattox, A.R.; Chappell, J.A.; Hurley, M.Y. New-onset vitiligo during long-term, stable infliximab treatment of pityriasis rubra pilaris. J. Drugs Dermatol. 2013, 12, 217–219. [Google Scholar] [PubMed]
- Ismail, W.A.; Al-Enzy, S.A.; Alsurayei, S.A.; Ismail, A.E. Vitiligo in a patient receiving infliximab for refractory ulcerative colitis. Arab J. Gastroenterol. 2011, 12, 109–111. [Google Scholar] [CrossRef]
- Ramírez-Hernández, M.; Marras, C.; Martínez-Escribano, J.A. Infliximab-induced vitiligo. Dermatology 2005, 210, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, K.M.; Khurrum, H.; Taieb, A.; Ezzedine, K. Treatment of generalized vitiligo with anti-TNF-α Agents. J. Drugs Dermatol. 2012, 11, 534–539. [Google Scholar]
- Webb, K.C.; Tung, R.; Winterfield, L.S.; Gottlieb, A.B.; Eby, J.M.; Henning, S.W.; Le Poole, I.C. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br. J. Dermatol. 2015, 173, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Torchia, D.; Rouhani, P.; Roberts, B.; Romanelli, P. Tumor necrosis factor-α in vitiligo: Direct correlation between tissue levels and clinical parameters. Cutan. Ocul. Toxicol. 2011, 30, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Giuliodori, K.; Ganzetti, G.; Liberati, G.; Offidani, A.M. A patient with psoriasis and vitiligo treated with etanercept. Am. J. Clin. Dermatol. 2010, 11 (Suppl. 1), 46–48. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, D.; Gregoriou, S.; Larios, G.; Moustou, E.; Belayeva-Karatza, E.; Kalogeromitros, D. Etanercept in the treatment of vitiligo. Dermatology 2007, 215, 84–85. [Google Scholar] [CrossRef]
- Vu, M.; Heyes, C.; Robertson, S.J.; Varigos, G.A.; Ross, G. Oral tofacitinib: A promising treatment in atopic dermatitis, alopecia areata and vitiligo. Clin. Exp. Dermatol. 2017, 42, 942–944. [Google Scholar] [CrossRef]
- Komnitski, M.; Komnitski, A.; Komnitski Junior, A.; Silva de Castro, C.C. Partial repigmentation of vitiligo with tofacitinib, without exposure to ultraviolet radiation. An. Bras. Dermatol. 2020, 95, 473–476. [Google Scholar] [CrossRef]
- Scheinberg, M.; Ferreira, S.B.; Santos, D.D.C.B. Tofacitinib-induced remission simultaneously in arthritis and vitiligo. Eur. J. Rheumatol. 2021, 8, 55–56. [Google Scholar] [CrossRef]
- Mobasher, P.; Guerra, R.; Li, S.J.; Frangos, J.; Ganesan, A.K.; Huang, V. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br. J. Dermatol. 2020, 182, 1047–1049. [Google Scholar] [CrossRef]
- Craiglow, B.G.; King, B.A. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015, 151, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Heaton, H.; Liu, L.Y.; King, B.A. Rapid Repigmentation of Vitiligo Using Tofacitinib Plus Low-Dose, Narrowband UV-B Phototherapy. JAMA Dermatol. 2018, 154, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Tajalli, M.; Kabir, S.; Vance, T.M.; Qureshi, A.A. Effective use of oral tofacitinib and phototherapy in a patient with concomitant alopecia areata, vitiligo, and plaque and inverse psoriasis. Clin. Case Rep. 2020, 8, 819–822. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Roccia, M.G.; Fioranelli, M.; Lotti, J.; Rovesti, M.; Satolli, F.; Valle, Y.; Goren, A.; et al. Micro—Focused Phototherapy Associated to Janus Kinase Inhibitor: A Promising Valid Therapeutic Option for Patients with Localized Vitiligo. Open Access Maced. J. Med. Sci. 2018, 6, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Strassner, J.P.; Refat, M.A.; Harris, J.E.; King, B.A. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J. Am. Acad. Dermatol. 2017, 77, 675–682. [Google Scholar] [CrossRef] [PubMed]
