GnRH Antagonists with or without Add-Back Therapy: A New Alternative in the Management of Endometriosis?
Abstract
1. Introduction
2. Why Are Estroprogestins and Progestins Only Effective in Two-Thirds of Women?
3. Why Do We Need New Options?
- Among new drugs, selective progesterone receptor modulators (SPRMs) are not a viable option, as they also induce endometrial changes in ectopic foci [35,36,37]. Moreover, their use for fibroids is strictly limited to defined indications due to the possibility of triggering liver disease, while their use in endometriosis is off-label.
- GnRH agonists are effective at treating endometriosis symptoms, but have numerous limitations, including a delayed therapeutic impact because of the flare-up effect, suppression of E2 to less than 20 pg/mL, inability to titrate E2 levels, and unpredictable reversibility of treatment when injectable depot forms of GnRH agonists are used [38,39,40,41,42,43].
4. How Do We Achieve Partial E2 Suppression? Is Gnrh Antagonist the Best New Option?
- Oral administration.
- Immediate suppression of FSH and LH secretion.
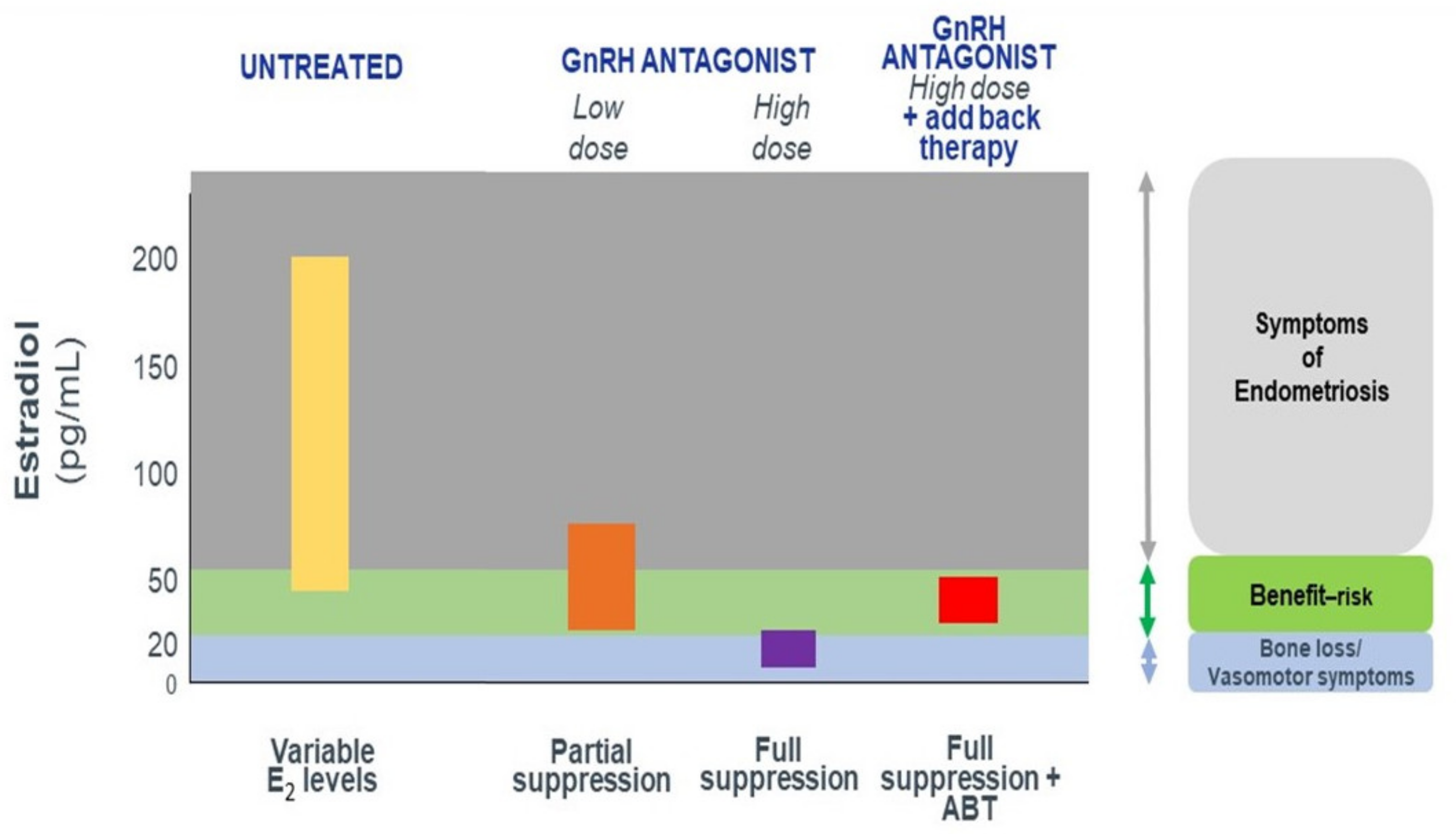
- Dose-dependent estrogen suppression, from partial suppression at lower doses to full suppression at higher doses (Figure 1), suggesting the possibility of individual tailoring according to the symptoms and wishes of the patient.
- Rapid reversibility and recovery of hormone secretion after stopping treatment.
