Pathogenesis of Endometriosis: New Insights into Prospective Therapies
Abstract
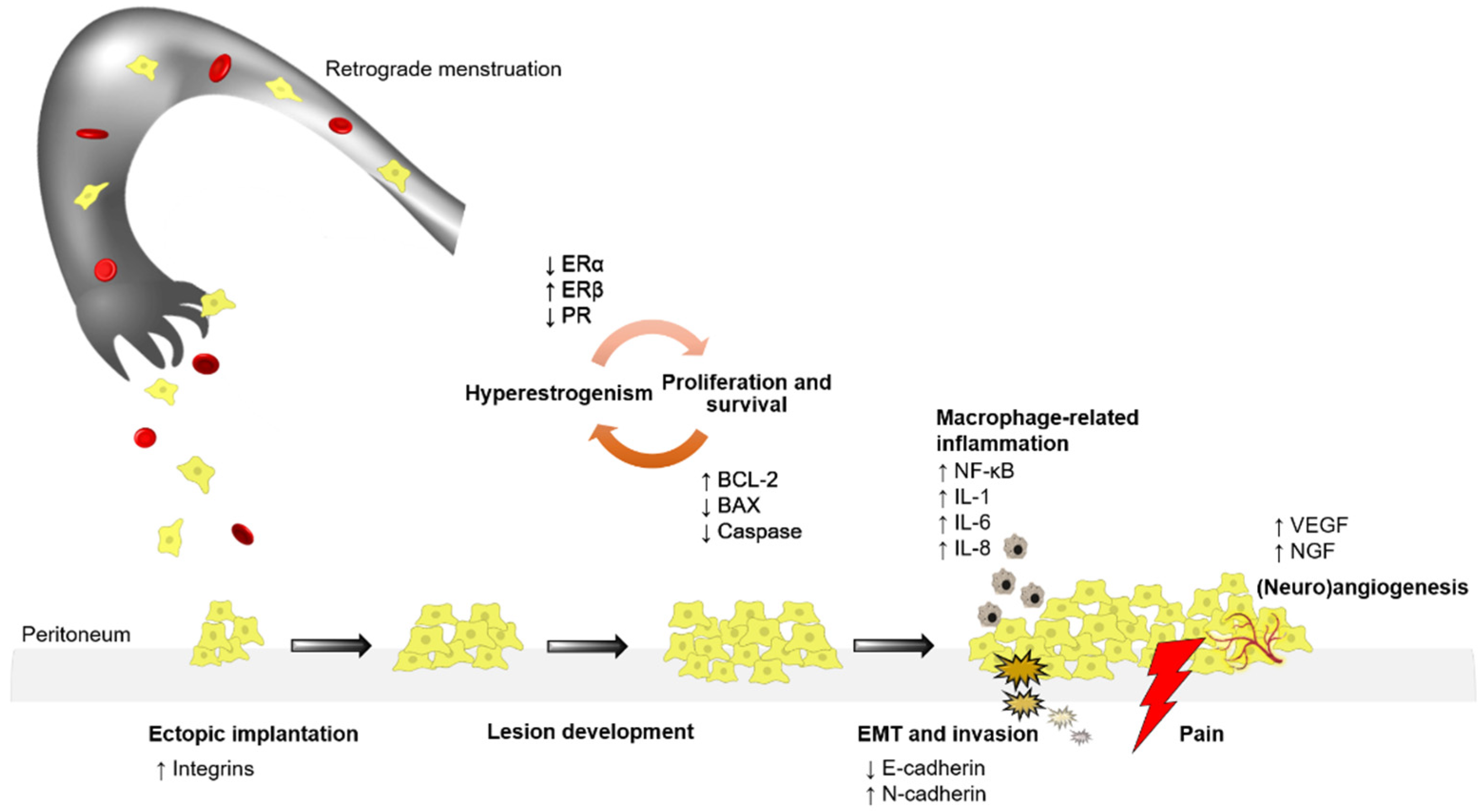
1. Introduction
2. Inflammatory Molecules
2.1. Macrophages
2.2. Dendritic Cells
2.3. Natural Killer Cells
2.4. Modulators: Interleukins, Cytokines, Interferons
2.5. Proinflammatory Cytokines
3. Hormones: Estrogen Receptor Alpha (ERα) and Beta (ERβ)
4. Apoptotic, Autophagic, and Tumor-Promoting Genes/Proteins
5. Epithelial-Mesenchymal Transition
6. Angiogenesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machairiotis, N.; Stylianaki, A.; Dryllis, G.; Zarogoulidis, P.; Kouroutou, P.; Tsiamis, N.; Katsikogiannis, N.; Sarika, E.; Courcoutsakis, N.; Tsiouda, T.; et al. Extrapelvic endometriosis: A rare entity or an under diagnosed condition? Diagn. Pathol. 2013, 8, 194. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93. [Google Scholar]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 10276, 839–852. [Google Scholar] [CrossRef]
- García-Gómez, E.; Vázquez-Martínez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbón, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2019, 10, 935. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef]
- Delbandi, A.-A.; Mahmoudi, M.; Shervin, A.; Heidari, S.; Kolahdouz-Mohammadi, R.; Zarnani, A.-H. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y.; Tan, X.; Li, M. Upregulation of fibroblast growth factor 2 contributes to endometriosis through SPRYs/DUSP6/ERK signaling pathway. Acta Histochem. 2021, 123, 151749. [Google Scholar] [CrossRef]
- Akoum, A.; Kong, J.; Metz, C.; Beaumont, M.C. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil. Steril. 2002, 77, 989–994. [Google Scholar] [CrossRef]
- Akoum, A.; Metz, C.N.; Al-Akoum, M.; Kats, R. Macrophage migration inhibitory factor expression in the intrauterine endometrium of women with endometriosis varies with disease stage, infertility status, and pelvic pain. Fertil. Steril. 2006, 85, 1379–1385. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, L. Association between macrophage migration inhibitory factor in the endometrium and estrogen in endometriosis. Exp. Ther. Med. 2015, 10, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Bengtsson, A.K.; Ryan, E.J.; Giordano, D.; Magaletti, D.M.; Clark, E.A. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 2004, 104, 1404–1410. [Google Scholar] [CrossRef]
- Schulke, L.; Berbic, M.; Manconi, F.; Tokushige, N.; Markham, R.; Fraser, I.S. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum. Reprod. 2009, 24, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Jeung, I.; Cheon, K.; Kim, M.-R. Decreased Cytotoxicity of Peripheral and Peritoneal Natural Killer Cell in Endometriosis. BioMed Res. Int. 2016, 2016, 2916070. [Google Scholar] [CrossRef] [PubMed]
- Machairiotis, N.; Vasilakaki, S.; Thomakos, N. Inflammatory Mediators and Pain in Endometriosis: A Systematic Review. Biomedicines 2021, 9, 54. [Google Scholar] [CrossRef]
- Malvezzi, H.; Hernandes, C.; Piccinato, C.A.; Podgaec, S. Interleukin in endometriosis-associated infertility-pelvic pain: Systematic review and meta-analysis. Reproduction 2019, 158, 1–12. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.-C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Fusco, R.; et al. The Methyl Ester of 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic Acid Reduces Endometrial Lesions Development by Modulating the NFkB and Nrf2 Pathways. Int. J. Mol. Sci. 2021, 22, 3991. [Google Scholar] [CrossRef]
