Gastric Helicobacter suis Infection Partially Protects against Neurotoxicity in A 6-OHDA Parkinson’s Disease Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Successful Gastric H. suis Colonization Is Associated with Gastric Inflammation
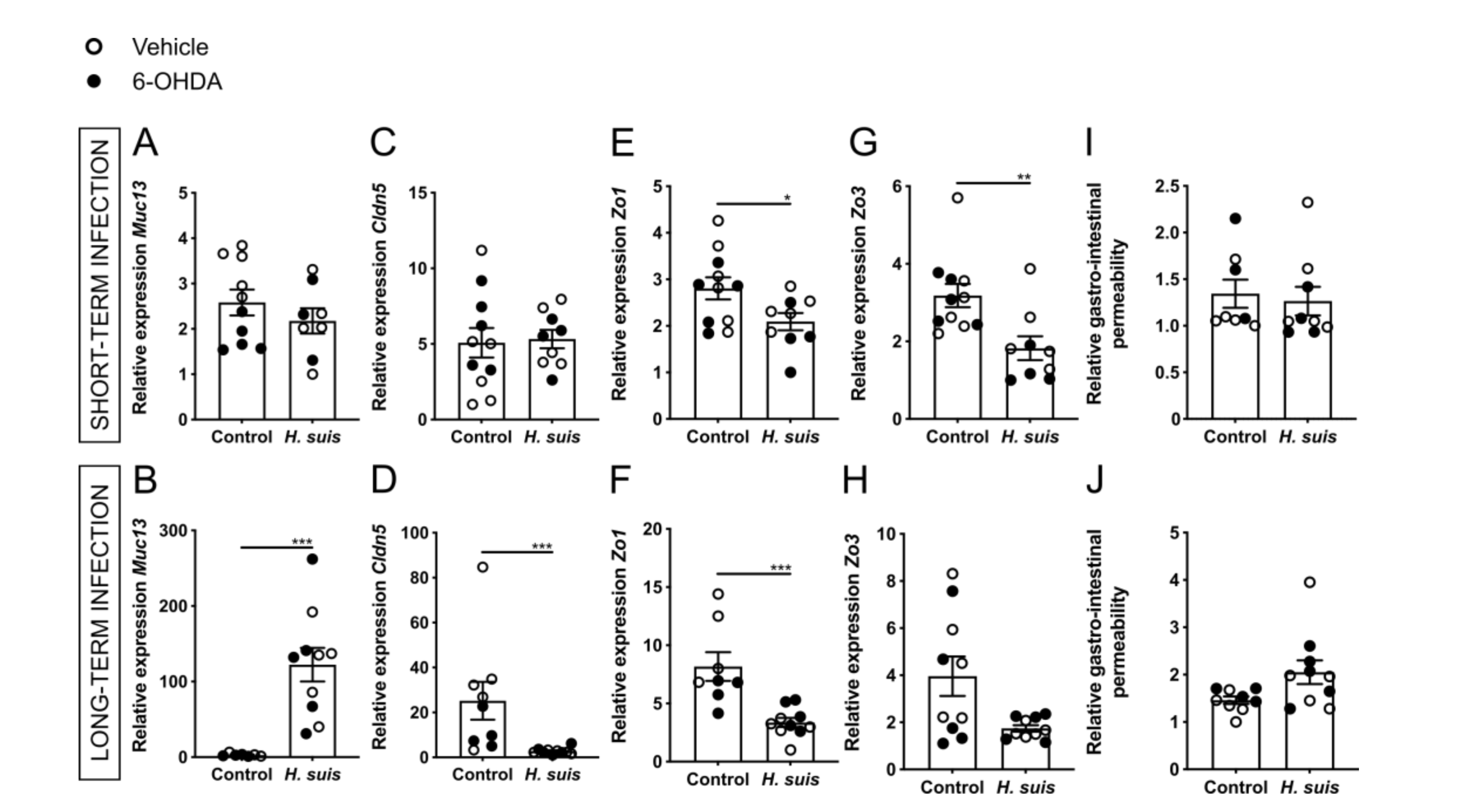
2.2. Gastric H. suis Infection Is Associated with A Limited Decrease in Integrity of the Gastrointestinal Barrier
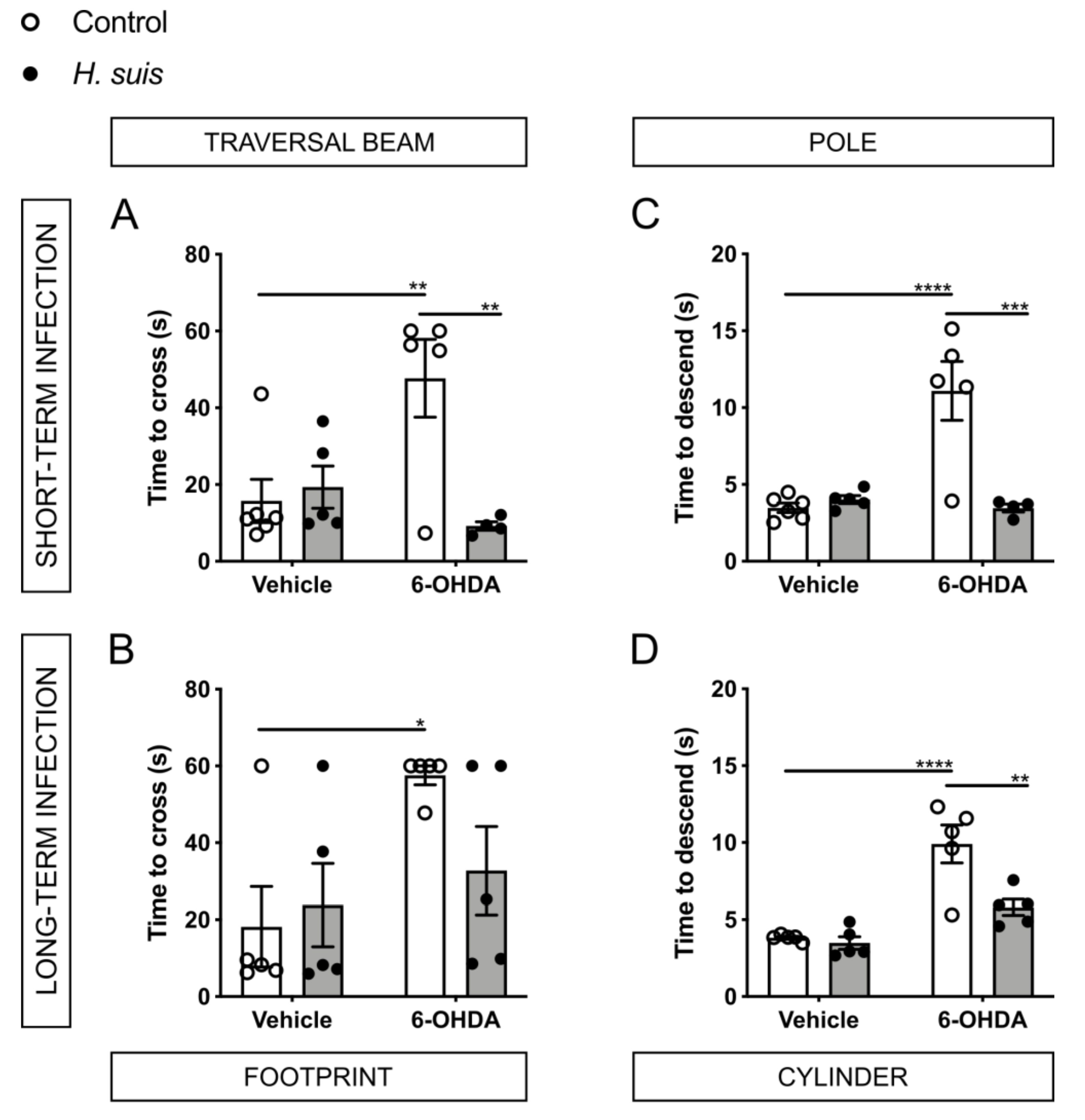
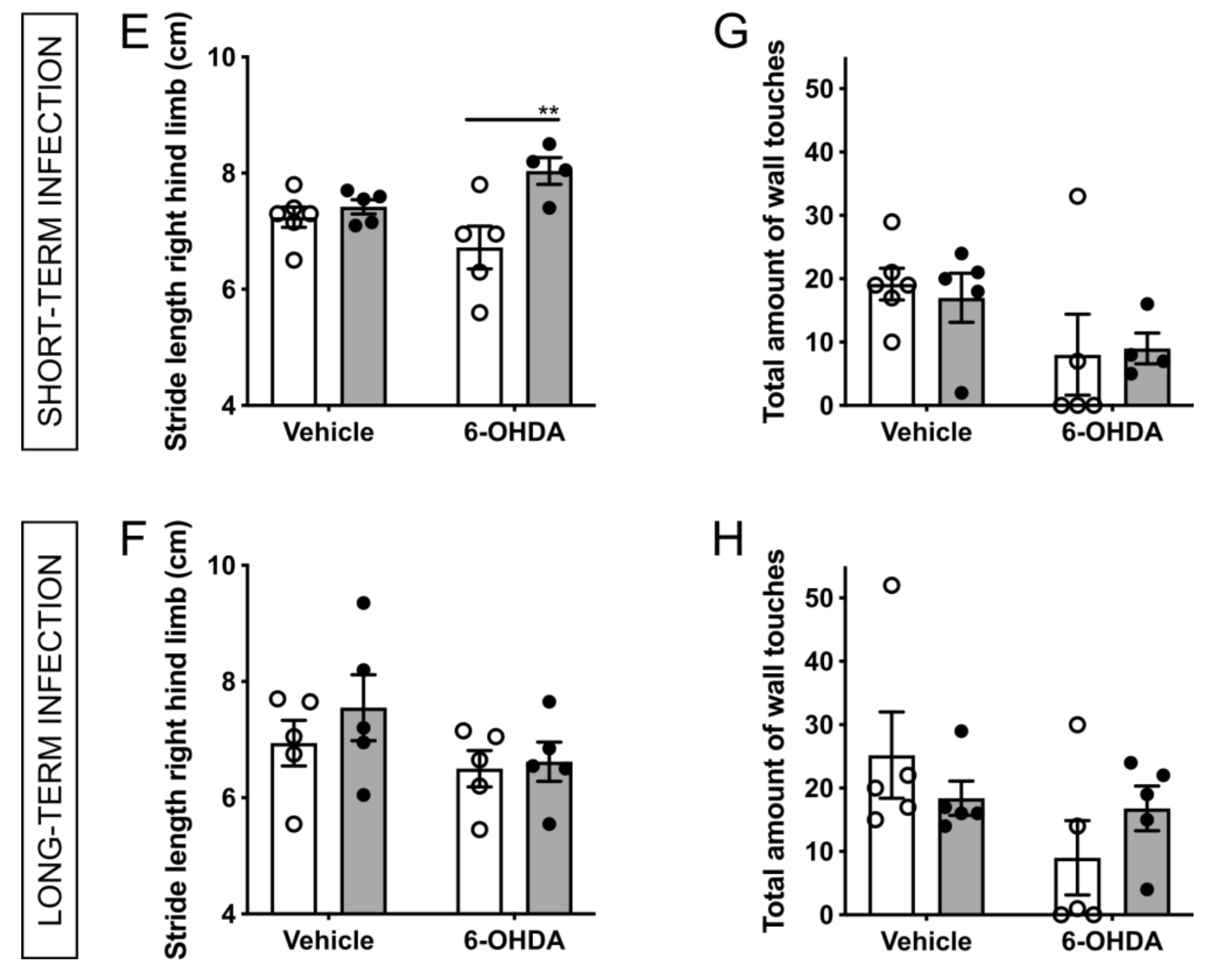
2.3. H. suis-Infected Mice Are Partially Protected against the Motor Deficits Induced by Intrastriatal 6-OHDA Injection
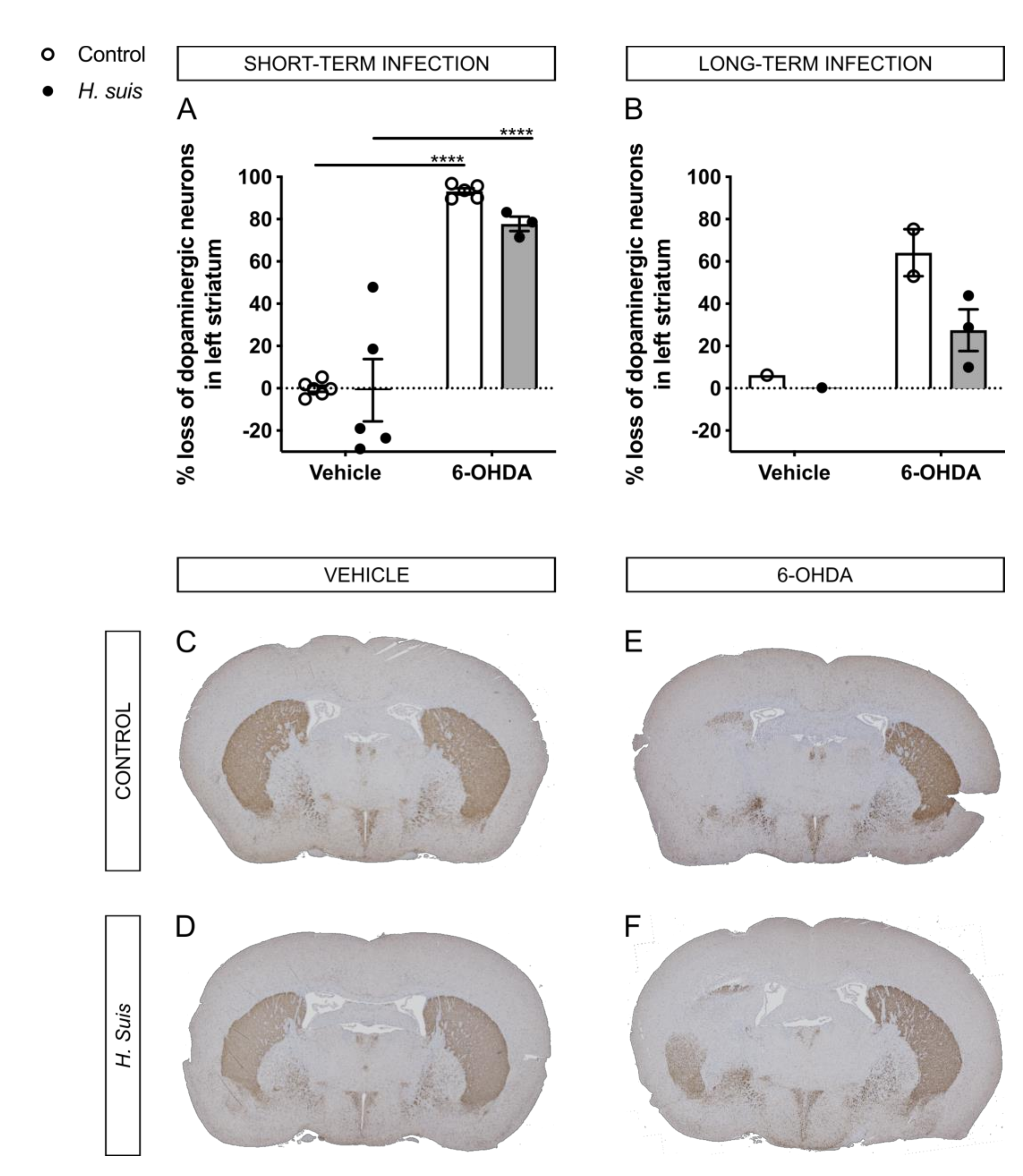
2.4. H. suis-Infected Mice Are Partially Protected against the Loss of Dopaminergic Neurons Induced by Intrastriatal 6-OHDA Injection
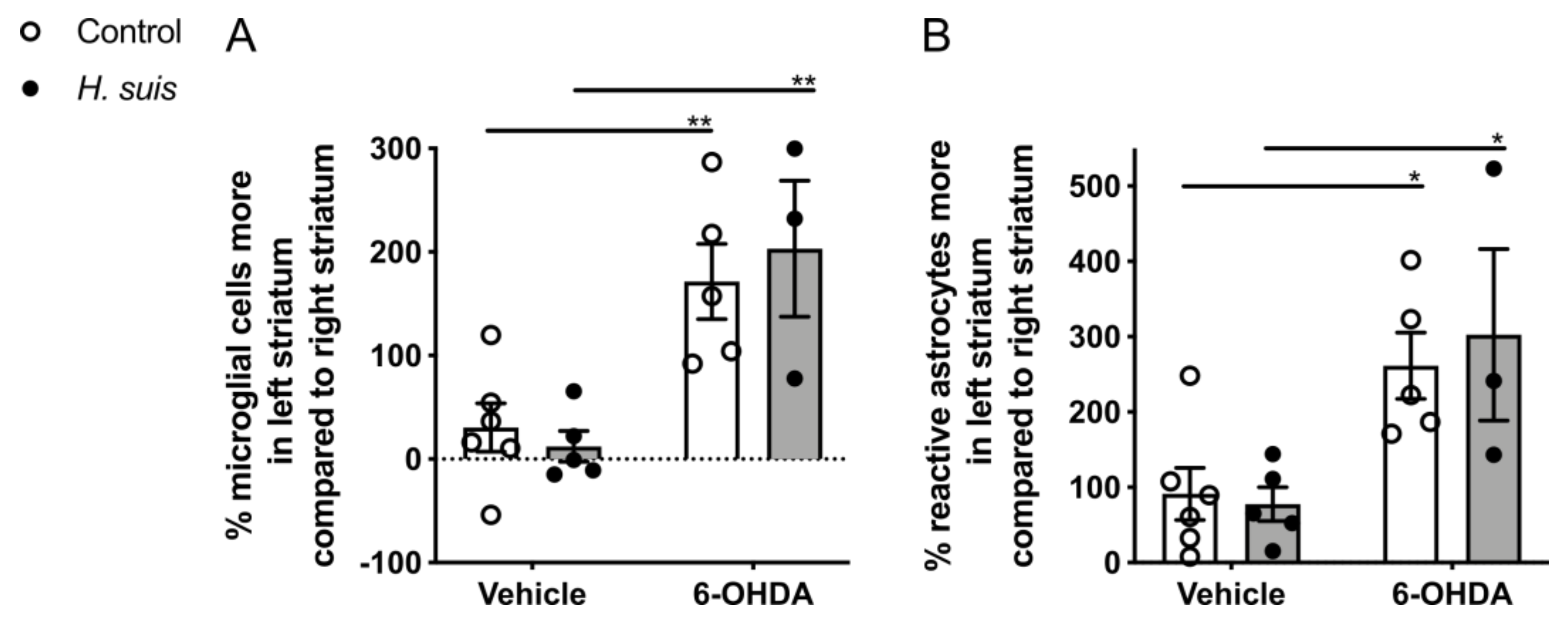
2.5. 6-OHDA-Induced Gliosis Is not Influenced by H. suis Infection
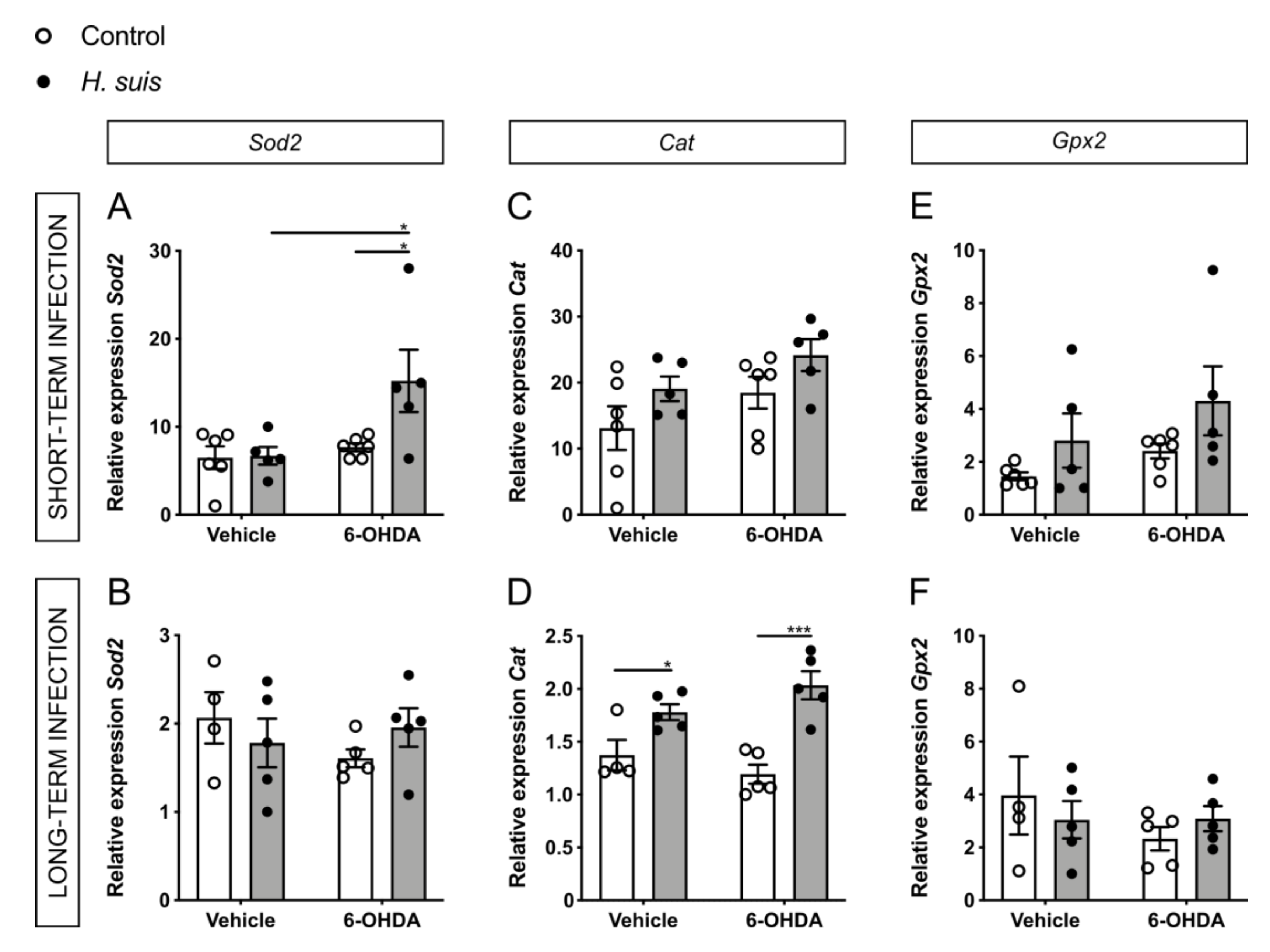
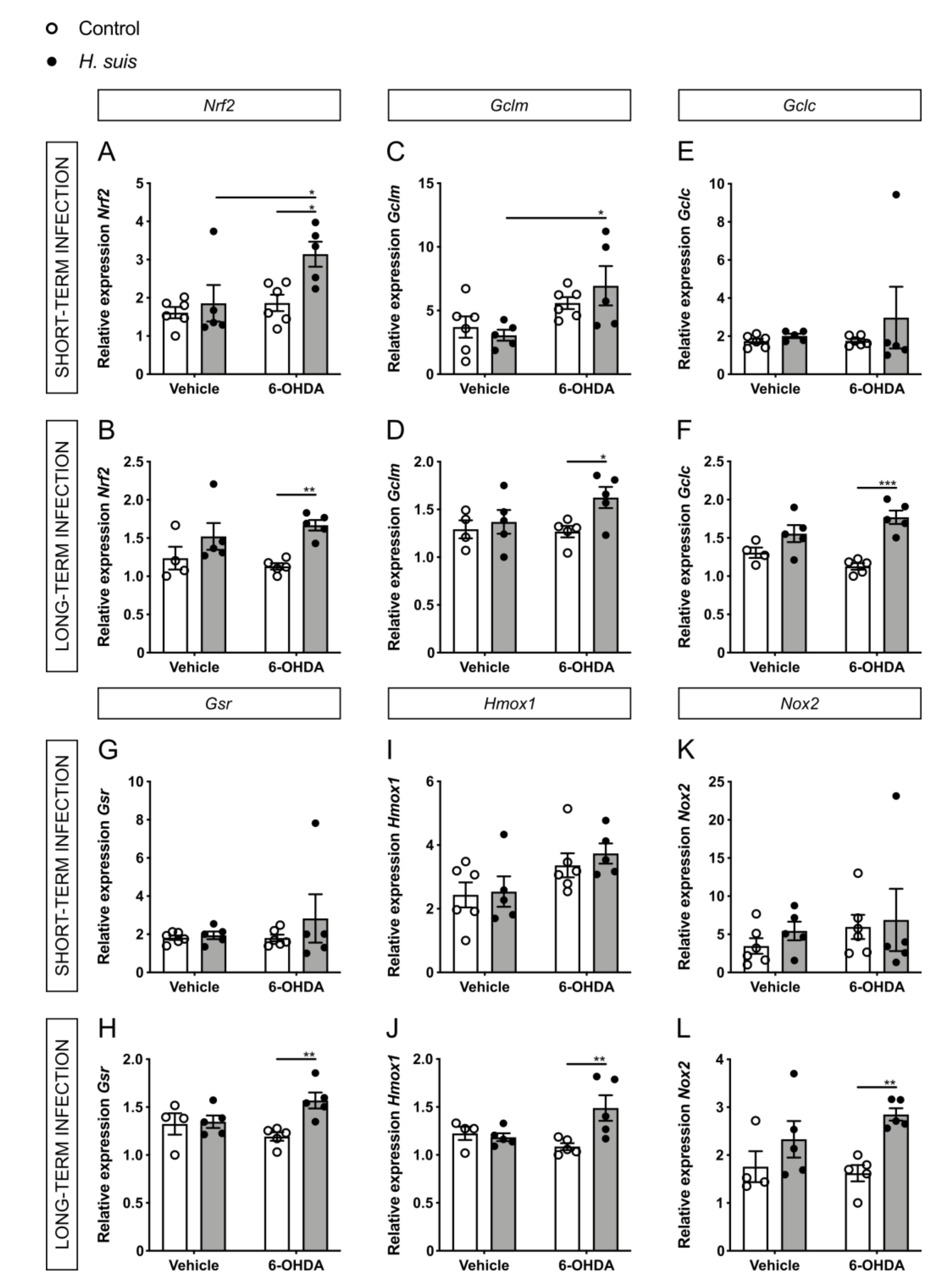
2.6. Changes in Oxidative Stress Pathways in the CNS could Explain the Neuroprotective Effect seen in H. suis-Infected Mice
3. Discussion
4. Materials and Methods
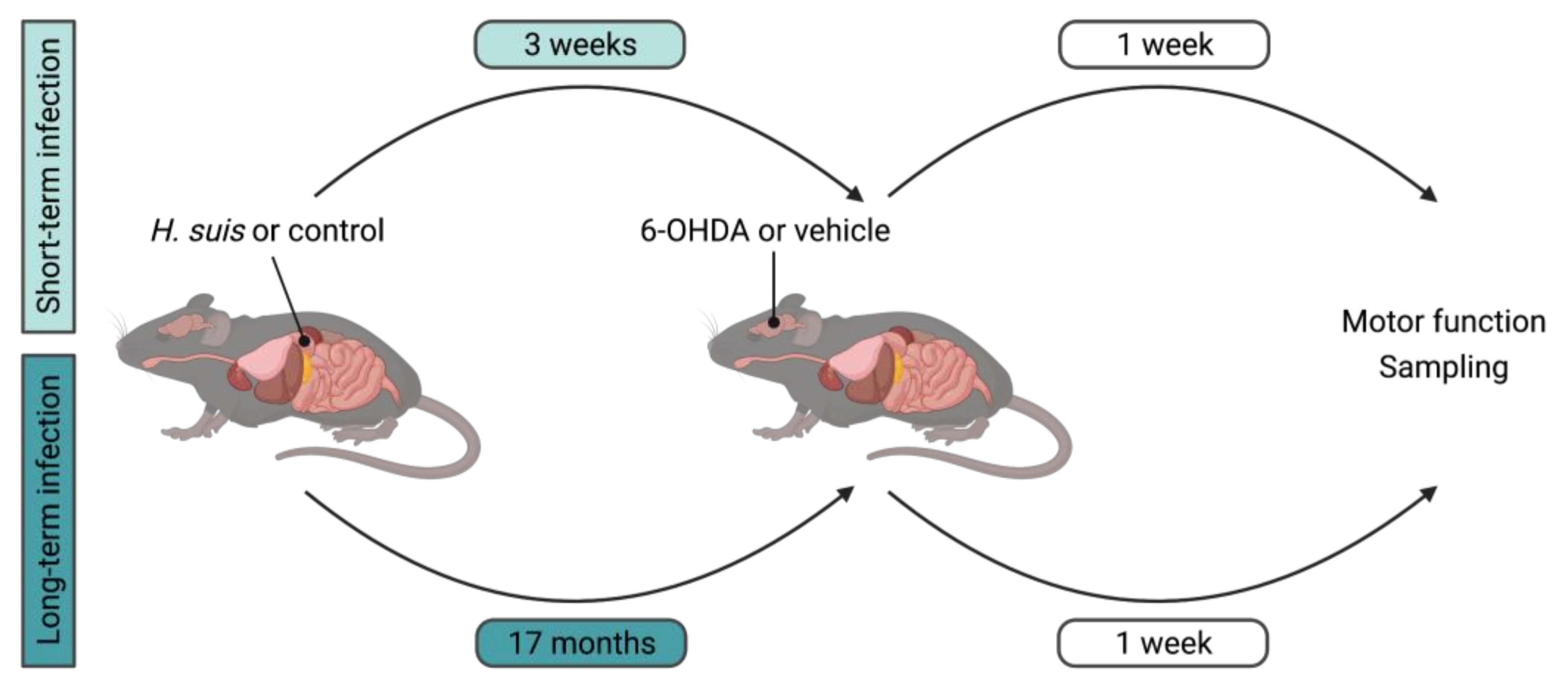
4.1. Animals
4.2. Cultivation of H. suis and In Vivo Infection Procedure
4.3. Intrastriatal 6-OHDA Injection Procedure
4.4. Behavior and Motor Function Tests
4.5. Collection and Processing of Samples
4.6. DNA Extraction and Quantification of Colonizing H. suis in the Stomach
4.7. RNA Extraction and RT-qPCR for Gene Expression Analysis
4.8. Quantification of the Gastrointestinal Permeability
4.9. Cytokine/Chemokine Measurements
4.10. Histopathology and Immunohistochemistry
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.; Dorsey, R.E.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.; Brayne, C.; Choi, J.-Y.J.; et al. Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, R.E.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [Green Version]
- Wirdefeldt, K.; Adami, H.-O.; Cole, P.; Trichopoulos, D.; Mandel, J. Epidemiology and Etiology of Parkinson’s Disease: A Review of the Evidence. Eur. J. Epidemiol. 2011, 26, 1. [Google Scholar] [CrossRef]
- Johnson, M.E.; Stecher, B.; Labrie, V.; Brundin, L.; Brundin, P. Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends Neurosci. 2018, 42, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.E.; Lang, A.E. Parkinson Disease. Nature reviews. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Dickson, D.W. Parkinson’s Disease and Parkinsonism: Neuropathology. Csh. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Tredici, K.D. Idiopathic Parkinson’s Disease: Possible Routes by Which Vulnerable Neuronal Types May Be Subject to Neuroinvasion by an Unknown Pathogen. J. Neural Transm. (Vienna Austria: 1996) 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.; Kim, W.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? NPJ Parkinson’s Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-First versus Body-First Parkinson’s Disease: A Multimodal Imaging Case-Control Study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef]