4.1. Elagolix
4.2. Linzagolix
4.3. Relugolix
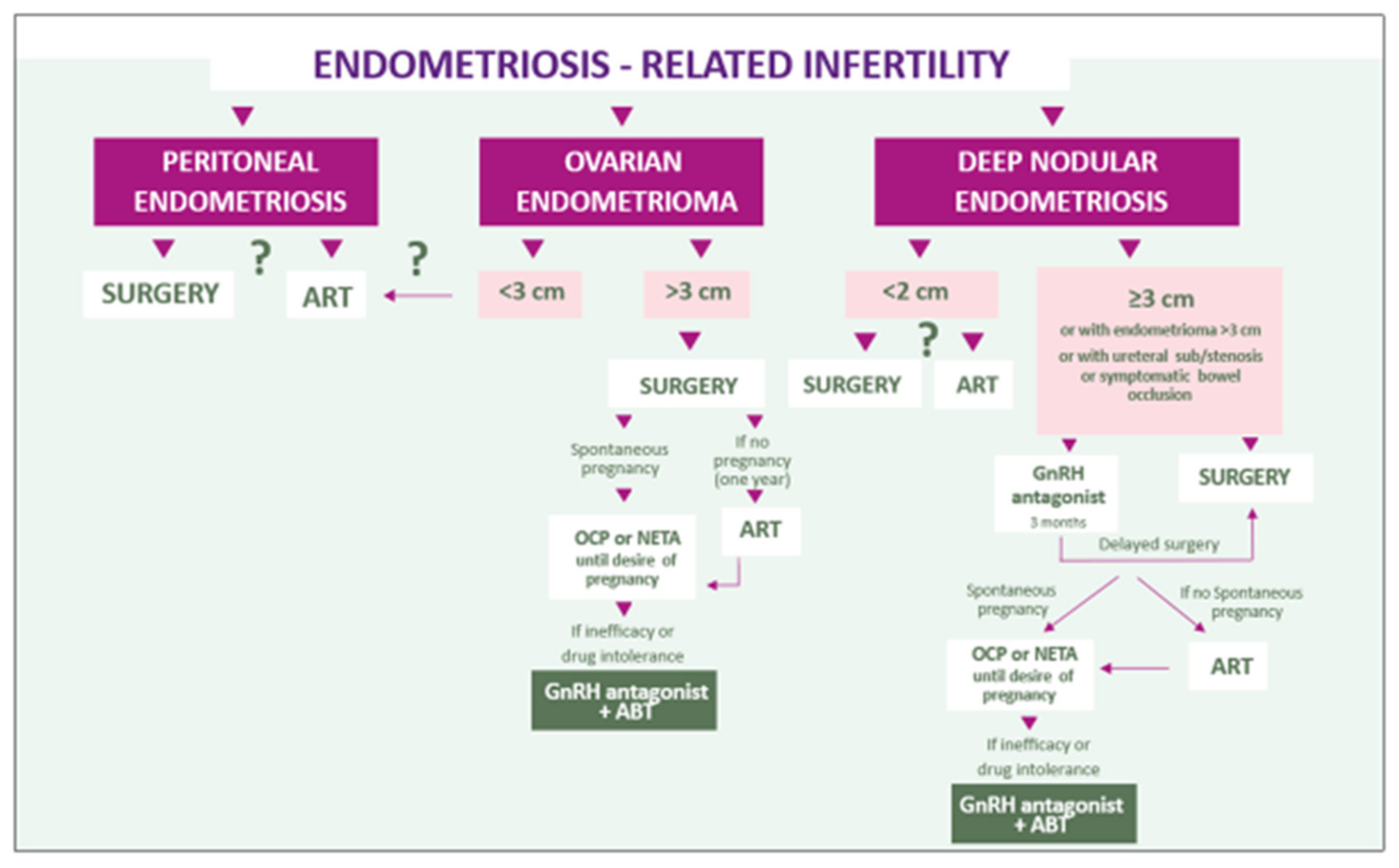
5. Discussion and Conclusion: A Combined Symptom-Oriented and Phenotype-Adapted Approach
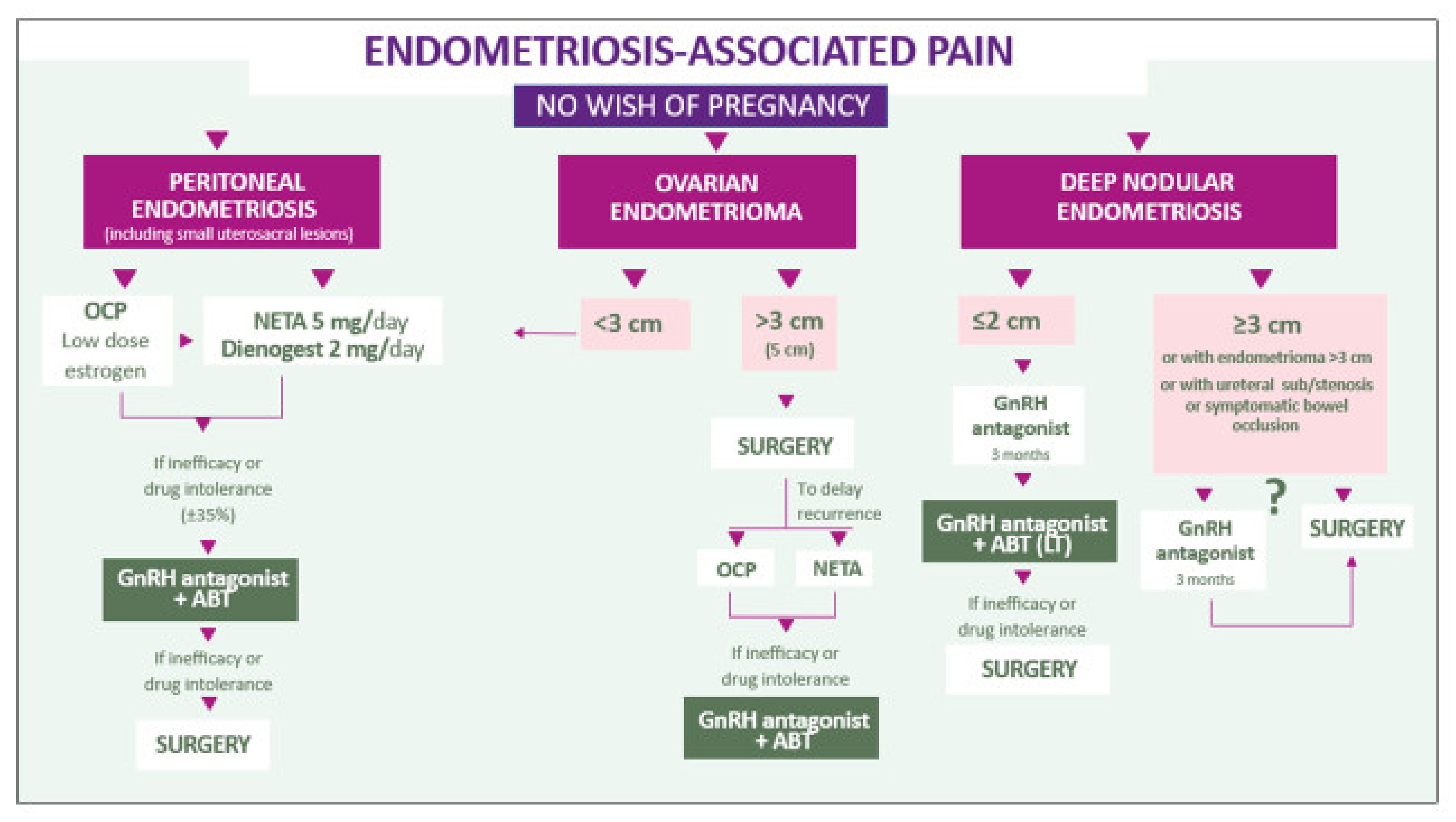
5.1. Peritoneal Lesions
5.2. Endometriomas
5.3. Deep Endometriosis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donnez, J.; Chantraine, F.; Nisolle, M. The efficacy of medical and surgical treatment of endometriosis-associated infertility: Arguments in favour of a medico-surgical approach. Hum. Reprod. Update 2002, 8, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharm. 2018, 19, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L. Hormone treatment of endometriosis: The estrogen threshold hypothesis. Am. J. Obstet. Gynecol. 1992, 166, 740–745. [Google Scholar] [CrossRef]
- Casper, R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017, 107, 533–536. [Google Scholar] [CrossRef]
- Vercellini, P. Are combined hormonal contraceptives the neglected treatment for symptomatic endometriosis? Fertil. Steril. 2018, 110, 61–62. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Berlanda, N.; Barbara, G.; Somigliana, E.; Bosari, S. Estrogen-progestins and progestins for the management of endometriosis. Fertil. Steril. 2016, 106, 1552–1571. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Frattaruolo, M.P.; Borghi, A.; Dridi, D.; Somigliana, E. Medical treatment of endometriosis-related pain. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 68–91. [Google Scholar] [CrossRef]
- Vercellini, P.; Facchin, F.; Buggio, L.; Barbara, G.; Berlanda, N.; Frattaruolo, M.P.; Somigliana, E. Management of endometriosis: Toward value-based, cost-effective, affordable care. J. Obstet. Gynaecol. Can. 2018, 40, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Donati, A.; Ottolini, F.; Frassineti, A.; Fiorini, J.; Nebuloni, V.; Frattaruolo, M.P.; Roberto, A.; Mosconi, P.; Somigliana, E. A steppedcare approach to symptomatic endometriosis management: A participatory research initiative. Fertil. Steril. 2018, 109, 1086–1096. [Google Scholar] [CrossRef]
- Vercellini, P.; Frattaruolo, M.P.; Buggio, L. Toward minimally disruptive management of symptomatic endometriosis: Reducing low-value care and the burden of treatment. Expert Rev. Pharm. Outcomes Res. 2018, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Taylor, R.N.; Taylor, H.S. Partial suppression of estradiol: A new strategy in endometriosis management? Fertil. Steril. 2017, 107, 568–570. [Google Scholar] [CrossRef]
- Harada, T.; Momoeda, M.; Taketani, Y.; Hoshiai, H.; Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: A placebo-controlled, double-blind, randomized trial. Fertil. Steril. 2008, 90, 1583–1588. [Google Scholar] [CrossRef]
- Brown, J.; Farquhar, C. Endometriosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2014, 2014, CD009590. [Google Scholar] [CrossRef] [PubMed]