- Horie, S.; Harada, T.; Mitsunari, M.; Taniguchi, F.; Iwabe, T.; Terakawa, N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertil. Steril. 2005, 83, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.-C.; Mettlen, M.; Guillet, A.; Donnez, J. Agents blocking the nuclear factor-kappaB pathway are effective inhibitors of endometriosis in an in vivo experimental model. Gynecol. Obstet. Investig. 2008, 65, 174–186. [Google Scholar] [CrossRef]
- Yagyu, T.; Kobayashi, H.; Matsuzaki, H.; Wakahara, K.; Kondo, T.; Kurita, N.; Sekino, H.; Inagaki, K.; Suzuki, M.; Konayama, N.; et al. Thalidomide inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in endometriotic stromal cells, possibly through suppression of nuclear factor-kappaB activation. J. Clin. Endocrinol. Metab. 2005, 90, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, O.; Mori, T.; Ito, F.; Okimura, H.; Kataoka, H.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Shigehiro, S.; Amami, T.; et al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid Biochem. Mol. Biol. 2018, 181, 125–132. [Google Scholar] [CrossRef]
- Huang, R.; Chen, S.; Zhao, M.; Li, Z.; Zhu, L. Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor-kappaB signaling pathway. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101642. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J.; et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chern, B.S.M.; Barton-Smith, P.; Phoon, J.W.L.; Tan, T.Y.; Viardot-Foucault, V.; Ku, C.W.; Tan, H.H.; Chan, J.K.Y.; Lee, Y.H. Peritoneal Fluid Cytokines Reveal New Insights of Endometriosis Subphenotypes. Int. J. Mol. Sci. 2020, 21, 3515. [Google Scholar] [CrossRef] [PubMed]
- Anastasiu, C.V.; Moga, M.A.; Neculau, A.E.; Bălan, A.; Scârneciu, I.; Dragomir, R.M.; Dull, A.-M.; Chicea, L.-M. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef]
- Peng, B.; Alotaibi, F.T.; Sediqi, S.; Bedaiwy, M.A.; Yong, P.J. Role of interleukin-1β in nerve growth factor expression, neurogenesis and deep dyspareunia in endometriosis. Hum. Reprod. 2020, 35, 901–912. [Google Scholar] [CrossRef]
- Sikora, J.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Association of the Precursor of Interleukin-1β and Peritoneal Inflammation-Role in Pathogenesis of Endometriosis. J. Clin. Lab. Anal. 2016, 30, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, W.; Pu, D.; Li, Z.; Huang, Q.; Chen, Q. The inhibitory effect of 15-R-LXA4 on experimental endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 200–204. [Google Scholar] [CrossRef]
- Zhuang, M.; Cao, Y.; Shi, Y.; Yu, L.; Niu, Y.; Zhang, T.; Sun, Z. Caulis Sargentodoxae Prescription Plays a Therapeutic Role with Decreased Inflammatory Cytokines in Peritoneal Fluid in the Rat Endometriosis Model. Evid.-Based Complement. Altern. Med. 2020, 2020, 9627907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Hu, M.; Chen, J.-M.; Sun, W.; Zhu, M.-B. Effects of gene polymorphism and serum levels of IL-2 and IL-6 on endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4635–4641. [Google Scholar] [PubMed]
- Qiu, X.-M.; Lai, Z.-Z.; Ha, S.-Y.; Yang, H.-L.; Liu, L.-B.; Wang, Y.; Shi, J.-W.; Ruan, L.-Y.; Ye, J.-F.; Wu, J.-N.; et al. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction 2020, 159, 251–260. [Google Scholar] [CrossRef]
- El-Zayadi, A.A.; Mohamed, S.A.; Arafa, M.; Mohammed, S.M.; Zayed, A.; Abdelhafez, M.S.; Badawy, A.M. Anti-IL-6 receptor monoclonal antibody as a new treatment of endometriosis. Immunol. Res. 2020, 68, 389–397. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Jin, D.; Zhang, Y. Serum miR-17, IL-4, and IL-6 levels for diagnosis of endometriosis. Medicine 2018, 97, e10853. [Google Scholar] [CrossRef] [PubMed]
