Identification of Tumor Antigens in Ovarian Cancers Using Local and Circulating Tumor-Specific Antibodies
Abstract
:1. Introduction
2. Results
2.1. Antibody Reactivity Profiles of ASC probes against Ovarian Cancer Cell Line
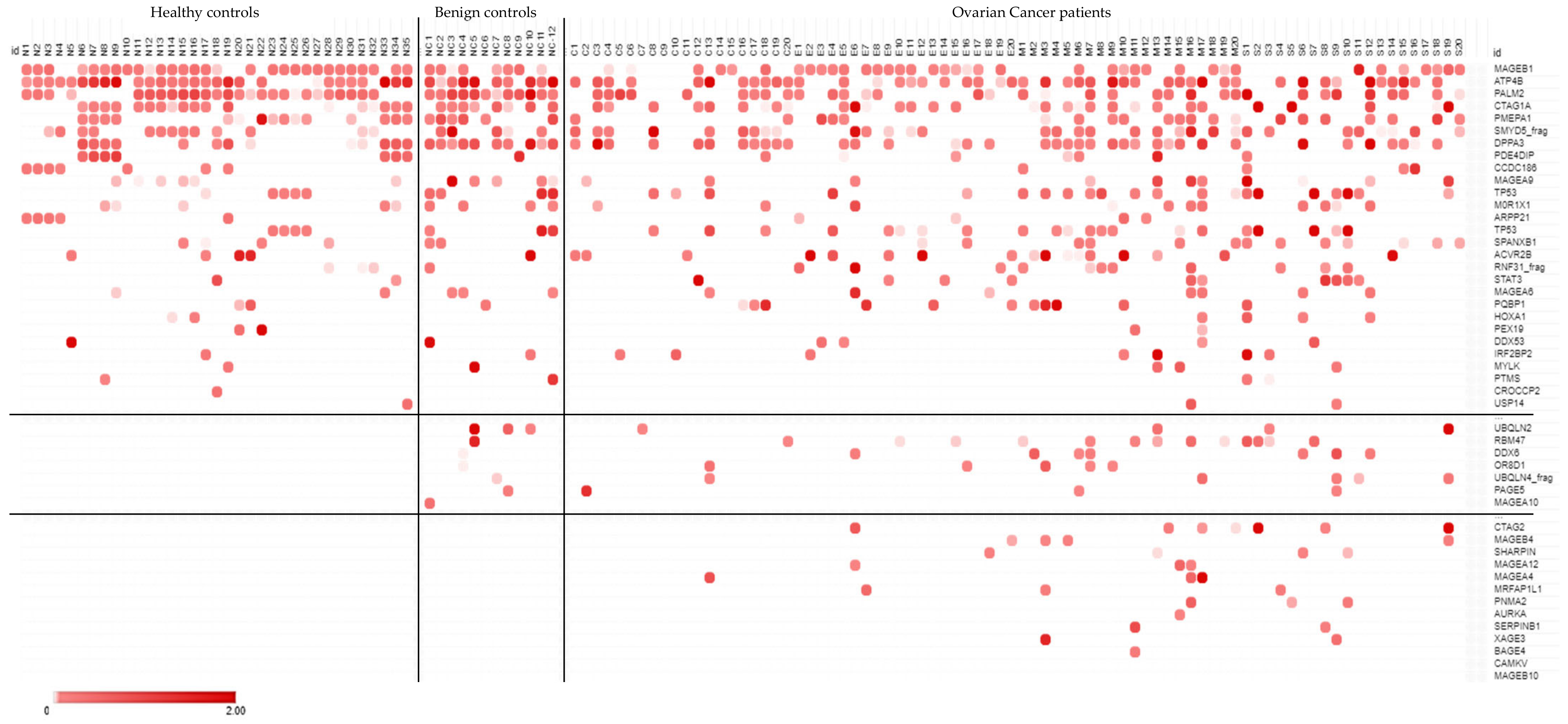
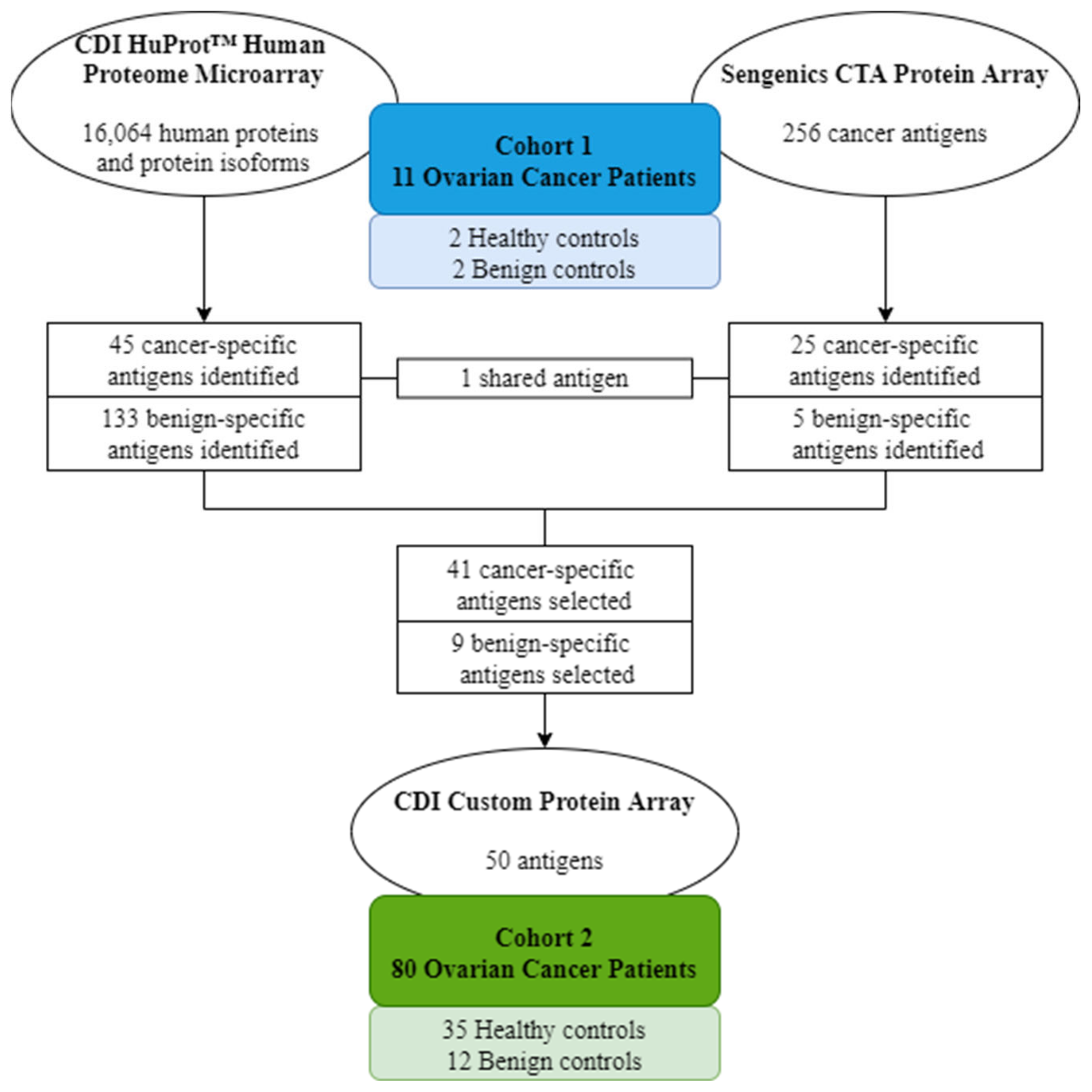
2.2. Candidate Antigen Selection Using High-Density Protein Microarrays
2.3. Diagnostic Screen Using a Custom Protein Microarray
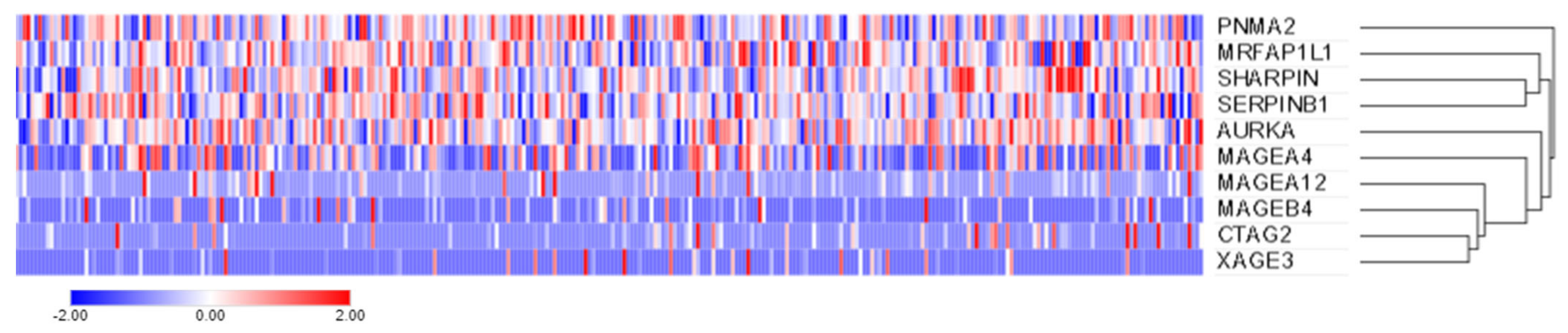
2.4. Candidate Gene Expression Using the Cancer Genome Atlas (TCGA)
3. Discussion
4. Materials and Methods
4.1. Patient Cohorts
4.2. Generation of ASC Probes and IgG Quantification
4.3. Western Blotting
4.4. Antibody Profiling Using Protein Microarrays
4.5. Gene Expression Profiling Using the Cancer Genome Atlas (TCGA)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, T.I.; Toups, K.L.; Saggese, D.A.; Kalli, K.R.; Cliby, W.A.; Muddiman, D.C. Epithelial Ovarian Cancer: Disease Etiology, Treatment, Detection, and Investigational Gene, Metabolite, and Protein Biomarkers. J. Proteome Res. 2007, 6, 2936–2962. [Google Scholar] [CrossRef]
- Bast, R.C.; Matulonis, U.A.; Sood, A.K.; Ahmed, A.A.; Amobi, A.E.; Balkwill, F.R.; Wielgos-Bonvallet, M.; Bowtell, D.D.L.; Brenton, J.D.; Brugge, J.S.; et al. Critical Questions in Ovarian Cancer Research and Treatment: Report of an American Association for Cancer Research Special Conference. Cancer 2019, 125, 1963–1972. [Google Scholar] [CrossRef]
- Ferraro, S.; Braga, F.; Lanzoni, M.; Boracchi, P.; Biganzoli, E.M.; Panteghini, M. Serum Human Epididymis Protein 4 Vs Carbohydrate Antigen 125 for Ovarian Cancer Diagnosis: A Systematic Review. J. Clin. Pathol. 2013, 66, 273–281. [Google Scholar] [CrossRef]