- Menozzi, E.; Macnaughtan, J.; Schapira, A.H.V. The Gut-Brain Axis and Parkinson Disease: Clinical and Pathogenetic Relevance. Ann. Med. 2021, 53, 611–625. [Google Scholar] [CrossRef]
- McGee, D.J.; Lu, X.-H.; Disbrow, E.A. Stomaching the Possibility of a Pathogenic Role for Helicobacter pylori in Parkinson’s Disease. J. Park Dis. 2018, 8, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.Y.; Yoon, W.T.; Shin, H.Y.; Jeon, S.H.; Rhee, P. Helicobacter pylori Infection and Motor Fluctuations in Patients with Parkinson’s Disease. Mov. Disord. 2008, 23, 1696–1700. [Google Scholar] [CrossRef]
- Hashim, H.; Azmin, S.; Razlan, H.; Yahya, N.W.; Tan, H.J.; Manaf, M.R.A.; Ibrahim, N.M. Eradication of Helicobacter pylori Infection Improves Levodopa Action, Clinical Symptoms and Quality of Life in Patients with Parkinson’s Disease. PLoS ONE 2014, 9, e112330. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.H.; Mahadeva, S.; Marras, C.; Thalha, A.M.; Kiew, C.K.; Yeat, C.M.; Ng, S.W.; Ang, S.P.; Chow, S.K.; Loke, M.F.; et al. Helicobacter pylori Infection Is Associated with Worse Severity of Parkinson’s Disease. Parkinsonism Relat. Disord. 2015, 21, 221–225. [Google Scholar] [CrossRef]
- Mridula, K.R.; Borgohain, R.; Reddy, V.C.; Bandaru, V.C.S.; Suryaprabha, T. Association of Helicobacter pylori with Parkinson’s Disease. J. Clin. Neurol. 2017, 13, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Yang, H.; Wu, Y.; Zhang, D.; Jiang, H. Meta-Analysis: Association of Helicobacter pylori Infection with Parkinson’s Diseases. Helicobacter 2017, 22, e12398. [Google Scholar] [CrossRef]
- Fu, P.; Gao, M.; Yung, K.K.L. Association of Intestinal Disorders with Parkinson’s Disease and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. ACS Chem. Neurosci. 2020, 11, 395–405. [Google Scholar] [CrossRef]
- Nyholm, D.; Hellström, P.M. Effects of Helicobacter pylori on Levodopa Pharmacokinetics. J. Park Dis. 2021, 11, 61–69. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Pietroiusti, A.; Brusa, L.; Galati, S.; Stefani, A.; Lunardi, G.; Fedele, E.; Sancesario, G.; Bernardi, G.; Bergamaschi, A.; et al. Helicobacter pylori Eradication and L-Dopa Absorption in Patients with PD and Motor Fluctuations. Neurology 2006, 66, 1824–1829. [Google Scholar] [CrossRef]
- Rees, K.; Stowe, R.; Patel, S.; Ives, N.; Breen, K.; Clarke, C.E.; Ben-Shlomo, Y. Helicobacter pylori Eradication for Parkinson’s Disease. Cochrane Database Syst. Rev. 2011, 11, CD008453. [Google Scholar] [CrossRef]