- Buggio, L.; Somigliana, E.; Barbara, G.; Frattaruolo, M.P.; Vercellini, P. Oral and depot progestin therapy for endometriosis: Towards a personalised medicine. Expert Opin. Pharmacother. 2017, 18, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Bracco, B.; Mosconi, P.; Roberto, A.; Alberico, D.; Dhouha, D.; Somigliana, E. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: A before and after study. Fertil. Steril. 2016, 105, 734–743.e3. [Google Scholar] [CrossRef]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone receptor isoform A but not B is expressed in endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Cheng, Y.H.; Pavone, M.E.; Yin, P.; Imir, G.; Utsunomiya, H.; Thung, S.; Xue, Q.; Marsh, E.E.; Tokunaga, H.; et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin. Reprod. Med. 2010, 28, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Cheng, Y.H.; Yin, P.; Imir, G.; Utsunomiya, H.; Attar, E.; Innes, J.; Kim, J.J. Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol. Cell. Endocrinol. 2006, 248, 94–10319. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Dahoud, W.; Skomorovska-Prokvolit, Y.; Yi, L.; Liu, J.H.; Falcone, T.; Hurd, W.W.; Mesiano, S. Abundance and Localization of Progesterone Receptor Isoforms in Endometrium in Women with and Without Endometriosis and in Peritoneal and Ovarian Endometriotic Implants. Reprod. Sci. 2015, 22, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Donnez, J.; Dolmans, M.M. Oxidative stress, mitochondria, and infertility: Is the relationship fully established? Fertil. Steril. 2021, 17, 320–324. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil. Steril. 2002, 78, 712–718. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Dolmans, M.-M.; Donnez, J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil. Steril. 2002, 77, 561–570. [Google Scholar] [CrossRef]
- Lousse, J.-C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. Elite Ed. 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Lousse, J.C.; Defrere, S.; Van Langendonckt, A.; Gras, J.; González-Ramos, R.; Colette, S.; Donnez, J. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil. Steril. 2009, 91, 1668–1675. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Hormone therapy for intramural myoma-related infertility from ulipristal acetate to GnRH antagonist: A review. Reprod. Biomed. Online 2020, 41, 431–442. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fibroids and medical therapy: Bridging the gap from selective progesterone receptor modulators to gonadotropin-releasing hormone antagonist. Fertil. Steril. 2020, 114, 739–741. [Google Scholar] [CrossRef]
- Chwalisz, K.; Surrey, E.; Stanczyk, F.Z. The hormonal profile of norethindrone acetate: Rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod. Sci. 2012, 19, 563–571. [Google Scholar] [CrossRef]
- European Society of Human Reproduction and Embryology. ESHRE Guideline for the Diagnosis and Treatment of Endometriosis. 2010. Available online: http://guidelines.endometriosis.org/index.html (accessed on 1 October 2020).
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev. 2010, 2010, CD008475. [Google Scholar] [CrossRef]
