Progress of Phototherapy Applications in the Treatment of Bone Cancer
Abstract
1. Introduction
2. PDT
3. Application of PDT in Bone Cancer
3.1. Preliminary Studies on the Therapeutic Effect of PDT on Bone Cancer
3.2. PDT Using New Generations of PSs for Bone Cancer
3.2.1. Dextran-Benzoporphyrin Derivatives (BPD)
3.2.2. Acridine Orange (AO)
3.2.3. Aminolevulinic Acid (ALA)
3.2.4. 5,10,15,20-Tetrakis(meta-hydroxyphenyl)chlorine (mTHPC)
3.2.5. Indocyanine Green (ICG)
3.2.6. Methylene Blue (MB)
3.2.7. Chlorin e6 (Ce6)
3.2.8. Chlorophyll Derivatives
3.2.9. Benzochloroporphyrin Derivatives (BCPDs)
3.2.10. Other Porphyrin Derivatives
3.2.11. Photodynamic Molecular Beacons (PMBs)
3.2.12. Other New PSs
3.3. Combination of PDT and Other Therapies for Bone Cancer
3.3.1. PDT Combined with Chemotherapy
3.3.2. PDT Combined with Immunotherapy
3.3.3. PDT Combined with Hyperthermia
3.3.4. PDT Combined with Radiotherapy
3.3.5. Other Applications of PDT for Clinical Bone Cancer
4. PTT
5. Application of PTT in Bone Cancer
5.1. Metal-Based PTAs
5.1.1. Au
5.1.2. Pt
5.1.3. Cu
5.1.4. Fe
5.2. Carbon-Based PTAs
5.2.1. Graphene-Family Materials
5.2.2. MWCNTs
5.2.3. Other Carbon-Based PTAs
5.3. Semiconductor-Based PTAs
5.3.1. MXene Nanaosheets
5.3.2. Oxide Semiconductor-Based Materials
5.3.3. Metal-Organic Frameworks
5.3.4. Other Semiconductor-Based Materials
5.4. Organic Molecule-Based PTAs
5.4.1. Organic NIR Dyes
5.4.2. Conductive Polymers
5.5. Combination of PTT and PDT
6. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rainusso, N.; Wang, L.L.; Yustein, J.T. The adolescent and young adult with cancer: State of the art—Bone tumors. Curr. Oncol. Rep. 2013, 15, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Gorlick, R.; Janeway, K.; Lessnick, S.; Randall, R.L.; Marina, N.; Committee, C.O.G.B.T. Children’s Oncology Group’s 2013 blueprint for research: Bone tumors. Pediatr. Blood Cancer 2013, 60, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Siclari, V.A.; Qin, L. Targeting the osteosarcoma cancer stem cell. J. Orthop. Surg. Res. 2010, 5, 78. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Z.; Huang, Z.; Chen, D.C.; Zhu, X.X.; Wang, Y.Z.; Yan, Y.W.; Tang, S.; Madhavan, S.; Ni, W.; et al. Super enhancer inhibitors suppress MYC driven transcriptional amplification and tumor progression in osteosarcoma. Bone Res. 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef]
- Italiano, A.; Mir, O.; Cioffi, A.; Palmerini, E.; Piperno-Neumann, S.; Perrin, C.; Chaigneau, L.; Penel, N.; Duffaud, F.; Kurtz, J.E.; et al. Advanced chondrosarcomas: Role of chemotherapy and survival. Ann. Oncol. 2013, 24, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.C.; Kovar, H.; Grimer, R.; Whelan, J.; Claude, L.; et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2011, 12, 39–50. [Google Scholar] [CrossRef]
- Yuasa, T.; Urakami, S. Kidney cancer: Decreased incidence of skeletal-related events in mRCC. Nat. Rev. Urol. 2014, 11, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Fukutomia, M.; Yokotaa, M.; Chumanb, H.; Haradab, H.; Zaitsub, Y.; Funakoshia, A.; Wakasugia, H.; Iguchi, H. Increased incidence of bone metastases in hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1083–1088. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef]
- Suva, L.J.; Washam, C.; Nicholas, R.W.; Griffin, R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011, 7, 208–218. [Google Scholar] [CrossRef]
- Heck, R.K.; Peabody, T.D.; Simon, M.A. Staging of Primary Malignancies of Bone. CA Cancer J. Clin. 2006, 56, 366–375. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Nezafati, N.; Saber-Samandari, S. The Effective Role of Hydroxyapatite-Based Composites in Anticancer Drug-Delivery Systems. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 41–75. [Google Scholar] [CrossRef]
- Van der Bij, G.J.; Oosterling, S.J.; Beelen, R.H.; Meijer, S.; Coffey, J.C.; van Egmond, M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann. Surg. 2009, 249, 727–734. [Google Scholar] [CrossRef]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The Future of Cancer Diagnosis and Therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Shakhar, G.; Ben-Eliyahu, S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann. Surg. Oncol. 2003, 10, 972–992. [Google Scholar] [CrossRef] [PubMed]
- Letfullin, R.R.; Rice, C.E.; George, T.F. Theoretical study of bone cancer therapy by plasmonic nanoparticles. Ther. Deliv. 2011, 2, 1259–1273. [Google Scholar] [CrossRef]
- Au, C.M.; Luk, S.K.; Jackson, C.J.; Ng, H.K.; Yow, C.M.; To, S.S. Differential effects of photofrin, 5-aminolevulinic acid and calphostin C on glioma cells. J. Photochem. Photobiol. B 2006, 85, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, T.; Gao, J.; Wang, Y.; Li, D.; Zhao, Z.; Jiang, B.; Dong, Z.; Liu, H. Albumin-bioinspired iridium oxide nanoplatform with high photothermal conversion efficiency for synergistic chemo-photothermal of osteosarcoma. Drug Deliv. 2019, 26, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Wang, Y.; Chen, X.; Fang, F. Novel strategy in giant cutaneous squamous cell carcinoma treatment: The case experience with a combination of photodynamic therapy and surgery. Photodiagn. Photodyn. Ther. 2017, 19, 116–118. [Google Scholar] [CrossRef]
- Castilho-Fernandes, A.; Lopes, T.G.; Primo, F.L.; Pinto, M.R.; Tedesco, A.C. Photodynamic process induced by chloro-aluminum phthalocyanine nanoemulsion in glioblastoma. Photodiagn. Photodyn. Ther. 2017, 19, 221–228. [Google Scholar] [CrossRef]
- Fahey, J.M.; Korytowski, W.; Girotti, A.W. Upstream signaling events leading to elevated production of pro-survival nitric oxide in photodynamically-challenged glioblastoma cells. Free Radic. Biol. Med. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-containing bioactive glasses and glass-ceramics: From tissue regeneration to cancer therapeutic strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xing, Y.; Li, X.; Du, X.; Xu, T.; Zhang, X. Cancer Cell Membrane Camouflaged Semi-Yolk@Spiky-Shell Nanomotor for Enhanced Cell Adhesion and Synergistic Therapy. Small 2020, 16, e2003834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhang, R.Y.; Yang, X.Q.; Zhang, X.S.; Zhang, F.; An, J.; Wang, Z.Y.; Dong, Y.; Liu, B.; Zhao, Y.D.; et al. One-for-All Nanoplatform for Synergistic Mild Cascade-Potentiated Ultrasound Therapy Induced with Targeting Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interface 2020, 12, 40052–40066. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, S.; Dai, Z.; Zhang, H.; Zheng, X. A novel theranostic nano-platform (PB@FePt-HA-g-PEG) for tumor chemodynamic-photothermal co-therapy and triple-modal imaging (MR/CT/PI) diagnosis. J. Mater. Chem. B 2020, 8, 5351–5360. [Google Scholar] [CrossRef]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef]
- Du, P.; Hu, S.; Cheng, Y.; Li, F.; Li, M.; Li, J.; Yi, L.; Feng, H. Photodynamic therapy leads to death of C6 glioma cells partly through AMPAR. Brain Res. 2012, 1433, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.; van Doeveren, T.E.; Tan, I.B.; Dilci, A.; van Veen, R.L.; Karakullukcu, B. The use of photodynamic therapy as adjuvant therapy to surgery in recurrent malignant tumors of the paranasal sinuses. Photodiagn. Photodyn. Ther. 2015, 12, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lei, Q.; Qiu, W.X.; Liu, L.H.; Zheng, D.W.; Fan, J.X.; Rong, L.; Sun, Y.X.; Zhang, X.Z. Mitochondria-targeting “Nanoheater” for enhanced photothermal/chemo-therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef]
- Wang, D.; Dong, H.; Li, M.; Cao, Y.; Yang, F.; Zhang, K.; Dai, W.; Wang, C.; Zhang, X. Erythrocyte-Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma. ACS Nano 2018, 12, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Mota, H.C.; Sibata, C.H. Clinical PD/PDT in North America: An historical review. Photodiagn. Photodyn. Ther. 2004, 1, 263–277. [Google Scholar] [CrossRef]
- Dougherty, T.J. Photodynamic therapy (PDT) of malignant tumors. Crit. Rev. Oncol. Hematol. 1984, 2, 83–116. [Google Scholar] [CrossRef]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagn. Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Cruz, P.M.; Mo, H.; McConathy, W.J.; Sabnis, N.; Lacko, A.G. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013, 4, 119. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Sattler, U.G.; Mueller-Klieser, W. The anti-oxidant capacity of tumour glycolysis. Int. J. Radiat. Biol. 2009, 85, 963–971. [Google Scholar] [CrossRef]