- Liu, H.; Su, W.; Li, S.; Du, W.; Ma, X.; Jin, Y.; Li, K.; Chen, H. Eradication of Helicobacter pylori Infection Might Improve Clinical Status of Patients with Parkinson’s Disease, Especially on Bradykinesia. Clin. Neurol. Neurosurg. 2017, 160, 101–104. [Google Scholar] [CrossRef]
- Lolekha, P.; Sriphanom, T.; Vilaichone, R.-K. Helicobacter pylori Eradication Improves Motor Fluctuations in Advanced Parkinson’s Disease Patients: A Prospective Cohort Study (HP-PD Trial). PLoS ONE 2021, 16, e0251042. [Google Scholar] [CrossRef]
- Tan, A.; Lim, S.; Mahadeva, S.; Loke, M.; Tan, J.; Ang, B.; Chin, K.; Adnan, A.; Ong, S.; Ibrahim, A.; et al. Helicobacter pylori Eradication in Parkinson’s Disease: A Randomized Placebo-Controlled Trial. Mov. Disord. 2020, 35, 2250–2260. [Google Scholar] [CrossRef]
- Blaecher, C.; Smet, A.; Flahou, B.; Pasmans, F.; Ducatelle, R.; Taylor, D.; Weller, C.; Bjarnason, I.; Charlett, A.; Lawson, A.J.; et al. Significantly Higher Frequency of Helicobacter suis in Patients with Idiopathic Parkinsonism than in Control Patients. Aliment. Pharmacol. Ther. 2013, 38, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Dobbs, R.J.; Dobbs, S.M.; Weller, C.; Bjarnason, I.T.; Bjarnason, I.T.; Oxlade, N.L.; Charlett, A.; Al-Janabi, M.A.; Kerwin, R.W.; Mahler, R.F.; et al. Role of Chronic Infection and Inflammation in the Gastrointestinal Tract in the Etiology and Pathogenesis of Idiopathic Parkinsonism. Part 1: Eradication of Helicobacter in the Cachexia of Idiopathic Parkinsonism. Helicobacter 2005, 10, 267–275. [Google Scholar] [CrossRef]
- Haesebrouck, F.; Pasmans, F.; Flahou, B.; Chiers, K.; Baele, M.; Meyns, T.; Decostere, A.; Ducatelle, R. Gastric Helicobacters in Domestic Animals and Nonhuman Primates and Their Significance for Human Health. Clin. Microbiol. Rev. 2009, 22, 202–223. [Google Scholar] [CrossRef] [Green Version]
- De Cooman, L.D.; Flahou, B.; Houf, K.; Smet, A.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F. Survival of Helicobacter suis Bacteria in Retail Pig Meat. Int. J. Food Microbiol. 2013, 166, 164–167. [Google Scholar] [CrossRef]
- Gorlé, N.; Blaecher, C.; Bauwens, E.; Vandendriessche, C.; Balusu, S.; Vandewalle, J.; Cauwenberghe, C.V.; Wonterghem, E.V.; Imschoot, G.V.; Liu, C.; et al. The Choroid Plexus Epithelium as a Novel Player in the Stomach-Brain Axis during Helicobacter Infection. Brain Behav. Immun. 2018, 69, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef] [Green Version]
- Andrew, R.; Watson, D.G.; Best, S.A.; Midgley, J.M.; Wenlong, H.; Petty, R.K.H. The Determination of Hydroxydopamines and Other Trace Amines in the Urine of Parkinsonian Patients and Normal Controls. Neurochem. Res. 1993, 18, 1175–1177. [Google Scholar] [CrossRef]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-Hydroxydopamine Model of Parkinson’s Disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef]
