The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium
Abstract
1. Introduction
2. Results
2.1. FLI Cytokines Promote the Maturation and Developmental Capacity of Porcine Oocytes
2.2. Time Course of MAPK3/1 Activation in COCs Cultured in Control and FLI Medium
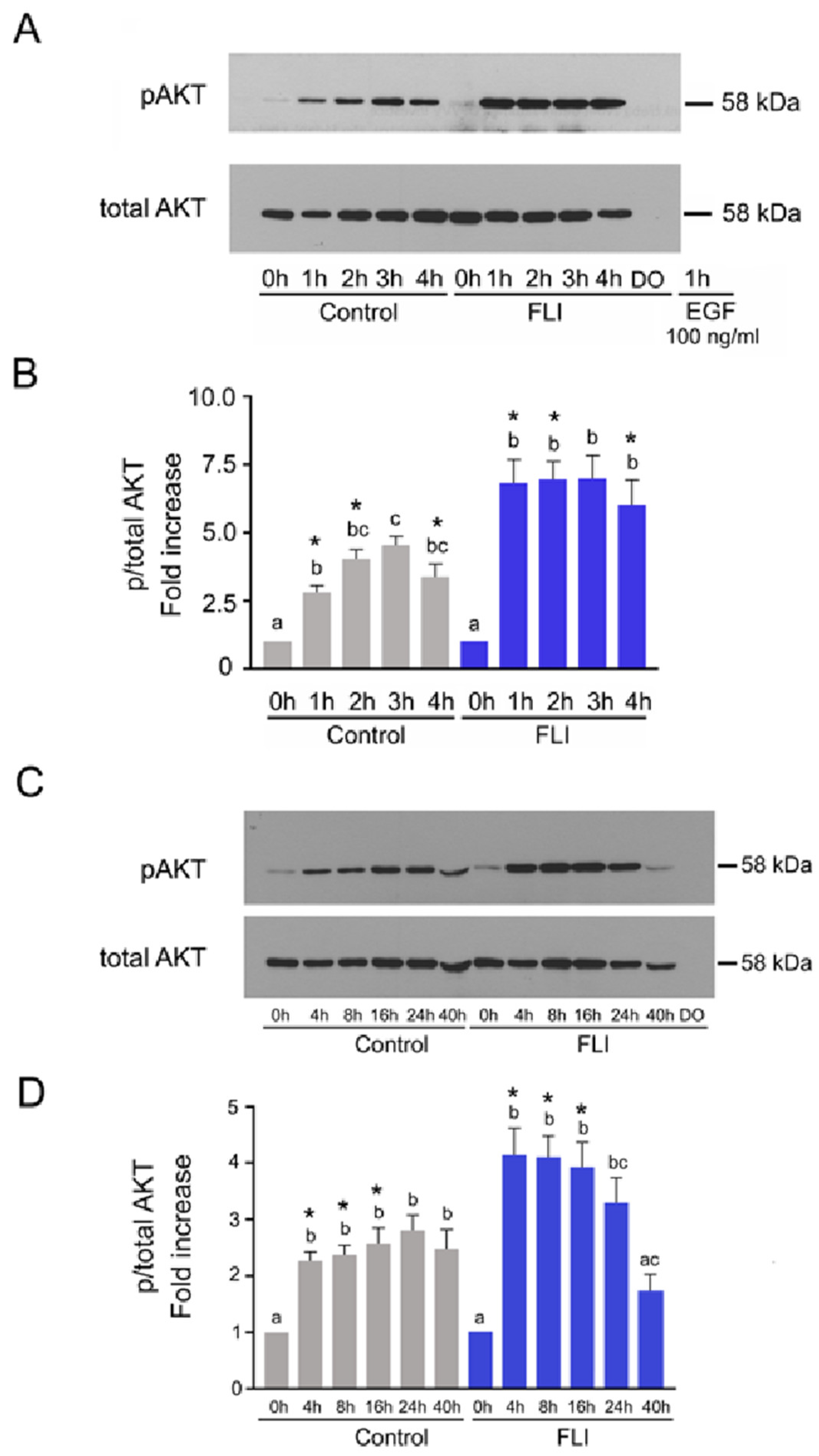
2.3. Time Course of AKT Activation in COCs Cultured in Control and FLI Medium
2.4. Expression of Cumulus Expansion and Signaling Related Genes in COCs Cultured in Control and FLI Medium
3. Discussion
4. Materials and Methods
4.1. Culture Media and Reagents
4.2. Collection and Culture of Cumulus-Oocyte Complexes
4.3. Assessment of Oocyte Maturation
4.4. Parthenogenetic Activation and Culture of Embryos
4.5. Immunoblotting
4.6. Expression Analysis of Predicted Target and Quality-Related Genes Using RT-qPCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, M.; Hsieh, M.; Musa Zamah, A.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Thompson, J.G. Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007, 67, 6–15. [Google Scholar] [CrossRef]
- Albuz, F.K.; Sasseville, M.; Lane, M.; Armstrong, D.T.; Thompson, J.G.; Gilchrist, R.B. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 2010, 25, 2999–3011. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, I.; Okazaki, T.; Noma, N.; Nishibori, M.; Yamashita, Y.; Shimada, M. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction 2008, 136, 9–21. [Google Scholar] [CrossRef]
- Prochazka, R.; Blaha, M.; Němcová, L. Significance of epidermal growth factor receptor signaling for acquisition of meiotic and developmental competence in mammalian oocytes. Biol. Reprod. 2017, 97, 537–549. [Google Scholar] [CrossRef]
- Buratini, J., Jr.; Pinto, M.G.; Castilho, A.C.; Amorim, R.L.; Giometti, I.C.; Portela, V.M.; Nicola, E.S.; Price, C.A. Expression and function of fibroblast growth factor 10 and its receptor, fibroblast growth factor receptor 2B, in bovine follicles. Biol. Reprod. 2007, 77, 743–750. [Google Scholar] [CrossRef]
- Zhang, K.; Hansen, P.J.; Ealy, A.D. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction 2010, 140, 815–826. [Google Scholar] [CrossRef]
- Diógenes, M.N.; Guimarães, A.L.S.; Leme, L.O.; Maurício, M.F.; Dode, M.A.N. Effect of prematuration and maturation with fibroblast growth factor 10 (FGF10) on in vitro development of bovine oocytes. Theriogenology 2017, 102, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Lee, S.E.; Hyun, H.; Shin, M.Y.; Park, Y.G.; Jeong, S.G.; Kim, E.Y.; Park, S.P. Fibroblast growth factor 10 markedly improves in vitro maturation of porcine cumulus-oocyte complexes. Mol. Reprod. Dev. 2017, 84, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, M.; Baloch, A.R.; Zhang, Q.; Wang, J.; Ma, R.; Xu, G.; Kashif, J.; Wang, L.; Fan, J.; et al. FGF10 enhances yak oocyte fertilization competence and subsequent blastocyst quality and regulates the levels of CD9, CD81, DNMT1, and DNMT3B. J. Cell. Physiol. 2019, 234, 17677–17689. [Google Scholar] [CrossRef]
