1. Introduction
Various lesions in the oral cavity have been related to the Human Papilloma Virus (HPV) infection: verruca vulgaris (VV), squamous cell papilloma (SP), condyloma acuminatum (CA), and multifocal epithelial hyperplasia (MFEH) [
1]. All of them are a benign hyperplastic exophytic proliferation of the oral epithelium [
2], caused by different HPV genotypes. Subtypes 6 and 11, with a low-oncogenic risk, are the most commonly found and cause CA in both the oral cavity [
2] and in the anogenital region [
3]. Labial mucosa, soft palate and lingual frenum are the most common locations of CA [
4] and koilocytes can be observed in histopathologic sections [
5]. All HPV-related oral lesions present clinical similarities, and therefore, a biopsy is necessary for a precise diagnosis.
Although CA is considered a benign lesion, clinical infections with the high-risk genotypes 16 and 18 have been found to cause oral and genital CA and have been associated with malignant lesions [
6]. Spontaneous remission of oral CA is possible [
5], but if this is not the case, there are different treatments to eliminate it. Surgical therapy seems to be the preferred treatment over Trichloroacetic acid (TCA), cryotherapy, and CO
2 laser because these methods often induce artifactual changes that compromise the diagnostic capabilities of the pathologist [
1]. Surgical or ablation treatments have the advantage in that the lesion (s) are removed in a single session and, typically, are quick interventions. However, they are procedures that generally require anesthesia and the control of pain and inflammation. The possibility of recurrences should not be ruled out. In addition to lesions caused by HPV, there are a variety of conditions and diseases of the oral cavity requiring surgical, ablative, or extractive interventions that involve mild to severe pain and inflammation, such as certain tumors of the oral mucosa, temporomandibular joint pathologies, facial trauma, and which could require dental interventions, etc. Pain management in minor surgical and ablative treatments does not always attract the attention it deserves, even though it is crucial for patient satisfaction in that they feel well and are mightily encouraged to follow treatment adherence. Surgery and ablative techniques usually require prior local anesthesia, and the postoperative pain and inflammation should also be controlled. For pain management of these processes, analgesics, anesthetics, and anti-inflammatory drugs can be combined in various regimens.
Ketorolac is a non-steroidal anti-inflammatory drug (NSAID) with a potent analgesic effect and a moderate anti-inflammatory action. It is indicated to treat moderate to severe pain. The analgesic ketorolac potency has been equated to that of several opioids [
7] without presenting the problems associated with these drugs, such as tolerance or sedation [
8]. Its use both pre [
9] and postoperatively [
10] has been analyzed, showing successful results. Ketorolac is marketed as tromethamine salt and can be administered orally, intramuscularly, intravenously, and by nasal or ophthalmic processes. Several authors have studied the analgesic safety and efficacy of ketorolac tromethamine (KT) after its topical application in different mucoadhesive formulations on the oral mucosa [
11,
12]. The results were satisfactory and promising. Therefore, we are able to propose its use during the removal of oral condyloma.
Mucoadhesive topical formulations have advantages over the most common routes, such as a simple and painless application and a better bioavailability of the active ingredient, allowing formulations with lower doses and inducing fewer side effects [
13]. However, when formulating drugs intended to be applied to the oral mucosa, certain aspects need to be considered and may limit the success of our formula. One of them is the biology and histology of the mucosa. The lining of the oral cavity includes the buccal (cheeks), sublingual, gingival, palatal, and labial mucosa. These are made up of closely compacted epithelial cells, which help fulfill the mucosa’s primary function: to protect the underlying tissues from external agents and fluid loss [
14]. The drug to be designed must be able to cross the mucosal barrier. There are different factors to consider, such as the tissue’s permeability, the drug’s molecular weight, the partition coefficient (octanol/water) log P, and all aspects that are related to the formulation: the release capacity of the drug from the vehicle to tissue, pH, and the formulation’s biocompatibility with the target tissue.
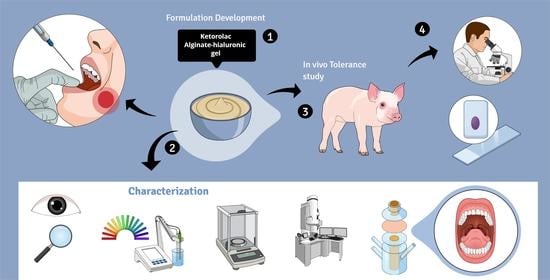
In this work, we designed and formulated a 2% ketorolac tromethamine hydrogel composed of sodium alginate as a polymer to be applied to the buccal and sublingual mucosae to treat pain and inflammation before, during, and after surgical, ablative, or extractive procedures. In order to reduce damage to the mucous membranes, hyaluronic acid (HA) was incorporated into the formulation, taking advantage of its well-known and well-documented regenerative and moisturizing action, as well as its role in strengthening cell resistance to mechanical damage [
15]. In addition, HA also has gelling properties, which are excellent for the type of formulation to be made. High and low molecular weight HA have been used. The low-molecular-weight HA can penetrate to slightly deeper layers and there it acts regeneratively, while the high-molecular-weight HA acts at a more superficial level. The physicochemical, mechanical, and morphological characteristics of the gel have been analyzed, and the biopharmaceutical properties were examined by ex vivo permeation tests and in vivo administration.
