Impact of Host Immune Status on Discordant Anti-SARS-CoV-2 Circulating B Cell Frequencies and Antibody Levels
Abstract
:1. Introduction
2. Results
2.1. Plasma Titers of Anti-SARS-CoV-2 Antibodies
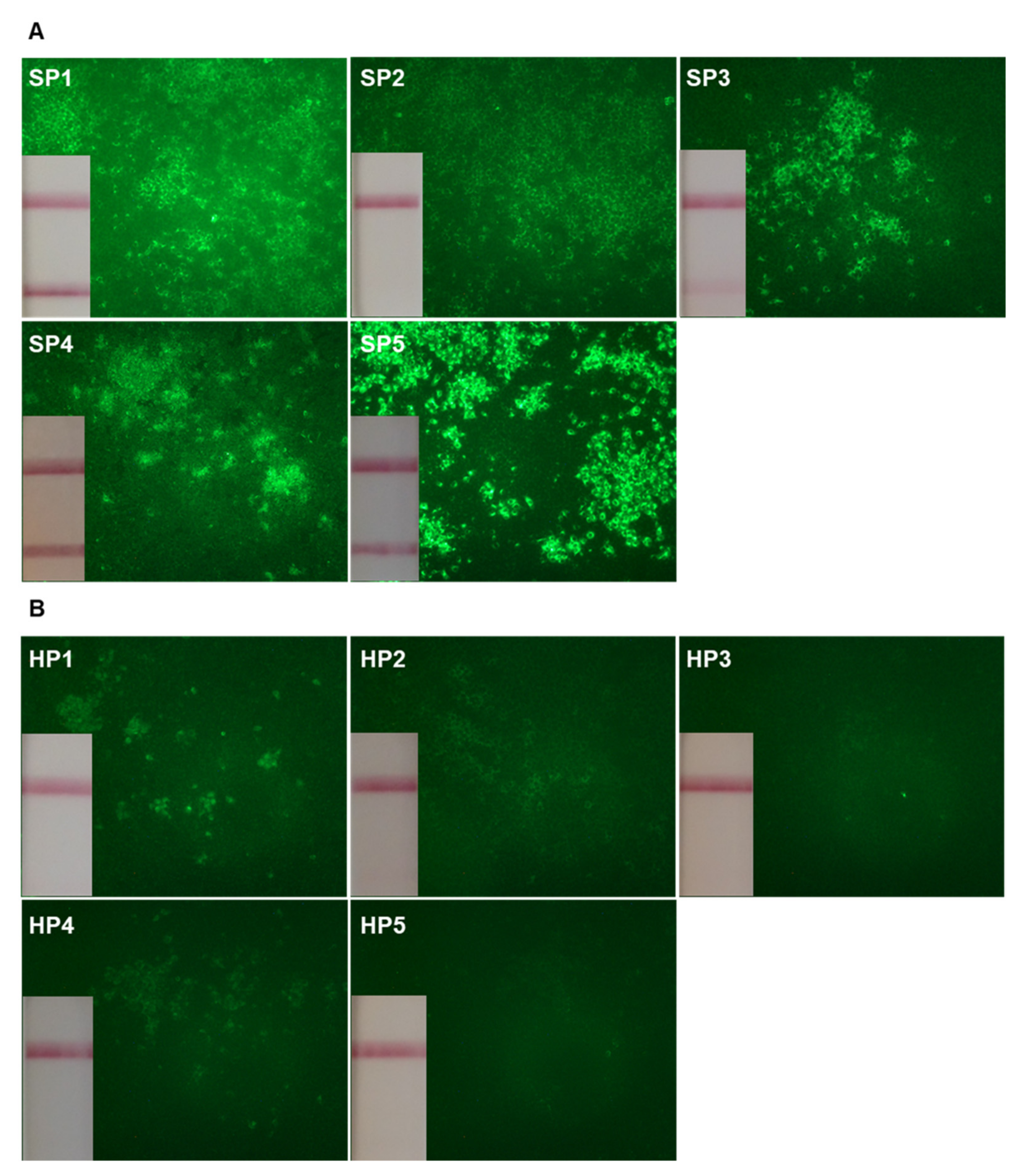
2.2. Frequencies of Circulating SARS-CoV-2 Reactive B cells
2.3. Neutralization Activity of Circulating SARS-CoV-2 Specific B Cell Clones
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Generation of Immortalized B Cell Clones
4.3. Indirect Immunofluorescence Assay
4.4. Rapid Antibody Test
4.5. ELISA
4.6. Seroneutralization Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the Human Innate Immune System. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Chen, Z.; John Wherry, E. T Cell Responses in Patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Shuwa, H.A.; Shaw, T.N.; Knight, S.B.; Wemyss, K.; McClure, F.A.; Pearmain, L.; Prise, I.; Jagger, C.; Morgan, D.J.; Khan, S.; et al. Alterations in T and B Cell Function Persist in Convalescent COVID-19 Patients. Med 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Yu, K.K.; Fischinger, S.; Smith, M.T.; Atyeo, C.; Cizmeci, D.; Wolf, C.R.; Layton, E.D.; Logue, J.K.; Aguilar, M.S.; Shuey, K.; et al. Comorbid Illnesses Are Associated with Altered Adaptive Immune Responses to SARS-CoV-2. JCI Insight 2021, 6, e146242. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Oldridge, D.A.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep Immune Profiling of COVID-19 Patients Reveals Patient Heterogeneity and Distinct Immunotypes with Implications for Therapeutic Interventions. bioRxiv 2020. [Google Scholar] [CrossRef]
- Oja, A.E.; Saris, A.; Ghandour, C.A.; Kragten, N.A.M.; Hogema, B.M.; Nossent, E.J.; Heunks, L.M.A.; Cuvalay, S.; Slot, E.; Linty, F.; et al. Divergent SARS-CoV-2-Specific T- and B-Cell Responses in Severe but Not Mild COVID-19 Patients. Eur. J. Immunol. 2020, 50, 1998–2012. [Google Scholar] [CrossRef]
- Terpos, E.; Politou, M.; Sergentanis, T.N.; Mentis, A.; Rosati, M.; Stellas, D.; Bear, J.; Hu, X.; Felber, B.K.; Pappa, V.; et al. Anti-SARS-CoV-2 Antibody Responses in Convalescent Plasma Donors Are Increased in Hospitalized Patients; Subanalyses of a Phase 2 Clinical Study. Microorganisms 2020, 8, 1885. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.B.; Gualberto, A.; Rezende, C.; Percegoni, N.; Gameiro, J.; Hottz, E.D. The Weight of Obesity in Immunity from Influenza to COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 638852. [Google Scholar] [CrossRef]
- Busetto, L.; Bettini, S.; Fabris, R.; Serra, R.; Dal Pra, C.; Maffei, P.; Rossato, M.; Fioretto, P.; Vettor, R. Obesity and COVID-19: An Italian Snapshot. Obesity 2020, 28, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Sales-Peres, S.H.d.C.; de Azevedo-Silva, L.J.; Bonato, R.C.S.; Sales-Peres, M.d.C.; Pinto, A.C.d.S.; Santiago Junior, J.F. Coronavirus (SARS-CoV-2) and the Risk of Obesity for Critically Illness and ICU Admitted: Meta-Analysis of the Epidemiological Evidence. Obes. Res. Clin. Pract. 2020, 14, 389–397. [Google Scholar] [CrossRef]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the Aging Immune System. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2021, 11, 571416. [Google Scholar] [CrossRef]
