Therapeutic Effect of α-MSH in Primary Cultured Orbital Fibroblasts Obtained from Patients with Thyroid Eye Disease
Abstract
1. Introduction
2. Results
2.1. Characterization of Fibroblasts and Endothelial Cells in TED Patients and Normal Control
2.2. Effect of α-MSH on the Viability of Orbital Fibroblasts
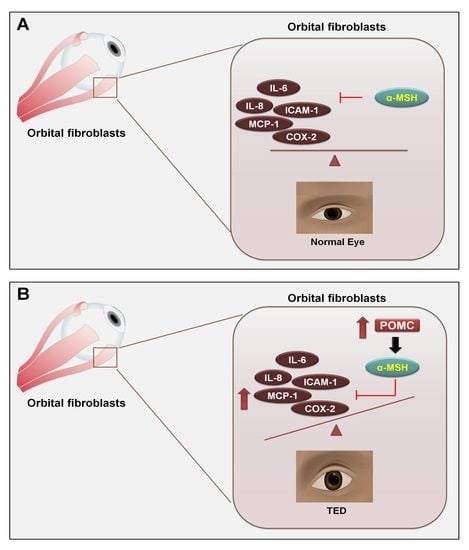
2.3. α-MSH Attenuates the IL-1βInduced Pro-Inflammatory Cytokines Both in Normal and TED Orbital Fibroblasts
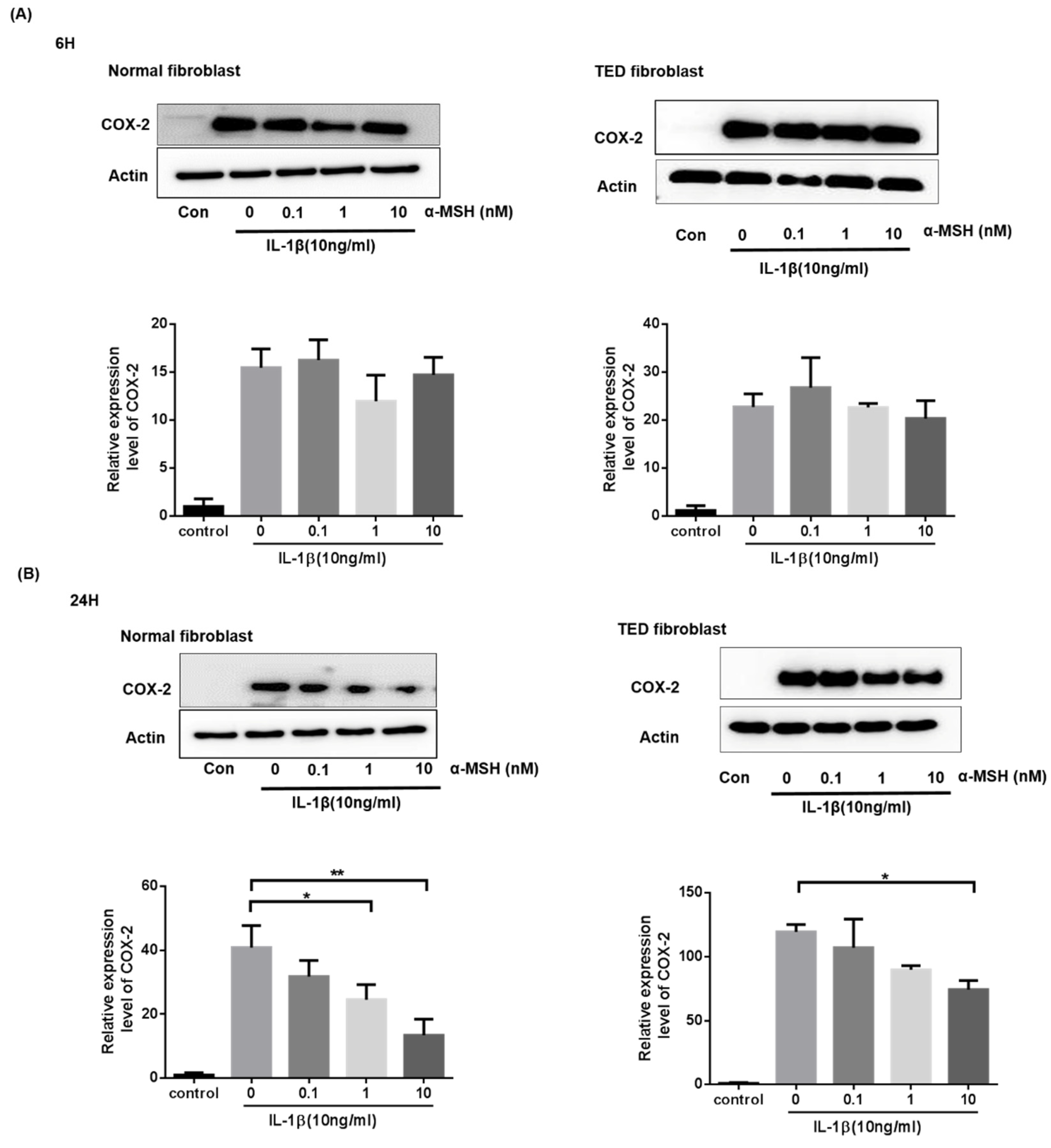
2.4. α-MSH Attenuates the IL-1βInduced Expression of COX-2 Proteins Both in Normal and TED Orbital Fibroblasts
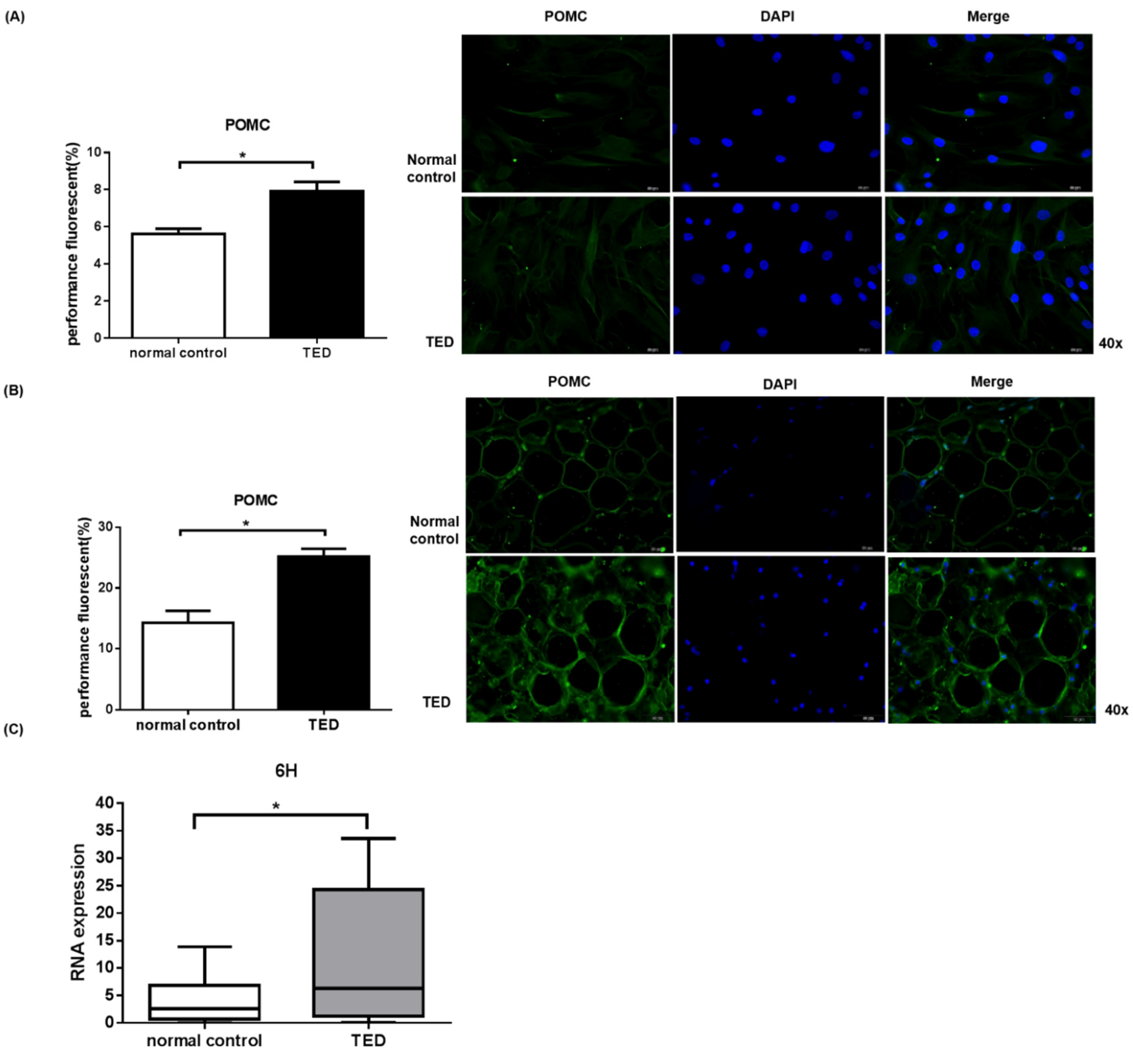
2.5. POMC Expression in Primary Orbital Fibroblasts and Orbital Tissue
3. Discussion
4. Materials and Methods
4.1. Cell and Tissue Culture Protocols
4.2. Characterization of Orbital Fibroblasts
4.3. MTT Assays
4.4. Lactate Dehydrogenase (LDH) Assay
4.5. Apoptosis Assays
4.6. Extraction of RNA
4.7. Quantitative Real-Time PCR
4.8. Western Blot Assay
4.9. Immunofluorescence Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajendram, R.; Bunce, C.; Adams, G.G.; Dayan, C.; Rose, G.E. Smoking and strabismus surgery in patients with thyroid eye disease. Ophthalmology 2011, 118, 2493–2497. [Google Scholar] [CrossRef]
- Weiler, D.L. Thyroid eye disease: A review. Clin. Exp. Optom. 2017, 100, 20–25. [Google Scholar] [CrossRef]
- Alkawas, A.A.; Hussein, A.M.; Shahien, E.A. Orbital steroid injection versus oral steroid therapy in management of thyroid-related ophthalmopathy. Clin. Exp. Ophthalmol. 2010, 38, 692–697. [Google Scholar] [CrossRef]
- Lehmann, G.M.; Feldon, S.; Smith, T.; Phipps, R.P. Immune Mechanisms in Thyroid Eye Disease. Thyroid 2008, 18, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, B.S.; Bahn, R.S.; Smith, T. Current Perspective on the Pathogenesis of Graves’ Disease and Ophthalmopathy. Endocr. Rev. 2003, 24, 802–835. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Weetman, A.P. New understanding of the role of cytokines in the pathogenesis of Graves’ ophthalmopathy. J. Endocrinol. Investig. 2004, 27, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Y.; Zhang, Y.; He, Q.; Xu, R.; Ge, F.; Wu, C. Celastrol inhibits IL-1beta-induced inflammation in orbital fibroblasts through the suppression of NF-kappaB activity. Mol. Med. Rep. 2016, 14, 2799–2806. [Google Scholar] [CrossRef]