- Barros, R.G.; Lima, P.F.; Soares, A.C.S.; Sanches, L.; Price, C.A.; Buratini, J. Fibroblast growth factor 2 regulates cumulus differentiation under the control of the oocyte. J. Assist. Reprod. Genet. 2019, 36, 905–913. [Google Scholar] [CrossRef]
- Singh, B.; Armstrong, D.T. Insulin-like growth factor-1, a component of serum that enables porcine cumulus cells to expand in response to follicle-stimulating hormone in vitro. Biol. Reprod. 1997, 56, 1370–1375. [Google Scholar] [CrossRef]
- Nemcova, L.; Nagyova, E.; Petlach, M.; Tomanek, M.; Prochazka, R. Molecular mechanisms of insulin-like growth factor 1 promoted synthesis and retention of hyaluronic acid in porcine oocyte-cumulus complexes. Biol. Reprod. 2007, 76, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Purohit, G.N.; Brady, M.S.; Sharma, S.S. Influence of epidermal growth factor and insulin-like growth factor 1 on nuclear maturation and fertilization of buffalo cumulus oocyte complexes in serum free media and their subsequent development in vitro. Anim. Reprod. Sci. 2005, 87, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, J.; Li, M.; Yan, L.; Zhao, Y.; Lian, Y.; Li, R.; Liu, P.; Qiao, J. Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development. Hum. Reprod. 2012, 27, 2146–2159. [Google Scholar] [CrossRef]
- Kaya, A.; Sağirkaya, H.; Misirlioğlu, M.; Gümen, A.; Parrish, J.J.; Erdoğan Memili, E. Leptin and IGF-I improve bovine embryo quality in vitro. Anim. Reprod. 2017, 14, 1151–1160. [Google Scholar] [CrossRef]
- Sato, A.; Sarentonglaga, B.; Ogata, K.; Yamaguchi, M.; Hara, A.; Atchalalt, K.; Sugane, N.; Fukumori, R.; Nagao, Y. Effects of insulin-like growth factor-1 on the in vitro maturation of canine oocytes. J. Reprod. Dev. 2018, 64, 83–88. [Google Scholar] [CrossRef]
- Dang-Nguyen, T.Q.; Haraguchi, S.; Kikuchi, K.; Somfai, T.; Bodó, S.; Nagai, T. Leukemia inhibitory factor promotes porcine oocyte maturation and is accompanied by activation of signal transducer and activator of transcription 3. Mol. Reprod. Dev. 2014, 81, 230–239. [Google Scholar] [CrossRef]
- Mo, X.; Wu, G.; Yuan, D.; Jia, B.; Liu, C.; Zhu, S.; Hou, Y. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2014, 81, 608–618. [Google Scholar] [CrossRef]
- Vendrell-Flotats, M.; García-Martínez, T.; Martínez-Rodero, I.; López-Béjar, M.; LaMarre, J.; Yeste, M.; Mogas, T. In vitro maturation in the presence of leukemia inhibitory factor modulates gene and miRNA expression in bovine oocytes and embryos. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Neira, J.A.; Tainturier, D.; Peña, M.A.; Martal, J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-β1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Ansari, M.M.; Parmar, M.S.; Chandra, V.; Sharma, G.T. Stem cell conditioned media contains important growth factors and improves in vitro buffalo embryo production. Anim. Biotechnol. 2016, 27, 118–125. [Google Scholar] [CrossRef]