- Kolopp-Sarda, M.; Miossec, P. High Oligoclonality of Immunoglobulins in SARS-CoV2 Positive Patients. Ann. Rheum. Dis. 2020, 80, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Liu, F.; Zheng, N.S.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Chiappetta, S.; Sharma, A.M.; Bottino, V.; Stier, C. COVID-19 and the Role of Chronic Inflammation in Patients with Obesity. Int. J. Obes. 2020, 44, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Gubbi, S. COVID-19 Pandemic, Coronaviruses, and Diabetes Mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Galarza, J.; Prócel, C.; Cañadas, C.; Aguirre, D.; Pibaque, R.; Bedón, R.; Sempértegui, F.; Drexhage, H.; Baldeón, L. Immune Response to SARS-CoV-2 Infection in Obesity and T2D: Literature Review. Vaccines 2021, 9, 102. [Google Scholar] [CrossRef]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banchereau, J. Generation of Human B-Cell Lines Dependent on CD40-Ligation and Interleukin-4. Front. Immunol. 2015, 6, 55. [Google Scholar] [CrossRef]
- Banchereau, J.; de Paoli, P.; Vallé, A.; Garcia, E.; Rousset, F. Long-Term Human B Cell Lines Dependent on Interleukin-4 and Antibody to CD40. Science 1991, 251, 70–72. [Google Scholar] [CrossRef]
- Weidner, L.; Gänsdorfer, S.; Unterweger, S.; Weseslindtner, L.; Drexler, C.; Farcet, M.; Witt, V.; Schistal, E.; Schlenke, P.; Kreil, T.R.; et al. Quantification of SARS-CoV-2 Antibodies with Eight Commercially Available Immunoassays. J. Clin. Virol. 2020, 129, 104540. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic Changes in Anti-SARS-CoV-2 Antibodies during SARS-CoV-2 Infection and Recovery from COVID-19. Nat. Commun. 2020, 11, 6044. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.E.; Edwards, E.S.J.; Aui, P.M.; Varese, N.; Stojanovic, S.; McMahon, J.; Peleg, A.Y.; Boo, I.; Drummer, H.E.; Hogarth, P.M.; et al. Rapid Generation of Durable B Cell Memory to SARS-CoV-2 Spike and Nucleocapsid Proteins in COVID-19 and Convalescence. Sci. Immunol. 2020, 5, eabf8891. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-Existing Comorbidities and Therapeutic Interventions on COVID-19. Front. Immunol. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Miossec, P. Synergy Between Cytokines and Risk Factors in the Cytokine Storm of COVID-19: Does Ongoing Use of Cytokine Inhibitors Have a Protective Effect? Arthritis Rheumatol. 2020, 72, 1963–1966. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why Does COVID-19 Disproportionately Affect Older People? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Khan, M.S.H.; Dhurandhar, N.V.; Hegde, V. The Triumvirate: Why Hypertension, Obesity, and Diabetes Are Risk Factors for Adverse Effects in Patients with COVID-19. Acta Diabetol. 