Abstract
Paracetamol is commonly used to treat fever and pain in pregnant women, but there are growing concerns that this may cause attention deficit hyperactivity disorder and autism spectrum disorder in the offspring. A growing number of epidemiological studies suggests that relative risks for these disorders increase by an average of about 25% following intrauterine paracetamol exposure. The data analyzed point to a dose–effect relationship but cannot fully account for unmeasured confounders, notably indication and genetic transmission. Only few experimental investigations have addressed this issue. Altered behavior has been demonstrated in offspring of paracetamol-gavaged pregnant rats, and paracetamol given at or prior to day 10 of life to newborn mice resulted in altered locomotor activity in response to a novel home environment in adulthood and blunted the analgesic effect of paracetamol given to adult animals. The molecular mechanisms that might mediate these effects are unknown. Paracetamol has diverse pharmacologic actions. It reduces prostaglandin formation via competitive inhibition of the peroxidase moiety of prostaglandin H2 synthase, while its metabolite N-arachidonoyl-phenolamine activates transient vanilloid-subtype 1 receptors and interferes with cannabinoid receptor signaling. The metabolite N-acetyl-p-benzo-quinone-imine, which is pivotal for liver damage after overdosing, exerts oxidative stress and depletes glutathione in the brain already at dosages below the hepatic toxicity threshold. Given the widespread use of paracetamol during pregnancy and the lack of safe alternatives, its impact on the developing brain deserves further investigation.
1. Introduction
Paracetamol (acetaminophen, N-acetyl-para-aminophenol) is among the most popular painkillers used by mothers during pregnancy [1] and by young children worldwide. It is also used to treat fever and has been advocated for pharmacological closure of a patent ductus arteriosus in preterm infants [2,3]. Until recently, paracetamol had been considered safe for use in pregnancy. However, there is mounting (albeit controversial) evidence that it may have long-term negative effects on the offspring when used by pregnant women, increasing the risks for attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). This narrative review presents retrieved data and views which at times are difficult to reconcile but open avenues to further research.
2. Pharmacology of Paracetamol
2.1. Inhibition of Prostaglandin Synthesis
Despite its popularity and use for many years, the safety of its application and its mechanism of action are not fully understood. Paracetamol is a manifold drug, and several complex metabolic pathways are involved in its antipyretic and analgesic action (see schematic overview in Figure 1 and Figure 2). Some of the effects of paracetamol are mediated by reduced prostaglandin formation [4] via competitive inhibition of the peroxidase moiety of prostaglandin-endoperoxide synthase, also called prostaglandin H2 (PG H2) synthase [5,6]. Prostaglandin-endoperoxide synthase is a bifunctional enzyme that consists of a cyclooxygenase (COX) site and a peroxidase site that work in series. The COX site oxidizes arachidonic acid to prostaglandin G2 (PG G2). PG G2 is then rapidly converted by the peroxidase site to PG H2, which goes on to serve as a substrate for several isomerases/synthases that ultimately result in the release of biologically active compounds such as thromboxane A2, prostaglandin I2, or prostaglandin E2 [7]. The COX site can be inhibited by non-steroidal anti-inflammatory drugs such as ibuprofen or indomethacin, while paracetamol acts as a reducing co-substrate of the peroxidase site, lowering the rate of conversion of PG G2 to PG H2 [8].
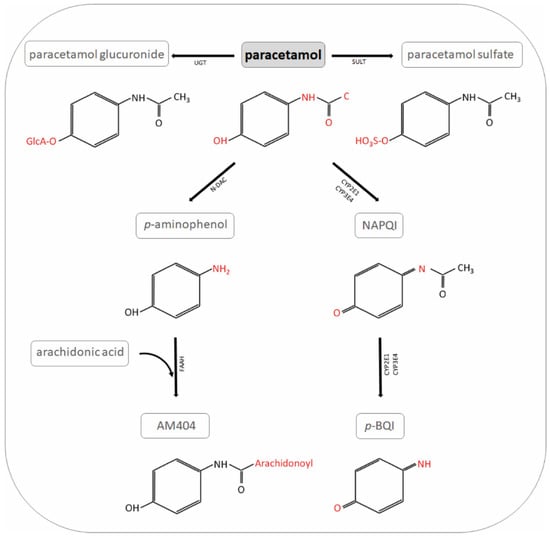
Figure 1.
Paracetamol metabolites. NAPQI: N-acetyl-p-benzo-quinone-imine; UGT: UDP-glucuronyl transferase; SULT: sulfotransferase; N-DAC: N-deacetylase; GST: glutathione S-transferase; p-BQI: p-benzoquinone; FAAH: fatty acid amide hydrolase; AM404: N-arachidonoyl-phenolamine.
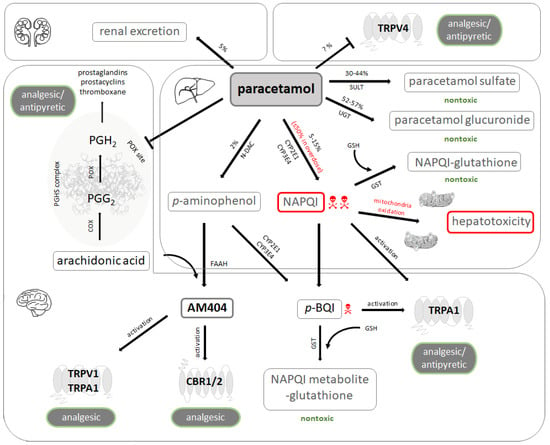
Figure 2.
