Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data
Abstract
:1. Introduction
2. Results
2.1. Preclinical Safety and Efficacy Results
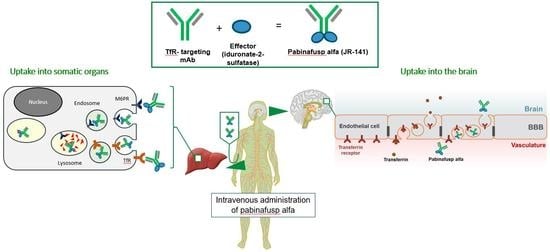
2.1.1. Mechanism of Action: Cellular Uptake and BBB Penetration
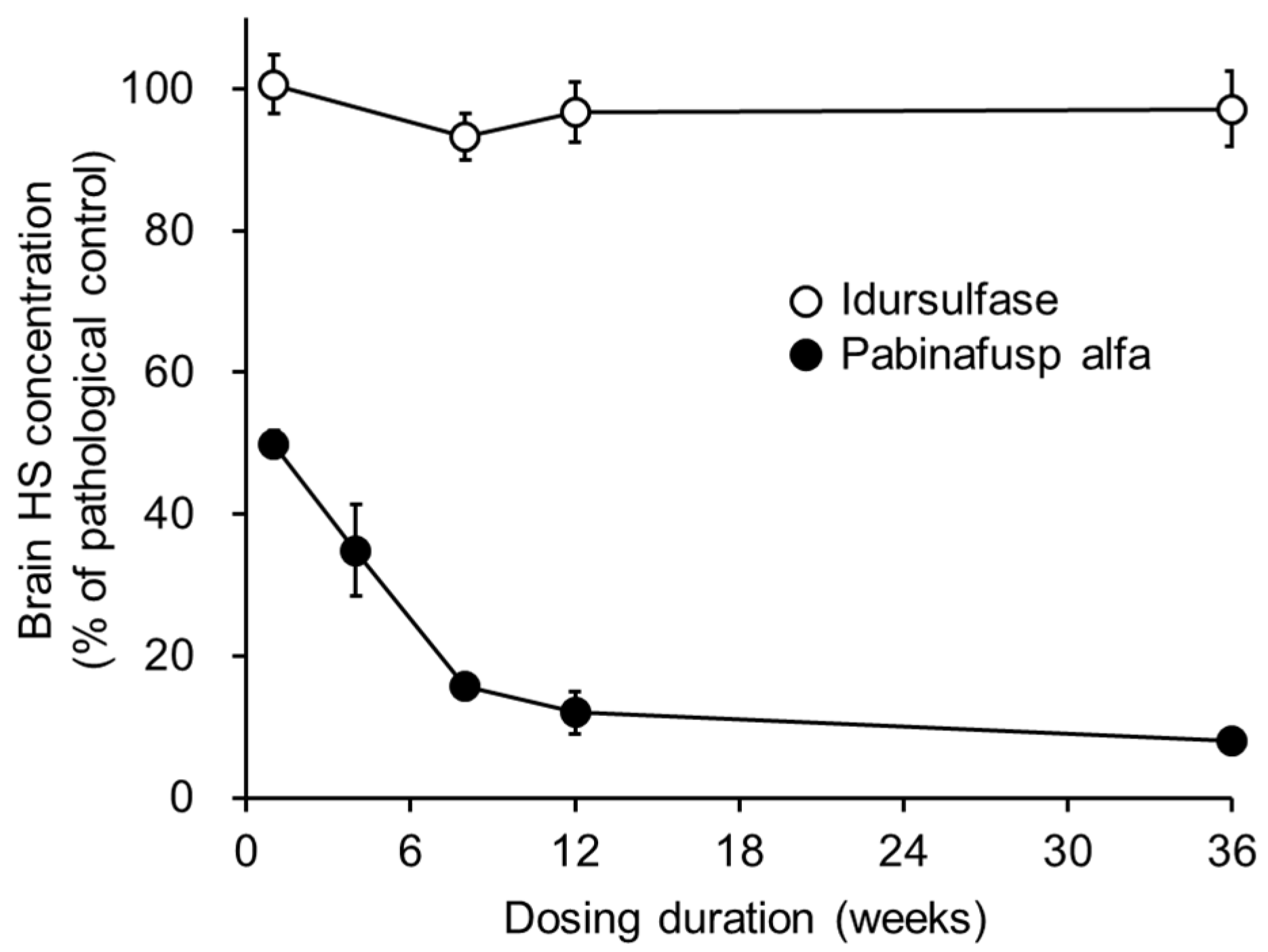