- Dragoman, M.V.; Jatlaoui, T.; Nanda, K.; Curtis, K.M.; Gaffield, M.E. Research gaps identified during the 2014 update of the WHO medical eligibility criteria for contraceptive use and selected practice recommendations for contraceptive use. Contraception 2016, 94, 195–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donnez, J.; Pirard, C.; Smets, M.; Jadoul, P.; Squifflet, J. Surgical management of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 329–348. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Clerckx, F.; Casanas-Roux, F.; Saussoy, P.; Gillerot, S. Advanced endoscopic techniques used in dysfunctional bleeding, fibroids and endometriosis, and the role of gonadotrophin-releasing hormone agonist treatment. Br. J. Obstet. Gynaecol. 1994, 101 (Suppl. S10), 2–9. [Google Scholar] [CrossRef]
- Lamb, Y.N. Elagolix: First Global Approval. Drugs 2018, 78, 1501–1508. [Google Scholar] [CrossRef]
- Ng, J.; Chwalisz, K.; Carter, D.C.; Klein, C.E. Dose-Dependent Suppression of Gonadotropins and Ovarian Hormones by Elagolix in Healthy Premenopausal Women. J. Clin. Endocrinol. Metab. 2017, 102, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
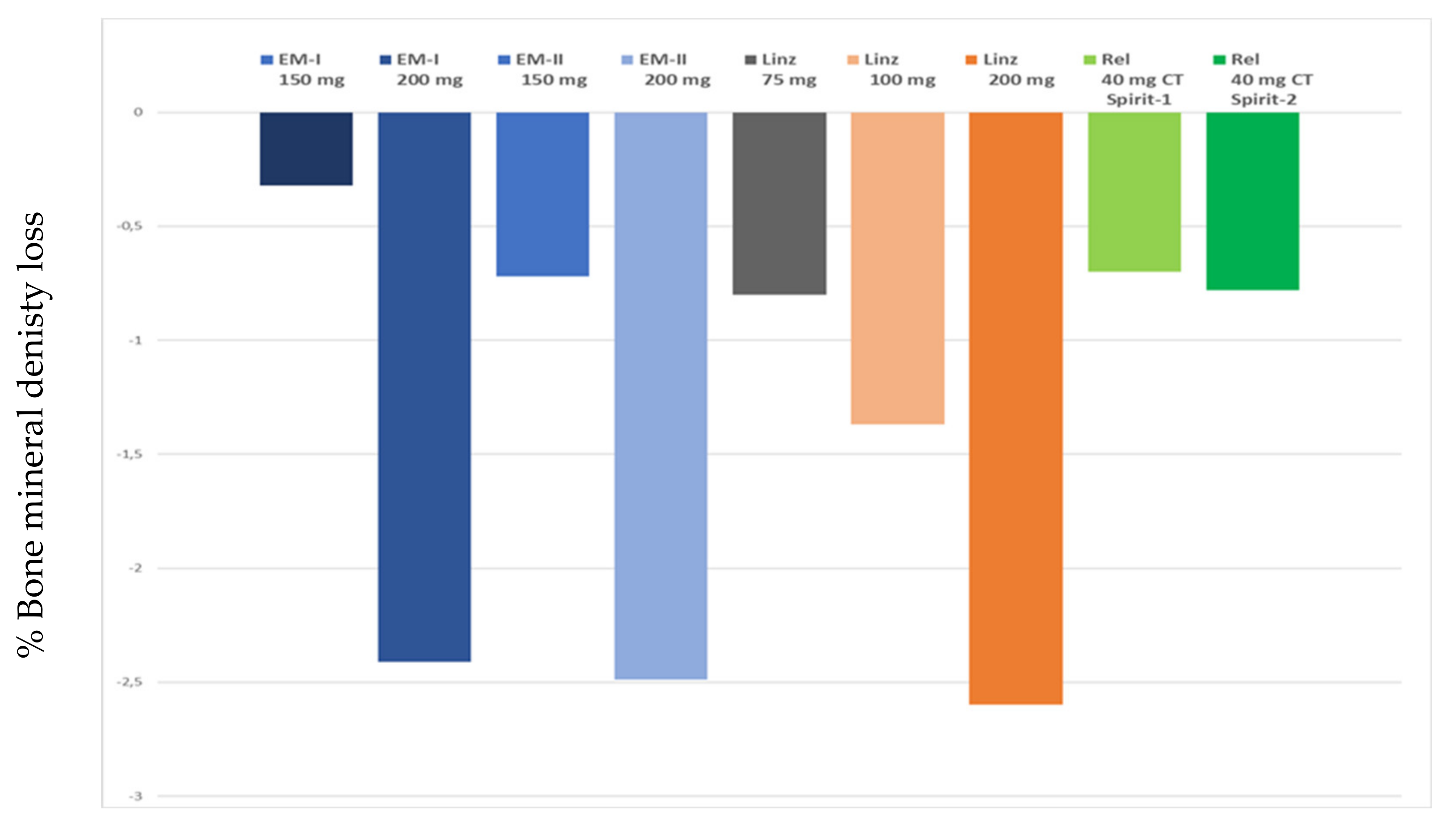
- Carr, B.; Dmowski, W.P.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: Effects on bone mineral density. Reprod. Sci. 2014, 21, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Carr, B.; Dmowski, W.P.; Koltun, W.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix treatment for endometriosis-associated pain: Results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod. Sci. 2014, 21, 363–371. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Surrey, E.; Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Archer, D.F.; Diamond, M.P.; Johnson, N.P.; Watts, N.B.; Gallagher, J.C.; et al. Long-Term Outcomes of Elagolix in Women with Endometriosis: Results from Two Extension Studies. Obstet. Gynecol. 2018, 132, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Dun, E.C.; Chwalisz, K. Clinical evaluation of the oral gonadotropin-releasing hormone-antagonist elagolix for the management of endometriosis-associated pain. Pain Manag. 2019, 9, 497–515. [Google Scholar] [CrossRef]
- Barra, F.; Scala, C.; Ferrero, S. Elagolix sodium for the treatment of women with moderate to severe endometriosis-associated pain. Drugs Today 2019, 55, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywinski, R.M.; Soliman, A.M.; Chen, J.; Snabes, M.C.; Coyne, K.S.; Surrey, E.S.; Taylor, H.S. Achieving clinically meaningful response in endometriosis pain symptoms is associated with improvements in health-related quality of life and work productivity: Analysis of 2 phase III clinical trials. Am. J. Obstet. Gynecol. 2020, 222, 592.e1–592.e10. [Google Scholar] [CrossRef]
- Taylor, H.S.; Soliman, A.M.; Johns, B.; Pokrzywinski, R.M.; Snabes, M.; Coyne, K.S. Health-Related Quality of Life Improvements in Patients with Endometriosis Treated with Elagolix. Obstet. Gynecol. 2020, 136, 501–509. [Google Scholar] [CrossRef]