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian Carcinoma Subtypes are Different Diseases: Implications for Biomarker Studies. PLoS Med. 2008, 5, 1749–1760. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking Ovarian Cancer: Recommendations for Improving Outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toss, A.; Tomasello, C.; Razzaboni, E.; Contu, G.; Grandi, G.; Cagnacci, A.; Schilder, R.J.; Cortesi, L. Hereditary Ovarian Cancer: Not only BRCA 1 and 2 Genes. Biomed Res. Int. 2015, 2015, 341723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, E.; Lim, E.; Mathivanan, S. Secreted Tumor Antigens—Immune Biomarkers for Diagnosis and Therapy. Proteomics 2017, 17, 1600442. [Google Scholar] [CrossRef] [PubMed]
- Da Gama Duarte, J.; Peyper, J.M.; Blackburn, J.M. B Cells and Antibody Production in Melanoma. Mamm. Genome 2018, 3, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Gentry-Maharaj, A.; Simmons, A.; Ryan, A.; Fourkala, E.O.; Lu, Z.; Baggerly, K.A.; Zhao, Y.; Lu, K.H.; Bowtell, D.; et al. Elevation of TP53 Autoantibody before CA125 in Preclinical Invasive Epithelial Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5912–5922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladd, J.J.; Chao, T.; Johnson, M.M.; Qiu, J.; Chin, A.; Israel, R.; Pitteri, S.J.; Mao, J.; Wu, M.; Amon, L.M.; et al. Autoantibody Signatures Involving Glycolysis and Splicesome Proteins Precede a Diagnosis of Breast Cancer Among Postmenopausal Women. Cancer Res. 2013, 73, 1502–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaenker, P.; Gray, E.S.; Ziman, M.R. Autoantibody Production in Cancer-The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun. Rev. 2016, 15, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Rossi, J.F.; Lu, Z.Y.; Massart, C.; Levon, K. Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer. Front. Immunol. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Da Gama Duarte, J.; Parakh, S.; Andrews, M.C.; Woods, K.; Pasam, A.; Tutuka, C.; Ostrouska, S.; Blackburn, J.M.; Behren, A.; Cebon, J. Autoantibodies May Predict Immune-Related Toxicity: Results from a Phase I Study of Intralesional Bacillus Calmette-Guérin followed by Ipilimumab in Patients with Advanced Metastatic Melanoma. Front. Immunol. 2018, 9, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowen, M.F.; Giles, K.M.; Simpson, D.; Tchack, J.; Zhou, H.; Moran, U.; Dawood, Z.; Pavlick, A.C.; Hu, S.; Wilson, M.A.; et al. Baseline Antibody Profiles Predict Toxicity in Melanoma Patients Treated with Immune Checkpoint Inhibitors. J. Transl. Med. 2018, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Meeusen, E.N.T.; Brandon, M.R. Antibody Secreting Cells as Specific Probes for Antigen Identification. J. Immunol. Methods 1994, 172, 71–76. [Google Scholar] [CrossRef]
- McWilliam, H.E.G.; Driguez, P.; Piedrafita, D.; Maupin, K.A.; Haab, B.B.; McManus, D.P.; Meeusen, E.N.T. The Developing Schistosome Worms Elicit Distinct Immune Responses in Different Tissue Regions. Immunol. Cell Biol. 2013, 91, 477–485. [Google Scholar] [CrossRef]
- Young, A.R.; Duarte, J.D.G.; Coulson, R.; O’Brien, M.; Deb, S.; Lopata, A.; Behren, A.; Mathivanan, S.; Lim, E.; Meeusen, E.; et al. Immunoprofiling of Breast Cancer Antigens Using Antibodies Derived from Local Lymph Nodes. Cancers 2019, 11, 682. [Google Scholar] [CrossRef] [Green Version]