- Invitti, A.L.; Schor, E.; Parreira, R.M.; Kopelman, A.; Kamergorodsky, G.; Gonçalves, G.A.; Batista Castello Girão, M.J. Inflammatory cytokine profile of co-cultivated primary cells from the endometrium of women with and without endometriosis. Mol. Med. Rep. 2018, 18, 1287–1296. [Google Scholar]
- Wu, G.; Bersinger, N.A.; Mueller, M.D.; von Wolff, M. Intrafollicular inflammatory cytokines but not steroid hormone concentrations are increased in naturally matured follicles of women with proven endometriosis. J. Assist. Reprod. Genet. 2017, 34, 357–364. [Google Scholar] [CrossRef]
- Miller, J.E.; Monsanto, S.P.; Ahn, S.H.; Khalaj, K.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Interleukin-33 modulates inflammation in endometriosis. Sci. Rep. 2017, 7, 17903. [Google Scholar] [CrossRef]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Akiyama, I.; Sato, E.; Ito, M.; Kobayashi, M.; Nakashima, A.; Wada, S.; Onda, T.; et al. IL-33 Exacerbates Endometriotic Lesions via Polarizing Peritoneal Macrophages to M2 Subtype. Reprod. Sci. 2020, 27, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Borghese, B.; Chouzenoux, S.; Vaiman, D.; Borderie, D.; Streuli, I. Serum and peritoneal interleukin-33 levels are elevated in deeply infiltrating endometriosis. Hum. Reprod. 2012, 27, 2001–2009. [Google Scholar] [CrossRef]
- Wang, X.M.; Ma, Z.Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharm. Sci. 2018, 22, 2513–2518. [Google Scholar]
- Kondo, W.; dal Lago, E.A.; de Noronha, L.; Olandoski, M.; Kotze, P.G.; do Amaral, V.F. Effect of anti-TNF-α on peritoneal endometrial implants of rats. Rev. Col. Bras. Cir. 2011, 38, 266–273. [Google Scholar] [CrossRef]
- Iwabe, T.; Harada, T.; Tsudo, T.; Nagano, Y.; Yoshida, S.; Tanikawa, M.; Terakawa, N. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J. Clin. Endocrinol. Metab. 2000, 85, 824–829. [Google Scholar] [PubMed]
- Grund, E.M.; Kagan, D.; Tran, C.A.; Zeitvogel, A.; Starzinski-Powitz, A.; Nataraja, S.; Palmer, S.S. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol. Pharm. 2008, 73, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A.-A. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J. Cell Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Karamian, A.; Paktinat, S.; Esfandyari, S.; Nazarian, H.; Ziai, S.A.; Zarnani, A.-H.; Salehpour, S.; Hosseinirad, H.; Novin, M.G. Pyrvinium pamoate induces in-vitro suppression of IL-6 and IL-8 produced by human endometriotic stromal cells. Hum. Exp. Toxicol. 2021, 40, 649–660. [Google Scholar] [CrossRef]
- Wei, X.; Shao, X. Nobiletin alleviates endometriosis via down-regulating NF-κB activity in endometriosis mouse model. Biosci. Rep. 2018, 38, BSR20180470. [Google Scholar] [CrossRef] [PubMed]
- Veillat, V.; Carli, C.; Metz, C.N.; Al-Abed, Y.; Naccache, P.H.; Akoum, A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J. Clin. Endocrinol. Metab. 2010, 95, E403–E412. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.-M. GnRH antagonists with or without add-back therapy: A new alternative in the management of endometriosis? Int. J. Mol. Sci. 2021, 22, 11342. [Google Scholar] [CrossRef]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, T.; Hu, P.; Qi, C.; Qian, L. miR-143-3p inhibits endometriotic stromal cell proliferation and invasion by inactivating autophagy in endometriosis. Mol. Med. Rep. 2021, 23, 356. [Google Scholar] [CrossRef]