- Osuga, Y.; Seki, Y.; Tanimoto, M.; Kusumoto, T.; Kudou, K.; Terakawa, N. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain in a dose-response manner: A randomized, double-blind, placebo-controlled study. Fertil. Steril. 2021, 115, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Relugolix: First Global Approval. Drugs 2019, 79, 675–679. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Becker, C.M.; Johnson, N.; Lessey, B.A.; Abrao, M.S.; Brown, E.L.; Wilk, K.; Ferreira, J.C.A.; Mathur, V.; Li, Y.; et al. Efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: Phase 3 randomized, double-blind, placebo-controlled study (spirit 2). Fertil. Steril. 2020, 114, 397–405. [Google Scholar] [CrossRef]
- Zandvliet, A.S.; Ouerdani, A.; Lee, T.Y.; Migoya, E.M.; Ferreira, J.C.A.; De Greef, R. Simulated long-term effects of relugolix combination therapy on bone mineral density at the lumbar spine as predicted by a validated semi-mechanistic exposure-response model. Fertil. Steril. 2020, 114, e350. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, H.S.; Taylor, R.N.; Akin, M.D.; Tatarchuk, T.; Wilk, K.; Gotteland, J.-P.; Lecomte, V.; Bestel, E. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: A randomized clinical trial. Fertil. Steril. 2020, 114, 44–55. [Google Scholar] [CrossRef]
- Pohl, O.; Marchand, L.; Bell, D.; Gotteland, J.P. Effects of combined GnRH receptor antagonist linzagolix and hormonal add-back therapy on vaginal bleeding-delayed add-back onset does not improve bleeding pattern. Reprod. Sci. 2020, 27, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Donnez, J. Gonadotropin-releasing hormone antagonist (linzagolix): A new therapy for uterine adenomyosis. Fertil. Steril. 2020, 114, 640–645. [Google Scholar] [CrossRef]
- Taylor, R.N.; Bestel, E.; Gotteland, J.P.; LeComte, V.; Dubouloz, R.; Terrill, P.; Humberstone, A.; Loumaye, E. Long term treatment of endometriosis associated pain (EAP) with linzagolix: Efficacy and safety after 12 months of treatment. Fertil Steril. 2019, 112, e323. [Google Scholar] [CrossRef]
- Bestel, E.; Gotteland, J.P.; Donnez, J.; Taylor, R.N.; Garner, E.I. Quality of Life Results After 52 Weeks of Treatment with Linzagolix for Endometriosis-Associated Pain. Obstet. Gynecol. 2020, 135, 26S–27S. [Google Scholar] [CrossRef]
- Borini, A.; Coticchio, G. Gonadotropin-releasing hormone antagonist linzagolix: Possible treatment for assisted reproduction patients presenting with adenomyosis and endometriosis? Fertil. Steril. 2020, 114, 517–518. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: A committee opinion. Fertil. Steril. 2014, 101, 927–935. [Google Scholar] [CrossRef]
- Vercellini, P.; Vigano, P.; Barbara, G.; Buggio, L.; Somigliana, E. ‘Luigi Mangiagalli’ Endometriosis Study Group. Elagolix for endometriosis: All that glitters is not gold. Hum. Reprod. 2019, 34, 193–199. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Smoes, P.; Gillet, N.; Beguin, S.; Casanas-Roux, F. Peritoneal endometriosis and “endometriotic” nodules of the rectovaginal septum are two different entities. Fertil. Steril. 1996, 66, 362–368. [Google Scholar] [CrossRef]