- Smith, T.J. Orbital Fibroblasts Exhibit a Novel Pattern of Responses to Proinflammatory Cytokines: Potential Basis for the Pathogenesis of Thyroid-Associated Ophthalmopathy. Thyroid 2002, 12, 197–203. [Google Scholar] [CrossRef]
- Yoon, J.S.; Chae, M.K.; Lee, S.Y.; Lee, E.J. Anti-inflammatory effect of quercetin in a whole orbital tissue culture of Graves’ orbitopathy. Br. J. Ophthalmol. 2012, 96, 1117–1121. [Google Scholar] [CrossRef]
- Konuk, E.B.Y.; Konuk, O.; Misirlioglu, M.; Menevse, A.; Unal, M. Expression of cyclooxygenase-2 in orbital fibroadipose connective tissues of Graves’ ophthalmopathy patients. Eur. J. Endocrinol. 2006, 155, 681–685. [Google Scholar] [CrossRef][Green Version]
- Heufelder, A.E.; Bahn, R.S. Elevated expression in situ of selectin and immunoglobulin superfamily type adhesion molecules in retroocular connective tissues from patients with Graves’ ophthalmopathy. Clin. Exp. Immunol. 1993, 91, 381–389. [Google Scholar] [CrossRef]
- Melanocortin in the Pathogenesis of Inflammatory Eye Diseases: Considerations for Treatment. Retina 2018, 38 (Suppl. 1), 1–12. [CrossRef]
- Gatti, S.; Colombo, G.; Buffa, R.; Turcatti, F.; Garofalo, L.; Carboni, N.; Ferla, L.; Fassati, L.R.; Lipton, J.M.; Catania, A. α-Melanocyte-stimulating hormone protects the allograft in experimental heart transplantation 1. Transplantation 2002, 74, 1678–1684. [Google Scholar] [CrossRef]
- Brzoska, T.; Luger, T.A.; Maaser, C.; Abels, C.; Böhm, M. α-Melanocyte-Stimulating Hormone and Related Tripeptides: Biochemistry, Antiinflammatory and Protective Effects in Vitro and in Vivo, and Future Perspectives for the Treatment of Immune-Mediated Inflammatory Diseases. Endocr. Rev. 2008, 29, 581–602. [Google Scholar] [CrossRef]
- Catania, A.; Gatti, S.; Colombo, G.; Lipton, J.M. Targeting Melanocortin Receptors as a Novel Strategy to Control Inflammation. Pharmacol. Rev. 2004, 56, 1–29. [Google Scholar] [CrossRef]