2.1.2. Substrate Reduction
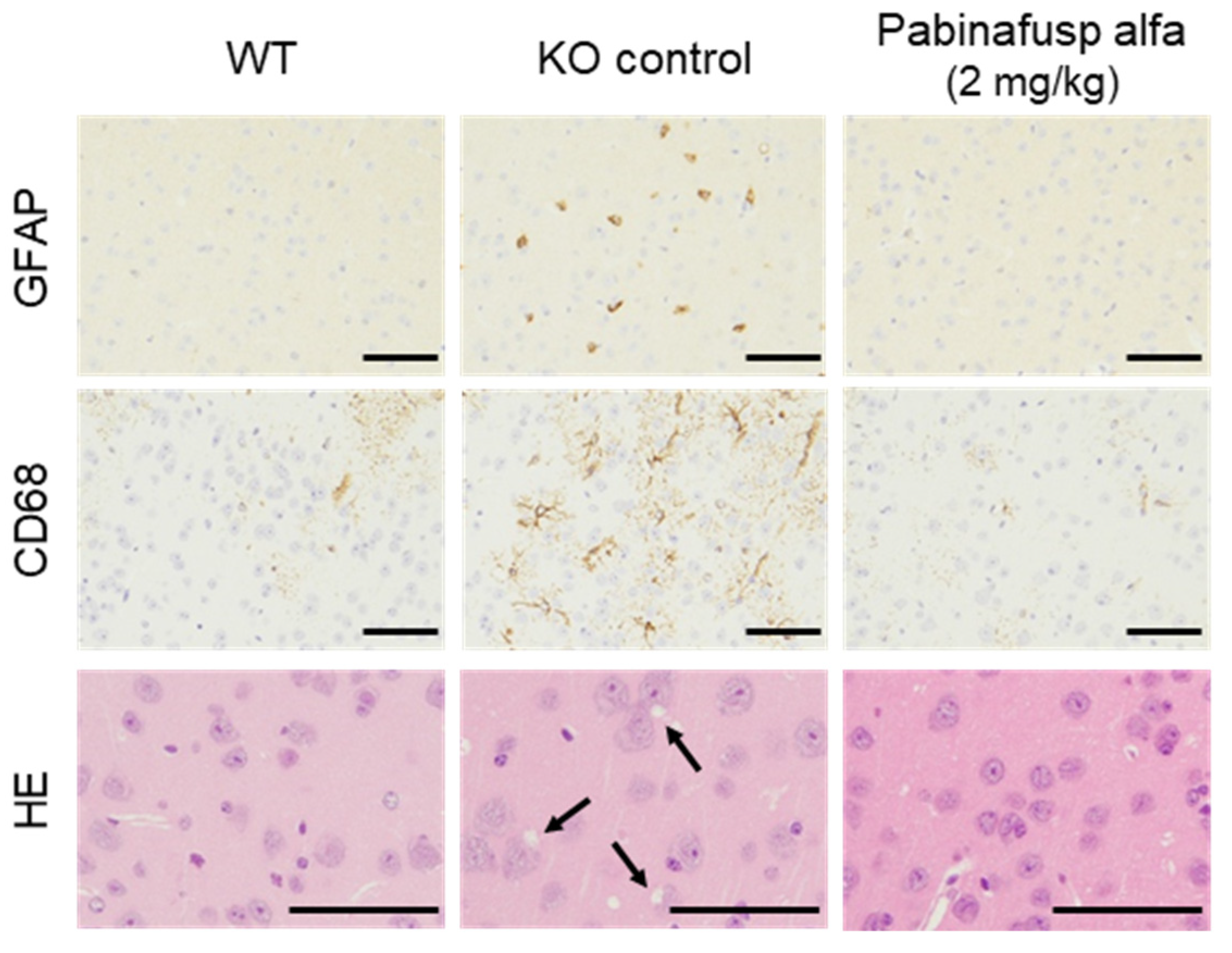
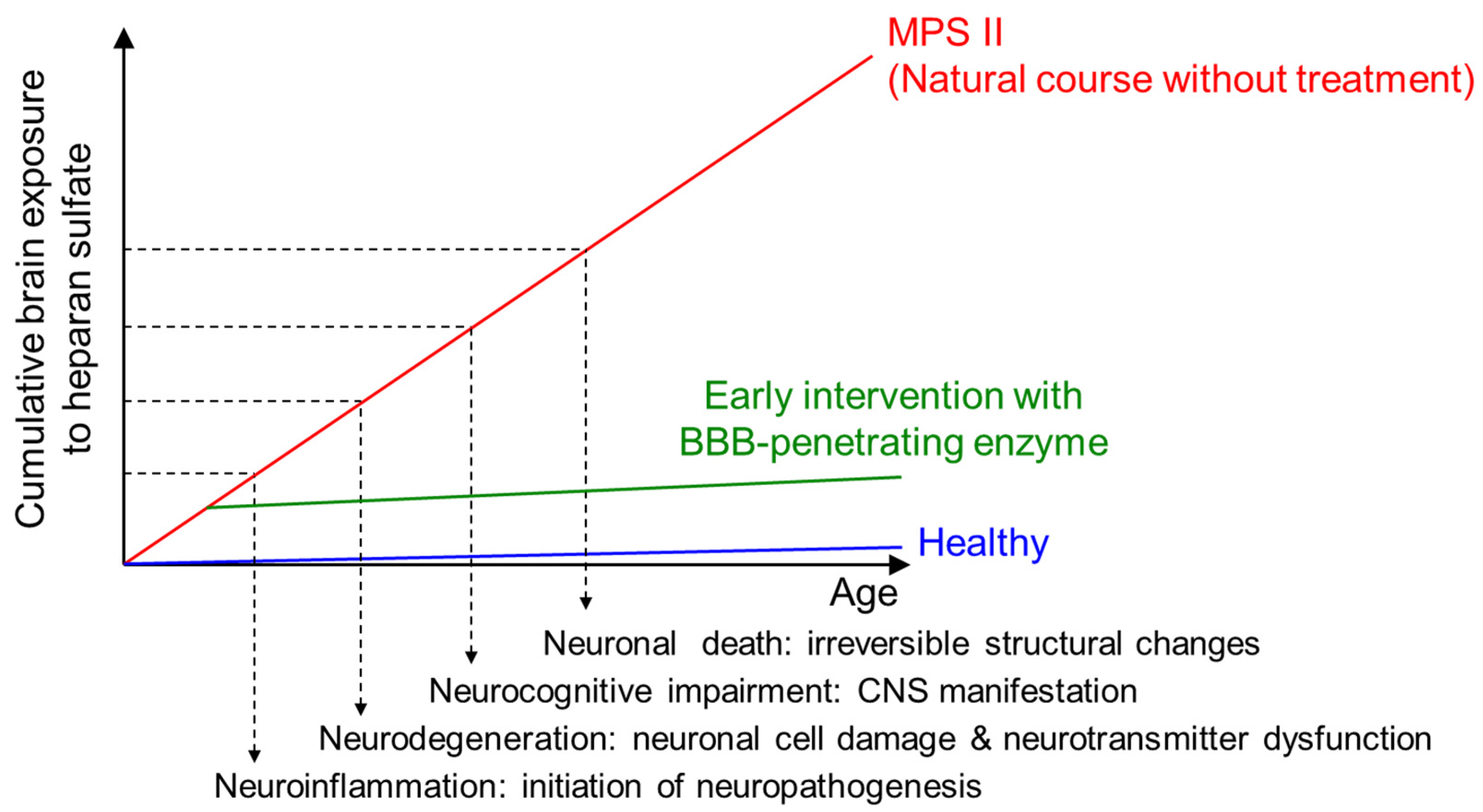
2.1.3. Prevention of Neuroinflammation and