- Donnez, J.; Smoes, P.; Gillerot, S.; Casanas-Roux, F.; Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 1998, 13, 1686–1690. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef]
- Nisolle, M.; Paindaveine, B.; Bourdon, A.; Berlière, M.; Casanas-Roux, F.; Donnez, J. Histologic study of peritoneal endometriosis in infertile women. Fertil. Steril. 1990, 53, 984–988. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroni, M.; Bounous, V.E.; Clarizia, R.; Mautone, D.; Mabrouk, M. Recurrent endometriosis: A battle against an unknown enemy. Eur. J. Contracept. Reprod. Health Care 2019, 24, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Donnez, J. Deep endometriosis: The place of laparoscopic shaving. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 5, 100–113. [Google Scholar] [CrossRef]
- Biberoglu, K.O.; Behrman, S.J. Dosage aspects of danazol therapy in endometriosis: Short-term and long-term effectiveness. Am. J. Obstet. Gynecol. 1981, 139, 645–654. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Lessey, B.A.; Gordts, S.; Donnez, O.; Somigliana, E.; Chapron, C.; Garcia-Velasco, J.A.; Donnez, J. Ovarian endometriosis and infertility: In vitro fertilization (IVF) or surgery as the first approach? Fertil. Steril. 2018, 110, 1218–1226. [Google Scholar] [CrossRef]
- Donnez, J. Women with endometrioma-related infertility face a dilemma when choosing the appropriate therapy: Surgery or in vitro fertilization. Fertil. Steril. 2018, 110, 1216–1217. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Gillet, N.; Smets, M.; Bassil, S.; Casanas-Roux, F. Large ovarian endometriomas. Hum. Reprod. 1996, 11, 641–646. [Google Scholar] [CrossRef]
- Nisolle-Pochet, M.; Casanas-Roux, F.; Donnez, J. Histologic study of ovarian endometriosis after hormonal therapy. Fertil. Steril. 1988, 49, 423–426. [Google Scholar] [CrossRef]
- Kitajima, M.; Defrère, S.; Dolmans, M.M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 932–940. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Donnez, J. Fertility preservation in women for medical and social reasons: Oocytes vs ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 63–80. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Practice bulletin no. 114: Management of endometriosis. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar] [CrossRef]
- Leyland, N.; Casper, R.; Laberge, P.; Singh, S.S. Endometriosis: Diagnosis and management. J. Obstet. Gynecol. Can. 2010, 32, S1–S3. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycka, A.; Zubrzycki, M.; Perdas, E.; Zubrzycka, M. Genetic, epigenetic, and steroidogenic modulation mechanisms in endometriosis. J. Clin. Med. 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Roman, H. Choosing the right surgical technique for deep endometriosis: Shaving, disc excision, or bowel resection? Fertil. Steril. 2017, 108, 931–942. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef]