- Lipton, J.M.; Catania, A. Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol. Today 1997, 18, 140–145. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.; Liu, X.; Jiang, Y.; Zhang, L.; Zhang, X.; Li, X.; Zhang, Y. α-Melanocyte-Stimulating Hormone Protects Retinal Vascular Endothelial Cells from Oxidative Stress and Apoptosis in a Rat Model of Diabetes. PLoS ONE 2014, 9, e93433. [Google Scholar] [CrossRef]
- Clemson, C.M.; Yost, J.; Taylor, A.W. The Role of Alpha-MSH as a Modulator of Ocular Immunobiology Exemplifies Mechanistic Differences between Melanocortins and Steroids. Ocul. Immunol. Inflamm. 2017, 25, 179–189. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, T.J. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest. Ophthalmol. Vis. Sci. 2014, 55, 1735–1748. [Google Scholar] [CrossRef]
- Chen, B.; Tsui, S.; Smith, T. IL-1β Induces IL-6 Expression in Human Orbital Fibroblasts: Identification of an Anatomic-Site Specific Phenotypic Attribute Relevant to Thyroid-Associated Ophthalmopathy. J. Immunol. 2005, 175, 1310–1319. [Google Scholar] [CrossRef]
- Khong, J.J.; McNab, A.A.; Ebeling, P.R.; Craig, J.; Selva, D. Pathogenesis of thyroid eye disease: Review and update on molecular mechanisms. Br. J. Ophthalmol. 2016, 100, 142–150. [Google Scholar] [CrossRef]
- Wang, Y.; Patel, A.; Douglas, R.S. Thyroid Eye Disease: How A Novel Therapy May Change The Treatment Paradigm. Ther. Clin. Risk Manag. 2019, 15, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M. On behalf of the European Group on Graves’ Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur. Thyroid. J. 2016, 5, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Drui, D.; Fediaevski, L.D.P.; Clermont, C.V.; Daumerie, C. Graves’ orbitopathy: Diagnosis and treatment. Ann. D’endocrinol. 2018, 79, 656–664. [Google Scholar] [CrossRef]
- Hodgson, N.M.; Rajaii, F. Current Understanding of the Progression and Management of Thyroid Associated Orbitopathy: A Systematic Review. Ophthalmol. Ther. 2019, 9, 21–33. [Google Scholar] [CrossRef]