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-specific B Cell Responses in Patients with Head and Neck Cancer. Nature 2020, 597, 274–278. [Google Scholar] [CrossRef]
- Duarte, J.G.; Blackburn, J.M. Advances in the Development of Human Protein Microarrays. Expert Rev. Proteom. 2017, 14, 627–641. [Google Scholar] [CrossRef]
- Chatterjee, M.; Tainsky, M.A. Autoantibodies as Biomarkers for Ovarian Cancer. Cancer Biomark. 2010, 8, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Coronella-Wood, J.A.; Hersh, E.M. Naturally Occurring B-Cell Responses to Breast Cancer. Cancer Immunol. Immunother. 2003, 52, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Shokat, K.M.; Goodnow, C.C. Antigen-Induced B-Cell Death and Elimination During Germinal-Centre Immune Responses. Nature 1995, 375, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating Cell Lines as Tumour Models by Comparison of Genomic Profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.R.; Pero, S.C.; Voss, W.N.; Shukla, G.S.; Sun, Y.; Schaetzle, S.; Lee, C.; Horton, A.P.; Harlow, S.; Gollihar, J.; et al. Identification of Tumor-Reactive B Cells and Systemic Igg in Breast Cancer Based on Clonal Frequency in the Sentinel Lymph Node. Cancer Immunol. Immunother. 2018, 67, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Cramer, D.W.; Sibani, S.; Wallstrom, G.; Wong, J.; Park, J.; Qiu, J.; Vitonis, A.; Labaer, J. Autoantibody Signature for the Serologic Detection of Ovarian Cancer. J. Proteome Res. 2015, 14, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Katchman, B.A.; Chowell, D.; Wallstrom, G.; Vitonis, A.F.; LaBaer, J.; Cramer, D.W.; Anderson, K.S. Autoantibody Biomarkers for the Detection of Serous Ovarian Cancer. Gynecol. Oncol. 2017, 146, 129–136. [Google Scholar] [CrossRef]