Subsequent Neurodegeneration
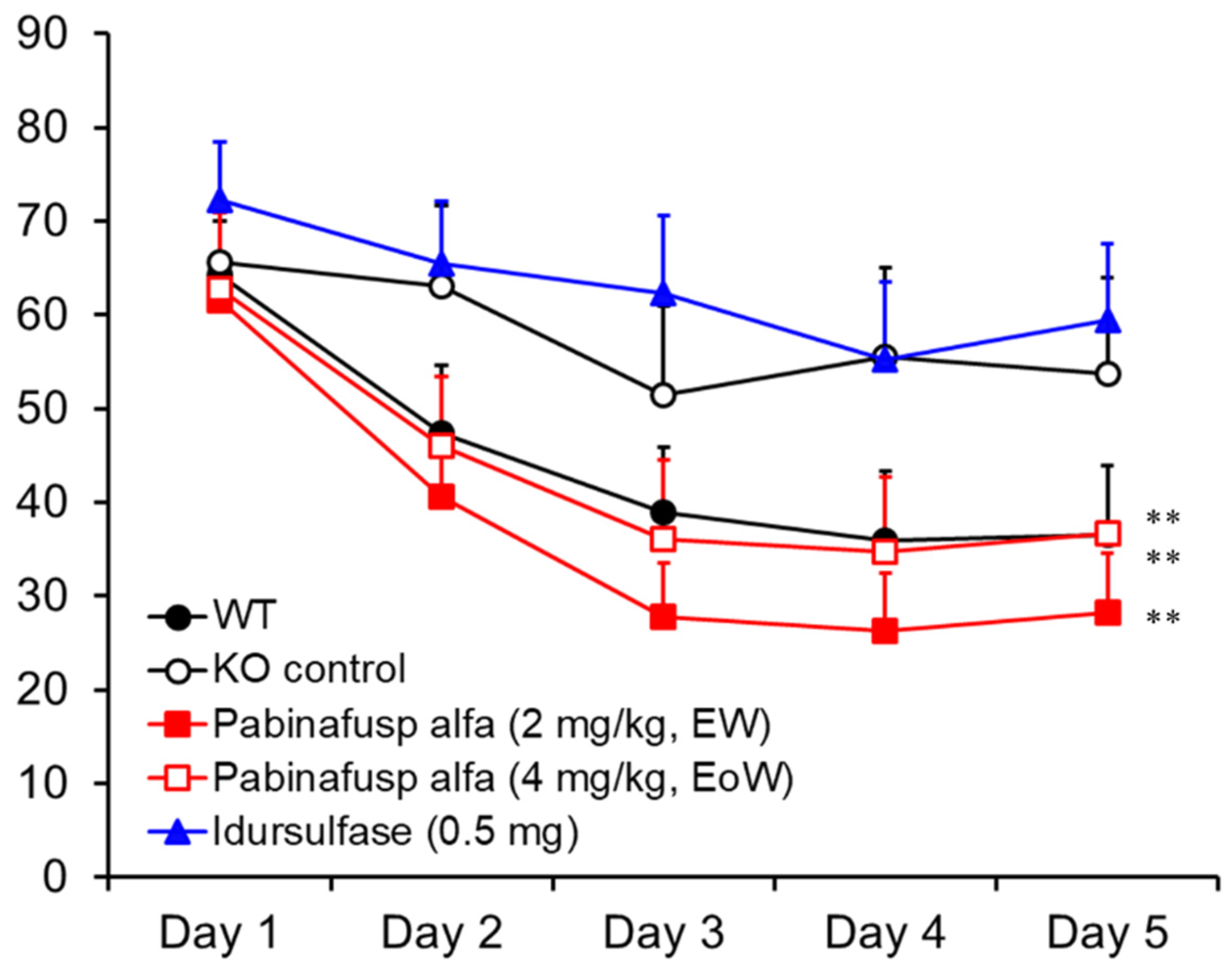
2.1.4. Prevention of Neurocognitive Abnormalities
2.1.5. Identification of biomarker for CNS efficacy