Paracetamol metabolism and pharmacology. Unchanged paracetamol (acetaminophen) is excreted in the urine to a small extent. It may also act as an antagonist of the vanilloid-subtype 4 receptors (TRPV4) in various tissues. Paracetamol is mainly metabolized in the liver. The metabolites generated by glucuronidation and sulfation are not toxic and subject to urinary excretion, while a minor fraction undergoes oxidative metabolism. This results in formation of N-acetyl-p-benzo-quinone-imine (NAPQI), a highly toxic intermediary produced by cytochrome P450 enzymes. NAPQI builds adducts with mitochondrial proteins and induces oxidative stress, nuclear DNA fragmentation, and subsequent cell necrosis. Paracetamol is becoming de-acetylated to p-aminophenol, which in turn is metabolized by the hepatic microsomal cytochrome P450 enzyme system to the toxic compound p-benzoquinone (p-BQI). Under physiological conditions, detoxification of NAPQI and p-BQI occurs by binding to glutathione (GSH) and subsequent renal excretion. The prostaglandin endoperoxide H synthase (PGHS) complex consists of a cyclooxygenase (COX) and a peroxidase (POX) moiety. Arachidonic acid is first transformed to the unstable prostaglandin G2 (PGG2) by COX, which is further reduced to prostaglandin H2 (PGH2) by POX. PGH2 gives rise to various endogenous regulators such as prostaglandins, prostacyclins, and thromboxane. Paracetamol induces analgesia and antipyresis by blocking prostaglandin synthesis at the POX site of PGHS complex. p-aminophenol undergoes conjugation with arachidonic acid by fatty acid amide hydrolase (FAAH) to N-arachidonoyl-phenolamine (AM404), which is a potent activator of TRPV1 and transient receptor potential ankyrin 1 (TRPA1) as well as a weak agonist of cannabinoid receptors type 1 and 2 (CBR1/2). Activation of these receptors by AM404 may mediate analgesic effects. NAPQI and p-BQI may also produce analgesic and antipyretic effects by activating TRPA1. UGT: UDP-glucuronyl transferase; SULT: sulfotransferase; N-DAC: N-deacetylase; GST: glutathione S-transferase.
There are two prostaglandin-endoperoxide synthase isoenzymes, formerly called COX1 (constitutively expressed) and COX2 (inducible). Paracetamol is a partially selective COX2 inhibitor; concentrations of paracetamol necessary to achieve 50% inhibition of the prostaglandin-endoperoxide synthase activity by the inducible isoenzyme (26 µM) are approximately 25% of that by the constitutively expressed isoenzyme (114 µM) [9]. Paracetamol has little anti-inflammatory effect [10] because it inhibits intracellular prostaglandin-endoperoxide synthase but not molecules released from damaged cells [11]. Of note, COX2 knockout mice display autism-related behavior [12].
2.2. Interaction with Central Receptors Involved in Nociception
An additional mechanism has been proposed to mediate paracetamol-induced central analgesia and lowering of body temperature. While paracetamol itself acts as an antagonist of transient vanilloid-subtype 4 receptor (TRPV4) [13], the paracetamol metabolite N-arachidonoyl-phenolamine (AM404) activates transient vanilloid-subtype 1 receptors (TRPV1) [14] and transient receptor potential ankyrin 1 (TRPA1) [15]. AM404 is generated by de-acetylation of paracetamol to p-amino-phenol (in the liver) and subsequent conjugation with arachidonic acid by the enzyme fatty acid amide hydrolase (FAAH; in brain and spinal cord) [16,17]. AM404 can be detected in cerebrospinal fluid after administration of paracetamol [18] and mediates central analgesia by increasing local concentrations of γ-amino-butyric acid (GABA), glutamate, and endocannabinoids, thereby decreasing the connectivity of cortex, amygdala, hypothalamus, and periaqueductal grey [19]. Synaptic endocannabinoid availability is achieved by AM404-mediated inhibition of the anandamide membrane transporter, while AM404 itself acts as a weak agonist of the cannabinoid receptors type 1 and 2. The paracetamol metabolites N-acetyl-p-benzo-quinone-imine (NAPQI) and p-benzoquinone (p-BQI) generated by the CYP450 isoform CYP2E1 expressed in brain and spinal cord [20] is also a direct stimulator of TRPA1 [21], but this interaction is limited by its short half-life.
2.3. Pharmacokinetics and Toxicology
Paracetamol is mainly excreted following conjugation with glucuronic acid or sulfate. A variable fraction, however, is oxidized in the liver by a number of CYP450 isoforms (CYP2E1, CYP1A2, CYP3A4, and CYP2A6) to NAPQI. NAPQI is a highly reactive compound neutralized by reduced glutathione (GSH), resulting in the generation of L-cysteinyl-S-acetaminophen. If concentrations of NAPQI exceed the available GSH, NAPQI wreaks havoc by covalently binding to thiol groups of various cellular proteins and lipids. The involvement of mitochondria triggers an oxidative stress cascade that leads to accumulation of reactive oxygen species, formation of peroxynitrite, mitochondrial membrane permeability transition, and ultimately cell death [22]. Acute paracetamol toxicity may be antagonized by restoration of GSH stores via early administration of high-dose N-acetyl-cysteine administration [23]. At a later stage, moderate hypothermia to induce RNA-binding motif protein 3 [24] appears to be a promising strategy that is awaiting clinical evaluation.
Metabolization of paracetamol to NAPQI occurs mostly in hepatocytes, and acute liver failure constitutes the principal cause of death following intentional (suicidal) or unintentional (unsupervised prolonged administration) paracetamol overdose. Encephalopathy seen in this situation is therefore attributed to liver failure. However, NAPQI is also generated in the brain by the CYP450 isoform CYP2E1 [20]. As NAPQI is covalently bound to GSH, it depletes GSH in the brain and may aggravate oxidative stress. In rats, cortical neuronal death involving cytochrome c release and caspase 3 activation is induced by paracetamol at doses below those required to produce hepatotoxicity [25].
It is also relevant that paracetamol crosses both the placental barrier and the fetal blood–brain barrier and remains in the bloodstream of the infant for prolonged periods time [26,27], increasing the risks of altered development of the fetal brain.
3. Epidemiological Studies Investigating the Impact of Paracetamol on the Developing Brain
3.1. Paracetamol Use during Pregnancy
Paracetamol has been widely recommended for the treatment of pain and fever in pregnant women, and it is being estimated that about every other pregnant woman resorts to the use of paracetamol during pregnancy. However, there are a number of prospective cohort studies to suggest that intake of paracetamol increases the likelihood of autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) in the offspring (Table 1). The seminal analysis of the Danish National Birth Cohort comprised 64,322 pregnant women who had been recruited between 1996 and 2002 [28] and answered two telephone interviews before (at 12 and 30 weeks of gestation) and a further interview 6 months after delivery. There was a moderately increased risk of physician-diagnosed ADHD, prescription of ADHD medication, or parental reports of ADHD-like behavior at 7 years of age when the child was ever exposed to paracetamol before birth (average hazard ratios [HR] 1.37, 1.29 and 1.13, respectively). HR increased for all three outcome variables when children were exposed to paracetamol for more than 20 weeks (1.84, 1.53 and 1.46). Prenatal paracetamol was also associated with an increased risk of ASD with, but not without, hyperkinetic traits (HR 1.51 and 1.06, respectively) [29]. ASD risks were increased in children whose mothers had taken paracetamol during 3 trimesters (HR 1.77 and 1.25). In a small sub-cohort of 1491 children assessed at 5 years of age by trained psychologists, intrauterine paracetamol exposure was also associated with poorer cognitive [30] and attention scores [31].