- Perez-Moreiras, J.V.; Gomez-Reino, J.J.; Maneiro, J.R.; Perez-Pampin, E.; Lopez, A.R.; Alvarez, F.M.R.; Laguarta, J.M.C.; Cabello, A.D.E.; Sorroche, M.G.; Gregori, E.E.; et al. Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 195, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, R.A.; Bussey, M.R.; Shayesteh, Y.; Jay, W.M. Rituximab in the Treatment of Thyroid Eye Disease: A Review. Neuro-Ophthalmol. 2015, 39, 109–115. [Google Scholar] [CrossRef]
- Stan, M.; Garrity, J.A.; Leon, B.G.C.; Prabin, T.; Bradley, E.A.; Bahn, R.S. Randomized Controlled Trial of Rituximab in Patients With Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2015, 100, 432–441. [Google Scholar] [CrossRef]
- Markham, A. Teprotumumab: First Approval. Drugs 2020, 80, 509–512. [Google Scholar] [CrossRef]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef]
- Chen, H.; Mester, T.; Raychaudhuri, N.; Kauh, C.Y.; Gupta, S.; Smith, T.; Douglas, R.S. Teprotumumab, an IGF-1R Blocking Monoclonal Antibody Inhibits TSH and IGF-1 Action in Fibrocytes. J. Clin. Endocrinol. Metab. 2014, 99, E1635–E1640. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marino, M.; Vaidya, B.; Wiersinga, W.M. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 1, G43–G67. [Google Scholar]
- Cai, S.; Yang, Q.; Hou, M.; Han, Q.; Zhang, H.; Wang, J.; Qi, C.; Bo, Q.; Ru, Y.; Yang, W.; et al. Alpha-Melanocyte-Stimulating Hormone Protects Early Diabetic Retina from Blood-Retinal Barrier Breakdown and Vascular Leakage via MC4R. Cell Physiol. Biochem. 2018, 45, 505–522. [Google Scholar] [CrossRef]
- Taylor, A.W.; Lee, D. Applications of the Role of α-MSH in Ocular Immune Privilege. Adv. Exp. Med. Biol. 2010, 681, 143–149. [Google Scholar]
- Shiratori, K.; Ohgami, K.; Ilieva, I.B.; Koyama, Y.; Yoshida, K.; Ohno, S. Inhibition of Endotoxin-Induced Uveitis and Potentiation of Cyclooxygenase-2 Protein Expression by α-Melanocyte–Stimulating Hormone. Investig. Opthalmol. Vis. Sci. 2004, 45, 159–164. [Google Scholar] [CrossRef][Green Version]
- Naveh, N.; Kaplan-Messas, A.; Marshall, J. Mechanism related to reduction of intraocular pressure by melanocortins in rabbits. Br. J. Ophthalmol. 2000, 84, 1411–1414. [Google Scholar] [CrossRef][Green Version]
- Rinne, P.; Lyytikäinen, L.-P.; Raitoharju, E.; Kadiri, J.J.; Kholova, I.; Kähönen, M.; Lehtimäki, T.; Oksala, N. Pro-opiomelanocortin and its Processing Enzymes Associate with Plaque Stability in Human Atherosclerosis–Tampere Vascular Study. Sci. Rep. 2018, 8, 15078. [Google Scholar] [CrossRef]