- Xie, K.; Fu, C.; Wang, S.; Xu, H.; Liu, S.; Shao, Y.; Gong, Z.; Wu, X.; Xu, B.; Han, J.; et al. Cancer-Testis Antigens in Ovarian Cancer: Implication for Biomarkers and Therapeutic Targets. J. Ovarian Res. 2019, 12, 1. [Google Scholar] [CrossRef]
- Yakirevich, E.; Sabo, E.; Lavie, O.; Mazareb, S.; Spagnoli, G.C.; Resnick, M.B. Expression of the MAGE-A4 and NY-ESO-1 Cancer-Testis Antigens in Serous Ovarian Neoplasms. Clin. Cancer Res. 2003, 9, 6453–6460. [Google Scholar]
- Daudi, S.; Eng, K.H.; Mhawech-Fauceglia, P.; Morrison, C.; Miliotto, A.; Beck, A.; Matsuzaki, J.; Tsuji, T.; Groman, A.; Gnjatic, S.; et al. Expression and Immune Responses to MAGE Antigens Predict Survival in Epithelial Ovarian Cancer. PLoS ONE 2014, 9, e104099. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, H.; Yamada, A.; Matsumoto, H.; Ito, M.; Ushijima, K.; Nishida, T.; Yakushiji, M.; Itoh, K. Serum MAGE-4 Protein in Ovarian Cancer Patients. Gynecol. Oncol. 2000, 76, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Odunsi, K.; Jungbluth, A.A.; Stockert, E.; Qian, F.; Gnjatic, S.; Tammela, J.; Intengan, M.; Beck, A.; Keitz, B.; Santiago, D.; et al. NY-ESO-1 and LAGE-1 Cancer-Testis Antigens are Potential Targets for Immunotherapy in Epithelial Ovarian Cancer. Cancer Res. 2003, 63, 6076–6083. [Google Scholar]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
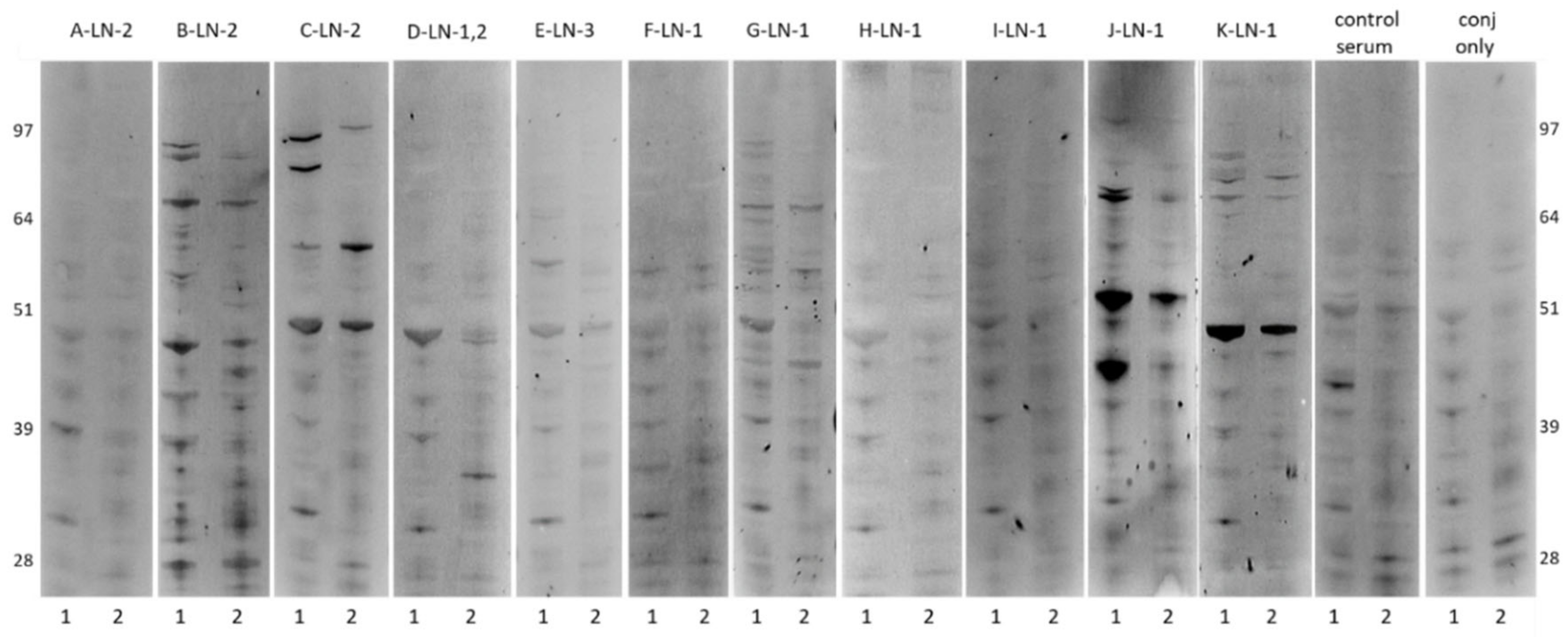
| Patient LN/µg/mL | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LN1 | 0.14 | 0.12 | 15.00 | 0.28 | 0.24 | 0.18 | 0.45 | 0.26 | 0.47 | 1.05 | 0.39 |
| LN2 | 0.28 | 0.34 | 0.58 | 0.65 | 0.04 | 0.04 | 0.06 | ||||
| LN3 | 0.88 | 0.33 | 0.14 |