- Golab, J.; Nowis, D.; Skrzycki, M.; Czeczot, H.; Baranczyk-Kuzma, A.; Wilczynski, G.M.; Makowski, M.; Mroz, P.; Kozar, K.; Kaminski, R.; et al. Antitumor effects of photodynamic therapy are potentiated by 2-methoxyestradiol. A superoxide dismutase inhibitor. J. Biol. Chem. 2003, 278, 407–414. [Google Scholar]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Li, Z.; Zhang, X. Progress of photodynamic therapy applications in the treatment of musculoskeletal sarcoma (Review). Oncol. Lett. 2014, 8, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- TJ, D.; MT, C.; TS, M. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg. Med. 1990, 10, 485–488. [Google Scholar]
- Zhang, J.; Jiang, C.; Figueiro Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B. 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 1992, 14, 275–292. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef]
- Beharry, A.A. Next-Generation Photodynamic Therapy: New Probes for Cancer Imaging and Treatment. Biochemistry 2018, 57, 173–174. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Savellano, M.D.; Hasan, T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem. Photobiol. 2003, 74, 431–439. [Google Scholar] [CrossRef]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Juzenas, P.; Ma, L.W.; Iani, V.; Moan, J. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med. Sci. 2004, 19, 139–149. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Calin, M.A.; Diaconeasa, A.; Savastru, D.; Tautan, M. Photosensitizers and light sources for photodynamic therapy of the Bowen’s disease. Arch. Dermatol. Res. 2011, 303, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Jayadevappa, R.; Chhatre, S.; Soukiasian, H.J.; Murgu, S. Outcomes of patients with advanced non-small cell lung cancer and airway obstruction treated with photodynamic therapy and non-photodynamic therapy ablation modalities. J. Thorac. Dis. 2019, 11, 4389–4399. [Google Scholar] [CrossRef]
- Saravana-Bawan, S.; David, E.; Sahgal, A.; Chow, E. Palliation of bone metastases-exploring options beyond radiotherapy. Ann. Palliat. Med. 2019, 8, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Fingar, V.H.; Wieman, T.J.; Doak, K.W. Role of Thromboxane and Prostacyclin Release on Photodynamic Therapy-induced Tumor Destruction. Cancer Res. 1990, 50, 2599–2603. [Google Scholar]
- Fingar, V.H.; Wieman, T.J.; Doak, K.W. Changes in tumor interstitial pressure induced by photodynamic therapy. Photochem. Photobiol. 1991, 53, 763–768. [Google Scholar] [CrossRef]
- Meyer, M.; Speight, P.; Bown, S.G. A study of the effects of photodynamic therapy on the normal tissues of the rabbit jaw. Br. J. Cancer 1991, 64, 1093–1097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hourigan, A.J.; Kells, A.F.; Schwartz, H.S. In vitro Photodynamic Therapy of Musculoskeletal Neoplasms. J. Orthop. Res. 1993, 11, 633–637. [Google Scholar] [CrossRef]
- Fingar, V.H.; Kik, P.K.; Haydon, P.S.; Cerrito, P.B.; Tseng, M.; Abang, E.; Wieman, T.J. Analysis of acute vascular damage after photodynamic therapy using benzoporphyrin derivative (BPD). Br. J. Cancer 1999, 79, 1702–1708. [Google Scholar] [CrossRef]
- Burch, S.; London, C.; Seguin, B.; Rodriguez, C.; Wilson, B.C.; Bisland, S.K. Treatment of canine osseous tumors with photodynamic therapy: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 1028–1034. [Google Scholar] [CrossRef]
- Burch, S.; Bogaards, A.; Siewerdsen, J.; Moseley, D.; Yee, A.; Finkelstein, J.; Weersink, R.; Wilson, B.C.; Bisland, S.K. Photodynamic therapy for the treatment of metastatic lesions in bone: Studies in rat and porcine models. J. Biomed. Opt. 2005, 10, 034011. [Google Scholar] [CrossRef]
- Bisland, S.K.; Burch, S. Photodynamic therapy of diseased bone. Photodiagn. Photodyn. Ther. 2006, 3, 147–155. [Google Scholar] [CrossRef]
- Akens, M.K.; Yee, A.J.; Wilson, B.C.; Burch, S.; Johnson, C.L.; Lilge, L.; Bisland, S.K. Photodynamic therapy of vertebral metastases: Evaluating tumor-to-neural tissue uptake of BPD-MA and ALA-PpIX in a murine model of metastatic human breast carcinoma. Photochem. Photobiol. 2007, 83, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Akens, M.K.; Hardisty, M.R.; Wilson, B.C.; Schwock, J.; Whyne, C.M.; Burch, S.; Yee, A.J. Defining the therapeutic window of vertebral photodynamic therapy in a murine pre-clinical model of breast cancer metastasis using the photosensitizer BPD-MA (Verteporfin). Breast Cancer Res. Treat. 2010, 119, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Akens, M.K.; Hardisty, M.R.; Burch, S.; Bisland, S.K.; Yee, A.J.M.; Wilson, B.C.; Whyne, C.M. Effects of Photodynamic Therapy on the Structural Integrity of Vertebral Bone. Spine 2010, 35, 272–277. [Google Scholar] [CrossRef]
- Wise-Milestone, L.; Akens, M.K.; Lo, V.C.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. Local treatment of mixed osteolytic/osteoblastic spinal metastases: Is photodynamic therapy effective? Breast Cancer Res. Treat. 2012, 133, 899–908. [Google Scholar] [CrossRef]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Murata, H.; Marunaka, Y.; Hosogi, S.; Uchida, A.; Sudo, A. Photodynamic therapy with acridine orange in musculoskeletal sarcomas. J. Bone Jt. Surg. 2010, 92-B, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Shintani, K.; Satonaka, H.; Uchida, A. Acridine Orange Used for Photodynamic Therapy Accumulates in Malignant Musculoskeletal Tumors Depending on pH Gradient. Anticancer Res. 2006, 26, 187–194. [Google Scholar] [PubMed]
- Kusuzaki, K.; Aomori, K.; Suginoshita, T.; Minami, G.; Takeshita, H.; Murata, H.; Hashiguchi, S.; Ashihara, T.; Hirasaw, Y. Total Tumor Cell Elimination with Minimum Damage to Normal Tissues in Musculoskeletal Sarcomas following Photodynamic Therapy with Acridine Orange. Oncology 2000, 59, 174–180. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Minami, G.; Takeshita, H.; Murata, H.; Hashiguchi, S.; Nozaki, T.; Ashihara, T.; Hirasawa, Y. Photodynamic Inactivation with Acridine Orange on a Multidrug-resistant Mouse Osteosarcoma Cell Line. Jpn. J. Cancer Res. 2000, 91, 439–445. [Google Scholar] [CrossRef]
- Ueda, H.; Murata, H.; Takeshita, H.; Minami, G.; Hashiguchi, S.; Kubo, T. Unfiltered Xenon Light is Useful for Photodynamic Therapy with Acridine Orange. Anticancer Res. 2005, 25, 3979–3984. [Google Scholar] [PubMed]
- Satonaka, H.; Kusuzaki, K.; Matubara, T.; Shintani, K.; Wakabayashi, T.; Nakamura, T.; Matsumine, A.; Uchida, A. Flash Wave Light Strongly Enhanced the Cytocidal Effect of Photodynamic Therapy with Acridine Orange on a Mouse Osteosarcoma Cell Line. Anticancer Res. 2007, 27, 3339–3344. [Google Scholar]
- Dietze, A.; Berg, K. ALA-induced porphyrin formation and fluorescence in synovitis tissue. Photodiagn. Photodyn. Ther. 2005, 2, 299–307. [Google Scholar] [CrossRef]
- White, B.; Rossi, V.; Baugher, P.J. Aminolevulinic Acid-Mediated Photodynamic Therapy Causes Cell Death in MG-63 Human Osteosarcoma Cells. Photomed. Laser Surg. 2016, 34, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Piette, J. RIP3 expression induces a death profile change in U2OS osteosarcoma cells after 5-ALA-PDT. Lasers Surg. Med. 2011, 43, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Campanile, C.; Botter, S.M.; Born, W.; Fuchs, B. Cytotoxic efficacy of photodynamic therapy in osteosarcoma cells in vitro. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Reidy, K.; Campanile, C.; Muff, R.; Born, W.; Fuchs, B. mTHPC-mediated photodynamic therapy is effective in the metastatic human 143B osteosarcoma cells. Photochem. Photobiol. 2012, 88, 721–727. [Google Scholar] [CrossRef]
- Meier, D.; Botter, S.M.; Campanile, C.; Robl, B.; Grafe, S.; Pellegrini, G.; Born, W.; Fuchs, B. Foscan and foslip based photodynamic therapy in osteosarcoma in vitro and in intratibial mouse models. Int. J. Cancer 2017, 140, 1680–1692. [Google Scholar] [CrossRef]
- Sakka, S.G. Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients. J. Clin. Monit. Comput. 2018, 32, 787–796. [Google Scholar] [CrossRef]
- Maarek, J.-M.I.; Holschneider, D.P.; Rubinstein, E.H. Fluorescence dilution technique for measurement of cardiac output and circulating blood volume in healthy human subjects. Anesthesiology 2007, 106, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bozkulak, O.; Yamaci, R.F.; Tabakoglu, O.; Gulsoy, M. Photo-toxic effects of 809-nm diode laser and indocyanine green on MDA-MB231 breast cancer cells. Photodiagn. Photodyn. Ther. 2009, 6, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Skrivanova, K.; Skorpikova, J.; Svihalek, J.; Mornstein, V.; Janisch, R. Photochemical properties of a potential photosensitiser indocyanine green in vitro. J. Photochem. Photobiol. B 2006, 85, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, W.; Abels, C.; Karrer, S.; Weiß, T.; Messmann, H.; Landthaler, M.; Szeimies, R.-M. Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br. J. Cancer 1999, 80, 360–363. [Google Scholar] [CrossRef]