The results show that alginate-HA hydrogel with 2% KT is a promising formulation for combating pain and inflammation, the two common side effects of surgical, ablative, and extractive treatments of the oral cavity.
3. Discussion
We elaborated a 2% ketorolac tromethamine hydrogel composed of sodium alginate as a polymer to be applied to the buccal and sublingual mucosae with the aim of treating pain and inflammation before, during, and after surgical, ablative, or extractive procedures in the oral cavity. The hyaluronic acid was incorporated into the formulation because of its well-known regenerative, moisturizing and strengthening properties [
15]. Both, low and high molecular weight hyaluronic acids have been used. The low-molecular-weight HA can penetrate to slightly deeper layers, and there, it can act regeneratively, while the high-molecular-weight HA acts at a more superficial level [
26].
The physicochemical, mechanical, and morphological characteristics of the gel have been analyzed. The pH was within the normal intraoral pH range (6.8–7.8) [
16], thus no disruptions, neither the biota nor the functions of saliva in the oral cavity, are expected. The alginate-HA gel showed to be hygroscopic in nature since it is able to uptake 15-fold its weigh in solvent, and their components can disperse in the medium relatively quickly compared to other polymer-based hydrogels. Mallandrich et al. studied the degradation of a 2% carbopol hydrogel, which required 24 h to be thoroughly degraded [
27].
When formulating gels, determining the extensibility is crucial to ensure that the formulation is pleasant to use and has a comfortable application. That is why very high (very fluid) or very low (very viscous) extensibility should be avoided. The patient’s compliance will be affected by the sensory feeling of the formulation. Inoue and co-workers investigated the correlation between the physical properties of different formulations and the sensory feeling [
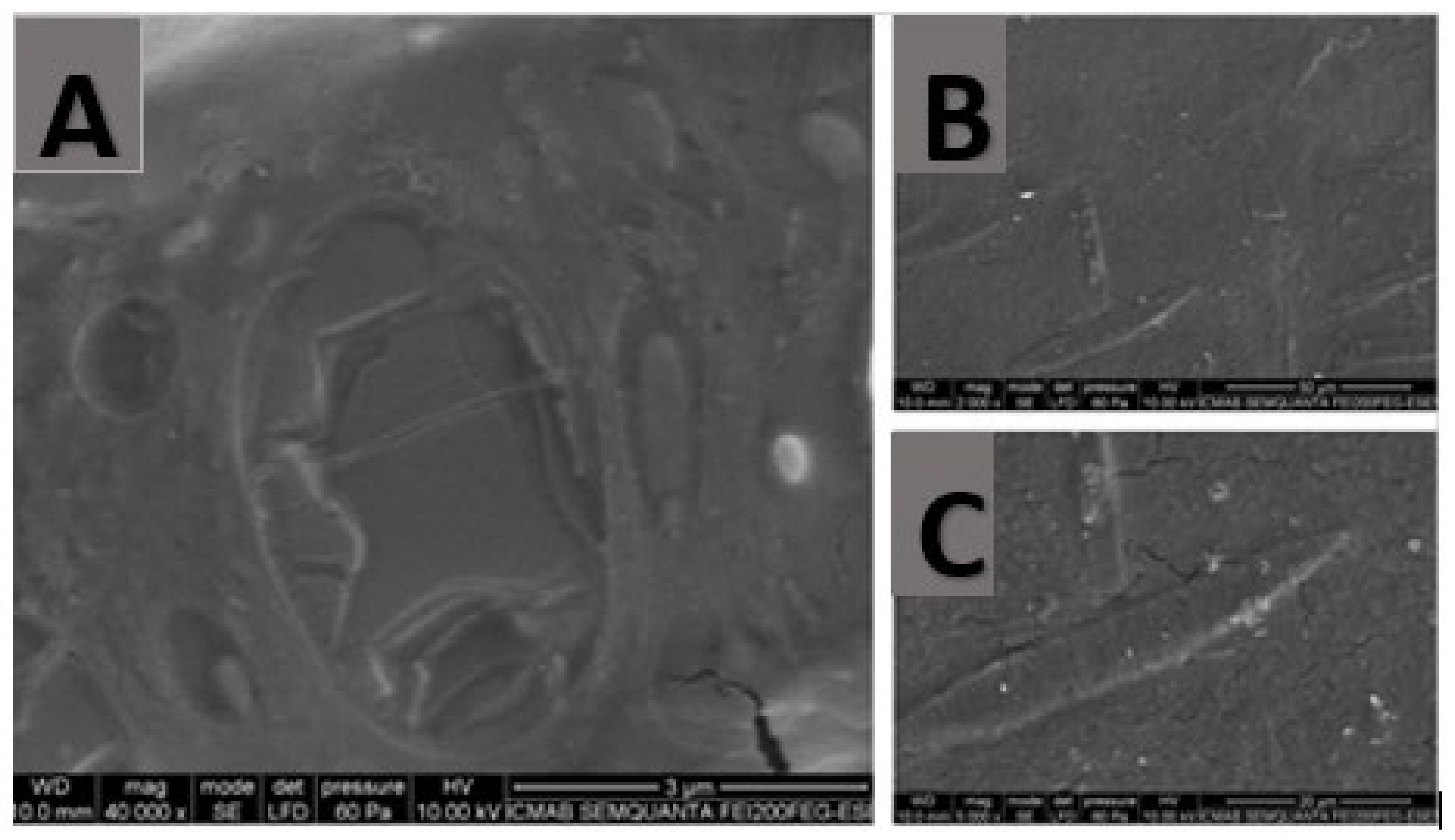
28]. This means that rheological studies are essential to evaluate the galenic features and the suitability of a formulation. Despite the compact structure of the alginate-HA 2% KT gel observed by SEM, the gel exhibited good extensibility and an ideal rheological behavior for the indication of the hydrogel. Pseudoplastic behavior is interesting because it allows a smooth and easy extension application by dabbing without high pressure and, therefore, painlessly. Furthermore, thixotropy also displays interesting behavior in semi-solid products because the formulation’s change in structure results in fluidization that facilitates the product’s application. This is an interesting outcome since the mucous membranes are already sensitive tissues per se and even more so after a surgical, ablative, or extractive intervention.