- Xu, H.; Gao, Y.; Shu, Y.; Wang, Y.; Shi, Q. EPHA3 enhances macrophage autophagy and apoptosis by disrupting the mTOR signaling pathway in mice with endometriosis. Biosci. Rep. 2019, 39, BSR20182274. [Google Scholar] [CrossRef]
- Sui, X.; Li, Y.; Sun, Y.; Li, C.; Li, X.; Zhang, G. Expression and significance of autophagy genes LC3, Beclin1 and MMP-2 in endometriosis. Exp. Ther. Med. 2018, 16, 1958–1962. [Google Scholar] [CrossRef]
- Yang, H.-L.; Mei, J.; Chang, K.-K.; Zhou, W.J.; Huang, L.-Q.; Li, M.-Q. Autophagy in endometriosis. Am. J. Transl. Res. 2017, 9, 4707–4725. [Google Scholar]
- Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Salinaro, A.; Raffone, E.; Genovese, T.; et al. Autophagy and Mitophagy Promotion in a Rat Model of Endometriosis. Int. J. Mol. Sci. 2021, 22, 5074. [Google Scholar] [CrossRef]
- Ruiz, A.; Rockfield, S.; Taran, N.; Haller, E.; Engelman, R.W.; Flores, I.; Panina-Bordignon, P.; Nanjundan, M. Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis. Cell Death Dis. 2016, 7, e2059. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.; Prentice, A.; Charnock-Jones, D.S.; Sharkey, A.M.; Smith, S.K. Immunolocalization of the apoptosis regulating proteins Bcl-2 and Bax in human endometrium and isolated peritoneal fluid macrophages in endometriosis. Hum. Reprod. 1997, 12, 146–152. [Google Scholar] [CrossRef][Green Version]
- Johnson, M.C.; Torres, M.; Alves, A.; Bacallao, K.; Fuentes, A.; Vega, M.; Boric, M.A. Augmented cell survival in eutopic endometrium from women with endometriosis: Expression of c-myc, TGF-beta1 and bax genes. Reprod. Biol. Endocrinol. 2005, 3, 45. [Google Scholar] [CrossRef]
- García-Solares, J.; Dolmans, M.-M.; Squifflet, J.-L.; Donnez, J.; Donnez, O. Invasion of human deep nodular endometriotic lesions is associated with collective cell migration and nerve development. Fertil. Steril. 2018, 110, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Orellana, R.; Van Kerk, O.; Dehoux, J.-P.; Donnez, J.; Dolmans, M.-M. Invasion process of induced deep nodular endometriosis in an experimental baboon model: Similarities with collective cell migration? Fertil. Steril. 2015, 104, 491–497. [Google Scholar] [CrossRef]
- Yoshida, K.; Yoshihara, K.; Adachi, S.; Haino, K.; Nishino, K.; Yamaguchi, M.; Nishikawa, N.; Kashima, K.; Yahata, T.; Masuzaki, H.; et al. Possible involvement of the E-cadherin gene in genetic susceptibility to endometriosis. Hum. Reprod. 2012, 27, 685–689. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiong, Y.; Liu, Y.; Xiong, W.; Zhang, L.; Liu, H.; Du, Y.; Li, N. Hypoxia-inducible factor 1α-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum. Reprod. 2016, 31, 1327–1338. [Google Scholar] [CrossRef]
- Bartley, J.; Jülicher, A.; Hotz, B.; Mechsner, S.; Hotz, H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obstet. 2014, 289, 871–881. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C.; Maleysson, E.; Canis, M.; Mage, G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J. Clin. Endocrinol. Metab. 2010, 95, 3437–3445. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-P.; Chen, L.M.; Chen, F.; Jiang, N.-H.; Sui, L. Smad signaling coincides with epithelial-mesenchymal transition in a rat model of intrauterine adhesion. Am. J. Transl. Res. 2019, 11, 4726–4737. [Google Scholar]