- Spate, L.D.; Murphy, S.L.; Benne, J.A.; Giraldo, A.; Hylan, D.; Prather, R.S. In vitro-matured gilt oocytes can have equal or better developmental competence than sow oocytes with new maturation media. Reprod. Fertil. Dev. 2016, 29, 150. [Google Scholar] [CrossRef]
- Lucas-Hahn, A.; Petersen, B.; Nowak-Imialek, M.; Baulain, U.; Becker, R.; Eylers, H.-M.; Hadeler, K.-G.; Hassel, P.; Niemann, H. A new maturation medium improves porcine embryo production in vitro. Reprod. Fertil. Dev. 2018, 30, 200–201. [Google Scholar] [CrossRef]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef]
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef]
- Su, Y.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002, 143, 2221–2232. [Google Scholar] [CrossRef]
- Su, Y.Q.; Denegre, J.M.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev. Biol. 2003, 263, 126–138. [Google Scholar] [CrossRef]
- Siddappa, D.; Beaulieu, É.; Gévry, N.; Roux, P.P.; Bordignon, V.; Duggavathi, R. Effect of the transient pharmacological inhibition of Mapk3/1 pathway on ovulation in mice. PLoS ONE 2015, 10, e0119387. [Google Scholar] [CrossRef]
- Meinecke, B.; Krischek, C. MAPK/ERK kinase (MEK) signalling is required for resumption of meiosis in cultured cumulus-enclosed pig oocytes. Zygote 2003, 11, 7–16. [Google Scholar] [CrossRef]
- Prochazka, R.; Petlach, M.; Nagyova, E.; Nemcova, L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: Comparison with gonadotropins. Reproduction 2011, 141, 425–435. [Google Scholar] [CrossRef]
- Prochazka, R.; Blaha, M. Regulation of mitogen-activated protein kinase 3/1 activity during meiosis resumption in mammals. J. Reprod. Dev. 2015, 61, 495–502. [Google Scholar] [CrossRef]
- Hashimoto, N.; Watanabe, N.; Furuta, Y.; Tamemoto, H.; Sagata, N.; Yokoyama, M.; Okazaki, K.; Nagayoshi, M.; Takeda, N.; Ikawa, Y. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 1994, 370, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Gordo, A.C.; He, C.L.; Smith, S.; Fissore, R.A. Mitogen activated protein kinase plays a significant role in metaphase II arrest, spindle morphology, and maintenance of maturation promoting factor activity in bovine oocytes. Mol. Reprod. Dev. 2001, 59, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Fan, H.Y.; Chen, D.Y.; Song, X.F.; Schatten, H.; Sun, Q.Y. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: Microtuble organization, asymmetric division and metaphase II arrest. Cell. Res. 2003, 13, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, R.; Nemcova, L. Mechanisms of FSH- and amphiregulin-induced MAP-kinase 3/1 activation in pig cumulus-oocyte complexes during maturation in vitro. Int. J. Mol. Sci. 2019, 20, 1179. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Morrow, J.D.; Wang, H.; Dey, S.K. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. Biol. Chem. 2006, 281, 37117–37129. [Google Scholar] [CrossRef]