- Varcin, M.; Bentea, E.; Mertens, B.; Haute, C.V.D.; Baekelandt, V.; Michotte, Y.; Sarre, S. Acute versus Long-Term Effects of 6-Hydroxydopamine on Oxidative Stress and Dopamine Depletion in the Striatum of Mice. J. Neurosci. Meth. 2011, 202, 128–136. [Google Scholar] [CrossRef]
- Berger, K.; Przedborski, S.; Cadet, J.L. Retrograde Degeneration of Nigrostriatal Neurons Induced by Intrastriatal 6-Hydroxydopamine Injection in Rats. Brain Res. Bull. 1991, 26, 301–307. [Google Scholar] [CrossRef]
- Przedbroski, S.; Leviver, M.; Jiang, H.; Ferreira, M.; Jackson-Lewis, V.; Donaldson, D.; Togasaki, D.M. Dose-Dependent Lesions of the Dopaminergic Nigrostriatal Pathway Induced by Instrastriatal Injection of 6-Hydroxydopamine. Neuroscience 1995, 67, 631–647. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the Mucosal Barrier to Infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Kufe, D.W. Mucins in Cancer: Function, Prognosis and Therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [Green Version]
- Breugelmans, T.; Spaendonk, H.V.; Man, J.G.D.; Schepper, H.U.D.; Jauregui-Amezaga, A.; Macken, E.; Lindén, S.K.; Pintelon, I.; Timmermans, J.-P.; Winter, B.Y.D.; et al. In-Depth Study of Transmembrane Mucins in Association with Intestinal Barrier Dysfunction During the Course of T Cell Transfer and DSS-Induced Colitis. J. Crohn’s Colitis 2020, 14, 974–994. [Google Scholar] [CrossRef]
- Vermette, D.; Hu, P.; Canarie, M.F.; Funaro, M.; Glover, J.; Pierce, R.W. Tight Junction Structure, Function, and Assessment in the Critically Ill: A Systematic Review. Intensive Care Med. Exp. 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Stride Length Regulation in Parkinson’s Disease Normalization Strategies and Underlying Mechanisms. Brain 1996, 119, 551–568. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A Modulator of Parkinson’s Disease? J. Neural. Transm. 2016, 123, 611–619. [Google Scholar] [CrossRef]
- Petrillo, S.; Schirinzi, T.; Lazzaro, G.D.; D’Amico, J.; Colona, V.L.; Bertini, E.; Pierantozzi, M.; Mari, L.; Mercuri, N.B.; Piemonte, F.; et al. Systemic Activation of Nrf2 Pathway in Parkinson’s Disease. Mov. Disord. 2020, 35, 180–184. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid. Med. Cell Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Augustin, A.D.; Savio, A.; Nevel, A.; Ellis, R.J.; Weller, C.; Taylor, D.; Tucker, R.M.; Ibrahim, M.A.; Bjarnason, I.; Dobbs, S.M.; et al. Helicobacter suis Is Associated with Mortality in Parkinson’s Disease. Front. Med. 2019, 6, 188. [Google Scholar] [CrossRef]