- Funayama, T.; Sakane, M.; Abe, T.; Ochiai, N. Photodynamic therapy with indocyanine green injection and near-infrared light irradiation has phototoxic effects and delays paralysis in spinal metastasis. Photomed. Laser Surg. 2012, 30, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Funayama, T.; Tsukanishi, T.; Hara, I.; Ozeki, E.; Sakane, M. Tumor-selective near-infrared photodynamic therapy with novel indocyanine green-loaded nanocarrier delays paralysis in rats with spinal metastasis. Photodiagn. Photodyn. Ther. 2013, 10, 374–378. [Google Scholar] [CrossRef] [PubMed]
- GS, T.; SL, F.; VM, R.; MA, C. Methylene blue reverts multidrug resistance: Sensitivity of multidrug resistant cells to this dye and its photodynamic action. Cancer Lett. 2000, 151, 161–167. [Google Scholar]
- Rice, L.; Wainwright, M.; Phoemix, D.A. Phenothiazine photosensitizers. III. Activity of methylene blue derivatives against pigmented melanoma cell lines. J. Chemother. 2000, 12, 94–104. [Google Scholar]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Satonaka, H.; Shintani, K.; Nakamura, T.; Uchida, A. Methylene blue in place of acridine orange as a photosensitizer in photodynamic therapy of osteosarcoma. In Vivo 2008, 22, 297–304. [Google Scholar]
- Guan, J.; Lai, X.; Wang, X.; Leung, A.W.; Zhang, H.; Xu, C. Photodynamic action of methylene blue in osteosarcoma cells in vitro. Photodiagn. Photodyn. Ther. 2014, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, S.A.; Elsayed, A.; Moawad, M.; Ahmed, W.A. Hydroxyapatite nanocomposite as a potential agent in osteosarcoma PDT. Photodiagn. Photodyn. Ther. 2020, 32, 102056. [Google Scholar] [CrossRef] [PubMed]
- Darwish, K.M.; Salama, I.; Mostafa, S.; El-Sadek, M. RP-HPLC/pre-column derivatization for analysis of omeprazole, tinidazole, doxycycline and clarithromycin. J. Chromatogr. Sci. 2013, 51, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef]
- Mohsenian, N.B.; Shanei, A.; Alavi, S.J.; Kheirollahi, M.; Nia, A.H.; Tavakoli, M.B. Mn-doped ZnS quantum dots-chlorin e6 shows potential as a treatment for chondrosarcoma: An in vitro study. IET Nanobiotechnol. 2019, 13, 387–391. [Google Scholar] [CrossRef]
- Lee, E.; Park, J.; Youn, Y.S.; Oh, K.T.; Kim, D.; Lee, E.S. Alendronate/cRGD-Decorated Ultrafine Hyaluronate Dot Targeting Bone Metastasis. Biomedicines 2020, 8, 492. [Google Scholar] [CrossRef]
- Iriuchishima, T.; Saito, A.; Aizawa, S.; Taira, K.; Yamamoto, T.; Ryu, J. The minimum influences for murine normal joint tissue by novel bactericidal treatment and photodynamic therapy using na-pheophorbide a for septic arthritis. Photomed. Laser Surg. 2008, 26, 153–158. [Google Scholar] [CrossRef]
- Nagai, Y.; Aizawa, S.; Iriuchishima, T.; Goto, B.; Nagaoka, M.; Tokuhashi, Y.; Saito, A. Phototoxic effect of na-pheophorbide a toward osteosarcoma cells in vitro using a laser diode. Photomed. Laser Surg. 2014, 32, 481–489. [Google Scholar] [CrossRef]
- Schreiber, S.; Gross, S.; Brandis, A.; Harmelin, A.; Rosenbach-Belkin, V.; Scherz, A.; Salomon, Y. Local photodynamic therapy (PDT) of rat C6 glioma xenografts with Pd-bacteriopheophorbide leads to decreased metastases and increase of animal cure compared with surgery. Int. J. Cancer 2002, 99, 279–285. [Google Scholar] [CrossRef]
- Koudinova, N.V.; Pinthus, J.H.; Brandis, A.; Brenner, O.; Bendel, P.; Ramon, J.; Eshhar, Z.; Scherz, A.; Salomon, Y. Photodynamic therapy with Pd-Bacteriopheophorbide (TOOKAD): Successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int. J. Cancer 2003, 104, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ou, Y.S.; Tao, Y.; Yin, H.; Tu, P.H. Apoptosis and autophagy induced by pyropheophorbide-alpha methyl ester-mediated photodynamic therapy in human osteosarcoma MG-63 cells. Apoptosis 2016, 21, 749–760. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, H.; Tao, Y.; Zhong, S.; Yu, H.; Li, J.; Bai, Z.; Ou, Y. Antitumor effects and mechanisms of pyropheophorbidealpha methyl estermediated photodynamic therapy on the human osteosarcoma cell line MG. Int. J. Mol. Med. 2020, 45, 971–982. [Google Scholar]
- Yao, J.; Zhang, W.; Sheng, C.; Miao, Z.; Yang, F.; Yu, J.; Zhang, L.; Song, Y.; Zhou, T.; Zhou, Y. Design, synthesis, and in vitro photodynamic activities of benzochloroporphyrin derivatives as tumor photosensitizers. Bioorg. Med. Chem. Lett. 2008, 18, 293–297. [Google Scholar] [CrossRef]
- Gong, H.Y.; Sun, M.X.; Hu, S.; Tao, Y.Y.; Gao, B.; Li, G.D.; Cai, Z.D.; Yao, J.Z. Benzochloroporphyrin derivative induced cytotoxicity and inhibition of tumor recurrence during photodynamic therapy for osteosarcoma. Asian Pac. J. Cancer Prev. 2013, 14, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, C.; Zeng, H.; Yin, F.; Wang, Z.; Yao, J.; Hua, Y.; Cai, Z. Benzochloroporphyrin derivative photosensitizer-mediated photodynamic therapy for Ewing sarcoma. J. Photochem. Photobiol. B 2016, 160, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, M.; Zhou, C.; Yin, F.; Wang, Z.; Hua, Y.; Ca, Z. Hematoporphyrin Monomethyl Ether-Mediated Photodynamic Therapy Selectively Kills Sarcomas by Inducing Apoptosis. PLoS ONE 2013, 8, e77727. [Google Scholar] [CrossRef]
- Huang, Z. An update on the regulatory status of PDT photosensitizers in China. Photodiagn. Photodyn. Ther. 2008, 5, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, C.; Zeng, H.; Puebla-Osorio, N.; Damiani, E.; Chen, J.; Wang, H.; Li, G.; Yin, F.; Shan, L.; et al. Hiporfin-mediated photodynamic therapy in preclinical treatment of osteosarcoma. Photochem. Photobiol. 2015, 91, 533–544. [Google Scholar] [CrossRef]
- Serra, A.; Pineiro, M.; Santos, C.I.; Gonsalves, A.M.d.R.; Abrantes, M.; Laranjo, M.; Botelho, M.F. In vitro photodynamic activity of 5,15-bis(3-hydroxyphenyl)porphyrin and its halogenated derivatives against cancer cells. Photochem. Photobiol. 2010, 86, 206–212. [Google Scholar] [CrossRef]
- De Miguel, G.C.; Abrantes, A.M.; Laranjo, M.; Grizotto, A.Y.K.; Camporeze, B.; Pereira, J.A.; Brites, G.; Serra, A.; Pineiro, M.; Rocha-Gonsalves, A.; et al. A new therapeutic proposal for inoperable osteosarcoma: Photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Makhadmeh, G.N.; Abdul Aziz, A. Photodynamic application of protoporphyrin IX as a photosensitizer encapsulated by silica nanoparticles. Artif. Cell. Nanomed. B 2018, 46, S1043–S1046. [Google Scholar] [CrossRef] [PubMed]
- Duchi, S.; Sotgiu, G.; Lucarelli, E.; Ballestri, M.; Dozza, B.; Santi, S.; Guerrini, A.; Dambruoso, P.; Giannini, S.; Donati, D.; et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: Effective photoinduced in vitro killing of osteosarcoma. J. Control Release 2013, 168, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Chen, J.; Stefflova, K.; Jarvi, M.; Li, H.; Wilson, B.C. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc. Natl. Acad. Sci. USA 2007, 104, 8989–8994. [Google Scholar] [CrossRef]
- Liu, T.W.; Akens, M.K.; Chen, J.; Wise-Milestone, L.; Wilson, B.C.; Zheng, G. Imaging of specific activation of photodynamic molecular beacons in breast cancer vertebral metastases. Bioconjug. Chem. 2011, 22, 1021–1030. [Google Scholar] [CrossRef]
- Liu, T.W.; Akens, M.K.; Chen, J.; Wilson, B.C.; Zheng, G. Matrix metalloproteinase-based photodynamic molecular beacons for targeted destruction of bone metastases in vivo. Photoch. Photobio. Sci. 2016, 15, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, J.; Hu, Y.; Wang, S.; Chen, M.; Wang, Y. Potential antineoplastic effects of Aloe-emodin: A comprehensive review. Am. J. Chin. Med. 2014, 42, 275–288. [Google Scholar] [CrossRef]
- Vargas, F.; Rivas, C.; Medrano, M. Interaction of emodin, aloe-emodin, and rhein with human serum albumin: A fluorescence spectroscopic study. Toxicol. Mech. Methods 2004, 14, 227–231. [Google Scholar] [CrossRef]
- Lee, H.Z.; Yang, W.H.; Hour, M.J.; Wu, C.Y.; Peng, W.H.; Bao, B.Y.; Han, P.H.; Bau, D.T. Photodynamic activity of aloe-emodin induces resensitization of lung cancer cells to anoikis. Eur. J. Pharmacol. 2010, 648, 50–58. [Google Scholar] [CrossRef]