- Raffin-Sanson, M.L.; De Keyzer, Y.; Bertagna, X. Proopiomelanocortin, a polypeptide precursor with multiple functions: From physiology to pathological conditions. Eur. J. Endocrinol. 2003, 149, 79–90. [Google Scholar] [CrossRef]
- Garfield, A.S.; Lam, D.D.; Marston, O.J.; Przydzial, M.J.; Heisler, L.K. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol. Metab. 2009, 20, 203–215. [Google Scholar] [CrossRef]
- Wang, W.; Guo, D.-Y.; Lin, Y.-J.; Tao, Y.-X. Melanocortin Regulation of Inflammation. Front. Endocrinol. 2019, 10, 683. [Google Scholar] [CrossRef]
- Luger, T.A.; Scholzen, T.E.; Brzoska, T.; Böhm, M. New Insights into the Functions of α-MSH and Related Peptides in the Immune System. Ann. N. Y. Acad. Sci. 2003, 994, 133–140. [Google Scholar] [CrossRef]
- Tsai, H.-E.; Liu, G.-S.; Kung, M.-L.; Liu, L.-F.; Wu, J.-C.; Tang, C.-H.; Huang, C.-H.; Chen, S.-C.; Lam, H.-C.; Wu, C.-S.; et al. Downregulation of Hepatoma-Derived Growth Factor Contributes to Retarded Lung Metastasis via Inhibition of Epithelial–Mesenchymal Transition by Systemic POMC Gene Delivery in Melanoma. Mol. Cancer Ther. 2013, 12, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Shi, Z.X.; Levy, A.; Lightman, S.L. Thyroid hormone-mediated regulation of corticotropin-releasing hormone messenger ribonucleic acid in the rat. Endocrinology 1994, 134, 1577–1580. [Google Scholar] [CrossRef] [PubMed]
- Rittenhouse, P.A.; Redei, E. Thyroxine Administration Prevents Streptococcal Cell Wall-Induced Inflammatory Responses. Endocrinology 1997, 138, 1434–1439. [Google Scholar] [CrossRef]
- Kamilaris, T.C.; Debold, C.R.; Johnson, E.O.; Mamalaki, E.; Listwak, S.J.; Calogero, A.E.; Kalogeras, K.T.; Gold, P.W.; Orth, D.N. Effects of Short and Long Duration Hypothyroidism and Hyperthyroidism on the Plasma Adrenocorticotropin and Corticosterone Responses to Ovine Corticotropin-Releasing Hormone in Rats. Endocrinology 1991, 128, 2567–2576. [Google Scholar] [CrossRef]