- Gorlé, N.; Bauwens, E.; Haesebrouck, F.; Smet, A.; Vandenbroucke, R.E. Helicobacter and the Potential Role in Neurological Disorders: There Is More Than Helicobacter pylori. Front. Immunol. 2021, 11, 584165. [Google Scholar] [CrossRef]
- Knott, C.; Wilkin, G.P.; Stern, G. Astrocytes and Microglia in the Substantia Nigra and Caudate-Putamen in Parkinson’s Disease. Parkinsonism Relat. Disord. 1999, 5, 115–122. [Google Scholar] [CrossRef]
- Rodrigues, R.W.P.; Gomide, V.C.; Chadi, G. Astroglial and Microglial Reaction After A Partial Nigrostriatal Degeneration Induced by The Striatal Injection of Different Doses of 6-Hydroxydopamine. Int. J. Neurosci. 2001, 109, 91–126. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA Damage in the Parkinsonian Brain: An Apparent Selective Increase in 8-Hydroxyguanine Levels in Substantia Nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Dexter, D.; Carter, C.; Agid, F.; Agid, Y.; Lees, A.J.; Jenner, P.; Marsden, C.D. Lipid Peroxidation as Cause of Nigral Cell Death in Parkinson’s Disease. Lancet 1986, 328, 639–640. [Google Scholar] [CrossRef]
- Saggu, H.; Cooksey, J.; Dexter, D.; Wells, F.R.; Lees, A.; Jenner, P.; Marsden, C.D. A Selective Increase in Particulate Superoxide Dismutase Activity in Parkinsonian Substantia Nigra. J. Neurochem. 1989, 53, 692–697. [Google Scholar] [CrossRef]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in Glutathione Levels in Parkinson’s Disease and Other Neurodegenerative Disorders Affecting Basal Ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Nagata, K.; Yu, H.; Nishikawa, M.; Kashiba, M.; Nakamura, A.; Sato, E.F.; Tamura, T.; Inoue, M. Helicobacter pylori Generates Superoxide Radicals and Modulates Nitric Oxide Metabolism. J. Biol. Chem. 1998, 273, 14071–14073. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Naito, Y. The Role of Neutrophils and Inflammation in Gastric Mucosal Injury. Free Radical Res. 2009, 33, 785–794. [Google Scholar] [CrossRef]
- Baek, H.Y.; Lim, J.W.; Kim, H.; Kim, J.M.; Kim, J.S.; Jung, H.C.; Kim, K.H. Oxidative-Stress-Related Proteome Changes in Helicobacter pylori-Infected Human Gastric Mucosa. Biochem. J. 2004, 379, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-Z.; Minohara, Y.; Fan, X.J.; Wang, J.; Reyes, V.E.; Patel, J.; Dirden-Kramer, B.; Boldogh, I.; Ernst, P.B.; Crowe, S.E. Helicobacter pylori Infection Induces Oxidative Stress and Programmed Cell Death in Human Gastric Epithelial Cells. Infect. Immun. 2007, 75, 4030–4039. [Google Scholar] [CrossRef] [Green Version]
- Flahou, B.; Haesebrouck, F.; Chiers, K.; Deun, K.V.; Smet, L.D.; Devreese, B.; Vandenberghe, I.; Favoreel, H.; Smet, A.; Pasmans, F.; et al. Gastric Epithelial Cell Death Caused by Helicobacter suis and Helicobacter pylori Γ-glutamyl Transpeptidase Is Mainly Glutathione Degradation-dependent. Cell Microbiol. 2011, 13, 1933–1955. [Google Scholar] [CrossRef]