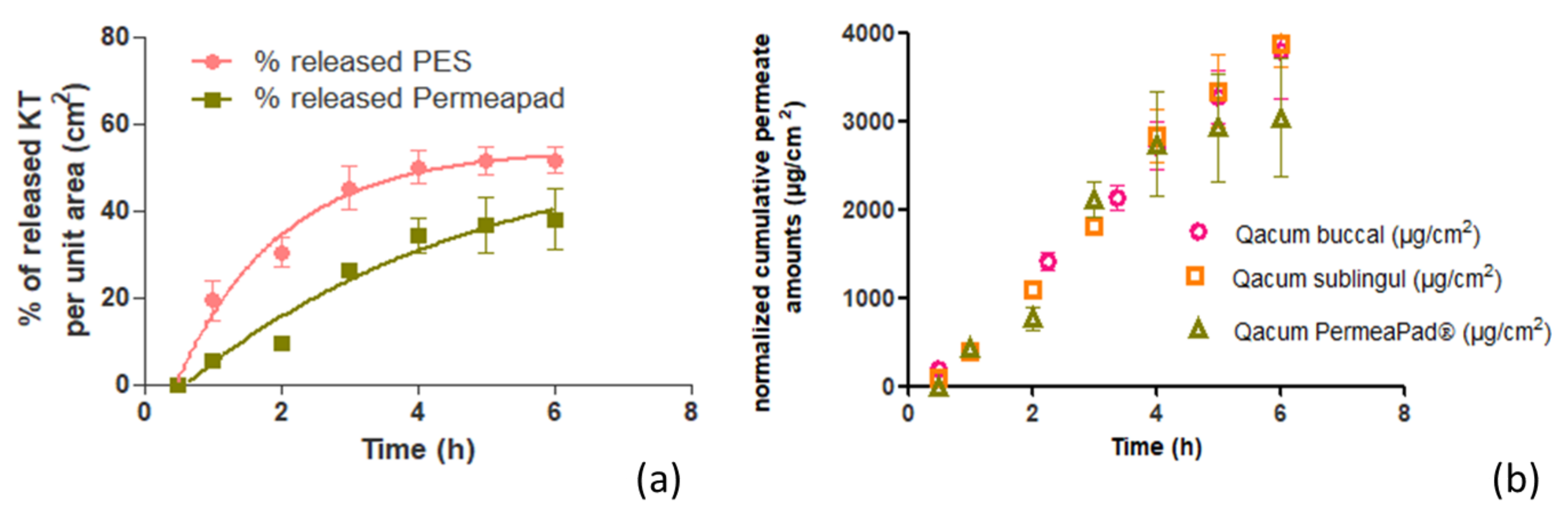
The biomimetic membrane PermeaPad
® was tested and compared to the buccal and sublingual mucosae. It was observed that the biomimetic membrane correlated well with both mucosae. These results are in agreement with other researchers’ work. Bibi et al. [
19] investigated the use of PermeaPad
® as a predictor in the buccal absorption of Metoprolol solution. The authors compared the apparent permeability obtained with PermeaPad
® to the previous works performed by other authors, which evaluated the apparent permeability of metroprolol solution in cell culture, in ex porcine buccal mucosa and, finally, in in vivo studies conducted on minipigs. Bibi et al. found good in vitro–in vivo correlation between PermeaPad
® and all the three systems evaluated.