2021, 58, 831–843. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Y.; Chai, Y.; Cheng, W.; Wang, K.; Cao, J.; Hu, X. Association of Clinical and Immunological Characteristics With Disease Severity and Outcomes in 211 Patients With COVID-19 in Wuhan, China. Front. Cell. Infect. Microbiol. 2021, 11, 667487. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Adamo, S.; Chevrier, S.; Cervia, C.; Zurbuchen, Y.; Raeber, M.E.; Yang, L.; Sivapatham, S.; Jacobs, A.; Baechli, E.; Rudiger, A.; et al. Profound Dysregulation of T Cell Homeostasis and Function in Patients with Severe COVID-19. Allergy 2021, 76, 2866–2881. [Google Scholar] [CrossRef]
- Schub, D.; Klemis, V.; Schneitler, S.; Mihm, J.; Lepper, P.M.; Wilkens, H.; Bals, R.; Eichler, H.; Gärtner, B.C.; Becker, S.L.; et al. High Levels of SARS-CoV-2-Specific T Cells with Restricted Functionality in Severe Courses of COVID-19. JCI Insight 2020, 5, 142167. [Google Scholar] [CrossRef]
- Mann, E.R.; Menon, M.; Knight, S.B.; Konkel, J.E.; Jagger, C.; Shaw, T.N.; Krishnan, S.; Rattray, M.; Ustianowski, A.; Bakerly, N.D.; et al. Longitudinal Immune Profiling Reveals Key Myeloid Signatures Associated with COVID-19. Sci. Immunol. 2020, 5, eabd6197. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive Mapping of Immune Perturbations Associated with Severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.; Ramonell, R.; Cashman, K.; Nguyen, D.; Ley, A.; Kyu, S.; Saini, A.; Haddad, N.; Chen, W.; Howell, J.C.; et al. Critically Ill SARS-CoV-2 Patients Display Lupus-like Hallmarks of Extrafollicular B Cell Activation. medRxiv 2020. [Google Scholar] [CrossRef]
- Farris, A.D.; Guthridge, J.M. Overlapping B Cell Pathways in Severe COVID-19 and Lupus. Nat. Immunol. 2020, 21, 1478–1480. [Google Scholar] [CrossRef]
- Choe, J.; Choi, Y.S. IL-10 Interrupts Memory B Cell Expansion in the Germinal Center by Inducing Differentiation into Plasma Cells. Eur. J. Immunol. 1998, 28, 508–515. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.-W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Fischinger, S.; Zohar, T.; Slein, M.D.; Burke, J.; Loos, C.; McCulloch, D.J.; Newman, K.L.; Wolf, C.; Yu, J.; et al. Distinct Early Serological Signatures Track with SARS-CoV-2 Survival. Immunity 2020, 53, 524–532. [Google Scholar] [CrossRef]
- Legros, V.; Denolly, S.; Vogrig, M.; Boson, B.; Siret, E.; Rigaill, J.; Pillet, S.; Grattard, F.; Gonzalo, S.; Verhoeven, P.; et al. A Longitudinal Study of SARS-CoV-2-Infected Patients Reveals a High Correlation between Neutralizing Antibodies and COVID-19 Severity. Cell. Mol. Immunol. 2021, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
| Health Care Personnel a | Severe Patients a | p-Value | |
|---|---|---|---|
| N | 5 | 5 | |
| Sex (M/F) | 2/3 | 4/1 | 0.524 |
| Age, years; median (min–max) | 54 (27–60) | 81 (48–91) | 0.048 |
| > 65 years (%) | 0 (0) | 4 (80) | 0.048 |