- Tu, P.; Huang, Q.; Ou, Y.; Du, X.; Li, K.; Tao, Y.; Yin, H. Aloe-emodin-mediated photodynamic therapy induces autophagy and apoptosis in human osteosarcoma cell line MG63 through the ROS/JNK signaling pathway. Oncol. Rep. 2016, 35, 3209–3215. [Google Scholar] [CrossRef]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of near-infrared photoactivable phthalocyanine-loaded nanoparticles to kill tumor cells: An improved tool for photodynamic therapy of solid cancers. Nanomedicine 2016, 12, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Bellotti, C.; Duchi, S.; Martella, E.; Columbaro, M.; Dozza, B.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Frisoni, T.; et al. Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J. Exp. Clin. Cancer Res. 2020, 39, 40. [Google Scholar] [CrossRef]
- Yousefi Sadeghloo, A.; Khorsandi, K.; Kianmehr, Z. Synergistic effect of photodynamic treatment and doxorubicin on triple negative breast cancer cells. Photochem. Photobiol. Sci. 2020, 19, 1580–1589. [Google Scholar] [CrossRef]
- Martinez de Pinillos Bayona, A.; Moore, C.M.; Loizidou, M.; MacRobert, A.J.; Woodhams, J.H. Enhancing the efficacy of cytotoxic agents for cancer therapy using photochemical internalisation. Int. J. Cancer 2016, 138, 1049–1057. [Google Scholar] [CrossRef]
- Ecker, R.D.; Endo, T.; Wetjen, N.M.; Krauss, W.E. Diagnosis and treatment of vertebral column metastases. Mayo Clin. Proc. 2005, 80, 1177–1186. [Google Scholar] [CrossRef]
- Kaijzel, E.L.; van der Pluijm, G.; Lowik, C.W. Whole-body optical imaging in animal models to assess cancer development and progression. Clin. Cancer Res. 2007, 13, 3490–3497. [Google Scholar] [CrossRef]
- Won, E.; Wise-Milestone, L.; Akens, M.K.; Burch, S.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. Beyond bisphosphonates: Photodynamic therapy structurally augments metastatically involved vertebrae and destroys tumor tissue. Breast Cancer Res. Treat. 2010, 124, 111–119. [Google Scholar] [CrossRef]
- Hojjat, S.P.; Won, E.; Hardisty, M.R.; Akens, M.K.; Wise-Milestone, L.M.; Whyne, C.M. Non-destructive evaluation of the effects of combined bisphosphonate and photodynamic therapy on bone strain in metastatic vertebrae using image registration. Ann. Biomed. Eng. 2011, 39, 2816–2822. [Google Scholar] [CrossRef] [PubMed]
- Akens, M.K.; Wise-Milestone, L.; Won, E.; Schwock, J.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. In vitro and in vivo effects of photodynamic therapy on metastatic breast cancer cells pre-treated with zoledronic acid. Photodiagn. Photodyn. Ther. 2014, 11, 426–433. [Google Scholar] [CrossRef]
- Heymann, P.G.; Ziebart, T.; Kammerer, P.W.; Mandic, R.; Saydali, A.; Braun, A.; Neff, A.; Draenert, G.F. The enhancing effect of a laser photochemotherapy with cisplatin or zolendronic acid in primary human osteoblasts and osteosarcoma cells in vitro. J. Oral Pathol. Med. 2016, 45, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kotagiri, N.; Cooper, M.L.; Rettig, M.; Egbulefu, C.; Prior, J.; Cui, G.; Karmakar, P.; Zhou, M.; Yang, X.; Sudlow, G.; et al. Radionuclides transform chemotherapeutics into phototherapeutics for precise treatment of disseminated cancer. Nat. Commun. 2018, 9, 275. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Debele, T.A.; Peng, S.; Tsai, H.C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Martella, E.; Ferroni, C.; Guerrini, A.; Ballestri, M.; Columbaro, M.; Santi, S.; Sotgiu, G.; Serra, M.; Donati, D.M.; Lucarelli, E.; et al. Functionalized Keratin as Nanotechnology-Based Drug Deliv System for the Pharmacological Treatment of Osteosarcoma. Int. J. Mol. Sci. 2018, 19, 3670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yan, X.; Yu, A.; Wang, Y. Doxycycline synergizes with doxorubicin to inhibit the proliferation of castration-resistant prostate cancer cells. Acta Bioch. Bioph. Sin. 2017, 49, 999–1007. [Google Scholar] [CrossRef]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Fujii, T.; Nagasue, N. Doxycycline inhibits cell proliferation and invasive potential: Combination therapy with cyclooxygenase-2 inhibitor in human colorectal cancer cells. J. Lab. Clin. Med. 2004, 143, 207–216. [Google Scholar] [CrossRef]
- Tong, F.; Ye, Y.; Chen, B.; Gao, J.; Liu, L.; Ou, J.; van Hest, J.C.M.; Liu, S.; Peng, F.; Tu, Y. Bone-Targeting Prodrug Mesoporous Silica-Based Nanoreactor with Reactive Oxygen Species Burst for Enhanced Chemotherapy. ACS Appl. Mater. Interface 2020, 12, 34630–34642. [Google Scholar] [CrossRef] [PubMed]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Ling, Y.H.; Liebes, L.; Zou, Y.; Perez-Soler, R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J. Biol. Chem. 2003, 278, 33714–33723. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, Z.; Guan, Z.; Shen, Y.; Jiang, Y.; Xu, X.; Huang, Z.; Zhao, C. A light-triggered self-reinforced nanoagent for targeted chemo-photodynamic therapy of breast cancer bone metastases via ER stress and mitochondria mediated apoptotic pathways. J. Control Release 2020, 319, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Wang, Y.H.; Liu, G.F.; Wang, L.; Li, Y.; Guo, Z.Y.; Cheng, C. Graphene Oxide Nanoparticle-Loaded Ginsenoside Rg3 Improves Photodynamic Therapy in Inhibiting Malignant Progression and Stemness of Osteosarcoma. Front. Mol. Biosci. 2021, 8, 663089. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 350. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, G.; Le, V.; Zhang, A.; Chen, S.; Liang, X.; Liu, J. Photodynamic-therapy Activates Immune Response by disrupting Immunity Homeostasis of Tumor Cells, which Generates Vaccine for Cancer Therapy. Int. J. Biol. Sci. 2016, 12, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, Y.; Chen, W.R.; Wang, X. DC vaccine generated by ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncoimmunology 2016, 5, e1072674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, Y.; Fan, G.; Hu, S. Photodynamic therapy reduces the inhibitory effect of osteosarcoma cells on dendritic cells by upregulating HSP. Oncol. Lett. 2018, 16, 5034–5040. [Google Scholar]
- Furi, I.; Sipos, F.; Germann, T.M.; Kalmar, A.; Tulassay, Z.; Molnar, B.; Muzes, G. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: Clinico-pathogenic aspects. World J. Gastroenterol. 2013, 19, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. CpG oligodeoxynucleotide-based therapy of lymphoid malignancies. Adv. Drug Deliv. Rev. 2009, 61, 263–267. [Google Scholar] [CrossRef]
- Salem, A.K.; Weiner, G.J. CpG oligonucleotides as immunotherapeutic adjuvants: Innovative applications and delivery strategies. Adv. Drug Deliv. Rev. 2009, 61, 193–194. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.K.; Castano, A.P.; Mroz, P.; Avci, P.; Hamblin, M.R. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J. Biophotonics 2014, 7, 897–905. [Google Scholar] [CrossRef]
- Marrache, S.; Choi, J.H.; Tundup, S.; Zaver, D.; Harn, D.A.; Dhar, S. Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr. Biol. 2013, 5, 215–223. [Google Scholar] [CrossRef]
- Ribas, A.; Shin, D.S.; Zaretsky, J.; Frederiksen, J.; Cornish, A.; Avramis, E.; Seja, E.; Kivork, C.; Siebert, J.; Kaplan-Lefko, P.; et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol. Res. 2016, 4, 194–203. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.; Zhu, J.; Jin, L.; Liu, B.; Xia, K.; Wang, J.; Gao, J.; Liang, C.; Tao, H. Autophagy inhibitor enhance ZnPc/BSA nanoparticle induced photodynamic therapy by suppressing PD-L1 expression in osteosarcoma immunotherapy. Biomaterials 2019, 192, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, J.; Wang, Y.; Wang, J.; Fang, W.; Xia, K.; Shao, J.; Wu, M.; Liu, B.; Liang, C.; et al. A review and outlook in the treatment of osteosarcoma and other deep tumors with photodynamic therapy: From basic to deep. Oncotarget 2017, 8, 39833–39848. [Google Scholar] [CrossRef]
- Harmon, B.V.; Corder, A.M.; Collins, R.J.; Gobé, G.C.; Allen, J.; Allan, D.J.; Kerr, J.F. Cell death induced in a murine mastocytoma by 42-47 degrees C heating in vitro: Evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int. J. Radiat. Biol. 1990, 58, 845–858. [Google Scholar] [CrossRef]
- Yuen, W.F.; Fung, K.P.; Lee, C.Y.; Choy, Y.M.; Kong, S.K.; Ko, S.; Kwok, T.T. Hyperthermia and tumour necrosis factor-alpha induced apoptosis via mitochondrial damage. Life Sci. 2020, 67, 725–732. [Google Scholar] [CrossRef]
- Nomura, J.; Yanase, S.; Matsumura, Y.; Nagai, K.; Tagawa, T. Efficacy of Combined Photodynamic and Hyperthermic Therapy with a New Light Source in an in Vivo Osteosarcoma Tumor Model. J. Clin. Laser Med. Surg. 2004, 22, 3–8. [Google Scholar] [CrossRef]
- Yanase, S.; Nomura, J.; Matsumura, Y.; Watanabe, Y.; Tagawa, T. Synergistic Increase in Osteosarcoma Cell Sensitivity to Photodynamic Therapy with Aminolevulinic Acid Hexyl Ester in the Presence of Hyperthermia. Photomed. Laser Surg. 2009, 27, 791–797. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. RadioTher. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W.M. Palliative radiotherapy trials for bone metastases: A systematic review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- MA, F.; FD, V.; MH, S. Spinal radiosurgery for metastatic disease of the spine. Cancer Control. 2007, 14, 405–411. [Google Scholar]