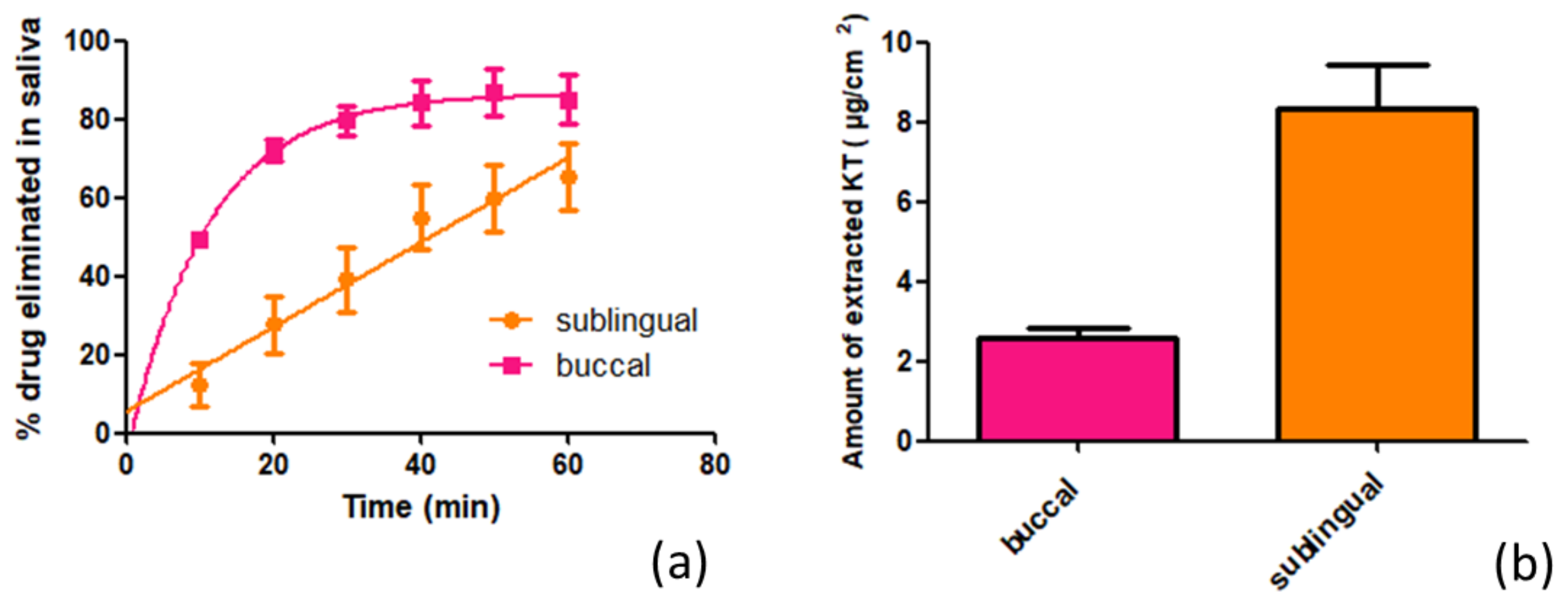
Ketorolac tromethamine rapidly diffuses across the mucous membranes of the oral cavity, especially through the buccal mucosa. Under infinite doses and an exposure time of 6 h, ketorolac tromethamine would achieve therapeutic plasma concentrations. Nevertheless, the impact of saliva on drug elimination should not be disregarded, since the main drawback in buccal delivery is that the patient may swallow part of the applied dose before the drug is absorbed, even if it has been released [
29]. The in vitro test showed that saliva dragged more than 60% of Ketorolac in 1 h, which is swallowed and follows on from an oral intake. Even despite the saliva’s effect, Ketorolac tromethamine was able to penetrate both mucosae. The alginate-HA-hydrogel was formulated with Ketorolac in the tromethamine salt since this is more hydrophilic and allows it to be better integrated into the hydrogel. However, it is to be expected that once the gel is applied to the mucous membranes, the KT changes and, due to the environment in which it is found, protonates and/or ionizes and that, during the process, the different forms coexist until reaching a balance. These changes will affect the physicochemical properties of the drug, such as its solubility in saliva or the value of the log P partition coefficient, thus modifying its tissue affinity [
18,
30]. Given that the alginate-HA-hydrogel was formulated with the tromethamine salt, it is likely that this was the predominant form at the beginning of the study when applying the gel on the mucosa and therefore that the process of dissolving KT in saliva was favored. It should not be forgotten, however, that the KT of the formulation is simultaneously being absorbed through the mucosa. This absorption depends on different factors. On one hand, the mucosa histology, and on the other, the KT physicochemical properties. Taking into account that both the buccal and sublingual mucosae consist of a non-keratinized stratified squamous epithelium and that the main difference is the thickness of the said epithelium (the sublingual being between 100 and 200 μm, 8–12 cells thick, and between 500 and 800 μm, 40–50 cells thick, the buccal [
24]), it is logical to think that the sublingual mucosa presents less resistance to the passage of KT. From the beginning, the amount of KT that is eliminated by the action of the saliva when the gel is applied on this mucosa is lower compared to when the gel is applied on the buccal mucosa. The kinetic dissolution profile is the result of the sum of these two processes. Through the buccal mucosa, it is observed how at the beginning, the KT dissolution in saliva predominates until after about 30 min, when a balance is reached between what is dissolved and what is absorbed by the mucosa. This behavior adjusts to first-order kinetics. On the other hand, in the sublingual mucosa, by presenting less resistance to the passage of KT through its structure, the dissolution and absorption processes are balanced from the beginning, and this therefore describes zero-order dissolution kinetics. These results show how, apart from the drug physicochemical properties and the mucosa physiological characteristics, other factors such as salivation play a significant role in the bioavailability of KT since, in both mucosae, much more than half of the applied dose was eliminated by the action of saliva, which can be explained, in part, by the high-water solubility of KT, which is eliminated from the mucosa by the action of saliva and ends up being ingested. It is therefore considered that the KT is administered orally. In contrast, the amount that is retained in the mucosa is responsible for the local analgesic and anti-inflammatory action.