Table 1.
Paracetamol intake estimated based on maternal self-report.
A statistically significant association between intrauterine paracetamol exposure, as recalled by mothers, and a diagnosis of ADHD was also observed in the Nurses’ Health Study [33] and the Norwegian Mother and Child Cohort Study [37]. In the Norwegian study, however, no increased likelihood of ADHD was observed when paracetamol had been taken for less than eight days or in only one trimester. Notably, an association also emerged between paternal use of paracetamol and offspring ADHD while maternal paracetamol six months before pregnancy had no effect.
Various other studies have linked intrauterine paracetamol exposure, as recalled and reported by mothers, to results of questionnaires as proxy measures of ADHD [32,34,35,36,38,39,40,41,42,44,45]. Four sequential systematic reviews concluded that the evidence available suggests that the risk of ADHD and ASD is increased following prenatal paracetamol exposure [46,47,48,49].
There are, however, several points of concern [50,51]. First, the questionnaires used have poor internal and external validity, as they were developed as screening instruments rather than diagnostic tools. This adds to the heterogeneity of the results, and some studies using questionnaires indeed failed to detect a significant impact of intrauterine paracetamol [43,44]. Second, ADHD and ASD are partially heritable traits which may go undiagnosed in adults. This source of confounding is difficult to control for in epidemiological studies. In a sample of 7921 genotyped mothers participating in the Avon Longitudinal Study of Parents and Children (ALSPAC) study, maternal polygenic risk scores for ADHD (but not ASD) were slightly but significantly linked both to infections (odds ratio [OR] 1.11) and use of acetaminophen during late pregnancy (OR 1.11) [52]. However, in the Nurses’ Health Study II cohort that included 8856 children (721 with ADHD), only paracetamol use at the time of pregnancy was associated with childhood ADHD (OR 1.34), while there was not effect for paracetamol ingestions during periods 4 years before or 4 years after the pregnancy [33]. Third, fever is one of the leading indications for use of paracetamol, and fever during pregnancy itself has been associated with lower performance intelligence quotients [30], increased risks of ASD [53,54] and ADHD [55]. These associations were similar whether the woman had used paracetamol or not. In separate investigations, maternal infections during pregnancy have been associated with ASD in the offspring [56]. None of the studies accounted for maternal migraine which may be another important confounding indication [57]. Fourth, the epidemiological studies mentioned rely on maternal reports to quantitate paracetamol intake during pregnancy which may lead to exposure misclassification.
The last concern has been addressed by an analysis of public health insurance data from Taiwan [58] and cohort studies measuring perinatal, fetal, or neonatal paracetamol and paracetamol metabolites [59,60,61,62] (Table 2). The first approach reported a weak association between prescription of paracetamol during pregnancy and physician-diagnosed ADHD in the offspring [58]. However, paracetamol may be obtained without prescription, and paracetamol prescribed before pregnancy or to other household members may be taken by a pregnant woman encountering fever or pain. Attempts to measure paracetamol and its metabolites met with the challenge that paracetamol has become an almost universal component of human blood or urine [63]. Unchanged paracetamol was indeed detected in all 140 urine samples provided by mothers participating in the Swedish Environmental Longitudinal, Mother and child, Asthma and allergy study [59]; all 1180 maternal plasma obtained 1–3 after birth of women of the Boston Birth cohort [60]; and in all 996 cord blood samples of infants enrolled in the same cohort [61]. While neither raw nor log-transformed urinary paracetamol concentrations displayed a normal distribution [63], data of the Swedish cohort study demonstrated a linear association between log-transformed urinary paracetamol concentrations and mother-reported paracetamol use during mid-pregnancy [59], as well as a small impact of paracetamol intake (by maternal report or urinary concentration) on language development at 3 years of age. In the Boston Birth cohort, paracetamol burden according to blood samples obtained from mothers and infants was related to physician-diagnosed ADHD [60,61]. Neonatal meconium collected after birth may actually be the best way to capture prolonged intrauterine exposure to paracetamol and other drugs, as it accumulates chemicals from the fetal bile and the fetal urine passed into the amniotic fluid which is ingested by the fetus. In the Canadian Gestation and the Environment Cohort, paracetamol in meconium was unrelated to the children’s intelligence examined at 6–8 years of age [64] but showed a dose–response association with physician-diagnosed ADHD [62]. Each doubling of exposure increased the odds of ADHD by 10% among 345 children analyzed, 199 (57.7%) of whom had detectable paracetamol in meconium, and 33 (9.6%) were diagnosed with ADHD. In a subset of 48 children who underwent resting-state functional magnetic resonance imaging (MRI) at 9–11 years of age, paracetamol detected in meconium was linked to altered brain connectivity between fronto-parietal and default mode network nodes to sensorimotor cortex clusters, mediating an association of intrauterine paracetamol exposure and ADHD [62].

Table 2.
Paracetamol intake measured by perinatal metabolites.
While in 2015 the Food and Drug Administration (FDA) continued to support the use of paracetamol for pain and fever during pregnancy [65], the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency (EMA/PRAC/157165/2019) stated in 2019 that a large amount of data on pregnant women indicated neither malformative nor fetal/neonatal toxicity, while epidemiological studies on neurodevelopment in children exposed to paracetamol in utero showed inconclusive results. It recommended that paracetamol can be used during pregnancy if clinically needed, but it should be used at the lowest effective dose for the shortest possible time and at the lowest possible frequency [66]. The cautious stance of the regulatory authorities in the USA and Europe partially reflects the lack of safe alternatives to treat fever and pain in pregnant women. A very recently published consensus statement supported by 91 scientists, clinicians, and public health professionals recommends to implement specific actions to caution pregnant women at the beginning of pregnancy to forego paracetamol unless its use is medically indicated, to consult with a physician or pharmacist if they are uncertain whether its use is indicated and before using paracetamol on a long-term basis, and to minimize exposure by using the lowest effective dose for the shortest possible time [67].