- Buommino, E.; Donnarumma, G.; Manente, L.; Filippis, A.; Silvestri, F.; Iaquinto, S.; Tufano, M.A.; Luca, A. The Helicobacter pylori Protein HspB Interferes with Nrf2/Keap1 Pathway Altering the Antioxidant Response of Ags Cells. Helicobacter 2012, 17, 417–425. [Google Scholar] [CrossRef]
- Cherdantseva, L.A.; Potapova, O.V.; Sharkova, T.V.; Belyaeva, Y.Y.; Shkurupiy, V.A. Association of Helicobacter pylori and INOS Production by Macrophages and Lymphocytes in the Gastric Mucosa in Chronic Gastritis. J. Immunol. Res. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Paik, J.Y.; Lee, H.G.; Piao, J.-Y.; Kim, S.-J.; Kim, D.-H.; Na, H.-K.; Surh, Y.-J. Helicobacter pylori Infection Promotes Autophagy through Nrf2-Mediated Heme Oxygenase Upregulation in Human Gastric Cancer Cells. Biochem. Pharmacol. 2019, 162, 89–97. [Google Scholar] [CrossRef]
- Iglesias-González, J.; Sánchez-Iglesias, S.; Méndez-Álvarez, E.; Rose, S.; Hikima, A.; Jenner, P.; Soto-Otero, R. Differential Toxicity of 6-Hydroxydopamine in SH-SY5Y Human Neuroblastoma Cells and Rat Brain Mitochondria: Protective Role of Catalase and Superoxide Dismutase. Neurochem. Res. 2012, 37, 2150–2160. [Google Scholar] [CrossRef]
- Jakel, R.J.; Townsend, J.A.; Kraft, A.D.; Johnson, J.A. Nrf2-Mediated Protection against 6-Hydroxydopamine. Brain Res. 2007, 1144, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological Targeting of the Transcription Factor Nrf2 at the Basal Ganglia Provides Disease Modifying Therapy for Experimental Parkinsonism. Antioxid. Redox Sign. 2011, 14, 2347–2360. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Vargas, M.R.; Johnson, D.A.; Johnson, J.A. Astrocyte-Specific Overexpression of Nrf2 Delays Motor Pathology and Synuclein Aggregation throughout the CNS in the Alpha-Synuclein Mutant (A53T) Mouse Model. J. Neurosci. 2012, 32, 17775–17787. [Google Scholar] [CrossRef] [Green Version]
- Lastres-Becker, I.; Ulusoy, A.; Innamorato, N.G.; Sahin, G.; Rábano, A.; Kirik, D.; Cuadrado, A. α-Synuclein Expression and Nrf2 Deficiency Cooperate to Aggravate Protein Aggregation, Neuronal Death and Inflammation in Early-Stage Parkinson’s Disease. Hum. Mol. Genet. 2012, 21, 3173–3192. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Koppula, S.; Kim, I.-S.; More, S.V.; Kim, B.-W.; Choi, D.-K. Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Parkinson Disease: A Promising Multi Therapeutic Target Against Oxidative Stress, Neuroinflammation and Cell Death. CNS Neurol. Disord.-Drug Targets 2013, 11, 1015–1029. [Google Scholar] [CrossRef]