Thus, it is observed that the study in live animals and under a finite dose regimen does not reach systemic concentrations of KT. Besides the lower dose, this is probably due to the effect of saliva, as demonstrated in the in vitro study, which can influence by reducing the time that the alginate-HA gel is in contact with the mucous membranes and consequently with the amount of KT that could be permeated. It should be noted that the present study was carried out by administering a single drug dose in order to minimize the test time as much as possible and, therefore, the stress that could be caused to the animals. Other studies applying the alginate-HA gel in a multiple-dose regimen are necessary to analyze the behavior of the hydrogel more accurately in terms of bioavailability and permeability through the mucous membranes of the oral cavity.
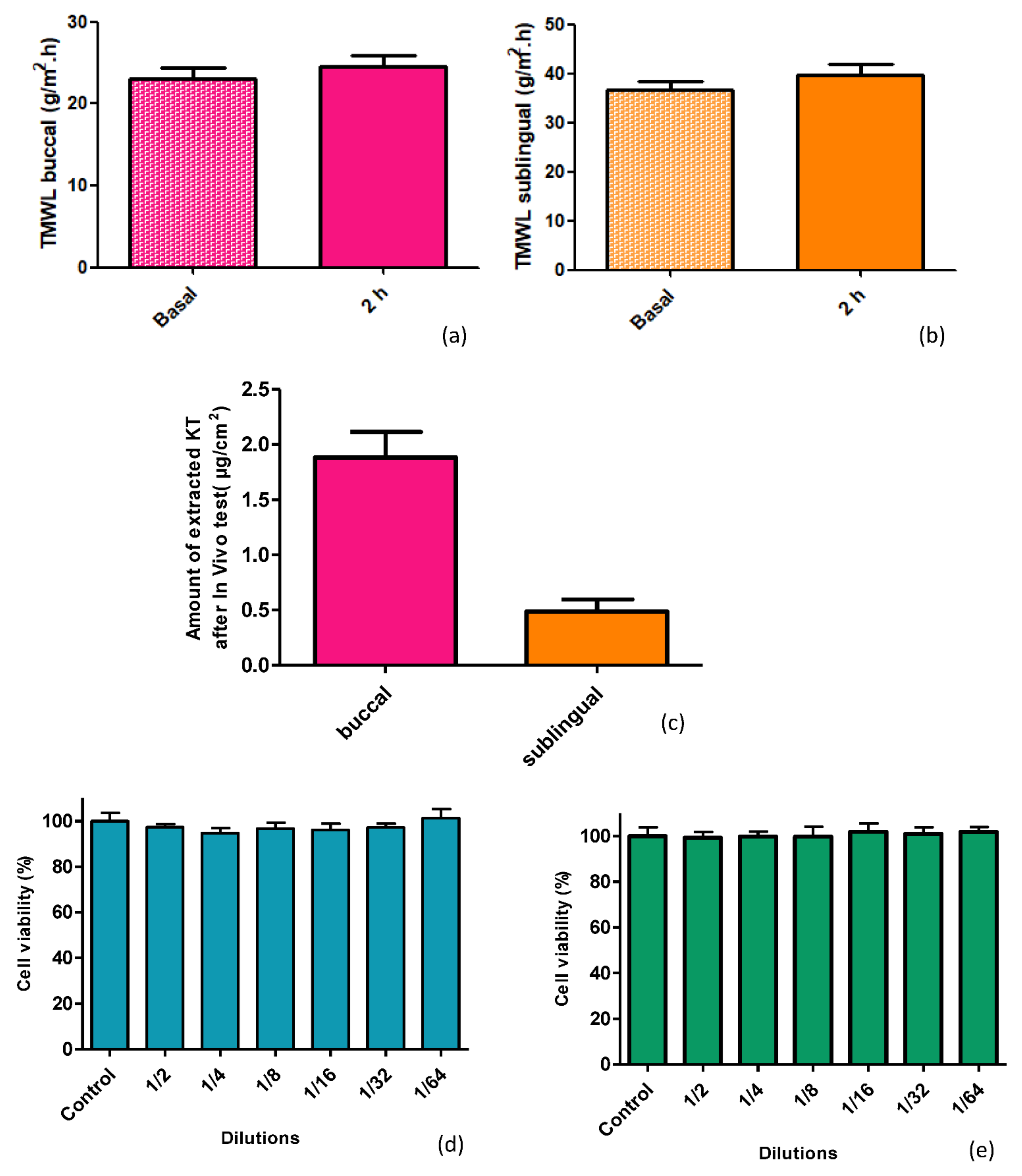
When mucosae are damaged, their barrier functions are impaired, resulting in higher water loss [
31]. This water loss can be measured by the transmucosal water loss (TMWL) method, which is well established in dermatology and used to assess the integrity of the mucosa barrier in vivo [
32]. In the TMWL measurement, the water density gradient that evaporates through the tissue is indirectly measured by placing the measuring device perpendicular to the site of interest and reaching a stable TMWL reading in about 60 s. Before exposing the mucosa to alginate-HA hydrogel, the basal TMWL value was measured. The formulation was then applied to the mucosae, and after 2 h, the TMWL value was measured again. The TMWL values obtained, both basal and 2 h post-application, (around 30 g/m
2·h for the buccal mucosa and around 40 g/m
2·h for the sublingual mucosa) show the excellent condition of the mucosa since both have values close to 30 g/m
2·h, which is considered acceptable for the integrity of the oral mucosa [
32]. Thus, the alginate-HA 2% KT does not cause mucosae disruption, being well-tolerated by the target tissues: the histological analysis revealed no differences between the treated and the untreated mucosae. Additionally, the cell viability showed that the alginate-HA 2% KT does not cause cytotoxicity in Caco-2 cells.