3.2. Postnatal Use of Paracetamol in Term and Preterm Newborn Infants
Paracetamol and ibuprofen have equal efficacy for the treatment of fever in infants [24], and paracetamol has evolved into a cornerstone of effective pain relief in neonates. Paracetamol allows for reduced dosing of opioids and may be used to effectively treat moderate pain after surgery, while there is insufficient evidence to support its use for painful procedures [68,69]. Paracetamol given after assisted vaginal birth may even increase the response to subsequent painful procedures [70]. Paracetamol has been furthermore advocated for the pharmacological closure of a patent ductus arteriosus in very preterm infants, although its efficacy is not superior to oral ibuprofen [2,3]. Follow-up examinations of preterm infants at 2 and 5 years of age currently do not point to altered neurodevelopmental outcome following postnatal paracetamol administration [71,72,73], while analysis of data from a survey among parents on 1515 US children found an increased risk of ASD following postnatal paracetamol administration before age two in boys but not in girls [74]. As a matter of concern, none of the trials employing paracetamol for closure of a patent ductus arteriosus in preterm infants registered with www.clinicialtrials.gov list ADHD and ASD as secondary outcome.
4. Animal Models
In contrast to the loads of epidemiological studies analyzing the associations between gestational exposure to paracetamol and behavior in offspring, very few investigations have addressed this issue in experimental animals. Injecting pregnant rats day 15–19 of pregnancy with low-dose paracetamol (15 mg/kg body weight) has been shown to result in a large number of genes up- or down-regulated in fetal brains [75], while injecting pregnant mice on day 12.5 of pregnancy with paracetamol at dosages causing acute liver toxicity, as shown by elevated plasma alanine transferase concentrations, has been shown to reduce birth weight, decrease the frequency of hematopoietic stem cells in offspring liver [76], and result in greater severity of airway inflammation in grown-up animals [77], demonstrating long-lasting effects in offspring. However, no behavioral assessment has been reported in these experiments. Offspring of pregnant mice receiving paracetamol at 150 mg/kg/d by gavage from gestational day 7 to delivery did not display altered open field locomotor activity at 30 days of life [78]. However, male mice showed reduced sexual behavior associated with decreased neuronal number in the sexually dimorphic nucleus of the preoptic area [79]. After 350 mg/kg/d, there was impaired nest-seeking behavior, augmented stereotypy, and decreased rostral grooming in male animals, as well as reduced exploratory behavior in three-chamber sociability in both sexes [80,81].
In rodents, developmental phases of brain development that take place during the last trimester of pregnancy in humans are being observed during the first 7–10 days of life, allowing to employ newborn rat or mouse pups to study human fetal intrauterine events [82,83,84]. Administration of paracetamol (30 or 60 mg/kg body weight) to 3- or 10-day-old mice was shown to result in altered locomotor activity in response to a novel home environment and impaired spatial learning in adulthood [85]. Neonatal paracetamol also blunted the analgesic effect of paracetamol given to adult animals. Notably, exposure on day 19 of age had no long-lasting effects [86]. These data point to a critical time window during brain development that corresponds to the last trimester of pregnancy in humans. Male mice exposed on day 10 of life to a single dose of paracetamol (30 mg/kg body weight) did not differ from controls while mice receiving a repeat dose 4 h apart showed altered locomotor and rearing activity when tested as adults [87]. In a separate series of experiments, effects of paracetamol could not be prevented by co-administration of cysteine and mannitol as antioxidants [88]. Low-dose paracetamol (5 or 15 mg/kg/d) during pregnancy, followed by postnatal administration until 60 days of life, also evoked changes in behavior and reduced social interaction of grown-up animals [89].
5. Chronic Exposure to Ultra-Low Concentrations of Paracetamol
The wide use of paracetamol as an analgesic and antipyretic available without prescription translates into paracetamol becoming a constant ingredient of sewage water [90]. Human urine samples in developed countries contain paracetamol at low concentrations irrespective of active paracetamol intake [63]. The common presence of paracetamol in the aquatic environment has prompted investigations on the effects of low concentrations of paracetamol in evolutionary distant marine species. Changes in development, behavior, enzyme activities, and DNA methylation patterns have been observed in zebrafish larvae and embryos exposed for several days to paracetamol at concentrations as low as 5 µg/L [91]. In small planktonic crustaceans of the genus Daphnia (water flea), paracetamol at 40 µg/L was found to alter glutathione S-transferase activity and behavior (swimming distance) [92], while sea mussels (Mytilus edulis) show altered gene expression patterns even at 40 ng/L [93]. While these observations bear little direct relevance for the developing human brain, they demonstrate that minute amounts of paracetamol may exert biological responses in evolutionary distant species.
6. Concluding Remarks
An array of diverse epidemiological studies link (prolonged) intrauterine exposure of paracetamol to ADHD and ASD in offspring, while there is little evidence that paracetamol taken during pregnancy is associated with brain function and development in a more general sense. Epidemiological studies cannot answer the question of whether or not this association represents a causal interference or is mediated by unaccounted confounders. The (few) experimental investigations published to date do show an impact of paracetamol on immature rodent animals, but the precise mechanisms are unknown. Despite decades of use for fever and pain, the actions of paracetamol on neurons have only recently been studied on a molecular level, and future work will have to elucidate how paracetamol may interfere with the developing brain.
Author Contributions
Writing the article: C.B. Preparing the figures: S.E. Review and editing the article: C.B., S.E., T.S. (Till Scheuer), T.S. (Thomas Schmitz). All authors have read and agreed to the published version of the manuscript.