- Skibinski, G.; Hwang, V.; Ando, D.M.; Daub, A.; Lee, A.K.; Ravisankar, A.; Modan, S.; Finucane, M.M.; Shaby, B.A.; Finkbeiner, S. Nrf2 Mitigates LRRK2- and α-Synuclein–Induced Neurodegeneration by Modulating Proteostasis. Proc. Natl. Acad. Sci. USA 2017, 114, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Inose, Y.; Izumi, Y.; Takada-Takatori, Y.; Akaike, A.; Koyama, Y.; Kaneko, S.; Kume, T. Protective Effects of Nrf2–ARE Activator on Dopaminergic Neuronal Loss in Parkinson Disease Model Mice: Possible Involvement of Heme Oxygenase-1. Neurosci. Lett. 2020, 736, 135268. [Google Scholar] [CrossRef]
- Anandhan, A.; Nguyen, N.; Syal, A.; Dreher, L.A.; Dodson, M.; Zhang, D.D.; Madhavan, L. NRF2 Loss Accentuates Parkinsonian Pathology and Behavioral Dysfunction in Human α-Synuclein Overexpressing Mice. Aging Dis. 2021, 12, 964–982. [Google Scholar] [CrossRef]
- Gumeni, S.; Papanagnou, E.-D.; Manola, M.S.; Trougakos, I.P. Nrf2 Activation Induces Mitophagy and Reverses Parkin/Pink1 Knock down-Mediated Neuronal and Muscle Degeneration Phenotypes. Cell Death Dis. 2021, 12, 671. [Google Scholar] [CrossRef]
- Schipper, H.M.; Liberman, A.; Stopa, E.G. Neural Heme Oxygenase-1 Expression in Idiopathic Parkinson’s Disease. Exp. Neurol. 1998, 150, 60–68. [Google Scholar] [CrossRef]
- Yoo, M.S.; Chun, H.S.; Son, J.J.; DeGiorgio, L.A.; Kim, D.J.; Peng, C.; Son, J.H. Oxidative Stress Regulated Genes in Nigral Dopaminergic Neuronal Cells: Correlation with the Known Pathology in Parkinson’s Disease. Mol. Brain Res. 2003, 110, 76–84. [Google Scholar] [CrossRef]
- Van Muiswinkel, F.L.; de Vos, R.A.I.; Bol, J.G.J.M.; Andringa, G.; Steur, E.N.H.J.; Ross, D.; Siegel, D.; Drukarch, B. Expression of NAD(P)H:Quinone Oxidoreductase in the Normal and Parkinsonian Substantia Nigra. Neurobiol. Aging 2004, 25, 1253–1262. [Google Scholar] [CrossRef]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Baele, M.; Decostere, A.; Vandamme, P.; Ceelen, L.; Hellemans, A.; Mast, J.; Chiers, K.; Ducatelle, R.; Haesebrouck, F. Isolation and Characterization of Helicobacter suis Sp. Nov. from Pig Stomachs. Int. J. Syst. Evol. Micr. 2008, 58, 1350–1358. [Google Scholar] [CrossRef] [Green Version]
- Fleming, S.M.; Salcedo, J.; Fernagut, P.-O.O.; Rockenstein, E.; Masliah, E.; Levine, M.S.; Chesselet, M.-F.F. Early and Progressive Sensorimotor Anomalies in Mice Overexpressing Wild-Type Human Alpha-Synuclein. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 9434–9440. [Google Scholar] [CrossRef] [Green Version]
- Flahou, B.; Deun, K.V.; Pasmans, F.; Smet, A.; Volf, J.; Rychlik, I.; Ducatelle, R.; Haesebrouck, F. The Local Immune Response of Mice after Helicobacter suis Infection: Strain Differences and Distinction with Helicobacter pylori. Vet. Res. 2012, 43, 75. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, J.L.; Solnick, J.V.; Neilan, B.A.; Seidel, K.; Hayter, R.; Hansen, L.M.; Lee, A. Description of ‘Candidatus Helicobacter heilmannii’ Based on DNA Sequence Analysis of 16S RRNA and Urease Genes. Int. J. Syst. Evol. Micr. 2004, 54, 2203–2211. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, R.E.; Dejonckheere, E.; Hauwermeiren, F.V.; Lodens, S.; Rycke, R.D.; Wonterghem, E.V.; Staes, A.; Gevaert, K.; López-Otin, C.; Libert, C. Matrix Metalloproteinase 13 Modulates Intestinal Epithelial Barrier Integrity in Inflammatory Diseases by Activating TNF. EMBO Mol. Med. 2013, 5, 1000–1016. [Google Scholar] [CrossRef] [Green Version]
- Flahou, B.; Haesebrouck, F.; Pasmans, F.; D’Herde, K.; Driessen, A.; Deun, K.V.; Smet, A.; Duchateau, L.; Chiers, K.; Ducatelle, R. Helicobacter suis Causes Severe Gastric Pathology in Mouse and Mongolian Gerbil Models of Human Gastric Disease. PLoS ONE 2010, 5, e14083. [Google Scholar] [CrossRef] [Green Version]
- Brkic, M.; Balusu, S.; Wonterghem, E.; Gorlé, N.; Benilova, I.; Kremer, A.; Hove, I.; Moons, L.; Strooper, B.; Kanazir, S.; et al. Amyloid β Oligomers Disrupt Blood-CSF Barrier Integrity by Activating Matrix Metalloproteinases. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 12766–12778. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlamont, H.; Bruggeman, A.; Bauwens, E.; Vandendriessche, C.; Clarebout, E.; Xie, J.; De Bruyckere, S.; Van Imschoot, G.; Van Wonterghem, E.; Ducatelle, R.; et al. Gastric Helicobacter suis Infection Partially Protects against Neurotoxicity in A 6-OHDA Parkinson’s Disease Mouse Model. Int. J. Mol. Sci. 2021, 22, 11328. https://doi.org/10.3390/ijms222111328
Berlamont H, Bruggeman A, Bauwens E, Vandendriessche C, Clarebout E, Xie J, De Bruyckere S, Van Imschoot G, Van Wonterghem E, Ducatelle R, et al. Gastric Helicobacter suis Infection Partially Protects against Neurotoxicity in A 6-OHDA Parkinson’s Disease Mouse Model. International Journal of Molecular Sciences. 2021; 22(21):11328. https://doi.org/10.3390/ijms222111328
Chicago/Turabian StyleBerlamont, Helena, Arnout Bruggeman, Eva Bauwens, Charysse Vandendriessche, Elien Clarebout, Junhua Xie, Sofie De Bruyckere, Griet Van Imschoot, Elien Van Wonterghem, Richard Ducatelle, and et al. 2021. "Gastric Helicobacter suis Infection Partially Protects against Neurotoxicity in A 6-OHDA Parkinson’s Disease Mouse Model" International Journal of Molecular Sciences 22, no. 21: 11328. https://doi.org/10.3390/ijms222111328
APA StyleBerlamont, H., Bruggeman, A., Bauwens, E., Vandendriessche, C., Clarebout, E., Xie, J., De Bruyckere, S., Van Imschoot, G., Van Wonterghem, E., Ducatelle, R., Santens, P., Smet, A., Haesebrouck, F., & Vandenbroucke, R. E. (2021). Gastric Helicobacter suis Infection Partially Protects against Neurotoxicity in A 6-OHDA Parkinson’s Disease Mouse Model. International Journal of Molecular Sciences, 22(21), 11328. https://doi.org/10.3390/ijms222111328