4. Materials and Methods
4.1. Materials
4.1.1. Reagents
Sodium alginate was purchased from Fagron Iberica (Terrassa, Spain). Ketorolac tromethamine and Nipagin were obtained from Sigma-Aldrich (Barcelona, Spain). Nipasol was acquired from Acofarma (Barcelona, Spain), Hyaluronic acids were obtained from Fagron Iberica (Terrassa, Spain); Na2HPO4 and KH2PO4 were supplied by Panreac (Barcelona, Spain), NaCl and KCl were from Merck (Darmstadt, Germany), CaCl from Ferosa (Spain) Hepes was obtained from Fagron Iberica (Terrassa, Spain) and glucose from Sigma-Aldrich (Barcelona, Spain). The Millipore Express® PLUS 0.45 µm PES Membrane was from Merck (Darmstadt, Germany), the Nylon membrane Filter with 0.45 µm pore size from Teknokroma (Barcelona, Spain) and the biomimetic membrane PermeaPad® from InnoME GmbH (Espelkamp, Germany).
The purified water was obtained from a Milli-Q1 Gradient A10 system apparatus (Millipore Iberica S.A.U., Madrid, Spain). All the other chemicals and reagents used in the study were of analytical grade.
4.1.2. Tissues for Ex Vivo Assays
The Bellvitge animal facility services provided the buccal and sublingual mucosae (Landrace Large White race). The Ethics Committee of Animal Experimentation of the University of Barcelona approved the Study Protocol (approved on 10 January 2019). A thickness of 500 µm was dermatomized (GA630, Aesculap, Tuttlingen, Germany) to carry out the test.
4.2. Preparation of the Sodium Alginate and Hyaluronic Acid Hydrogel
Drug-loaded hydrogel was prepared at laboratory scale with a KT concentration of 2% w/v. A concentration of 0.5% w/v of high molecular and 0.2% low molecular weight HA was used. Sodium alginate concentration was established at 4% w/v. Firstly, preservatives (nipagin and nipasol at 0.05% and 0.02%, respectively) and KT were added to purified water under continuous stirring. Separately, both HA were dispersed in ethanol (corresponding to a 5% of the final composition); then, the KT-preservatives solution was poured into the HA mixture, and finally, alginate was added gradually to avoid the formation of lumps. Then, the formulation was allowed to rest for 24 h at room temperature.
4.3. Gel Characterization
The alginate gel containing 2% ketorolac was characterized in terms of appearance, pH, extensibility, swelling and degradation ratio and rheological behavior.
4.3.1. Swelling and Degradation Tests
The swelling and degradation tests were carried out according to the methodology described by S. El Moussaoui et al. [
18]. The swelling test evaluates the hydrogel’s capacity to absorb water within its structure. First, the hydrogels were dehydrated at 40 °C until constant weight. The dehydrated hydrogels were immersed in PBS (pH 7.4) at room temperature for 14 min. The hydrogels were removed from the PBS at predetermined intervals (every 2 min). The excess PBS was removed, and the amount of captured PBS was weighed. The experiment was performed in triplicate. The swelling ratio (SR) was calculated using the following equation:
where
Wd is the weight of the dried hydrogel, and
Ws is the weight of the swollen hydrogel at different times.
The degradation test aims to monitor weight loss (WL) as a function of time. The WL was calculated by incubating known amounts of fresh hydrogel (3.017 g) in PBS (pH = 7.4) at 37 °C for 115 min. Three replicates of hydrogel were removed, blotted, and weighed at the following times: 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 55, 65, 75, 85, 100, and 115 min. The weight loss was expressed as the percentage of weight loss concerning the freshly prepared hydrogel. It was calculated based on Equation (2):
where
Wi is the initial weight of hydrogel and
Wd the weight of hydrogel at different times.
4.3.2. Extensibility
The extensibility was determined in triplicate at room temperature. The formulation was placed between two crystal platforms. Several weights (0, 2, 5, 10, and 20 g) were placed on the top platform for 2 min. The diameters (cm
2) of the circles which spread out were measured and recorded. The extensibility was calculated from the equation:
where
Ext = extensibility;
d = average diameter of the extended formulation.