Funding
German Research Council (DFG, Sche 20178/2-1), Förderverein für frühgeborene Kinder an der Charité.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| AM404 | N-arachidonoyl-phenolamine |
| ASD | Autism spectrum disorder |
| CBR1/2 | Cannabinoid receptors type 1 and 2 |
| COX | Cyclooxygenase |
| FAAH | Fatty acid amide hydrolase |
| FDA | Food and Drug Administration |
| GABA | γ-amino-butyric acid |
| GSH | Glutathione |
| GST | Glutathione S-transferase. |
| IQ | Intelligence quotient |
| HR | Hazard ratio |
| MRI | Magnetic resonance imaging |
| NAPQI | N-acetyl-p-benzo-quinone-imine |
| N-DAC | N-deacetylase |
| OR | Odds ratio |
| p-BQI | p-benzoquinone |
| PG G2 | Prostaglandin G2 |
| PG H2 | Prostaglandin H2 |
| PGHS | Prostaglandin endoperoxide-H synthase |
| POX | Peroxidase |
| SULT | Sulfotransferase |
| TRPA | Transient receptor potential ankyrin |
| TRPV | Transient vanilloid-subtype receptor |
| UGT | UDP-glucuronyl transferase |
References and Notes
- Bandoli, G.; Palmsten, K.; Chambers, C.D. Acetaminophen use in pregnancy: Examining prevalence, timing, and indication of use in a prospective birth cohort. Paediatr. Perinat. Epidemiol. 2019, 34, 237–246. [Google Scholar] [CrossRef]
- Mitra, S.; Florez, I.D.; Tamayo, M.E.; Mbuagbaw, L.; Vanniyasingam, T.; Veroniki, A.A.; Zea, A.M.; Zhang, Y.; Sadeghirad, B.; Thabane, L. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen with Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants. JAMA 2018, 319, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Shah, P.S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD010061. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2020, 48, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Weisz, D.; McNamara, P. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin. Perinatol. 2018, 42, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Seibold, S.A.; Rieke, C.J.; Song, I.; Cukier, R.I.; Smith, W.L. Prostaglandin Endoperoxide H Synthases. J. Biol. Chem. 2007, 282, 18233–18244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.L.; Urade, Y.; Jakobsson, P.-J. Enzymes of the Cyclooxygenase Pathways of Prostanoid Biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, A.; Ajmone-Cat, M.A.; Nicolini, A.; Sciulli, M.G.; Minghetti, L. Paracetamol effectively reduces prostaglandin E2 synthesis in brain macrophages by inhibiting enzymatic activity of cyclooxygenase but not phospholipase and prostaglandin E synthase. J. Neurosci. Res. 2003, 71, 844–852. [Google Scholar] [CrossRef]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2007, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Davies, M.; Day, R.; Mohamudally, A.; Scott, K. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Dominguez, R.; Warner, T.; Vojnovic, I.; Mitchell, J.A. Cellular mechanisms of acetaminophen: Role of cyclo-oxygenase. FASEB J. 2005, 19, 1–15. [Google Scholar] [CrossRef]
- Wong, C.T.; Bestard-Lorigados, I.; Crawford, D.A. Autism-related behaviors in the cyclooxygenase-2-deficient mouse model. Genes Brain Behav. 2018, 18, e12506. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, F.; Higashi, S.; Ando, E.; Ohsumi, T.; Watanabe, S.; Takeuchi, H. Modification of TRPV4 activity by acetaminophen. Heliyon 2020, 6, e03301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallet, C.; Barriere, D.; Ermund, A.; Jönsson, B.; Eschalier, A.; Zygmunt, P.M.; Högestätt, E.D. TRPV1 in Brain Is Involved in Acetaminophen-Induced Antinociception. PLoS ONE 2010, 5, e12748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, C.; Andersson, D.A.; Bevan, S.J. TRPA1 mediates the hypothermic action of acetaminophen. Sci. Rep. 2015, 5, 12771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Högestätt, E.D.; Jönsson, B.; Ermund, A.; Andersson, D.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of Acetaminophen to the Bioactive N-Acylphenolamine AM404 via Fatty Acid Amide Hydrolase-dependent Arachidonic Acid Conjugation in the Nervous System. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrière, D.A.; Mallet, C.; Blomgren, A.; Simonsen, C.; Daulhac, L.; Libert, F.; Chapuy, E.; Étienne, M.; Högestätt, E.D.; Zygmunt, P.M.; et al. Fatty Acid Amide Hydrolase-Dependent Generation of Antinociceptive Drug Metabolites Acting on TRPV1 in the Brain. PLoS ONE 2013, 8, e70690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Long, J.H.; Shah, S.; Rahman, J.; Perrett, D.; Ayoub, S.S.; Mehta, V. First evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J. Pain Res. 2017, 10, 2703–2709. [Google Scholar] [CrossRef] [Green Version]
- Barrière, D.A.; Boumezbeur, F.; Dalmann, R.; Cadeddu, R.; Richard, D.; Pinguet, J.; Daulhac, L.; Sarret, P.; Whittingstall, K.; Keller, M.; et al. Paracetamol is a centrally acting analgesic using mechanisms located in the periaqueductal grey. Br. J. Pharmacol. 2019, 177, 1773–1792. [Google Scholar] [CrossRef]
- Upadhya, S.C.; Tirumalai, P.S.; Boyd, M.R.; Mori, T.; Ravindranath, V. Cytochrome P4502E (CYP2E) in Brain: Constitutive Expression, Induction by Ethanol and Localization by Fluorescence in Situ Hybridization. Arch. Biochem. Biophys. 2000, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.; Andersson, A.; Bucher, B.; Galzi, J.-L.; Sterner, O.; Bevan, S.J.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, H.; Adelusi, O.B.; Ramachandran, A. Ferroptosis and Acetaminophen Hepatotoxicity: Are We Going Down Another Rabbit Hole? Gene Expr. 2021, 20, 169–178. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Hinson, J.A. The development and hepatotoxicity of acetaminophen: Reviewing over a century of progress. Drug Metab. Rev. 2020, 52, 472–500. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Braithwaite, I.; McKinlay, C.J.D.; Dalziel, S.R. Comparison of Acetaminophen (Paracetamol) With Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years. JAMA Netw. Open 2020, 3, e2022398. [Google Scholar] [CrossRef]