- Qi, S.; Yan, L.; Liu, Z.; Mu, Y.-L.; Li, M.; Zhao, X.; Chen, Z.-J.; Zhang, H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod. Biol. Endocrinol. 2018, 16, 62. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, L.; Liu, H.; Li, N.; Du, Y.; He, H.; Zhang, Z.; Liu, Y. E2-mediated EMT by activation of β-catenin/Snail signalling during the development of ovarian endometriosis. J. Cell. Mol. Med. 2019, 23, 8035–8045. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Masuda, H.; Hara, K.; Uchida, H.; Sato, K.; Sato, S.; Asada, H.; Maruyama, T.; Yoshimura, Y.; Katabuchi, H.; et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet. Gynecol. Scand. 2017, 96, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-W.; Chen, H.-Y.; Chiang, Y.-F.; Chang, L.-C.; Lin, P.-H.; Hsia, S.-M. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine 2020, 77, 153214. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Chiang, Y.-F.; Chen, H.-Y.; Huang, Y.-J.; Liu, A.-C.; Hsia, S.-M. The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study. Biomedicines 2020, 8, 528. [Google Scholar] [CrossRef]
- Yu, M.-M.; Zhou, Q.-M. 3,6-dihydroxyflavone suppresses the epithelial-mesenchymal transition, migration and invasion in endometrial stromal cells by inhibiting the Notch signaling pathway. Eur. Rev. Med. Pharm. Sci. 2018, 22, 4009–4017. [Google Scholar]
- Orellana, R.; García-Solares, J.; Donnez, J.; van Kerk, O.; Dolmans, M.-M.; Donnez, O. Important role of collective cell migration and nerve fiber density in the development of deep nodular endometriosis. Fertil. Steril. 2017, 107, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Smoes, P.; Gillerot, S.; Casanas-Roux, F.; Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 1998, 13, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Xiao, Z.-J.; Meng, X.; Wang, Y.; Yu, M.K.; Huang, W.Q.; Sun, X.; Chen, H.; Duan, Y.-G.; Jiang, C.; et al. MRP4 sustains Wnt/β-catenin signaling for pregnancy, endometriosis and endometrial cancer. Theranostics 2019, 9, 5049–5064. [Google Scholar] [CrossRef]
- Maharani, M.; Wahyuni, E.S.; Sutrisno, S. Effect of Genistein on Endometriosis Lesion, Matrix Metalloproteinase-2 and -9 Level of Endometriosis: In silico and In vivo Study. J. Clin. Mol. Endocrinol. 2015, 1, 4. [Google Scholar]
- Tsuno, A.; Nasu, K.; Kawano, Y.; Yuge, A.; Li, H.; Abe, W.; Narahara, H. Fasudil inhibits the proliferation and contractility and induces cell cycle arrest and apoptosis of human endometriotic stromal cells: A promising agent for the treatment of endometriosis. J. Clin. Endocrinol. Metab. 2011, 96, E1944–E1952. [Google Scholar] [CrossRef]
- Mendel, D.B.; Laird, A.D.; Xin, X.; Louie, S.G.; Christensen, J.G.; Li, G. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003, 9, 327–337. [Google Scholar] [PubMed]
- Abbas, M.A.; Disi, A.M.; Taha, M.O. Sunitinib as an anti-endometriotic agent. Eur. J. Pharm. Sci. 2013, 49, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Abad, A.; Delgado, F.; Tamarit, S.; Simón, C.; Pellicer, A. Effects of hyperprolactinemia treatment with the dopamine agonist quinagolide on endometriotic lesions in patients with endometriosis-associated hyperprolactinemia. Fertil. Steril. 2011, 95, 882–888. [Google Scholar] [CrossRef] [PubMed]
| NF-κB Role in Different Processes | Reported Key Players in the System | Specific Functions |
|---|---|---|
| Effect on angiogenic proteins [17,18,19] | Upregulates angiogenic factors including interleukin 8 (IL-8) and MIF in endometrial and endometriotic cells and VEGF | MIF stimulates endothelial cell proliferation |