- Prochazka, R.; Blaha, M.; Nemcova, L. Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction 2012, 144, 535–546. [Google Scholar] [CrossRef]
- Shimada, M.; Maeda, T.; Terada, T. Dynamic changes of connexin-43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3-kinase during meiotic resumption in porcine oocytes. Biol. Reprod. 2001, 64, 1255–1263. [Google Scholar] [CrossRef]
- Nagyová, E.; Procházka, R.; Vanderhyden, B.C. Oocytectomy does not influence synthesis of hyaluronic acid by pig cumulus cells: Retention of hyaluronic acid after insulin-like growth factor-I treatment in serum-free medium. Biol. Reprod. 1999, 61, 569–574. [Google Scholar] [CrossRef]
- Blaha, M.; Prochazka, R.; Adamkova, K.; Nevoral, J.; Nemcova, L. 2017 Prostaglandin E2 stimulates the expression of cumulus expansion-related genes in pigs: The role of protein kinase B. Prostag. Oth. Lipid M. 2017, 130, 38–46. [Google Scholar]
- Blaha, M.; Nemcova, L.; Kepkova, K.V.; Vodicka, P.; Prochazka, R. Gene expression analysis of pig cumulus-oocyte complexes stimulated in vitro with follicle stimulating hormone or epidermal growth factor-like peptides. Reprod. Biol. Endocrinol. 2015, 13, 113. [Google Scholar] [CrossRef][Green Version]
- Kalous, J.; Kubelka, M.; Solc, P.; Susor, A.; Motlík, J. AKT (protein kinase B) is implicated in meiotic maturation of porcine oocytes. Reproduction 2009, 138, 645–654. [Google Scholar] [CrossRef]
- Gearing, D.P.; Thut, C.J.; VandeBos, T.; Gimpel, S.D.; Delaney, P.B.; King, J.; Price, V.; Cosman, D.; Beckmann, M.P. Leukemia inhibitory factor receptor is structurally related to the IL-6 signal transducer, gp130. EMBO J. 1991, 10, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Oates, A.; Dunn, A.R. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J. Biol. Chem. 1996, 271, 30136–30143. [Google Scholar] [CrossRef] [PubMed]
- Burdon, T.; Stracey, C.; Chambers, I.; Nichols, J.; Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 1999, 210, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.N.; Kilgour, E.; Smith, P.D. Molecular pathways: Fibroblast growth factor signaling: A new therapeutic opportunity in cancer. Clin. Cancer Res. 2012, 18, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.H.; Quirion, R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006, 7, 51. [Google Scholar] [CrossRef]
- Wang, Z. Transactivation of Epidermal Growth Factor Receptor by G Protein-Coupled Receptors: Recent Progress, Challenges and Future Research. Int. J. Mol. Sci. 2016, 17, E95. [Google Scholar] [CrossRef] [PubMed]
- Panigone, S.; Hsieh, M.; Fu, M.; Persani, L.; Conti, M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol. Endocrinol. 2008, 22, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Richards, J.S. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: Key roles for prostaglandin synthase 2 and progesterone receptor. Mol. Endocrinol. 2006, 20, 1352–1365. [Google Scholar] [CrossRef]