- García-Solares, J.; Dolmans, M.M.; Squifflet, J.L.; Donnez, J.; Donnez, O. Invasion of human deep nodular endometriotic lesions is associated with collective cell migration and nerve development. Fertil. Steril. 2018, 110, 1318–1327. [Google Scholar] [CrossRef]
- Anaf, V.; Simon, P.; El Nakadi, I.; Fayt, I.; Simonart, T.; Buxant, F.; Noel, J.C. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum. Reprod. 2002, 17, 1895–1900. [Google Scholar] [CrossRef]
- Anaf, V.; El Nakadi, I.; De Moor, V.; Chapron, C.; Pistofidis, G.; Noel, J.C. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol. Obstet. Investig. 2011, 71, 112–117. [Google Scholar] [CrossRef]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noël, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef]
- Donnez, O.; Orellana, R.; Van Kerk, O.; Dehoux, J.P.; Donnez, J.; Dolmans, M.M. Invasion process of induced deep nodular endometriosis in an experimental baboon model: Similarities with collective cell migration? Fertil. Steril. 2015, 104, 491–497.e2. [Google Scholar] [CrossRef] [PubMed]
- Orellana, R.; García-Solares, J.; Donnez, J.; Van Kerk, O.; Dolmans, M.M.; Donnez, O. Important role of collective cell migration and nerve fiber density in the development of deep nodular endometriosis. Fertil. Steril. 2017, 107, 987–995.e5. [Google Scholar] [CrossRef]
- Donnez, O.; Soares, M.; Defrère, S.; Dehoux, J.P.; van Langendonckt, A.; Donnez, J.; Dolmans, M.M.; Colette, S. Nerve fiber density in deep nodular endometriotic lesions induced in a baboon experimental model. Fertil. Steril. 2013, 100, 1144–1150. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Somigliana, E. Role of medical therapy in the management of deep rectovaginal endometriosis. Fertil. Steril. 2017, 108, 913–930. [Google Scholar] [CrossRef]
- Ferrero, S.; Leone Roberti Maggiore, U.; Scala, C.; Di Luca, M.; Venturini, P.L.; Remorgida, V. Changes in the size of rectovaginal endometriotic nodules infiltrating the rectum during hormonal therapies. Arch. Gynecol. Obstet. 2013, 287, 447–453. [Google Scholar] [CrossRef]
- Darwish, B.; Roman, H. Surgical treatment of deep infiltrating rectal endometriosis: In favor of less aggressive surgery. Am. J. Obstet. Gynecol. 2016, 215, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, J.; Becker, C.M.; Canis, M.; Feki, A.; Grimbizis, G.F.; Hummelshoj, L.; Nisolle, M.; Roman, H.; Saridogan, E.; Tanos, V.; et al. Recommendations for the surgical treatment of endometriosis. Part 2: Deep endometriosis. Hum. Reprod. Open. 2020, 2020, hoaa002. [Google Scholar] [CrossRef] [PubMed]
- Ianieri, M.M.; Mautone, D.; Ceccaroni, M. Recurrence in Deep Infiltrating Endometriosis: A Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2018, 25, 786–793. [Google Scholar] [CrossRef]
- Roman, H. Endometriosis surgery and preservation of fertility, what surgeons should know. J. Visc. Surg. 2018, 155 (Suppl. S1), S31–S36. [Google Scholar] [CrossRef]
- Efficacité du cetrorelix depot en cas de récurrence de douleurs pelviennes et de dysménorrhée après chirurgie des nodules endometriosiques. In Proceedings of the GGOLFB-2007, Brussels, Belgium, 20 October 2007.