| Effect on invasion proteins [17,18,19] | Matrix metalloproteinases (MMPs), urokinase-type plasminogen activator, and tissue plasminogen activator | These are known to be implicated in remodeling the extracellular matrix, which could lead to endometrial invasion of the submesothelial space of the peritoneum |
| Effect on cell proliferation [19] | Endometriotic cell proliferation in eutopic and ectopic sites is governed by NF-κΒ, inhibiting apoptosis and favoring the development and maintenance of endometriosis | This primarily activates p50/p65 NF-κB dimers involved in the transcription of genes that regulate innate immunity, inflammation, and cell survival; intercellular adhesion molecule 1, B-cell lymphoma 2 (Bcl-2), and Bcl-XL (antiapoptotic proteins at the mitochondrial level); and caspase 3, caspase 8, and caspase 9 |
| Effect on the inflammatory process [17,18,19] | NF-κB-activated macrophages release proinflammatory cytokines and growth factors involved in boosting inducible nitric oxide synthase, cyclooxygenase-2 (COX-2), IL-1, IL-6, IL-8, tumor necrosis factor alpha (TNF-α), and VEGF | Activation of p65 NF-κB dimers in innate immunity |
| Name of Cytokine | Specific Functions | Study Model |
|---|---|---|
| IL-1/IL-1B [29,30,31,32] | IL-1 is basically responsible for and primarily functions to create a proinflammatory microenvironment in tissue, resulting in fever, inflammation, and even sepsis with the help of various integrins. IL-1 is present in endometriosis generation, where it facilitates the innervation process. Various studies applying different models report altered expression of IL-1 and its relation to disease progression and associated pain. IL-1 is widely linked to infertility caused by endometriosis | Rats Mice ESCs In vitro culture of peritoneal macrophages |
| IL-2 [32,33,34] | Promotes invasion and migration of ESCs, along with their growth | Women Rats |
| IL-6 [32,33,35,36] | IL-6 gene polymorphisms have been extensively studied in endometriosis patients, who demonstrate elevated levels of IL-6. Anti-IL-6 monoclonal antibody was found to be restorative against endometriosis in rats | Women Rats |
| IL-8 [17,37,38] | IL-8 is widely associated with adhesion and propagation of endometrial cells, along with increased expression of proteins involved in migration and invasion. It also promotes the progesterone resistance observed in endometriosis. IL-8 has an inverse relationship with apoptotic genes and proteins, thereby boosting lesion growth | Women Follicular fluid Primary ESC culture Rats |
| IL-33 [39,40,41] | IL-33 is one of the most crucial players in acute and chronic inflammation. It is known for its active role in all major processes, such as pain development, cell invasion, migration and adhesion, neovascularization, and many others, culminating in endometriosis. Its role is well established in DENs, especially at advanced stages of the disease | Women Rats Mice ESCs |
| TNF-α [42,43,44,45] | A proinflammatory cytokine known to impair glutathione, resulting in the accumulation of reactive oxygen species. Induces IL-6, IL-8, granulocyte-macrophage colony-stimulating factor, and MCP-1, while enhanced cell proliferation triggers COX-2 expression | Women Rats Mice ESCs |
| Apoptotic, Autophagic and Tumor-Promoting Proteins | Role in the Pathogenesis of Endometriosis/Reason for Interplay | Study Model |
|---|---|---|
| Autophagy-related gene 3 [55] | Component of the autophagic mechanism. Estrogen levels and progesterone resistance are also considered to be its major regulators | Mice |
| Beclin [56,57,58] | Induced by hypoxic conditions in endometrium. Activated in response to progesterone levels | Humans Rats Mice ESCs |