4.3.3. Rheological Profile
The rheological properties of the Alginate-HA hydrogel containing 2% KT were determined by a rotational Haake RheoStress 1 rheometer (Thermo Fisher Scientific, Karlsruhe, Germany) equipped with cone-plate geometry (Haake C60/2° Ti, 60 mm diameter, 0.105 mm gap between cone-plate). Measurements were performed in duplicate at 25 °C (Thermo Haake Phoenix II + Haake C25P). An oscillatory study was carried out, for which a stress sweep of 0 to 500 Pa, at 1 Hz, was first performed to determine the linear viscolastic zone. For the following test, the shear was set at a value between 0.4 and 100 Pa (linear zone), and 10 Pa was chosen to perform the Frequency Sweep test, which allows how the product behaves at low and high frequencies to be determined and recorded.
For the rotational study, the program was adjusted to the following conditions: ramp-up from 0 to 15.0 s
−1 for 3 min, constant shear rate at 15.0 s
−1 for 1 min, and ramp-down from 15.0 to 0 s
−1 for 3 min. The flow data obtained were fitted to different mathematical models (
Table A2) to describe the flow curve and characterize the flow properties.
4.3.4. Morphological Study
In order to examine the hydrogel structure, Scanning Electron Microscopy (SEM) was carried out in a JSM-7100F (JEOL Inc., Peabody, MA, USA). The sample was coated with a thin layer of carbon in an Emitech K950 coater (Quorum Technologies Ltd., Kent, UK).
4.4. In Vitro Release Assay on Membranes and Biomimetic Membranes
The in vitro release profile was assessed by vertical Franz diffusion cells (FDC 400, Crown Glass, Somerville, NY, USA) with an active diffusion area of 1.77 cm2. Hank’s solution pH (7.02) was used as receptor fluid, which was thermostated at 37 ± 1 °C. The stirring rate was set at 500 rpm, and sink conditions were held throughout the experiments. A total of 400 mg ± 10 mg of KT hydrogel was accurately applied to the membranes.
The membranes used were PermeaPad
®, and polyethersulfone (PES)—the selection of PES membrane was based on a previous study in which a solution of Ketorolac tromethamine was tested through Nylon and PES membranes. Samples of 300 μL from the receiver compartment were extracted over pre-established times (0.5, 1, 2, 3, 4, 5, and 6 h) and replaced with an equal volume of fresh solution. The KT content was analyzed by a validated HPLC method, described in
Section 4.8.
The experimental data (cumulative amount of KT per cm2) were fitted to different mathematical models (zero and first-order kinetics) so as to choose the best fitting model according to the correlation coefficient (r2) value.
4.5. Ex Vivo Transmucosal Permeation Assay
The ex vivo permeation tests were conducted on porcine oral mucosa from the pigs’ cheeks and sublingual tissues to evaluate the ability of KT to permeate through the mucosal membranes. The mucosae were dermatomed at a thickness of 500 µm.
The assays were performed on Franz diffusion cells with a diffusional area of 0.64 cm
2. They were conducted in the same conditions as in the release assay (
Section 4.4), adjusting the sampling times to 1, 2, 3, 4, 5, and 6 h. Samples were analyzed with the same HPLC method. From the permeated amounts analyzed by HPLC, the permeation parameters were calculated according to the equations described in
Appendix A (
Table A3).
4.5.1. Amount of Ketorolac Retained in the Mucosa
Once the permeation assay was finished, the KT retained in the mucosa was extracted. To do this effectively, the residual hydrogel on the mucosa was removed with a swab and cleaned with a gauze soaked in 0.05% solution of sodium lauryl sulfate and rinsed three times with distilled water. The permeation area of the mucosa was cut, weighed, perforated by a thin needle, and incubated with 1 mL of methanol:water (1:1) solution and sonicated for 20 min. The supernatants were analyzed by the HPLC method.
4.5.2. In Vitro—Ex Vivo Correlation
To determine if the PermeaPad® biomimetic membrane can be comparable to the buccal and sublingual mucosae, the ex vivo permeation results and parameters were compared with the release test results performed on the PermeaPad® membrane, and to achieve this, a one-way ANOVA statistical study was carried out.
Furthermore, the correlation between the amounts of KT permeated through PermeaPad® vs. through buccal and sublingual mucosae at each time was calculated with the help of GraphPad software.
4.6. In Vitro Study of the Influence of Saliva on Drug Elimination
To simulate the influence of saliva on the KT elimination once the hydrogel had been applied to the mucosa of the oral cavity, the buccal and sublingual mucosae were cut to an area of 2.83 cm
2 and placed vertically on a support point. A certain amount of KT hydrogel was carefully placed on the mucosa. Using a peristaltic pump, artificial saliva (
Table A4) was passed over the mucosa at a 0.24 mL/min flow rate. The study lasted 1 h, taking samples of the artificial saliva that dropped every 10 min. The collected samples were analyzed by HPLC to determine the amount of KT that had been eliminated by the action of saliva, and the amount of KT that had been retained in the mucosa was determined as described in
Section 4.5.1.
The KT extraction retained in the mucosa after the in vitro simulation of the influence of saliva on the elimination of KT was carried out according to
Section 4.5.1.