| ASC Probes | No. of Cancer-Specific Hits (RFU > 6000) | Top Hits (RFU > 6000) | Serum | No. of Positive Hits (Z-Score > 3) | No. of Cancer-Specific Hits (Z-Score > 3) | Shared ASC Probe and Serum Cancer-Specific Hits |
|---|---|---|---|---|---|---|
| A—LN2 | 3 | PLEKHA8 PEX19 PNMA2 | - | - | - | - |
| B—LN2 | 0 | - | B | 100 | 52 | - |
| C—LN3 | 9 | M0R1X1 IRF2BP2 USP5 PDE4DIP GTF2I EVC MFSD5 SLC25A22 GAD1 | C | 134 | 92 | IRF2BP2 USP5 PDE4DIP GTF2I GAD1 |
| D—LN1,2 | 5 | ATP4B PTMS GAGE10 SNX33 SLC25A22 | - | - | - | - |
| E—LN2 | 4 | OR8D1 ZIC2 PAGE5 CAMKV | - | - | - | - |
| F—LN1 | 3 | PLEKHA8 MAGEA4 SLC25A22 | - | - | - | - |
| G—LN1 | 8 | MAGEA4 STAT3 MAGEB10 MAGEA10 DDX6 MAGEA12 SERPINB1 MAGEA6 | G | 95 | 65 | MAGEA4 STAT3 MAGEB10 MAGEA10 DDX6 MAGEA12 |
| H—LN1 | 1 | MYLK | - | - | - | - |
| I—LN1 | 0 | - | - | - | - | - |
| J—LN1 | 13 | TP53 UBQLN4_frag UBQLN2 SHARPIN RBM47 RNF31_frag HOXA1 CCDC97 ZFYVE19 UBQLN1 RBCK1 DDX53 KCTD18 | J | 105 | 84 | TP53 UBQLN4_frag UBQLN2 SHARPIN RBM47 RNF31_frag HOXA1 ZFYVE19 UBQLN1 RBCK1 DDX53 KCTD18 |
| K—LN1 | 4 | PQBP1 MRFAP1L1 ARPP21 DDX53 | K | 136 | 86 | PQBP1 MRFAP1L1 ARPP21 DDX53 |
| ASC Probes | No. of Cancer-Specific Hits (Z-Score > 3) | Top Hits (Z-Score > 3) | Serum | No. of Cancer-Specific Hits (Z-Score > 3) | Top Hits (Z-Score > 3) | Shared ASC Probe and Serum Cancer-Specific Hits |
|---|---|---|---|---|---|---|
| A—LN2 | 2 | BAGE4 DSCR8/MMA1 | - | - | - | - |
| B—LN2 | 2 | BAGE4 DSCR8/MMA1 | B | 1 | SPANXN4 | 0 |
| C—LN1 | 4 | BAGE4 CTNNB1 DMRTC2 DSCR8/MMA1 | C | 1 | ACVR2A | 0 |
| C—LN2 | 6 | MAGEA4v3 MAGEB1 MAGEB2 OIP5 PRM2 XAGE3aV1 | ||||
| C—LN3 | 1 | DSCR8/MMA1 | ||||
| D—LN1,2 | 2 | BAGE3 p53L344P | - | - | - | - |
| E—LN2 | 2 | DSCR8/MMA1 MAGEA4v3 | - | - | - | - |
| E—LN3 | 4 | DMRTC2 DSCR8/MMA1 GRWD1 MAGEB4 | ||||