- Lo, V.C.; Akens, M.K.; Wise-Milestone, L.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. The benefits of photodynamic therapy on vertebral bone are maintained and enhanced by combination treatment with bisphosphonates and radiation therapy. J. Orthop. Res. 2013, 31, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Lo, V.C.; Akens, M.K.; Moore, S.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. Beyond radiation therapy: Photodynamic therapy maintains structural integrity of irradiated healthy and metastatically involved vertebrae in a pre-clinical in vivo model. Breast Cancer Res. Treat. 2012, 135, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.T.; Wang, L.; Zhang, P.; Liu, S.B. Photodynamic therapy in spinal metastases: A qualitative analysis of published results. Int. Surg. 2015, 100, 712–719. [Google Scholar] [CrossRef]
- Okcu, M.F.; Munsell, M.; Treuner, J.; Mattke, A.; Pappo, A.; Cain, A.; Ferrari, A.; Casanova, M.; Ozkan, A.; Raney, B. Synovial sarcoma of childhood and adolescence: A multicenter, multivariate analysis of outcome. J. Clin. Oncol. 2003, 21, 1602–1611. [Google Scholar] [CrossRef]
- Siegel, H.J.; Sessions, W.; Casillas, M.A., Jr.; Said-Al-Naief, N.; Lander, P.H.; Lopez-Ben, R. Synovial sarcoma: Clinicopathologic features, treatment, and prognosis. Orthopedics 2007, 30, 1020–1027. [Google Scholar]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; Miyazaki, S.; Shintani, K.; Seto, M.; Matsumine, A.; Hosoi, H.; Sugimoto, T.; Uchida, A. Clinical Outcome of a Novel Photodynamic Therapy Technique Using Acridine Orange for Synovial Sarcomas. Photochem. Photobiol. 2005, 81, 705–709. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; Miyazaki, S.; Okamura, A.; Seto, M.; Matsumine, A.; Hosoi, H.; Sugimoto, T.; Uchida, A. Clinical Trial of Photodynamic Therapy Using Acridine Orange with/without Low Dose Radiation as New Limb Salvage Modality in Musculoskeletal Sarcomas. Anticancer Res. 2005, 25, 1225–1236. [Google Scholar]
- Yoshida, K.; Kusuzaki, K.; Matsubara, T.; Matumine, A.; Kumamoto, T.; Komada, Y.; Naka, N.; Uchida, A. Periosteal Ewing’s sarcoma treated by photodynamic therapy with acridine orange. Oncol. Rep. 2005, 13, 279–282. [Google Scholar]
- Gelderblom, H.; Hogendoorn, P.C.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.; Bovee, J.V. The clinical approach towards chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Lin, C.Y.; Kuo, S.J.; Su, C.M.; Tang, C.H. An update on current and future treatment options for chondrosarcoma. Expert Rev. Anticancer Ther. 2019, 19, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Nhembe, F.; Jerjes, W.; Upile, T.; Hamdoon, Z.; Hopper, C. Chondrosarcoma of the hyoid treated with interstitial photodynamic therapy: Case study. Photodiagn. Photodyn. Ther. 2009, 6, 235–237. [Google Scholar] [CrossRef]
- Burch, S.; Bisland, S.K.; Bogaards, A.; Yee, A.J.M.; Finkelstein, J.A.; Wilson, B.C.; Whyne, C.M. Photodynamic therapy for the treatment of vertebral metastases in a rat model of human breast carcinoma. J. Orthop. Res. 2005, 23, 995–1003. [Google Scholar] [CrossRef]
- Fisher, C.; Ali, Z.; Detsky, J.; Sahgal, A.; David, E.; Kunz, M.; Akens, M.; Chow, E.; Whyne, C.; Burch, S.; et al. Photodynamic Therapy for the Treatment of Vertebral Metastases: A Phase I Clinical Trial. Clin. Cancer Res. 2019, 25, 5766–5776. [Google Scholar] [CrossRef]
- Selbo, P.K.; Weyergang, A.; Hogset, A.; Norum, O.J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control Release 2010, 148, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Nordstrand, S.; Selbo, P.K.; Tran, D.T.; Angell-Petersen, E.; Hogset, A. Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photoch. Photobio. Sci. 2011, 10, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Hamdoon, Z.; Berg, K.; Hogset, A.; Hopper, C. Recurrent chondroblastic osteosarcoma of the right mandible subjected to photochemical internalization. Photodiagn. Photodyn. Ther. 2019, 27, 288–290. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2021, 33, e2004788. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.; Dong, X.; Zhang, C.; Mei, L.; Zang, Y.; Yan, L.; Zhang, H.; Gu, Z. Enhanced radiosensitization of ternary Cu3BiSe3 nanoparticles by photo-induced hyperthermia in the second near-infrared biological window. Nanoscale 2019, 11, 7157–7165. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ma, Z.; Wang, H.; Zhou, B.; Zhu, S.; Zhong, Y.; Wang, J.; Wan, H.; Antaris, A.; Ma, R.; et al. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liang, F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, D.; Guo, D.; Wang, N.; Su, Y.; Jin, X.; Tong, G.; Zhu, X. Zwitterionic gold nanorods: Low toxicity and high photothermal efficacy for cancer therapy. Biomater. Sci. 2017, 5, 686–697. [Google Scholar] [CrossRef]
- Gazzi, A.; Fusco, L.; Khan, A.; Bedognetti, D.; Zavan, B.; Vitale, F.; Yilmazer, A.; Delogu, L.G. Photodynamic Therapy Based on Graphene and MXene in Cancer Theranostics. Front. Bioeng. Biotech. 2019, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Su, Y.L.; Hu, S.H.; Chen, S.Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 5890–5900. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Moorthy, M.S.; Manivasagan, P.; Xu, L.; Song, K.; Lee, K.D.; Kwak, M.; Oh, J.; Jin, J.-O. Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget 2018, 9, 12649–12661. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W.; et al. Multifunctional Photonic Nanomaterials for Diagnostic, Therapeutic, and Theranostic Applications. Adv. Mater. 2018, 30, 1701460. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Zharov, V.P. Circulating Tumor Cell Detection and Capture by Photoacoustic Flow Cytometry in Vivo and ex Vivo. Cancers 2013, 5, 1691–1738. [Google Scholar] [CrossRef]
- Qian, K.Y.; Song, Y.; Yan, X.; Dong, L.; Xue, J.; Xu, Y.; Wang, B.; Cao, B.; Hou, Q.; Peng, W.; et al. Injectable ferrimagnetic silk fibroin hydrogel for magnetic hyperthermia ablation of deep tumor. Biomaterials 2020, 259, 120299. [Google Scholar] [CrossRef]
- Yuan, P.; Luo, Y.; Luo, Y.; Ma, L. A “sandwich” cell culture platform with NIR-responsive dynamic stiffness to modulate macrophage phenotypes. Biomater. Sci. 2021, 9, 2553–2561. [Google Scholar] [CrossRef]
- Hou, X.; Tao, Y.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [Google Scholar] [CrossRef]
- Liao, J.; Han, R.; Wu, Y.; Qian, Z. Review of a new bone tumor therapy strategy based on bifunctional biomaterials. Bone Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265, 120404. [Google Scholar] [CrossRef] [PubMed]
- Lapotko, D.O.; Lukianova, E.; Oraevsky, A.A. Selective laser nano-thermolysis of human leukemia cells with microbubbles generated around clusters of gold nanoparticles. Lasers Surg. Med. 2006, 38, 631–642. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Zhang, J.; Wang, X.; Wang, Y.; Ge, H.; Yan, W.; Huang, Q.; Xiao, J.; Zhang, Q.; et al. Trifolium-like Platinum Nanoparticle-Mediated Photothermal Therapy Inhibits Tumor Growth and Osteolysis in a Bone Metastasis Model. Small 2015, 11, 2080–2086. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Li, J.; Chen, Y.; Chen, Y.; Kawazoe, N.; Chen, G. Bifunctional scaffolds for the photothermal therapy of breast tumor cells and adipose tissue regeneration. J. Mater. Chem. B 2018, 6, 7728–7736. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Kulkarni, S.; Pandey, A.; Mutalik, S. Liquid metal based theranostic nanoplatforms: Application in cancer therapy, imaging and biosensing. Nanomedicine 2020, 26, 102175. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Ma, H.; Zhai, D.; Deng, C.; Wang, J.; Zhuo, S.; Chang, J.; Wu, C. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018, 73, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Leed, T.-C.; Doua, Q.Q.; Deene, G.R. Utilising inorganic nanocarriers for gene delivery. Biomater Sci. 2016, 4, 70–86. [Google Scholar] [CrossRef]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T.-C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Jindal, A.B. The effect of particle shape on cellular interaction and Drug Deliv applications of micro- and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.I.; Khalid, M.U.; Abdullah, A.; Ali, A.; Bhatti, A.S.; Khan, S.U.; Ahmed, W. Facile synthesis of gold nanostars over a wide size range and their excellent surface enhanced Raman scattering and fluorescence quenching properties. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2018, 36, 03E101. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Kandler, M.; Baran, J.; Parlinska-Wojtan, M. From spherical to bone-shaped gold nanoparticles-Time factor in the formation of Au NPs, their optical and photothermal properties. Photodiagn. Photodyn. Ther. 2020, 30, 101670. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021, 6, 2221–2230. [Google Scholar] [CrossRef]