- Reizel, Y.; Elbaz, J.; Dekel, N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol. Endocrinol. 2010, 24, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Okamoto, M.; Kawashima, I.; Okazaki, T.; Nishimura, R.; Gunji, Y.; Hishinuma, M.; Shimada, M. Positive feedback loop between prostaglandin E2 and EGF-like factors is essential for sustainable activation of MAPK3/1 in cumulus cells during in vitro maturation of porcine cumulus oocyte complexes. Biol. Reprod. 2011, 85, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.J.; Sugimura, S.; Gilchrist, R.B. Oocyte induction of EGF responsiveness in somatic cells is associated with the acquisition of porcine oocyte developmental competence. Endocrinology 2015, 156, 2299–2312. [Google Scholar] [CrossRef]
- Prochazka, R.; Kalab, P.; Nagyova, E. Epidermal growth factor-receptor tyrosine kinase activity regulates expansion of porcine oocyte-cumulus cell-complexes in vitro. Biol. Reprod. 2003, 68, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, J.; Zhu, S.; Ahmed, J.Z.; Li, M.; Shi, D.; Huang, B. PI3K inhibitor reduces in vitro maturation and developmental competence of porcine oocytes. Theriogenology 2020, 157, 432–439. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, S.; Li, J.; Jam Zaheer, A.; Li, M.; Huang, B. PS48 promotes in vitro maturation and developmental competence of porcine oocytes through activating PI3K/Akt signalling pathway. Reprod. Domest. Anim. 2020, 55, 1678–1687. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.L.; Kong, I.K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- Artini, P.G.; Tatone, C.; Sperduti, S.; D’Aurora, M.; Franchi, S.; Di Emidio, G.; Ciriminna, R.; Vento, M.; Di Pietro, C.; Stuppia, L.; et al. Cumulus cells surrounding oocytes with high developmental competence exhibit down-regulation of phosphoinositol 1,3 kinase/protein kinase B (PI3K/AKT) signalling genes involved in proliferation and survival. Hum. Reprod. 2017, 32, 2474–2484. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011, 1813, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta. 2011, 1813, 1938–1945. [Google Scholar] [CrossRef]
- Yoshioka, K.; Suzuki, C.; Onishi, A. Defined system for in vitro production of porcine embryos using a single basic medium. J. Reprod. Dev. 2008, 54, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Surani, M.A.; Barton, S.C. Development of gynogenetic eggs in the mouse: Implications for parthenogenetic embryos. Science 1983, 222, 1034–1036. [Google Scholar] [CrossRef]
- Yoshioka, K.; Suzuki, C.; Tanaka, A.; Anas, I.M.-K.; Iwamura, S. Birth of Piglets Derived from Porcine Zygotes Cultured in a Chemically Defined Medium1. Biol. Reprod. 2002, 66, 112–119. [Google Scholar] [CrossRef] [PubMed]


| Type of | No. of Oocytes | % of Oocytes in | ||
|---|---|---|---|---|
| Medium | Examined | GV | GVBD | MII |
| M199+BSA | 154 | 63.65 ± 5.75 A | 9.18 ± 0.94 AB | 26.95 ± 5.79 A |
| +PMSG+hCG | 150 | 63.58 ± 6.93 A | 10.38 ± 3.55 AB | 26.08 ± 5.03 A |