| Microtubule-associated protein light chain 3 [56,58,59] | Decreased p62 with impaired inactivation of AKT, ERK1/2, and mechanistic target of rapamycin (mTOR) | Humans Mice |
| Bax/Bcl-2 [55,60,61] | ERβ plays a key role in anti-apoptosis, inflammation and invasion of ectopic lesions, activates mTOR, and demonstrates excessive expression of soluble Fas ligand. Constant source of TNF-α. Suppresses E-cadherin | Humans Mice Endometrial cells |
| Caspase intrinsic/extrinsic [25,26] | Reduced percentage of apoptotic cells and greater number of surviving cells entering the peritoneal cavity | Mice ESCs |
| EMT Regulators | Role in Endometriosis | Study Model |
|---|---|---|
| E-cadherin [62,63,65,66,67,68,69,70,71] | Allows endometrial cells to detach from their primary site, and also invasive endometrial cells to implant in pelvic sites. Loss of the epithelial cell phenotype, including the basement membrane junction | Humans Baboons Rats Endometrial cells |
| N-cadherin [62,63,65,66,67,68,69,70,71] | Elevated expression of N-cadherin possibly enhances cell motility by reducing the stability of cell-adhesion complexes | Humans Baboons Rats Endometrial cells |
| Twist [70] | Specific transcription factor involved in EMT and dedifferentiation, which maintains invasion and metastasis. Increased concentrations found in EMT | Humans |
| Snail/Slug [65,66,67,68,69,70] | Snail/Slug are known to be associated with loss of differentiation, tumor progression, and metastasis | Humans Rats Endometrial cells |
| ZEB [72] | ZEB1 is a downstream effector of the TGF-β signaling pathway, which inhibits E-cadherin expression for progression of epithelial tumors. Most commonly seen in DENs. Expressed in lesions but not endometrium | Humans |
| β-catenin [62,71] | β-catenin was detected in nuclei of epithelial cells in ovarian endometriosis, suggesting activation of the Wnt/β-catenin signaling pathway, a well-known EMT regulator during organ development. Inhibits E-cadherin expression | Humans Endometrial cells |
| Name of Inhibitor | Mainly Targeted Downstream/Upstream Proteins | Mode of Action | Study Model |
|---|---|---|---|
| Multidrug resistance protein 4 [78] | Wnt/β-catenin | Involved in embryo receptivity by stabilizing endometrial β-catenin | Endometrial cells Mice |
| Genistein [79] | MMP9, MMP2 | Reduces lesion size by targeting MMP signaling | Mice |
| Fasudil [80] | Rho/Rho-associated kinases | Attenuates myofibroblast differentiation and contractility, decreasing fibrosis. Regulates cell proliferation and apoptosis | EESCs |
| Sunitinib, SU6668, SU5416, sorafenib, and pazopanib [81,82] | VEGF, VEGF receptor (VEGFR), fibroblast growth factor receptor 1, MMP2 | Inhibit angiogenic pathways and reduce lesion size by activating apoptosis | Mice Rats |
| Quinagolide [83] | VEGF/VEGFR2 pathway | Shown to induce a considerable decrease in lesion size, potentially via regulation of angiogenesis | Humans |
| Name of Inhibitor | Mainly Targeted Downstream/Upstream Proteins | Mode of Action | Study Model |
|---|---|---|---|
| Methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid [20] | NF-κB | Shows antioxidant and anti-inflammatory action as well as decreased angiogenesis and increased apoptosis in endometriotic lesions | Rats with surgically induced endometriosis |
| Dienogest [21] | NF-κB, TNF-α, IL-8 | Attenuates expression of IL-8 by reducing TNFα-induced NF-κB activation and may confer a protective effect against endometriosis | Endometrial stromal cells (ESCs) |