- Posadas, I.; Santos, P.; Blanco, A.; Muñoz-Fernández, M.; Ceña, V. Acetaminophen Induces Apoptosis in Rat Cortical Neurons. PLoS ONE 2010, 5, e15360. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Garrettson, L.K.; Soda, D.M. Letter: Evidence of placental transfer of acetaminophen. Pediatrics 1975, 55, 895. [Google Scholar] [PubMed]
- Kumpulainen, E.; Kokki, H.; Halonen, T.; Heikkinen, M.; Savolainen, J.; Laisalmi, M. Paracetamol (Acetaminophen) Penetrates Readily into the Cerebrospinal Fluid of Children After Intravenous Administration. Pediatrics 2007, 119, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Rebordosa, C.; Lee, P.-C.; Olsen, J. Acetaminophen Use During Pregnancy, Behavioral Problems, and Hyperkinetic Disorders. JAMA Pediatr. 2014, 168, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Liew, Z.; Ritz, B.; Virk, J.; Olsen, J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Res. 2015, 9, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Virk, J.; Arah, O.A.; Olsen, J. Prenatal Use of Acetaminophen and Child IQ. Epidemiology 2016, 27, 912–918. [Google Scholar] [CrossRef]
- Liew, Z.; Bach, C.C.; Asarnow, R.F.; Ritz, B.; Olsen, J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int. J. Epidemiol 2016, 45, 2009–2017. [Google Scholar] [CrossRef]
- Inoue, K.; Ritz, B.; Ernst, A.; Tseng, W.L.; Yuan, Y.; Meng, Q.; Ramlau-Hansen, C.H.; Strandberg-Larsen, K.; Arah, O.A.; Obel, C.; et al. Behavioral Problems at Age 11 Years After Prenatal and Postnatal Exposure to Acetaminophen: Parent-Reported and Self-Reported Outcomes. Am. J. Epidemiol. 2020, 190, 1009–1020. [Google Scholar] [CrossRef]
- Liew, Z.; Kioumourtzoglou, M.-A.; Roberts, A.L.; O’Reilly, J.; Ascherio, A.; Weisskopf, M.G. Use of Negative Control Exposure Analysis to Evaluate Confounding: An Example of Acetaminophen Exposure and Attention-Deficit/Hyperactivity Disorder in Nurses’ Health Study II. Am. J. Epidemiol 2019, 188, 768–775. [Google Scholar] [CrossRef]
- Parker, S.E.; Collett, B.R.; Werler, M.M. Maternal acetaminophen use during pregnancy and childhood behavioural problems: Discrepancies between mother- and teacher-reported outcomes. Paediatr. Perinat. Epidemiol 2019, 34, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Brandlistuen, R.E.; Ystrom, E.; Nulman, I.; Koren, G.; Nordeng, H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int. J. Epidemiol 2013, 42, 1702–1713. [Google Scholar] [CrossRef] [Green Version]
- Vlenterie, R.; Wood, M.E.; Brandlistuen, R.E.; Roeleveld, N.; van Gelder, M.M.; Nordeng, H. Neurodevelopmental problems at 18 months among children exposed to paracetamolin utero: A propensity score matched cohort study. Int. J. Epidemiol. 2016, 45, 1998–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ystrom, E.; Gustavson, K.; Brandlistuen, R.E.; Knudsen, G.P.; Magnus, P.; Susser, E.; Smith, G.D.; Stoltenberg, C.; Surén, P.; Håberg, S.E.; et al. Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics 2017, 140, e20163840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trønnes, J.N.; Wood, M.; Lupattelli, A.; Ystrom, E.; Nordeng, H. Prenatal paracetamol exposure and neurodevelopmental outcomes in preschool-aged children. Paediatr. Perinat. Epidemiol 2019, 34, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.M.D.; Waldie, K.E.; Wall, C.; Murphy, R.; Mitchell, E.A.; The ABC Study Group. Associations between Acetaminophen Use during Pregnancy and ADHD Symptoms Measured at Ages 7 and 11 Years. PLoS ONE 2014, 9, e108210. [Google Scholar] [CrossRef] [Green Version]
- Stergiakouli, E.; Thapar, A.; Smith, G.D. Association of Acetaminophen Use During Pregnancy with Behavioral Problems in Childhood. JAMA Pediatr. 2016, 170, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Golding, J.; Gregory, S.; Clark, R.; Ellis, G.; Iles-Caven, Y.; Northstone, K. Associations between paracetamol (acetaminophen) intake between 18 and 32 weeks gestation and neurocognitive outcomes in the child: A longitudinal cohort study. Paediatr. Perinat. Epidemiol 2019, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Avella-Garcia, C.B.; Julvez, J.; Fortuny, J.; Rebordosa, C.; García-Esteban, R.; Riaño-Galan, I.; Tardon, A.; Rodríguez-Bernal, C.L.; Iñiguez, C.; Andiarena, A.; et al. Acetaminophen use in pregnancy and neurodevelopment: Attention function and autism spectrum symptoms. Int. J. Epidemiol. 2016, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovo-Rodrigues, L.; Carpena, M.X.; Martins-Silva, T.; Santos, I.S.; Anselmi, L.; Barros, A.J.D.; Barros, F.C.; Bertoldi, A.D.; Matijasevich, A. Low neurodevelopmental performance and behavioural/emotional problems at 24 and 48 months in Brazilian children exposed to acetaminophen during foetal development. Paediatr. Perinat. Epidemiol 2020, 34, 278–286. [Google Scholar] [CrossRef]