| F—LN1 | 4 | BAGE3 CCNA1 DSCR8/MMA1 MAGEA4v3 | - | - | - | - |
| G—LN1 | 1 | MAGEA4v2 | G | 2 | MAGEA10 MAGEA4v2 | MAGEA4v2 |
| H—LN1 | 2 | BAGE4 DSCR8/MMA1 | - | - | - | - |
| I—LN1 | 3 | BAGE4 DSCR8/MMA1 MAGEA4v3 | - | - | - | - |
| J—LN1 | 9 | p53C141Y p53K382R p53M133T p53S15A p53S392A p53S46A p53S6A p53T18A TP53 | J | 8 | p53K382R p53M133T p53S15A p53S392A p53S46A p53S6A p53T18A TP53 | p53K382R p53M133T p53S15A p53S392A p53S46A p53S6A p53T18A TP53 |
| K—LN1 | 1 | BAGE4 | K | 1 | CTAG2 | 0 |
| Cohort 1 (n = 11) | Cohort 2 (n = 80) | |
|---|---|---|
| Sample types | Pelvic lymph nodes, serum | Serum |
| Age—years | ||
| Median | 52 | 54 |
| Range | 31–69 | 23–83 |
| BMI—no. | ||
| Median | - | 24 |
| Range | - | 18–41 |
| Ethnicity—no. (%) | ||
| Asian | 11 (100.0) | 24 (30.0) |
| Caucasian | - | 56 (70.0) |
| Risk Factors—no. (%) | ||
| Smoking | - | 6 (7.5) |
| Familial cancers | - | 21 (26.3) |
| Hypertension | - | 22 (27.5) |
| Diabetes mellitus | - | 3 (3.8) |
| Stage—no. (%) | ||
| I | 3 (27.3) | 49 (61.2) |
| II | 1 (9.1) | 11 (13.8) |
| III | 7 (63.6) | 18 (22.5) |
| IV | 0 (0.0) | 2 (2.5) |
| Ovarian Cancer Subtype—no. (%) | ||
| Clear cell | 2 (18.2) | 20 (25.0) |
| Endometrioid | 0 (0.0) | 20 (25.0) |
| Mucinous | 2 (18.2) | 20 (25.0) |
| Serous | 5 (45.4) | 20 (25.0) |
| Mixed | 2 (18.2) | 0 (0.0) |
| CA-125 levels—U/mL | ||
| Median | 441 | 317 |
| Range | 5–16695 | 7–3088 |
| Healthy Cohort (n = 35) | Benign Conditions Cohort (n = 12) | |
|---|---|---|
| Sample types | Serum | Serum |
| Age—years | ||
| Median | 50 | 50 |
| Range | 36–61 | 36–71 |
| BMI—no. | ||
| Median | 26 | - |
| Range | 22–30 | - |
| Ethnicity—no. (%) | ||
| Asian | 0 (0.0) | 12 (100.0) |
| Caucasian | 35 (100.0) | 0 (0.0) |