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelley, C.; Winkel, C. The direct and indirect costs associated with endometriosis: A systematic literature review. Hum. Reprod. 2016, 31, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Surrey, E.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Real-World Evaluation of Direct and Indirect Economic Burden Among Endometriosis Patients in the United States. Adv. Ther. 2018, 35, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Johnson, S.J.; Mitchell, D.; Soliman, A.M.; Vora, J.B.; Agarwal, S.K. Cost-effectiveness of elagolix versus leuprolide acetate for treating moderate-to-severe endometriosis pain in the USA. J. Comp. Eff. Res. 2019, 8, 337–355. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Valbrun, T.G.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef]
- Leyland, N.; Estes, S.J.; Lessey, B.A.; Advincula, A.P.; Taylor, H.S. A Clinician’s Guide to the Treatment of Endometriosis with Elagolix. J. Womens Health 2021, 30, 569–578. [Google Scholar] [CrossRef]

| Type of Drug | Elagolix | Linzagolix | Relugolix + ABT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose | 150 mg | 200 mg (Twice Daily) | 150 mg | 200 mg (Twice Daily) | 75 mg | 100 mg | 200 mg | 40 mg + ABT | 40 mg + ABT |
| Assessments at Week 12 | |||||||||
| Dysmenorrhea (% responders) | 46.4 | 75.8 | 43.4 | 72.4 | 68.2 | 68.6 | 68.9 | NA | NA |
| NMPP (% responders) | 50.4 | 54.5 | 49.8 | 57.8 | 58.5 | 61.5 | 47.7 | NA | NA |
| Assessments at Week 24 | |||||||||
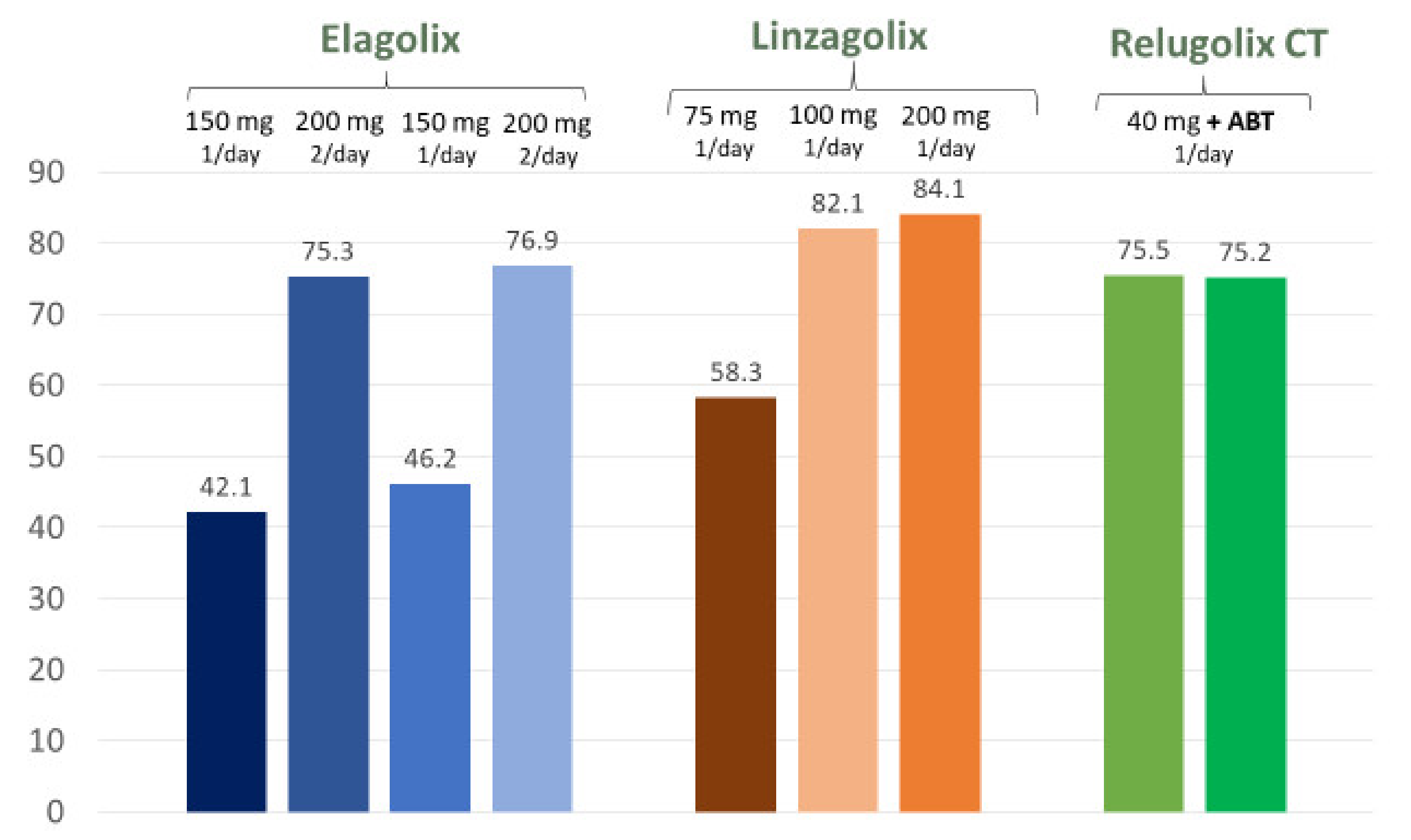
| Dysmenorrhea (% responders) | 42.1 | 75.3 | 46.2 | 76.9 | 58.3 | 82.1 | 84.1 | 75.5 | 75.2 |
| NMPP (% responders) | 45.7 | 62.1 | 51.6 | 62.2 | 72.9 | 64.1 | 72.7 | 58.5 | 66 |
| Assessments at Week 52 | |||||||||
| Dysmenorrhea (% responders) | 52.1 | 78.1 | 50.8 | 75.9 | 69.2 | 69.2 | 64.7 * | NA | NA |
| NMPP (% responders) | 67.8 | 69.1 | 66.4 | 67.2 | 69.2 | 53.8 | 76.5 * | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnez, J.; Dolmans, M.-M. GnRH Antagonists with or without Add-Back Therapy: A New Alternative in the Management of Endometriosis? Int. J. Mol. Sci. 2021, 22, 11342. https://doi.org/10.3390/ijms222111342
Donnez J, Dolmans M-M. GnRH Antagonists with or without Add-Back Therapy: A New Alternative in the Management of Endometriosis? International Journal of Molecular Sciences. 2021; 22(21):11342. https://doi.org/10.3390/ijms222111342
Chicago/Turabian StyleDonnez, Jacques, and Marie-Madeleine Dolmans. 2021. "GnRH Antagonists with or without Add-Back Therapy: A New Alternative in the Management of Endometriosis?" International Journal of Molecular Sciences 22, no. 21: 11342. https://doi.org/10.3390/ijms222111342
APA StyleDonnez, J., & Dolmans, M.-M. (2021). GnRH Antagonists with or without Add-Back Therapy: A New Alternative in the Management of Endometriosis? International Journal of Molecular Sciences, 22(21), 11342. https://doi.org/10.3390/ijms222111342