- Bertoldi, A.D.; Rifas-Shiman, S.L.; Boing, A.C.; Pizzol, T.D.S.D.; Miranda, V.I.A.; Silveira, M.P.T.; Silveira, M.F.; Domingues, M.R.; Santos, I.S.; Bassani, D.G.; et al. Associations of acetaminophen use during pregnancy and the first year of life with neurodevelopment in early childhood. Paediatr. Perinat. Epidemiol 2020, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Alemany, S.; Avella-García, C.; Liew, Z.; García-Esteban, R.; Inoue, K.; Cadman, T.; López-Vicente, M.; González, L.; Galán, I.R.; Andiarena, A.; et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur. J. Epidemiol 2021, 1–12. [Google Scholar] [CrossRef]
- Hoover, R.M.; Hayes, V.A.G.; Erramouspe, J. Association Between Prenatal Acetaminophen Exposure and Future Risk of Attention Deficit/Hyperactivity Disorder in Children. Ann. Pharmacother. 2015, 49, 1357–1361. [Google Scholar] [CrossRef]
- Masarwa, R.; Levine, H.; Gorelik, E.; Reif, S.; Perlman, A.; Matok, I. Prenatal Exposure to Acetaminophen and Risk for Attention Deficit Hyperactivity Disorder and Autistic Spectrum Disorder: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Cohort Studies. Am. J. Epidemiol 2018, 187, 1817–1827. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Kriebel, D.; Herbert, M.R.; Bornehag, C.-G.; Swan, S.H. Prenatal paracetamol exposure and child neurodevelopment: A review. Horm. Behav. 2018, 101, 125–147. [Google Scholar] [CrossRef]
- Gou, X.; Wang, Y.; Tang, Y.; Qu, Y.; Tang, J.; Shi, J.; Xiao, D.; Mu, D. Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: A meta-analysis. Aust. N. Z. J. Psychiatry 2019, 53, 195–206. [Google Scholar] [CrossRef]
- Damkier, P. Simple twist of fate: In utero exposure to acetaminophen and risk of childhood Attention Deficit Hyperactivity Disorder. Paediatr. Perinat. Epidemiol 2020, 34, 230–232. [Google Scholar] [CrossRef]
- Talge, N.M. Prenatal acetaminophen exposure and neurodevelopment: State of the evidence. Paediatr. Perinat. Epidemiol 2020, 34, 227–229. [Google Scholar] [CrossRef]
- Leppert, B.; Havdahl, A.; Riglin, L.; Jones, H.J.; Zheng, J.; Smith, G.D.; Tilling, K.; Thapar, A.; Reichborn-Kjennerud, T.; Stergiakouli, E. Association of Maternal Neurodevelopmental Risk Alleles with Early-Life Exposures. JAMA Psychiatry 2019, 76, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Brucato, M.; Ladd-Acosta, C.; Li, M.; Caruso, D.; Hong, X.; Kaczaniuk, J.; Stuart, E.A.; Fallin, M.D.; Wang, X. Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Res. 2017, 10, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Bresnahan, M.A.; Che, X.; Schultz, A.F.; Ukaigwe, J.E.; Eddy, M.L.; Hirtz, D.; Gunnes, N.; Lie, K.K.; Magnus, P.; et al. Prenatal fever and autism risk. Mol. Psychiatry 2017, 23, 759–766. [Google Scholar] [CrossRef]
- Gustavson, K.; Ask, H.; Ystrom, E.; Stoltenberg, C.; Lipkin, W.I.; Surén, P.; Håberg, S.E.; Magnus, P.; Knudsen, G.P.; Eilertsen, E.; et al. Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zerbo, O.; Qian, Y.; Yoshida, C.; Grether, J.K.; Van De Water, J.; Croen, L.A. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2013, 45, 4015–4025. [Google Scholar] [CrossRef] [Green Version]
- Masarwa, R.; Platt, R.W.; Filion, K.B. Acetaminophen use during pregnancy and the risk of attention deficit hyperactivity disorder: A causal association or bias? Paediatr. Perinat. Epidemiol 2020, 34, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Pan, T.-L.; Wang, P.-W.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Tsai, S.-J.; Chen, T.-J.; et al. Prenatal Exposure to Acetaminophen and the Risk of Attention-Deficit/Hyperactivity Disorder: A Nationwide Study in Taiwan. J. Clin. Psychiatry 2019, 80. [Google Scholar] [CrossRef] [PubMed]
- Bornehag, C.-G.; Reichenberg, A.; Hallerback, M.U.; Wikstrom, S.; Koch, H.; Jonsson, B.; Swan, S. Prenatal exposure to acetaminophen and children’s language development at 30 months. Eur. Psychiatry 2018, 51, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Riley, A.W.; Lee, L.-C.; Hong, X.; Wang, G.; Tsai, H.-J.; Mueller, N.T.; Pearson, C.; Thermitus, J.; Panjwani, A.; et al. Maternal Biomarkers of Acetaminophen Use and Offspring Attention Deficit Hyperactivity Disorder. Brain Sci. 2018, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Azuine, R.E.; Zhang, Y.; Hou, W.; Hong, X.; Wang, G.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure with Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry 2020, 77, 180–189. [Google Scholar] [CrossRef]
- Baker, B.H.; Lugo-Candelas, C.; Wu, H.; Laue, H.E.; Boivin, A.; Gillet, V.; Aw, N.; Rahman, T.; Lepage, J.-F.; Whittingstall, K.; et al. Association of Prenatal Acetaminophen Exposure Measured in Meconium with Risk of Attention-Deficit/Hyperactivity Disorder Mediated by Frontoparietal Network Brain Connectivity. JAMA Pediatr. 2020, 174, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Modick, H.; Weiss, T.; Dierkes, G.; Brüning, T.; Koch, H.M. Ubiquitous presence of paracetamol in human urine: Sources and implications. Reproduction 2014, 147, R105–R117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laue, H.E.; Cassoulet, R.; Abdelouahab, N.; Serme-Gbedo, Y.K.; Desautels, A.S.; Brennan, K.J.; Bellenger, J.P.; Burris, H.H.; Coull, B.A.; Weisskopf, M.G.; et al. Association Between Meconium Acetaminophen and Childhood Neurocognitive Development in GESTE, a Canadian Cohort Study. Toxicol. Sci. 2018, 167, 138–144. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Drug Safety Communications. FDA has reviewed possible risks of pain medicine use during pregnancy 1-9-2015.
- European Medicine Agency. PRAC recommendations on signals. EMA/PRAC/157165/2019. 8-4-2019.