| Hypertension (%) | 1 (20) | 5 (100) | 0.048 |
| Diabetes mellitus (%) | 0 (0) | 2 (40) | 0.444 |
| Obesity (%) | 1 (20) | 3 (60) | 0.524 |
| Symptoms onset to sampling, days; median (min–max) | 39 (27–45) | 20 (15–38) | 0.032 |
| Antiviral Ab titers b, log10 (IIF); median (min–max) | 1.7 (1.4–2.2) | 2.3 (2.2–2.6) | 0.024 |
| Anti-RBD total Ab, (rapid test) c; median (min–max) | 0 (0–1) | 3 (0–4) | 0.048 |
| Anti-RBD total Ab titers, log10 (ELISA); median (min–max) | 0.9 (0.8–0.9) | 1.9 (1.7–2.2) | 0.008 |
| Anti-NP total Ab titers, log10 (ELISA); median (min–max) | 1.7 (0.1–2.4) | 2.8 (2.6–3.6) | 0.008 |
| Frequency of antiviral B cell clones d; median (min–max) | 0.5 (0.3–1.8) | 1.0 (0.3–9.0) | 0.309 |
| Patient | Screened B Cell Clones | SARS-CoV-2 Reactive B Cell Clones | Neutralizing B Cell Clones | Clone | Mono-Clonal | Oligo-Clonal | Isotype | Antigenic Specificity |
|---|---|---|---|---|---|---|---|---|
| HP2 | 45,000 | 26 | 3 | 3E7 | ✔ | IgG3κ | RBD+S1+ S2−NP−E−PP− | |
| 2G5.B1 | ✔ | IgAM,κ | RBD−S1+ S2−NP−E−PP− | |||||
| 2D11.E1 | ✔ | IgAκ | RBD+S1− S2−NP−E−PP− | |||||
| HP4 | 15,500 | 28 | 1 | 5B9.A1 | ✔ | IgGAMκ | RBD−S1− S2−NP−E−PP− | |
| SP2 | 25,000 | 38 | 3 | 3D9 | ✔ | IgMκ | RBD−S1− S2−NP−E−PP− | |
| 3G10.A2 | ✔ | IgG4κ, IgMλ | RBD−S1− S2−NP−E−PP− | |||||
| 1F4.C2 | ✔ | IgG4κ | RBD+S1+ S2−NP−E−PP− | |||||
| SP4 | 49,000 | 15 | 3 | 3F7.F1 | ✔ | IgG4κ | RBD+S1+ S2−NP−E−PP− | |
| 3H2.F1 | ✔ | IgG1κ | RBD+S1+ S2−NP−E−PP− | |||||
| 2H2.F10 | ✔ | IgG1κ | RBD+S1+ S2−NP−E−PP | |||||
| SP5 | 36,000 | 34 | 1 | 3B12.A6 | ✔ | IgGAMκ | RBD+S1+ S2−NP−E−PP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutant, F.; Pin, J.-J.; Morfin-Sherpa, F.; Ferry, T.; Paul, S.; Pozzetto, B.; Normand, M.; Miossec, P. Impact of Host Immune Status on Discordant Anti-SARS-CoV-2 Circulating B Cell Frequencies and Antibody Levels. Int. J. Mol. Sci. 2021, 22, 11095. https://doi.org/10.3390/ijms222011095
Coutant F, Pin J-J, Morfin-Sherpa F, Ferry T, Paul S, Pozzetto B, Normand M, Miossec P. Impact of Host Immune Status on Discordant Anti-SARS-CoV-2 Circulating B Cell Frequencies and Antibody Levels. International Journal of Molecular Sciences. 2021; 22(20):11095. https://doi.org/10.3390/ijms222011095
Chicago/Turabian StyleCoutant, Frédéric, Jean-Jacques Pin, Florence Morfin-Sherpa, Tristan Ferry, Stéphane Paul, Bruno Pozzetto, Myriam Normand, and Pierre Miossec. 2021. "Impact of Host Immune Status on Discordant Anti-SARS-CoV-2 Circulating B Cell Frequencies and Antibody Levels" International Journal of Molecular Sciences 22, no. 20: 11095. https://doi.org/10.3390/ijms222011095
APA StyleCoutant, F., Pin, J.-J., Morfin-Sherpa, F., Ferry, T., Paul, S., Pozzetto, B., Normand, M., & Miossec, P. (2021). Impact of Host Immune Status on Discordant Anti-SARS-CoV-2 Circulating B Cell Frequencies and Antibody Levels. International Journal of Molecular Sciences, 22(20), 11095. https://doi.org/10.3390/ijms222011095