- Ceccatelli, S.; Giardino, L.; Calzá, L. Response of Hypothalamic Peptide mRNAs to Thyroidectomy. Neuroendocrinology 1992, 56, 694–703. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, H.J.; Choi, S.H.; Chang, E.-J.; Lee, S.Y.; Lee, E.J. Quercetin Inhibits IL-1β-Induced Inflammation, Hyaluronan Production and Adipogenesis in Orbital Fibroblasts from Graves’ Orbitopathy. PLoS ONE 2011, 6, e26261. [Google Scholar] [CrossRef]
- Paik, J.-S.; Cho, W.-K.; Oh, E.-H.; Lee, S.-B.; Yang, S.-W. Palmitate induced secretion of IL-6 and MCP-1 in orbital fibroblasts derived from patients with thyroid-associated ophthalmopathy. Mol. Vis. 2012, 18, 1467–1477. [Google Scholar]
- Pilling, D.; Fan, T.; Huang, D.; Kaul, B.; Gomer, R. Identification of Markers that Distinguish Monocyte-Derived Fibrocytes from Monocytes, Macrophages, and Fibroblasts. PLoS ONE 2009, 4, e7475. [Google Scholar] [CrossRef]
- Woeller, C.F.; Roztocil, E.; Hammond, C.; Feldon, S.; Phipps, R. The Aryl Hydrocarbon Receptor and Its Ligands Inhibit Myofibroblast Formation and Activation: Implications for Thyroid Eye Disease. Am. J. Pathol. 2016, 186, 3189–3202. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.-W.; Tsai, P.-J.; Tai, M.-H.; Bee, Y.-S. Therapeutic Effect of α-MSH in Primary Cultured Orbital Fibroblasts Obtained from Patients with Thyroid Eye Disease. Int. J. Mol. Sci. 2021, 22, 11225. https://doi.org/10.3390/ijms222011225
Cheng P-W, Tsai P-J, Tai M-H, Bee Y-S. Therapeutic Effect of α-MSH in Primary Cultured Orbital Fibroblasts Obtained from Patients with Thyroid Eye Disease. International Journal of Molecular Sciences. 2021; 22(20):11225. https://doi.org/10.3390/ijms222011225
Chicago/Turabian StyleCheng, Pei-Wen, Pei-Jhen Tsai, Ming-Hong Tai, and Youn-Shen Bee. 2021. "Therapeutic Effect of α-MSH in Primary Cultured Orbital Fibroblasts Obtained from Patients with Thyroid Eye Disease" International Journal of Molecular Sciences 22, no. 20: 11225. https://doi.org/10.3390/ijms222011225
APA StyleCheng, P.-W., Tsai, P.-J., Tai, M.-H., & Bee, Y.-S. (2021). Therapeutic Effect of α-MSH in Primary Cultured Orbital Fibroblasts Obtained from Patients with Thyroid Eye Disease. International Journal of Molecular Sciences, 22(20), 11225. https://doi.org/10.3390/ijms222011225