4.7. In Vivo Study and Analysis of Tolerance through Histology
With the aim of assessing the tolerability of the alginate-HA gel, the formulation was applied to female pigs (Yorkshire-Landrace) of 45–50 kg. Transmucosal water loss (TMWL) (TEWL-Dermalab) was measured at basal conditions (before applying the formulation) by placing the measuring device perpendicular to the site of interest and reaching a stable TMWL reading in about 60 s. Four pigs were used. Two had the hydrogel applied to the buccal mucosa and two to the sublingual mucosa. The hydrogel was applied on the mucosa covering the most accessible area to facilitate the process and cause the least possible stress to the animals. After two hours, the TMWL value was measured again, and a blood sample was taken to determine whether, by means of buccal and/or sublingual application, therapeutic systemic levels could be reached. Then, all animals were euthanized, and the mucosa tissues were obtained immediately afterwards. The tissues from two treated animals were fixed by immersing them overnight in 4% paraformaldehyde in phosphate-buffered 20 mM, pH 7.4. Then the tissues were processed so as to embed them in paraffin, and vertical histological sections were cut to be stained with hematoxylin and eosin and observed under a Leica DMD 108 optical microscope. The tissues from the two remaining treated animals underwent the extraction procedure for determining the Ketorolac retained in the mucosa, according to
Section 4.5.1. The study protocol was approved by the Animal Experimentation Ethics Committee of the University of Barcelona (code 10619, 10 January 2019).
4.8. Cytotoxicity
Cell viability was assessed by the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. This method is based on the mitochondrial reduction of tetrazolium to formazan which is directly proportional to the viable cell numbers. To achieve this, 1 × 104 Caco-2 cells in 100 μL of DMEM medium without phenol red were plated into each well in a 96-well plate and further incubated for five days at 37 °C before the addition of the compounds under study. The volume of each well was set to 0.1 mL, with eight duplicate wells for each specific sample under study. After 24 h, the cells were treated with 0.25% MTT (Sigma-Aldrich) in PBS and allowed to react for 2 h at 37 °C. The supernatant was then removed, and 0.1 mL of dimethyl sulfoxide (DMSO) was added (Applichem, Ecogen, Barcelona, Spain) to each well to fully dissolve the formazan produced by the living cell. For Cell viability determination, the optical density (OD) was measured at a wavelength of 570 nm in a Modulus™ Microplate Photometer (Turner BioSystems, Madrid, Spain). The results were expressed as percentage of cell survival relative to the control (untreated cells).
4.9. Analytical Method for the Determination of Ketorolac Tromethamine
To quantify the KT concentration described in previous sections, a HPLC method was used. The chromatographic conditions were the following: the column used was YMC-Pack Pro C18 (25 cm, 4.6 mm and 5 μm). The mobile phase consisted of acetonitrile (+0.065% triethylamine) and purified water (+0.165% acetic glacial acid), in an isocratic elution (1:1) at flux 1 mL/minute. The volume injected was 10 µL, and Ketorolac was determined at the wavelength of 314 nm. The standard range for the calibration line was from 0.39 to 300 μg/mL. Data were collected and processed using Empower Pro software (Walters, Milford, CT, USA).
4.10. Statistical Analysis
GraphPad Prism®, v. 5.00 software (San Diego, CA, USA), was used for all statistical calculations. If data followed a normal distribution, a t-test or an ANOVA (if more than two groups were being compared) was applied. If data followed a non-normal distribution, a Mann-Whitney (for two group comparison) or Kruskal-Wallis test (for more than two groups) was applied. The significance level was 0.05 in all cases.
5. Conclusions
The objective of this study was to comprehensively characterize a hydrogel based on sodium alginate and high and low molecular weight hyaluronic acid formulated with 2% ketorolac tromethamine. Organoleptic, morphological, and rheological studies showed the suitability of the formulation to be an excellent and easy topical application of the hydrogel on the mucosa of the oral cavity.
The release studies demonstrated the remarkable capacity of KT to be released from the alginate-HA hydrogel, releasing 51.59% of the drug per cm2 in 6 h.
Ex vivo permeation studies demonstrated good oral and sublingual mucosal patency for KT and predicted systemic steady-state concentrations within the therapeutic range. Furthermore, when comparing the results of both mucosae, no statistically significant differences were observed.
An additional positive finding was that comparative studies of the PermeaPad® biomimetic membrane with the buccal and sublingual mucosae showed an excellent correlation but a significantly lower drug retention capacity.
Through in vitro simulation, the influence of saliva on the bioavailability of the drug was observed. It was shown how in one hour, artificial saliva at a constant flow of 0.24 mL/min was capable of eliminating more than half of the initially applied dose on the mucous membranes, which would end up being swallowed and considered as oral administration.
In in vivo studies with pigs and under a finite regime dose, it was not possible to quantify the systemic concentrations of the drug, but the amounts of KT retained in both mucosae showed the feasibility of the gel to provide an analgesic and anti-inflammatory locally, which is very useful in surgical and/or ablative processes such as the elimination of papillomatous lesions, treatment of certain oral carcinomas, dental extractions, etc. Further studies are needed in a multi-dose regimen to characterize the hydrogel’s behavior better, and this would be more realistic since, in practice, a single application of the formulation would not be sufficient to obtain the desired effect.
Finally, the histological, cytotoxicity study and the measured TMWL values demonstrated the safety and innocuousness of the formulation, not showing any damage or alterations to the mucosal tissues.