- Sun, W.; Ge, K.; Jin, Y.; Han, Y.; Zhang, H.; Zhou, G.; Yang, X.; Liu, D.; Liu, H.; Liang, X.J.; et al. Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano 2019, 13, 7556–7567. [Google Scholar] [CrossRef]
- Tian, J.; Li, X.; Si, M.; Liu, T.; Li, J. CD271+ Osteosarcoma Cells Display Stem-Like Properties. PLoS ONE 2014, 9, e98549. [Google Scholar] [CrossRef]
- Schreiber, R.; Santiago, I.; Ardavan, A.; Turberfield, A.J. Ordering Gold Nanoparticles with DNA Origami Nanoflowers. ACS Nano 2016, 10, 7303–7306. [Google Scholar] [CrossRef]
- Couto, C.; Vitorino, R.; Daniel-da-Silva, A.L. Gold nanoparticles and bioconjugation: A pathway for proteomic applications. Crit. Rev. Biotechnol. 2017, 37, 238–250. [Google Scholar] [CrossRef]
- Tian, J.; Gu, Y.; Li, Y.; Liu, T. CD271 antibody-functionalized HGNs for targeted photothermal therapy of osteosarcoma stem cells. Nanotechnology 2020, 31, 305707. [Google Scholar] [CrossRef]
- Cui, G.; He, P.; Yu, L.; Wen, C.; Xie, X.; Yao, G. Oxygen self-enriched nanoplatform combined with US imaging and chemo/photothermal therapy for breast cancer. Nanomedicine 2020, 29, 102238. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic Acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Liu, Z.; Wang, L.; Luo, L.; Wang, M.; Wang, Q.; Gao, D. Gold nanoshell-based betulinic acid liposomes for synergistic chemo-photothermal therapy. Nanomedicine 2017, 13, 1891–1900. [Google Scholar] [CrossRef]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 85103. [Google Scholar] [CrossRef]
- Kajita, M.; Hikosaka, K.; Iitsuka, M.; Kanayama, A.; Toshima, N.; Miyamoto, Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic. Res. 2007, 41, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kajita, M.; Kim, J.; Kanayama, A.; Takahashi, K.; Mashino, T.; Miyamoto, Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology 2009, 20, 455105. [Google Scholar] [CrossRef]
- Manikandan, M.; Hasan, N.; Wu, H.F. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials 2013, 34, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Y.; Yan, Y.; Zhang, Q.; Cheng, Y. Dendrimer-Templated Ultrasmall and Multifunctional Photothermal Agents for Efficient Tumor Ablation. ACS Nano 2016, 10, 4863–4872. [Google Scholar] [CrossRef]
- Chen, C.L.; Kuo, L.R.; Lee, S.Y.; Hwu, Y.K.; Chou, S.W.; Chen, C.C.; Chang, F.H.; Lin, K.H.; Tsai, D.H.; Chen, Y.Y. Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 2013, 34, 1128–1134. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, X.; Zhang, S.; Wang, Y.; Zhou, Z.; Xiao, J.; Zhang, Q.; Cheng, Y. A Carboxyl-Terminated Dendrimer Enables Osteolytic Lesion Targeting and Photothermal Ablation of Malignant Bone Tumors. ACS Appl. Mater. Interface 2019, 11, 160–168. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, T.; Yan, Y.; Zhang, S.; Zhou, Y.; Deng, H.; Cai, X.; Xiao, J.; Song, D.; Zhang, Q.; et al. One stone with two birds: Phytic acid-capped platinum nanoparticles for targeted combination therapy of bone tumors. Biomaterials 2019, 194, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, F.; He, G.; Han, X.; Wang, X.; Qin, J.; Guo, Z.X.; Lu, X.; Wang, Q.; Parkin, I.P.; et al. Ultrasmall CuCo2S4Nanocrystals: All-in-One Theragnosis Nanoplatform with Magnetic Resonance/Near-Infrared Imaging for Efficiently Photothermal Therapy of Tumors. Adv. Funct. Mater. 2017, 27, 1606218. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Martinez-Alvarez, O.; Santos-Cruz, J.; Garcia-Contreras, R.; Acosta-Torres, L.S.; de la Fuente-Hernandez, J.; Arenas-Arrocena, M.C. Nanomaterials made of non-toxic metallic sulfides: A systematic review of their potential biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, C.; Zeng, J.; Sun, Q.; Wang, G.; Wang, Y.; Wu, Y.; Dou, S.; Gao, M.; Li, Z. Ambient Aqueous Synthesis of Ultrasmall PEGylated Cu2-x Se Nanoparticles as a Multifunctional Theranostic Agent for Multimodal Imaging Guided Photothermal Therapy of Cancer. Adv. Mater. 2016, 28, 8927–8936. [Google Scholar] [CrossRef]
- Chang, L.; Liu, Y.; Wu, C. Copper-Doped Mesoporous Bioactive Glass for Photothermal Enhanced Chemotherapy. J. Biomed. Nanotechnol. 2018, 14, 786–794. [Google Scholar] [CrossRef]
- Ma, H.; Ma, Z.; Chen, Q.; Li, W.; Liu, X.; Ma, X.; Mao, Y.; Yang, H.; Ma, H.; Wang, J. Bifunctional, Copper-Doped, Mesoporous Silica Nanosphere-Modified, Bioceramic Scaffolds for Bone Tumor Therapy. Front. Chem. 2020, 8, 610232. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Liu, H.; Wang, X.; Zhou, Z.; Huang, Q.; Song, D.; Cai, X.; Li, L.; Lin, K.; et al. Osteotropic peptide-mediated bone targeting for photothermal treatment of bone tumors. Biomaterials 2017, 114, 97–105. [Google Scholar] [CrossRef]
- Drager, J.; Sheikh, Z.; Zhang, Y.L.; Harvey, E.J.; Barralet, J.E. Local delivery of iron chelators reduces in vivo remodeling of a calcium phosphate bone graft substitute. Acta Biomater. 2016, 42, 411–419. [Google Scholar] [CrossRef]
- Katsumata, S.; Tsuboi, R.; Uehara, M.; Suzuki, K. Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci. Biotechnol. Biochem. 2006, 70, 2547–2550. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yan, T.; Xue, Y.; Guo, L.; Zhang, L.; Han, Y. Intrinsically ferromagnetic Fe-doped TiO2 coatings on titanium for accelerating osteoblast response in vitro. J. Mater. Chem. B 2018, 6, 5756–5767. [Google Scholar] [CrossRef]
- Jia, P.; Xu, Y.J.; Zhang, Z.L.; Li, K.; Li, B.; Zhang, W.; Yang, H. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J. Orthop. Res. 2012, 30, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Lin, R.; Liu, Y.; Zhang, M.; Zhai, D.; Huan, Z.; Wu, C. Three-Dimensional-Printed Bioceramic Scaffolds with Osteogenic Activity for Simultaneous Photo/Magnetothermal Therapy of Bone Tumors. ACS Biomater. Sci. Eng. 2019, 5, 6725–6734. [Google Scholar] [CrossRef]
- Sun, J.; Shakya, S.; Gong, M.; Liu, G.; Wu, S.; Xiang, Z. Combined Application of Graphene-Family Materials and Silk Fibroin in Biomedicine. ChemistrySelect 2019, 4, 5745–5754. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 764–780. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhu, C.; Xu, H.; Sun, D.; Chen, C.; Feng, G.; Liu, L.; Li, Y.; Zhang, L. Conducting Polyetheretherketone Nanocomposites with an Electrophoretically Deposited Bioactive Coating for Bone Tissue Regeneration and Multimodal Therapeutic Applications. ACS Appl. Mater. Interface 2020, 12, 56924–56934. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Deliv. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Cicuendez, M.; Silva, V.S.; Hortiguela, M.J.; Matesanz, M.C.; Vila, M.; Portoles, M.T. MC3T3-E1 pre-osteoblast response and differentiation after graphene oxide nanosheet uptake. Colloid Surf. B. 2017, 158, 33–40. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, N.; Moore, J.; McCoy, C.P.; Ziminska, M.; Rafferty, C.; Sarri, G.; Hamilton, A.R.; Li, Y.; Zhang, L.; et al. Nanoscale Hybrid Coating Enables Multifunctional Tissue Scaffold for Potential Multimodal Therapeutic Applications. ACS Appl. Mater. Interface 2019, 11, 27269–27278. [Google Scholar] [CrossRef]