- McKesey, J.; Pandya, A.G. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J. Am. Acad. Dermatol. 2019, 81, 646–648. [Google Scholar] [CrossRef]
- Olamiju, B.; Craiglow, B.G. Tofacitinib cream plus narrowband ultraviolet B phototherapy for segmental vitiligo in a child. Pediatr. Dermatol. 2020, 37, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Rosmarin, D.; Pandya, A.G.; Lebwohl, M.; Grimes, P.; Hamzavi, I.; Gottlieb, A.B.; Butler, K.; Kuo, F.; Sun, K.; Ji, T.; et al. Ruxolitinib cream for treatment of vitiligo: A randomised, controlled, phase 2 trial. Lancet 2020, 396, 110–120. [Google Scholar] [CrossRef]
- Rothstein, B.; Joshipura, D.; Saraiya, A.; Abdat, R.; Ashkar, H.; Turkowski, Y.; Sheth, V.; Huang, V.; Au, S.C.; Kachuk, C.; et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J. Am. Acad. Dermatol. 2017, 76, 1054–1060. [Google Scholar] [CrossRef]
- Harris, J.E.; Rashighi, M.; Nguyen, N.; Jabbari, A.; Ulerio, G.; Clynes, R.; Christiano, A.M.; Mackay-Wiggan, J. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J. Am. Acad. Dermatol. 2016, 74, 370–371. [Google Scholar] [CrossRef]
- Nadesalingam, K.; Goodfield, M.; Emery, P. Halo naevi, vitiligo and diffuse alopecia areata associated with tocilizumab therapy. Oxf. Med. Case Rep. 2016, 2016, omw027. [Google Scholar] [CrossRef][Green Version]
- Bunker, C.B.; Manson, J. Vitiligo remitting with tocilizumab. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e20. [Google Scholar] [CrossRef]
- Sachdeva, M.; Mufti, A.; Kashetsky, N.; Georgakopoulos, J.R.; Naderi-Azad, S.; Salsberg, J.; Yeung, J. A systematic review of vitiligo onset and exacerbation in patients receiving biologic therapy. JAAD Int. 2020, 2, 37–39. [Google Scholar] [CrossRef]
- Skurkovich, B.; Skurkovich, S. Inhibition of IFN-gamma as a method of treatment of various autoimmune diseases, including skin diseases. Ernst Schering Res. Found. Workshop 2006, 56, 1–27. [Google Scholar]
- Harris, J.E.; Harris, T.H.; Weninger, W.; Wherry, E.J.; Hunter, C.A.; Turka, L.A. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J. Investig. Dermatol. 2012, 132, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.M.; Strassner, J.P.; Zapata, L., Jr.; Garg, M.; Riding, R.L.; Refat, M.A.; Fan, X.; Azzolino, V.; Tovar-Garza, A.; Tsurushita, N.; et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018, 10, eaam7710. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Rashighi, M.; Agarwal, P.; Garg, M.; Essien, K.I.; Pell, L.S.; Harris, J.E. Resident Memory and Recirculating Memory T Cells Cooperate to Maintain Disease in a Mouse Model of Vitiligo. J. Investig. Dermatol. 2019, 139, 769–778. [Google Scholar] [CrossRef]
- Skon, C.N.; Lee, J.Y.; Anderson, K.G.; Masopust, D.; Hogquist, K.A.; Jameson, S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013, 14, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Xu, R.; Fan, B.; Chen, J.; Li, X.; Mao, W.; Hua, S.; Li, B. PD-L1 reverses depigmentation in Pmel-1 vitiligo mice by increasing the abundance of Tregs in the skin. Sci. Rep. 2018, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zdravković, T.P.; Wang, T.; Liu, Y.; Jin, H. Efficacy and safety of oral simvastatin in the treatment of patients with vitiligo. J. Investig. Med. 2021, 69, 393–396. [Google Scholar] [CrossRef]