| +PMSG+hCG+EGF (Control) | 146 | 13.63 ± 6.45 B | 16.53 ± 2.38 A | 69.90 ± 4.05 B |
| FLI | 163 | 1.20 ± 0.69 B | 1.3 ± 0.76 B | 97.5 ± 0.14 C |
| Type of | No. of Oocytes | % of Oocytes in | ||
|---|---|---|---|---|
| Medium | Examined | GV | GVBD | MII |
| Control | 91 | 22.11 ± 4.86 A | 12.41 ± 3.16 | 65.48 ± 3.12 A |
| +FGF2 | 97 | 0 ± 0 B | 8.03 ± 2.93 | 91.97 ± 2.94 B |
| +IGF1 | 97 | 4.80 ± 2.64 B | 4.13 ± 2.29 | 91.07 ± 4.47 B |
| +LIF | 92 | 0 ± 0 B | 4.67 ± 2.65 | 95.33 ± 2.45 B |
| FLI | 87 | 0 ± 0 B | 3.57 ± 0.50 | 96.43 ± 0.50 B |
| Type of | Time of | No. of Oocytes | % of Oocytes in | ||
|---|---|---|---|---|---|
| Medium | Culture (h) | Examined | GV | GVBD | MII |
| Control | 16 | 86 | 86.63 ± 5.95 A | 13.38 ± 5.95 A | 0 ± 0 A |
| FLI | 16 | 81 | 81.48 ± 6.25 A | 18.53 ± 6.25 A | 0 ± 0 A |
| Control | 24 | 97 | 71.93 ± 6.76 A | 22.40 ± 4.44 A | 5.68 ± 2.44 A |
| FLI | 24 | 99 | 30.83 ± 6.10 B | 56.03 ± 2.53 B | 13.15 ± 5.74 A |
| Control | 44 | 89 | 13.40 ± 6.61 A | 18.55 ± 7.45 A | 68.05 ± 5.72 A |
| FLI | 44 | 92 | 0 ± 0 A | 4.63 ± 2.67 A | 95.38 ± 2.67 B |
| Type of Medium | No. of Activated Oocytes | Cleavage Rate % ± SEM | Blastocyst Rate % ± SEM |
|---|---|---|---|
| Control | 95 | 70.51 ± 1.39 A | 20.69 ± 3.45 A |
| FLI | 100 | 87.88 ± 1.99 B | 34.07 ± 0.38 B |
| Component | Supplier | Control Medium | FLI Medium |
|---|---|---|---|
| TCM199 | Sigma, M7528 | TCM199 | TCM199 |
| Sodium pyruvate | Sigma, P4562 | 0.2 mM | 0.2 mM |
| L-glutamin | Sigma, G8540 | 6.85 mM | 6.85 mM |
| Cysteine | Sigma, C7352 | 0.57 mM | 0.57 mM |
| Gentamycin | Roth, 0233 | 50 µg/mL | 50 µg/mL |
| BSA | Sigma, A7030 | 1 mg/mL | 1 mg/mL |
| PMSG | Prospec 1, HOR-272 | 10 IU/mL | 10 IU/mL |
| hCG | Prospec 1, HOR-250 | 10 IU/mL | 10 IU/mL |
| EGF | PeproTech 2, AF-100-15 | 10 ng/mL | 10 IU/mL |
| human LIF | Merck 3, LIF1005 | − | 2 µL/mlL |
| human IGF1 | PeproTech 2, AF-100-11 | − | 20 ng/mL |
| human FGF2 | Sigma, F0291 | − | 40 ng/mL |
| Gene | Amplicon Length (bp) | Sequence 5´–3´ | Gene Accession No. | Tan |
|---|---|---|---|---|
| AKT1 | 157 | TAC TCC TTC CAG ACC CAC GA CGG AGT GCA GGT AGT CCA AG | NM_001159776.1 | 53 |
| ATP6 | 141 | AAT TCC TAT GCT CGT AAT ATG TTG AGT AGT GCT AAT | NC_000845 | 57 |
| BAX | 251 | AAG CGC ATT GGA GAT GAA CT CGA TCT CGA AGG AAG TCC AG | XM_003127290.5 | 55 |
| BCL2L1 | 196 | GAA ACC CCT AGT GCC ATC AA GGG ACG TCA GGT CAC TGA AT | XM_021077292.1 | 55 |
| CAT | 128 | CAA GAT TCT CCT GTG CTA CCC TAA CCT TCA CTT ACC | NM_214301.2 | 56 |
| CCND2 | 116 | CAG TGC TCC TAC TTC AAG ACC TCT TCT TCA CAC TTC | NM_214088.1 | 58 |
| CD44 | 218 | GAG GCG GCC CTG AAC ATA AAG GTA TTA GGC AGG TCT GTG AC | XM_013994425.2 | 58 |
| CDKN1B | 140 | AAG ACT GAT GCA CCG GAC AG TTC GGG GAA CCG TCT GAA AC | NM_214316.1 | 53 |
| EIF4E | 148 | GAATCTAATCAGGAGGTT AGTCTTCAACAGTATCAA | XM_003129314.3 | 53 |
| ERBB1 | 241 | CCC TCA AGG AGA TCA GCG AC CGC GGC TAA AGT TTC GAC AG | NM_214007 | 53 |