- Xu, C.; Ma, B.; Peng, J.; Gao, L.; Xu, Y.; Huan, Z.; Chang, J. Tricalcium silicate/graphene oxide bone cement with photothermal properties for tumor ablation. J. Mater. Chem. B 2019, 7, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.W.; Liu, X.L.; Yu, D.G.; Zhu, Z.A.; Ke, Q.F.; Mao, Y.Q.; Guo, Y.P.; Zhang, J.W. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability. J. Nanobiotechnol. 2021, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nie, W.; Chen, L.; McCoul, D.; Liu, D.; Zhang, X.; Ji, Y.; Yu, B.; He, C. Self-Assembled Hydroxyapatite-Graphene Scaffold for Photothermal Cancer Therapy and Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, C.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.; Chen, Q.; Wang, H. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Ding, X.; Singh, R.; Kraft, R.A.; Levi-Polyachenko, N.; Rylander, M.N.; Szot, C.; Buchanan, C.; Whitney, J.; Fisher, J.; et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc. Natl. Acad. Sci. USA 2009, 106, 12897–12902. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Wang, X.; Zhu, D.; Liu, L.; Leng, X. Simultaneous monitoring of the drug release and antitumor effect of a novel Drug Deliv system-MWCNTs/DOX/TC. Drug Deliv. 2017, 24, 143–151. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Wang, X.; Leng, X. An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer. Nanomedicine 2017, 13, 2271–2280. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Jing, H.; Dong, X.; Leng, X. MWCNT-mediated combinatorial photothermal ablation and chemo-immunotherapy strategy for the treatment of melanoma. J. Mater. Chem. B. 2020, 8, 4245–4258. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Y.; Ma, X.; Hu, S.; Zhang, J.; Wu, Q.; Ye, W.; Zhu, S.; Yang, D.; Qu, D.; et al. Photothermal ablation of bone metastasis of breast cancer using PEGylated multi-walled carbon nanotubes. Sci. Rep. 2015, 5, 11709. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Mohammadi-Aghdam, M.; Saber-Samandari, S. A novel magnetic bifunctional nanocomposite scaffold for photothermal therapy and tissue engineering. Int. J. Biol. Macromol. 2019, 138, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. Int. Ed. Engl. 2012, 51, 12215–12218. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Wang, X.; Wang, Z.; Zhang, C.; He, M.; Wang, K.; Chen, F.; Li, Z.; Shen, G.; et al. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy. Adv. Mater. 2012, 24, 5104–5110. [Google Scholar] [CrossRef]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice. Adv. Mater. 2015, 27, 4169–4177. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Li, M.; Lin, Z.; Wang, L.; Zhang, Y.; Yin, Q.; Xia, H.; Han, G. Zero-Dimensional Carbon Dots Enhance Bone Regeneration, Osteosarcoma Ablation, and Clinical Bacterial Eradication. Bioconjug. Chem. 2018, 29, 2982–2993. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, Y.N.; Wan, J.; Cui, R.; Yu, X.; Zhao, G.; Lin, K. A novel multifunctional carbon aerogel-coated platform for osteosarcoma therapy and enhanced bone regeneration. J. Mater. Chem. B 2020, 8, 368–379. [Google Scholar] [CrossRef]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Chang, Y.; Sun, C.; Shao, S.; Xie, Z.; Xiao, X.; Ma, P.; Zhang, H.; Cheng, Z.; et al. Intelligent Hollow Pt-CuS Janus Architecture for Synergistic Catalysis-Enhanced Sonodynamic and Photothermal Cancer Therapy. Nano Lett. 2019, 19, 4134–4145. [Google Scholar] [CrossRef]
- Tan, T.L.; Jin, H.M.; Sullivan, M.B.; Anasori, B.; Gogotsi, Y. High-Throughput Survey of Ordering Configurations in MXene Alloys Across Compositions and Temperatures. ACS Nano 2017, 11, 4407–4418. [Google Scholar] [CrossRef]
- Driscoll, N.; Richardson, A.G.; Maleski, K.; Anasori, B.; Adewole, O.; Lelyukh, P.; Escobedo, L.; Cullen, D.K.; Lucas, T.H.; Gogotsi, Y.; et al. Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces. ACS Nano 2018, 12, 10419–10429. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3 C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, e1701394. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yin, J.; Yu, L.; Zhang, C.; Zhu, Y.; Gao, Y.; Chen, Y. 2D MXene-Integrated 3D-Printing Scaffolds for Augmented Osteosarcoma Phototherapy and Accelerated Tissue Reconstruction. Adv. Sci. 2020, 7, 1901511. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef]
- Chung, M.F.; Liu, H.Y.; Lin, K.J.; Chia, W.T.; Sung, H.W. A pH-Responsive Carrier System that Generates NO Bubbles to Trigger Drug Release and Reverse P-Glycoprotein-Mediated Multidrug Resistance. Angew. Chem. Int. Ed. Engl. 2015, 54, 9890–9893. [Google Scholar] [CrossRef]
- Chachlaki, K.; Garthwaite, J.; Prevot, V. The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nat. Rev. Endocrinol. 2017, 13, 521–535. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van Oers, R.F.; Bakker, A.D.; Bacabac, R.G. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int. 2014, 25, 1427–1437. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, H.; Xu, T.; Zhu, D.; Yin, J.; Chen, Y.; Yu, X.; Gao, J.; Zhang, C.; Chen, Y.; et al. Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small 2020, 16, e1906814. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Han, Q.; Zhang, J.; Liu, Y.; Gan, X.; Xie, K.; Xie, L.; Deng, Y. MXene-Based Hydrogels Endow Polyetheretherketone with Effective Osteogenicity and Combined Treatment of Osteosarcoma and Bacterial Infection. ACS Appl. Mater. Interface 2020, 12, 45891–45903. [Google Scholar] [CrossRef]
- Chiang, C.W.; Chuang, E.Y. Biofunctional core-shell polypyrrole-polyethylenimine nanocomplex for a locally sustained photothermal with reactive oxygen species enhanced therapeutic effect against lung cancer. Int. J. Nanomed. 2019, 14, 1575–1585. [Google Scholar] [CrossRef]
- Giuliani, C. The Flavonoid Quercetin Induces AP-1 Activation in FRTL-5 Thyroid Cells. Antioxidants 2019, 8, 112. [Google Scholar] [CrossRef]
- Lu, J.W.; Yang, F.; Ke, Q.F.; Xie, X.T.; Guo, Y.P. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomedicine 2018, 14, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lu, J.; Ke, Q.; Peng, X.; Guo, Y.; Xie, X. Magnetic Mesoporous Calcium Sillicate/Chitosan Porous Scaffolds for Enhanced Bone Regeneration and Photothermal-Chemotherapy of Osteosarcoma. Sci. Rep. 2018, 8, 7345. [Google Scholar] [CrossRef]
- Jie, S.; Guo, X.; Ouyang, Z. Tumor ablation using novel photothermal NaxWO3 nanoparticles against breast cancer osteolytic bone metastasis. Int. J. Nanomed. 2019, 14, 7353–7362. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme Decorated Metal-Organic Frameworks for Enhanced Photodynamic Therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef]
- He, C.; Liu, D.; Lin, W. Nanomedicine Applications of Hybrid Nanomaterials Built from Metal-Ligand Coordination Bonds: Nanoscale Metal-Organic Frameworks and Nanoscale Coordination Polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef]
- Jian, M.; Liu, H.; Williams, T.; Ma, J.; Wang, H.; Zhang, X. Temperature-induced oriented growth of large area, few-layer 2D metal-organic framework nanosheets. Chem. Commun. 2017, 53, 13161–13164. [Google Scholar] [CrossRef]
- Qu, Y.; Zhuang, H.; Zhang, M.; Wang, Y.; Zhai, D.; Ma, B.; Wang, X.; Qin, C.; Huan, Z.; Wu, C. Bone cements for therapy and regeneration for minimally invasive treatment of neoplastic bone defects. J. Mater. Chem. B 2021, 9, 4355–4364. [Google Scholar] [CrossRef]
- Dang, W.; Ma, B.; Li, B.; Huan, Z.; Ma, N.; Zhu, H.; Chang, J.; Xiao, Y.; Wu, C. 3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication 2020, 12, 025005. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Ma, B.; Wu, J.; Chang, J.; Gelinsky, M.; Wu, C. Black Bioceramics: Combining Regeneration with Therapy. Adv. Mater. 2020, 32, e2005140. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Jiang, C.; Li, Y.; Song, X.; Lu, L. Plasmonic titanium nitride nanoparticles for in vivo photoacoustic tomography imaging and photothermal cancer therapy. Biomaterials 2017, 132, 37–47. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Jiao, Y.; Wu, C.; Guo, S.; Xiao, X.; Wei, X.; Wu, J.; Gao, P.; Wang, N.; et al. Biological Effects of a Three-Dimensionally Printed Ti6Al4V Scaffold Coated with Piezoelectric BaTiO3 Nanoparticles on Bone Formation. ACS Appl. Mater. Interface 2020, 12, 51885–51903. [Google Scholar] [CrossRef]
- Dang, W.; Yi, K.; Ju, E.; Jin, Y.; Xu, Y.; Wang, H.; Chen, W.C.; Wang, K.; Wang, Y.; Tao, Y.; et al. 3D Printed Bioceramic Scaffolds as a Universal Therapeutic Platform for Synergistic Therapy of Osteosarcoma. ACS Appl. Mater. Interface 2021, 13, 18488–18499. [Google Scholar] [CrossRef] [PubMed]
- Hamdadou, N.; Morsli, M.; Khelil, A.; Bernède, J.C. Fabrication of n- and p-type doped CuFeSe2thin films achieved by selenization of metal precursors. J. Phys. D Appl. Phys. 2006, 39, 1042–1049. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, J.; Ding, T.; Wang, C.; Zuo, J.; Yang, Q. Alternative synthesis of CuFeSe2 nanocrystals with magnetic and photoelectric properties. ACS Appl. Mater. Interface 2015, 7, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Li, T.; Li, B.; Ma, H.; Zhai, D.; Wang, X.; Chang, J.; Xiao, Y.; Wang, J.; Wu, C. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials 2018, 160, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2014, 8, 340–354. [Google Scholar] [CrossRef]