| Benign conditions—no. (%) | ||
| Endometriosis | - | 2 (16.7) |
| Adenomas | - | 4 (33.3) |
| Myomas | - | 2 (16.7) |
| Fibromas | 1 (8.3) | |
| Mucinous borderline tumor | - | 3 (25.0) |
| CA-125—U/mL | ||
| Median | - | 25 |
| Range | - | 4–65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Gama Duarte, J.; Quigley, L.T.; Young, A.R.; Hayashi, M.; Miyazawa, M.; Lopata, A.; Mancuso, N.; Mikami, M.; Behren, A.; Meeusen, E. Identification of Tumor Antigens in Ovarian Cancers Using Local and Circulating Tumor-Specific Antibodies. Int. J. Mol. Sci. 2021, 22, 11220. https://doi.org/10.3390/ijms222011220
Da Gama Duarte J, Quigley LT, Young AR, Hayashi M, Miyazawa M, Lopata A, Mancuso N, Mikami M, Behren A, Meeusen E. Identification of Tumor Antigens in Ovarian Cancers Using Local and Circulating Tumor-Specific Antibodies. International Journal of Molecular Sciences. 2021; 22(20):11220. https://doi.org/10.3390/ijms222011220
Chicago/Turabian StyleDa Gama Duarte, Jessica, Luke T. Quigley, Anna Rachel Young, Masaru Hayashi, Mariko Miyazawa, Alex Lopata, Nunzio Mancuso, Mikio Mikami, Andreas Behren, and Els Meeusen. 2021. "Identification of Tumor Antigens in Ovarian Cancers Using Local and Circulating Tumor-Specific Antibodies" International Journal of Molecular Sciences 22, no. 20: 11220. https://doi.org/10.3390/ijms222011220
APA StyleDa Gama Duarte, J., Quigley, L. T., Young, A. R., Hayashi, M., Miyazawa, M., Lopata, A., Mancuso, N., Mikami, M., Behren, A., & Meeusen, E. (2021). Identification of Tumor Antigens in Ovarian Cancers Using Local and Circulating Tumor-Specific Antibodies. International Journal of Molecular Sciences, 22(20), 11220. https://doi.org/10.3390/ijms222011220