| EREG | 129 | ATG GCT ACT GTT TGC ACG GA TGC TCA GAG GTT GTT GGA CG | XM_013978775.2 | 58 |
| FOXO1 | 149 | ATG GAG ACA CTT TGG ATT TAC TTC AAA TTA TCT GAC AG | NM_214014.3 | 53 |
| FOXO3 | 113 | ATT ATC CGT AGC GAA CTC AT TGC TTA GCA CCA GTG AAG | NM_001135959.1 | 57 |
| GJA1 | 227 | AGT GAT CCT TAC CAC GCC AC CGA TTC TGC TCG GCA CTG TA | NM_001244212.1 | 57 |
| GPX1 | 127 | GGT CTC CAG TGT GTC GCA AT GCT TCG ATG TCA GGC TCG AT | NM_214201.1 | 56 |
| HAS2 | 407 | GAA GTC ATG GGC AGG GAC AAT TC TGG CAG GCC CTT TCT ATG TTA | NM_214053.1 | 54 |
| JAK1 | 195 | GAC CGT CAC CTG CTT TGA GA ACG AAG CTG ATG TTG TCC GT | NM_214114.1 | 54 |
| MAPK1 | 157 | TAA GGT GCC ATG GAA CAG GC GGG CTC ATC ACT TGG GTC AT | NM_001198922.1 | 55 |
| MTOR | 151 | CGA TGG CCA GGG ATC TCT TC TCG GCC AAG TTT AAG AGC GT | XM_003127584.6 | 53 |
| PANX1 | 159 | CGC GCA GGA AAT CTC AAT CG TTA TGC AGG CAC AGT GGG AG | XM_003482597.4 | 57 |
| PCNA | 153 | TAA TGC AGA CAC CTT GGC ACT GCA AAT TCA CCA GAA GGC ATC | NM_001291925.1 | 55 |
| POLG | 152 | ACT GGC TGG ACA TCA GCA GT ACA GTA CCG CAT CAG GTC C | XM_001927064.5 | 55 |
| PTX3 | 208 | CGC CAA TAC TGT GAT TTC C TAT TTC ATC AAA GCC ACC AC | NM_001244783.1 | 54 |
| SOD1 | 139 | AAC ATG GTG GGC CAA AGG AT GTG CGG CCA ATG ATG GAA TG | NM_001190422.1 | 55 |
| SOD2 | 220 | GGT GGA GGC CAC ATC AAT CA AAC AAG CGG CAA TCT GCA AG | NM_214127.2 | 55 |
| STAT3 | 139 | CTT GCC AGT CGT GGT CAT CT CAC TTG ATC CCA CGT TCC GA | NM_001044580.1 | 57 |
| TNFAIP6 | 119 | TAT ACG ACA GTT ACG ACG AC GGA AGC ATC ACT TAG GAA T | NM_001159607.1 | 54 |
| TBP | 115 | ATA GCC TTC CAC CTT ACG CTC ATA GGC TGT GGA GTC AGT CCT | XM_021085483.1 | 58 |
| TUBA1B | 130 | AGT TTT CTG AGG CCC GTG AG TGC AGG GCT TAA AGG AAT GGT | NM_001044544.1 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procházka, R.; Bartková, A.; Němcová, L.; Murín, M.; Gad, A.; Marcollová, K.; Kinterová, V.; Lucas-Hahn, A.; Laurinčík, J. The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium. Int. J. Mol. Sci. 2021, 22, 11148. https://doi.org/10.3390/ijms222011148
Procházka R, Bartková A, Němcová L, Murín M, Gad A, Marcollová K, Kinterová V, Lucas-Hahn A, Laurinčík J. The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium. International Journal of Molecular Sciences. 2021; 22(20):11148. https://doi.org/10.3390/ijms222011148
Chicago/Turabian StyleProcházka, Radek, Alexandra Bartková, Lucie Němcová, Matej Murín, Ahmed Gad, Kateřina Marcollová, Veronika Kinterová, Andrea Lucas-Hahn, and Jozef Laurinčík. 2021. "The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium" International Journal of Molecular Sciences 22, no. 20: 11148. https://doi.org/10.3390/ijms222011148
APA StyleProcházka, R., Bartková, A., Němcová, L., Murín, M., Gad, A., Marcollová, K., Kinterová, V., Lucas-Hahn, A., & Laurinčík, J. (2021). The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium. International Journal of Molecular Sciences, 22(20), 11148. https://doi.org/10.3390/ijms222011148