| BAY 11-7085 and SN-50 [22] | NF-κB, adhesion molecule 1 | Reduces ICAM-1 expression and cell proliferation and increases apoptosis of endometriotic lesions, thereby diminishing their development | Nude mice |
| Thalidomide [23] | NF-κB, TNF-α, IL-8 | Attenuates the expression of IL-8 mRNA and protein by reducing TNF-α-induced NF-κB activation | Ectopic endometrial stromal cells (EESCs) |
| Genistein [24] | NF-κB, TNF-α, IL-6, IL-8 | Inhibits expression of inflammatory mediators and decreases proliferation in mouse lesions | EESCs Mice |
| Ginsenoside [25] | NF-κB, protein kinase B | Suppresses endometriosis by reducing the viability of human ectopic endometrial stromal cells via the NF-κB signaling cascade | EESCs |
| Gossypol [26] | TNF-α, IL-1β | Induces regression of ectopic lesions via inhibition of estrogen receptor | Mice |
| Name of Inhibitor | Mainly Targeted Downstream/Upstream Proteins | Mode of Action | Study Model |
|---|---|---|---|
| Resveratrol [46] | IL-6, IL-1B, MCP-1 | Downregulates expression of inflammatory markers in eutopic and, more markedly, ectopic endometrium | EESCs |
| Tocilizumab [35] | IL-6 | Monoclonal anti-IL-6 antibody shown to lead to lesion regression in rats | Rats |
| Pyrvinium pamoate [47] | IL-6, IL-8 | Suppresses mRNA expression of IL-6 and IL-8 in vitro | EESCs |
| Nobiletin [48] | NF-κB, IL-6, IL-1β | Reduces lesion size and pain by inhibiting cell proliferation, angiogenesis, and excess inflammation | Mice |
| (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester [49] | MIF, IL-8, MCP-1 | MIF inhibitor exhibits antiangiogenic effects in vitro | EESCs |
| Name of Inhibitor | Mainly Targeted Downstream/Upstream Proteins | Mode of Action | Study Models |
|---|---|---|---|
| Isoliquiritigenin [73] | E-cadherin, N-cadherin, Snail, Slug | Acts against viability, migration, and EMT in vitro. Reduces the volume and weight of mouse endometriotic lesions. Decreases the inflammatory response and triggers apoptosis | Endometrial cell lines Mice |
| Fucoidan [74] | Snail and Slug, Notch | Exerts anti-proliferative and anti-inflammatory effects, inhibiting EMT and inducing apoptosis | Endometrial cell lines Mice |
| Melatonin [69] | Notch homolog 1, Snail, Slug, N-cadherin, E-cadherin and Numb | Alleviates EMT and invasion by blocking estradiol and the Notch signaling pathway | Endometrial epithelial cells |
| 3,6-dihydroxyflavone [75] | Notch signaling pathway | Reduces EMT and in vitro cell migration via inhibition of Notch and downstream molecules | EESCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, R.; Stratopoulou, C.A.; Dolmans, M.-M. Pathogenesis of Endometriosis: New Insights into Prospective Therapies. Int. J. Mol. Sci. 2021, 22, 11700. https://doi.org/10.3390/ijms222111700
Kapoor R, Stratopoulou CA, Dolmans M-M. Pathogenesis of Endometriosis: New Insights into Prospective Therapies. International Journal of Molecular Sciences. 2021; 22(21):11700. https://doi.org/10.3390/ijms222111700
Chicago/Turabian StyleKapoor, Radhika, Christina Anna Stratopoulou, and Marie-Madeleine Dolmans. 2021. "Pathogenesis of Endometriosis: New Insights into Prospective Therapies" International Journal of Molecular Sciences 22, no. 21: 11700. https://doi.org/10.3390/ijms222111700
APA StyleKapoor, R., Stratopoulou, C. A., & Dolmans, M.-M. (2021). Pathogenesis of Endometriosis: New Insights into Prospective Therapies. International Journal of Molecular Sciences, 22(21), 11700. https://doi.org/10.3390/ijms222111700