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol use during pregnancy—a call for precautionary action. Nat. Rev. Endocrinol. 2021, 1–10. [Google Scholar] [CrossRef]
- Allegaert, K. A Critical Review on the Relevance of Paracetamol for Procedural Pain Management in Neonates. Front. Pediatr. 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlsson, A.; Shah, P.S. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database Syst. Rev. 2020, 1, CD011219. [Google Scholar] [CrossRef]
- Tinner, E.M.; Hoesli, I.; Jost, K.; Schöbi, N.; Megged, Y.U.; Burkhardt, T.; Krafft, A.; Bucher, H.U.; Surbek, D.; Nelle, M.; et al. Rectal Paracetamol in Newborn Infants after Assisted Vaginal Delivery May Increase Pain Response. J. Pediatr. 2013, 162, 62–66. [Google Scholar] [CrossRef]
- Eras, Z.; Uras, N.; Canpolat, F.E.; Erdeve, O.; Oguz, S.S.; Oncel, M.Y. Neurodevelopmental Outcomes of Preterm Infants Treated with Oral Paracetamol Versus Ibuprofen for Patent Ductus Arteriosus. Am. J. Perinatol. 2017, 34, 1185–1189. [Google Scholar] [CrossRef]
- Juujärvi, S.; Kallankari, H.; Pätsi, P.; Leskinen, M.; Saarela, T.; Hallman, M.; Aikio, O. Follow-up study of the early, randomised paracetamol trial to preterm infants, found no adverse reactions at the two-years corrected age. Acta Paediatr. 2018, 108, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Juujärvi, S.; Saarela, T.; Pokka, T.; Hallman, M.; Aikio, O. Intravenous paracetamol for neonates: Long-term diseases not escalated during 5 years of follow-up. Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 106, 178–183. [Google Scholar] [CrossRef]
- Bittker, S.S.; Bell, K.R. Postnatal Acetaminophen and Potential Risk of Autism Spectrum Disorder among Males. Behav. Sci. 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Koehn, L.M.; Huang, Y.; Habgood, M.D.; Kysenius, K.; Crouch, P.J.; Dziegielewska, K.M.; Saunders, N.R. Effects of paracetamol (acetaminophen) on gene expression and permeability properties of the rat placenta and fetal brain. F1000Research 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Thiele, K.; Solano, M.E.; Huber, S.; Flavell, R.A.; Kessler, T.; Barikbin, R.; Jung, R.; Karimi, K.; Tiegs, G.; Arck, P.C. Prenatal Acetaminophen Affects Maternal Immune and Endocrine Adaptation to Pregnancy, Induces Placental Damage, and Impairs Fetal Development in Mice. Am. J. Pathol. 2015, 185, 2805–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, K.; Keßler, T.; Thiele, K.; Ramisch, K.; Erhardt, A.; Huebener, P.; Barikbin, R.; Arck, P.; Tiegs, G. Prenatal acetaminophen induces liver toxicity in dams, reduces fetal liver stem cells, and increases airway inflammation in adult offspring. J. Hepatol. 2015, 62, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Hegde, S.; Kechichian, T.; Gamble, P.; Rahman, M.; Stutz, S.J.; Anastasio, N.C.; AlShehri, W.; Lei, J.; Mori, S.; et al. Is There a Causal Relation between Maternal Acetaminophen Administration and ADHD? PLoS ONE 2016, 11, e0157380. [Google Scholar] [CrossRef]
- Hay-Schmidt, A.; Finkielman, O.T.E.; Jensen, B.A.H.; Høgsbro, C.F.; Holm, J.B.; Johansen, K.H.; Jensen, T.K.; Andrade, A.M.; Swan, S.H.; Bornehag, C.-G.; et al. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction 2017, 154, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.M.; Rigobello, C.; Vidigal, C.B.; Moura, K.F.; Barbosa, D.S.; Gerardin, D.C.C.; Ceravolo, G.; Moreira, E.G. Gestational exposure to paracetamol in rats induces neurofunctional alterations in the progeny. Neurotoxicol Teratol. 2019, 77, 106838. [Google Scholar] [CrossRef]
- Rigobello, C.; Klein, R.M.; Debiasi, J.D.; Ursini, L.G.; Michelin, A.P.; Matsumoto, A.K.; Barbosa, D.S.; Moreira, E.G. Perinatal exposure to paracetamol: Dose and sex-dependent effects in behaviour and brain’s oxidative stress markers in progeny. Behav. Brain Res. 2021, 408, 113294. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Clancy, B.; Darlington, R.; Finlay, B. Translating developmental time across mammalian species. Neuroscience 2001, 105, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. J. Neurosci. 2013, 33, 7368–7383. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H.; Eriksson, P.; Gordh, T.; Fredriksson, A. Paracetamol (Acetaminophen) Administration During Neonatal Brain Development Affects Cognitive Function and Alters Its Analgesic and Anxiolytic Response in Adult Male Mice. Toxicol. Sci. 2013, 138, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippot, G.; Gordh, T.; Fredriksson, A.; Viberg, H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): Characterization of a critical period. J. Appl. Toxicol. 2017, 37, 1174–1181. [Google Scholar] [CrossRef]
- Philippot, G.; Hallgren, S.; Gordh, T.; Fredriksson, A.; Fredriksson, R.; Viberg, H. A Cannabinoid Receptor Type 1 (CB1R) Agonist Enhances the Developmental Neurotoxicity of Acetaminophen (Paracetamol). Toxicol. Sci. 2018, 166, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, N.; Hernandez, J.C.; Poulton, J.; Jones, J.P.; Konsoula, Z.; Smith, C.; Parker, W. Therapeutic doses of acetaminophen with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats. PLoS ONE 2021, 16, e0253543. [Google Scholar] [CrossRef]
- Blecharz-Klin, K.; Wawer, A.; Jawna-Zboińska, K.; Pyrzanowska, J.; Piechal, A.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Early paracetamol exposure decreases brain-derived neurotrophic factor (BDNF) in striatum and affects social behaviour and exploration in rats. Pharmacol. Biochem. Behav. 2018, 168, 25–32. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Silva, S.; Cardoso, V.V.; Benoliel, M.J.; Cardoso, E.; Coelho, M.R.; Martins, A.; Almeida, C.M.M. Screening and Seasonal Behavior of Analgesics, Non-steroidal Anti-inflammatory Drugs, and Antibiotics in Two Urban Wastewater Treatment Plants. Environ. Manag. 2021, 68, 411–425. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Pinto, G.; Correia, B.; Nunes, B. Embryonic development, locomotor behavior, biochemical, and epigenetic effects of the pharmaceutical drugs paracetamol and ciprofloxacin in larvae and embryos of Danio rerio when exposed to environmental realistic levels of both drugs. Environ. Toxicol. 2019, 34, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Nunes, B. Dangerous connections: Biochemical and behavioral traits in Daphnia magna and Daphnia longispina exposed to ecologically relevant amounts of paracetamol. Environ. Sci. Pollut. Res. 2021, 28, 38792–38808. [Google Scholar] [CrossRef]
- Koagouw, W.; Stewart, N.A.; Ciocan, C. Long-term exposure of marine mussels to paracetamol: Is time a healer or a killer? Environ. Sci. Pollut. Res. 2021, 28, 48823–48836. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).