- Cheng, L.; He, W.; Gong, H.; Wang, C.; Chen, Q.; Cheng, Z.; Liu, Z. PEGylated Micelle Nanoparticles Encapsulating a Non-Fluorescent Near-Infrared Organic Dye as a Safe and Highly-Effective Photothermal Agent for In Vivo Cancer Therapy. Adv. Funct. Mater. 2013, 23, 5893–5902. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Shi, W.; Liu, X.; Ruan, C.; Xue, M.; Ge, D. Affinity electromembrane with covalently coupled heparin for thrombin adsorption. J. Membr. Sci. 2013, 428, 70–77. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Liu, T.; Lv, F.; Qian, Z. Real-Time Fluorescence Tracking of Protoporphyrin Incorporated Thermosensitive Hydrogel and Its Drug Release in Vivo. ACS Appl. Mater. Interface 2016, 8, 5104–5113. [Google Scholar] [CrossRef] [PubMed]
- Biffi, S.; Voltan, R.; Rampazzo, E.; Prodi, L.; Zauli, G.; Secchiero, P. Applications of nanoparticles in cancer medicine and beyond: Optical and multimodal in vivo imaging, tissue targeting and Drug Deliv. Expert Opin. Drug Deliv. 2015, 12, 1837–1849. [Google Scholar] [CrossRef]
- Li, L.; Yang, S.; Song, L.; Zeng, Y.; He, T.; Wang, N.; Yu, C.; Yin, T.; Liu, L.; Wei, X.; et al. An Endogenous Vaccine Based on Fluorophores and Multivalent Immunoadjuvants Regulates Tumor Micro-Environment for Synergistic Photothermal and Immunotherapy. Theranostics 2018, 8, 860–873. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Chen, Z.; Wu, Y.; Gu, Y.; Lin, S.; Wang, Y. In Vivo Photoacoustic/Single-Photon Emission Computed Tomography Imaging for Dynamic Monitoring of Aggregation-Enhanced Photothermal Nanoagents. Anal. Chem. 2019, 91, 2128–2134. [Google Scholar] [CrossRef]
- Ferreira, L.P.; Gaspar, V.M.; Monteiro, M.V.; Freitas, B.; Silva, N.J.O.; Mano, J.F. Screening of dual chemo-photothermal cellular nanotherapies in organotypic breast cancer 3D spheroids. J. Control Release 2021, 331, 85–102. [Google Scholar] [CrossRef]
- Lin, W.; Li, C.; Xu, N.; Watanabe, M.; Xue, R.; Xu, A.; Araki, M.; Sun, R.; Liu, C.; Nasu, Y.; et al. Dual-Functional PLGA Nanoparticles Co-Loaded with Indocyanine Green and Resiquimod for Prostate Cancer Treatment. Int. J. Nanomed. 2021, 16, 2775–2787. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef]
- Yang, A.; Dong, X.; Liang, J.; Zhang, Y.; Yang, W.; Liu, T.; Yang, J.; Kong, D.; Lv, F. Photothermally triggered disassembly of a visible dual fluorescent poly(ethylene glycol)/alpha-cyclodextrin hydrogel. Soft Matter. 2018, 14, 4495–4504. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Chen, S.; Guo, Q.; Jing, Z.; Huang, B.; Pan, Y.; Wang, L.; Hu, Y. Zoledronate and SPIO dual-targeting nanoparticles loaded with ICG for photothermal therapy of breast cancer tibial metastasis. Sci. Rep. 2020, 10, 13675. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef]
- Bi, X.; Su, H.; Shi, W.; Liu, X.; He, Z.; Zhang, X.; Sun, Y.; Ge, D. BSA-modified poly(pyrrole-3-carboxylic acid) nanoparticles as carriers for combined chemo-photothermal therapy. J. Mater. Chem. B 2018, 6, 7877–7888. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Luo, J.; Sun, Z.; Xia, L.; Shi, M.; Liu, M.; Chang, J.; Wu, C. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 2016, 111, 138–148. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef]
- Le, Q.V.; Suh, J.; Choi, J.J.; Park, G.T.; Lee, J.W.; Shim, G.; Oh, Y.K. In Situ Nanoadjuvant-Assembled Tumor Vaccine for Preventing Long-Term Recurrence. ACS Nano 2019, 13, 7442–7462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, S.; Tian, Y.; Jiang, X.; Fu, D.; Shen, S.; Yang, W. Polydopamine-Coated Magnetic Composite Particles with an Enhanced Photothermal Effect. ACS Appl. Mater. Interface 2015, 7, 15876–15884. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; He, X.; Chen, H.; Zou, Y.; Li, Y.; Lin, K.; Cai, X.; Xiao, J.; Zhang, Q.; et al. Multifunctional melanin-like nanoparticles for bone-targeted chemo-photothermal therapy of malignant bone tumors and osteolysis. Biomaterials 2018, 183, 10–19. [Google Scholar] [CrossRef]
- Luo, S.; Wu, J.; Jia, Z.; Tang, P.; Sheng, J.; Xie, C.; Liu, C.; Gan, D.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, e1900047. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Xu, B.; Chen, H.; Wang, X.; Zhang, J.; Guo, R.; Shi, X.; Cao, X. Stem cell-mediated delivery of nanogels loaded with ultrasmall iron oxide nanoparticles for enhanced tumor MR imaging. Nanoscale 2019, 11, 4904–4910. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Liu, T.; Shao, P.; Duan, L.; Yan, J.; Mu, X.; Jiang, J. Polydopamine Nanoparticles Camouflaged by Stem Cell Membranes for Synergistic Chemo-Photothermal Therapy of Malignant Bone Tumors. Int. J. Nanomed. 2020, 15, 10183–10197. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zou, Q.; Zou, W.; Xie, Z.; Li, Z.; Zhao, X.; Du, C. Bifunctional scaffolds of hydroxyapatite/poly(dopamine)/carboxymethyl chitosan with osteogenesis and anti-osteosarcoma effect. Biomater. Sci. 2021, 9, 3319–3333. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, S.; Yong, Y.; Zhang, X.; Dong, X.; Du, J.; Xie, J.; Wang, Q.; Gu, Z.; Zhao, Y. Synthesis of BSA-Coated BiOI@Bi2 S3 Semiconductor Heterojunction Nanoparticles and Their Applications for Radio/Photodynamic/Photothermal Synergistic Therapy of Tumor. Adv. Mater. 2017, 29, 1704136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, Z.; Zhang, L.; Brown, E.M.; Wu, A. Layered bismuth oxyhalide nanomaterials for highly efficient tumor photodynamic therapy. Nanoscale 2016, 8, 12715–12722. [Google Scholar] [CrossRef]
- Hu, J.; Luo, H.; Qu, Q.; Liao, X.; Huang, C.; Chen, J.; Cai, Z.; Bao, Y.; Chen, G.; Li, B.; et al. Cell Membrane-Inspired Polymeric Vesicles for Combined Photothermal and Photodynamic Prostate Cancer Therapy. ACS Appl. Mater. Interface 2020, 12, 42511–42520. [Google Scholar] [CrossRef]
- Quadros, M.; Momin, M.; Verma, G. Design strategies and evolving role of biomaterial assisted treatment of osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111875. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, W.; Xu, X.; Lin, Z.; Xie, C.; Zhang, Y.; Zhang, T.; Li, L.; Lu, Y.; Li, Q. AgBiS2 nanoparticles with synergistic photodynamic and bioimaging properties for enhanced malignant tumor phototherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110324. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Luo, S.; Long, L.; Wang, Y.; Wang, D.; Fang, S.; Ouyang, Q.; Su, Y.; Cheng, T.; Shi, C. Structure-Guided Design and Synthesis of a Mitochondria-Targeting Near-Infrared Fluorophore with Multimodal Therapeutic Activities. Adv. Mater. 2017, 29, 1704196. [Google Scholar] [CrossRef]
- Shan, W.; Chen, R.; Zhang, Q.; Zhao, J.; Chen, B.; Zhou, X.; Ye, S.; Bi, S.; Nie, L.; Ren, L. Improved Stable Indocyanine Green (ICG)-Mediated Cancer Optotheranostics with Naturalized Hepatitis B Core Particles. Adv. Mater. 2018, 30, e1707567. [Google Scholar] [CrossRef]
- Zeng, W.N.; Yu, Q.P.; Wang, D.; Liu, J.L.; Yang, Q.J.; Zhou, Z.K.; Zeng, Y.P. Mitochondria-targeting graphene oxide nanocomposites for fluorescence imaging-guided synergistic phototherapy of drug-resistant osteosarcoma. J. Nanobiotechnol. 2021, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Gomer, C.J. Induction of Prosurvival Molecules During Treatment: Rethinking Therapy Options for Photodynamic Therapy. J. Natl. Compr. Cancer Netw. 2012, 10, S-35–S-39. [Google Scholar] [CrossRef] [PubMed]
- Brahim, W.; Mestiri, M.; Betrouni, N.; Hamrouni, K. Malignant pleural mesothelioma segmentation for photodynamic therapy planning. Comput. Med. Imaging Graph. 2018, 65, 79–92. [Google Scholar] [CrossRef] [PubMed]
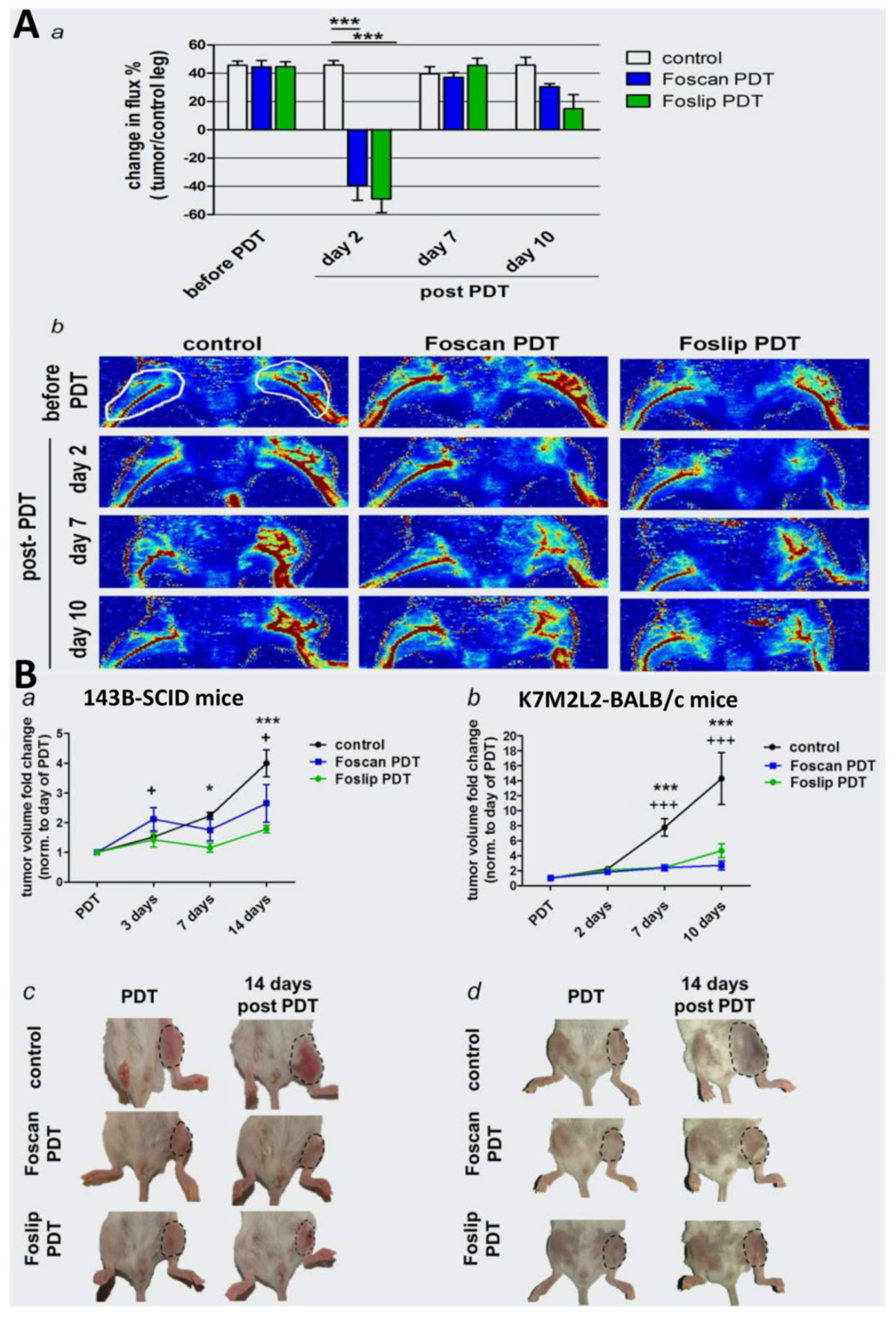
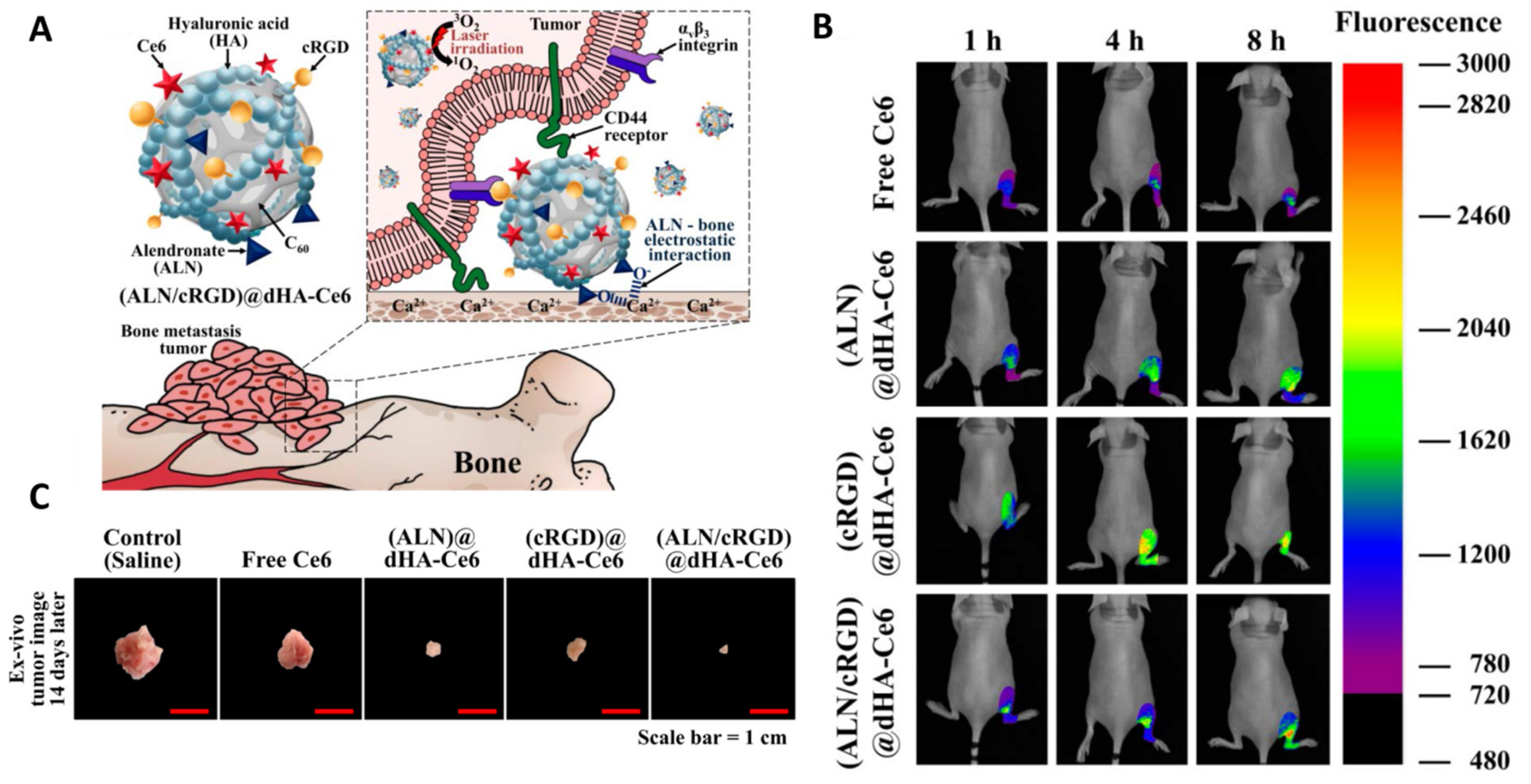
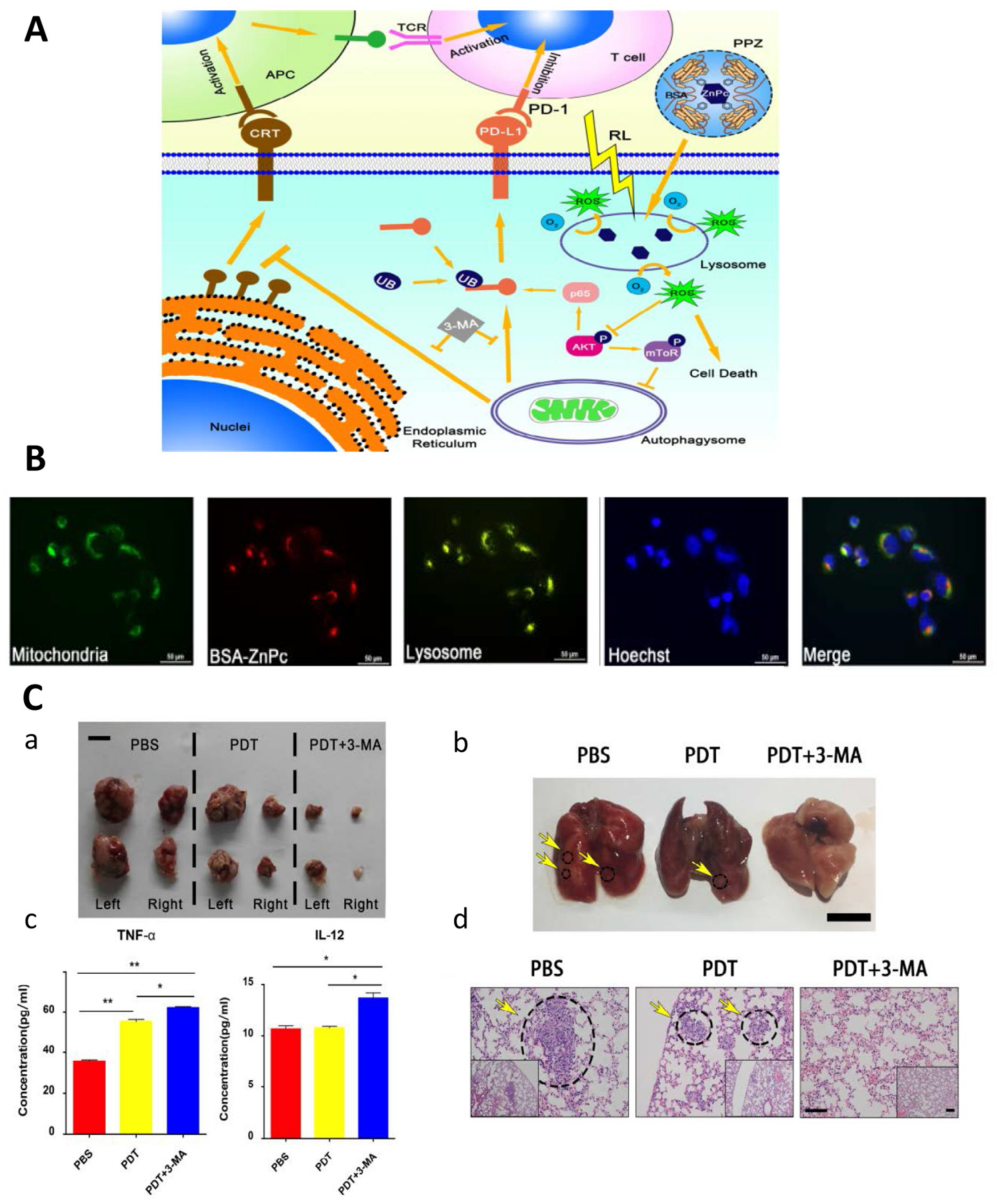

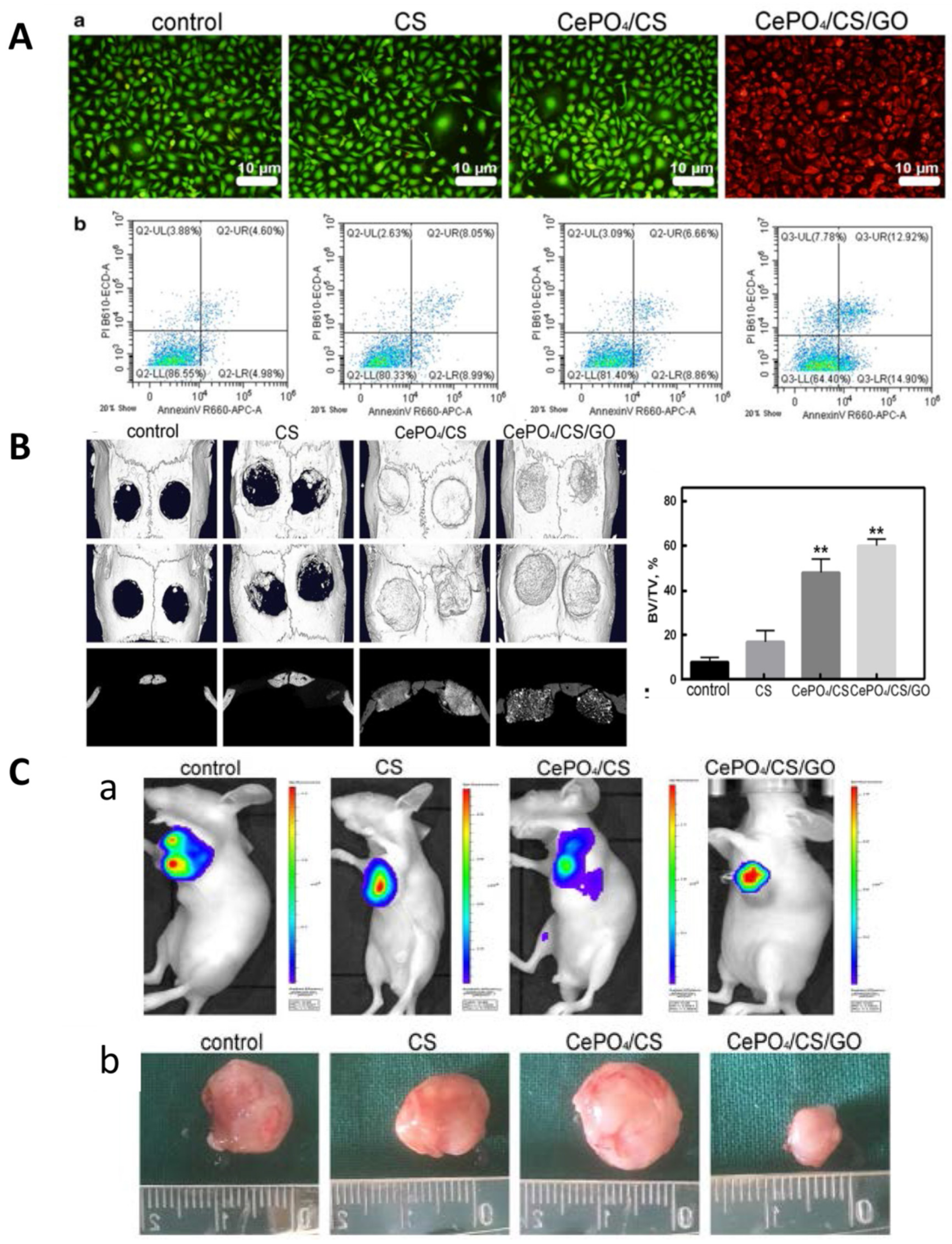

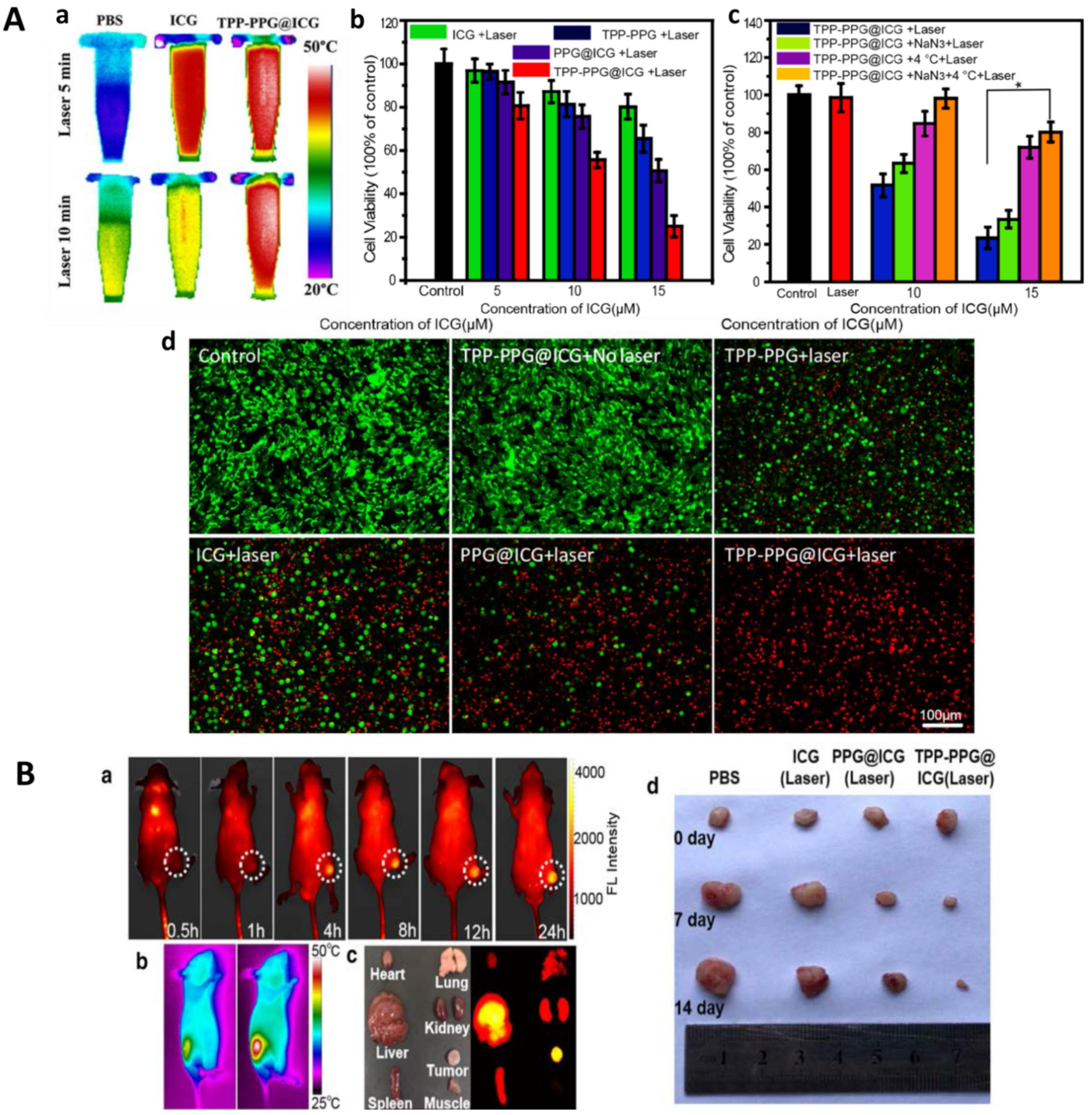
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Xing, F.; Braun, J.; Traub, F.; Rommens, P.M.; Xiang, Z.; Ritz, U. Progress of Phototherapy Applications in the Treatment of Bone Cancer. Int. J. Mol. Sci. 2021, 22, 11354. https://doi.org/10.3390/ijms222111354
Sun J, Xing F, Braun J, Traub F, Rommens PM, Xiang Z, Ritz U. Progress of Phototherapy Applications in the Treatment of Bone Cancer. International Journal of Molecular Sciences. 2021; 22(21):11354. https://doi.org/10.3390/ijms222111354
Chicago/Turabian StyleSun, Jiachen, Fei Xing, Joy Braun, Frank Traub, Pol Maria Rommens, Zhou Xiang, and Ulrike Ritz. 2021. "Progress of Phototherapy Applications in the Treatment of Bone Cancer" International Journal of Molecular Sciences 22, no. 21: 11354. https://doi.org/10.3390/ijms222111354
APA StyleSun, J., Xing, F., Braun, J., Traub, F., Rommens, P. M., Xiang, Z., & Ritz, U. (2021). Progress of Phototherapy Applications in the Treatment of Bone Cancer. International Journal of Molecular Sciences, 22(21), 11354. https://doi.org/10.3390/ijms222111354