| Target | Desired Effect | Real-Life Effect | |
|---|---|---|---|
| Ustekinumab [69,70,71,72,73] | IL-12 and -23 | Blocking of inflammation and Th17 polarization | Appearance of new vitiligo patches |
| Secukinumab [74,75,76] | IL-17A | Interrupting inflammation and production of other proinflammatory cytokines | Progression and appearance of new depigmentation areas |
| Adalimumab [77,78,79,80,81,89,90], infliximab [82,83,84,85,86,87,88,89,90], etanercept [91,92,93] | TNF-alpha | Stopping the progression of inflammation | Contrasting results in the appearance of new patches and the progression of already existing ones |
| Tildrakizumab | IL-23 | Blockage of the inflammatory network | Insufficient studies |
| Tocilizumab [108] | IL-6 receptor | Stopping the propagation of inflammation | Soluble form of IL-6 might be causative of new manifestations |
| Tofacitinib [94,95,96,97,98,99,100,101,102,103,104], ruxolitinib [105,106,107] | JAK1-3 and 1-2 | Halting inflammation cascade signals | Stopped progression. Might need concomitant UVB for repigmentation |
| Involvement | Possible Targets | Rationale | |
|---|---|---|---|
| Pd-1 | Immunity response and checkpoint function | PD-1, PD-L1 | Regulating T-cell activation |
| IFN-gamma | Inflammation and promotion of autophagy | IFN-gamma soluble, CXCL10-CXCR3 | Stopping specific CTLs killing of melanocytes |
| NOS | Production of oxygen radical species | Inducible synthase (iNOS) | Lower levels of oxidative stress |
| IL-15 | Regulates level of IL-17 | Soluble form and receptor CD122 | Stopping crosstalk between TRM cells and Tcm cells |
| S1PR1 | Transit from tissues to blood vessels of T lymphocytes | S1PR1 (receptor) or S1P (ligand) | Allowing the recirculation of T memory cells and preventing the maintenance of inflammation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custurone, P.; Di Bartolomeo, L.; Irrera, N.; Borgia, F.; Altavilla, D.; Bitto, A.; Pallio, G.; Squadrito, F.; Vaccaro, M. Role of Cytokines in Vitiligo: Pathogenesis and Possible Targets for Old and New Treatments. Int. J. Mol. Sci. 2021, 22, 11429. https://doi.org/10.3390/ijms222111429
Custurone P, Di Bartolomeo L, Irrera N, Borgia F, Altavilla D, Bitto A, Pallio G, Squadrito F, Vaccaro M. Role of Cytokines in Vitiligo: Pathogenesis and Possible Targets for Old and New Treatments. International Journal of Molecular Sciences. 2021; 22(21):11429. https://doi.org/10.3390/ijms222111429
Chicago/Turabian StyleCusturone, Paolo, Luca Di Bartolomeo, Natasha Irrera, Francesco Borgia, Domenica Altavilla, Alessandra Bitto, Giovanni Pallio, Francesco Squadrito, and Mario Vaccaro. 2021. "Role of Cytokines in Vitiligo: Pathogenesis and Possible Targets for Old and New Treatments" International Journal of Molecular Sciences 22, no. 21: 11429. https://doi.org/10.3390/ijms222111429
APA StyleCusturone, P., Di Bartolomeo, L., Irrera, N., Borgia, F., Altavilla, D., Bitto, A., Pallio, G., Squadrito, F., & Vaccaro, M. (2021). Role of Cytokines in Vitiligo: Pathogenesis and Possible Targets for Old and New Treatments. International Journal of Molecular Sciences, 22(21), 11429. https://doi.org/10.3390/ijms222111429