2.2. Preclinical Safety Results
2.3. Clinical Results
2.3.1. Clinical Efficacy Data
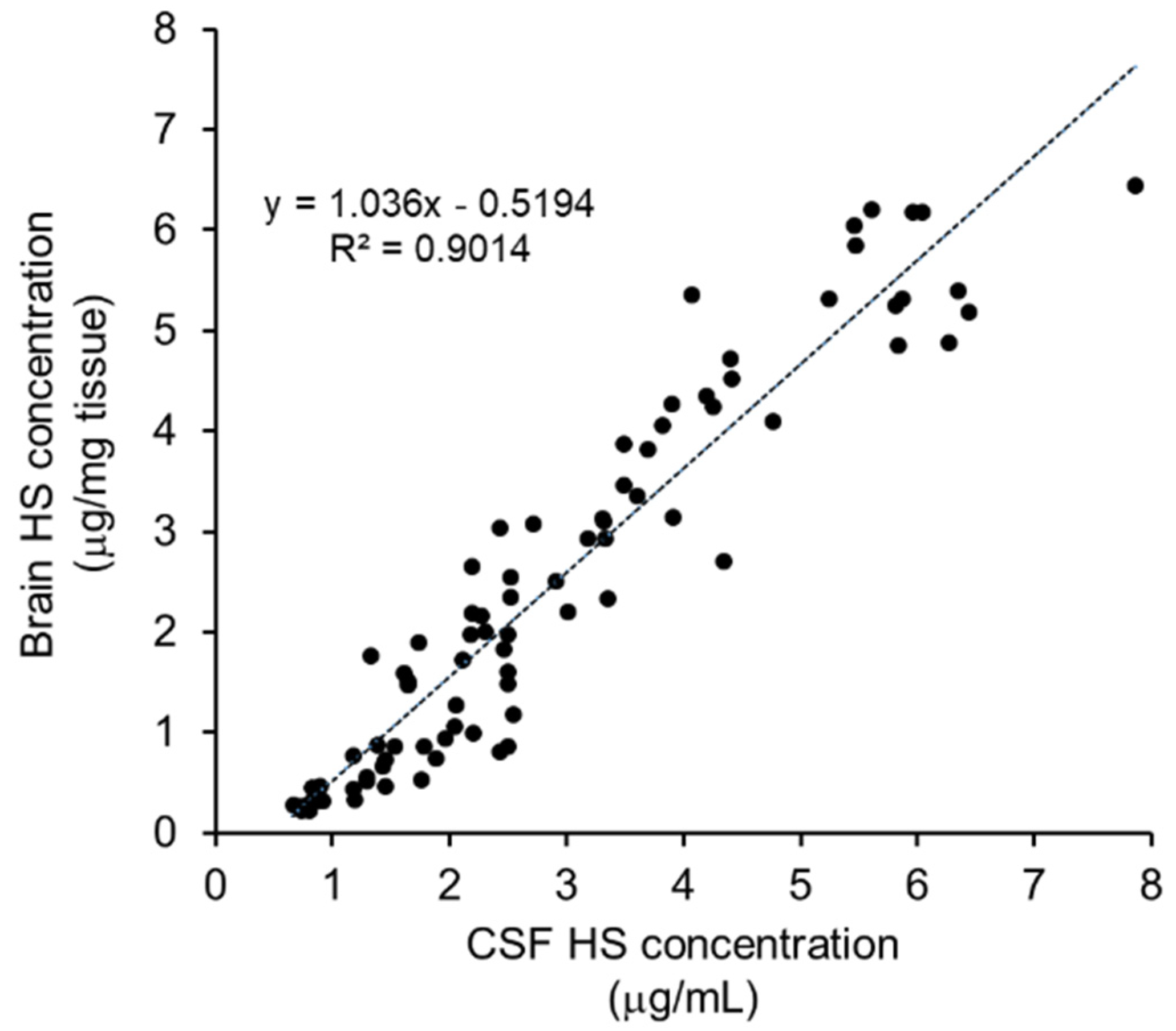
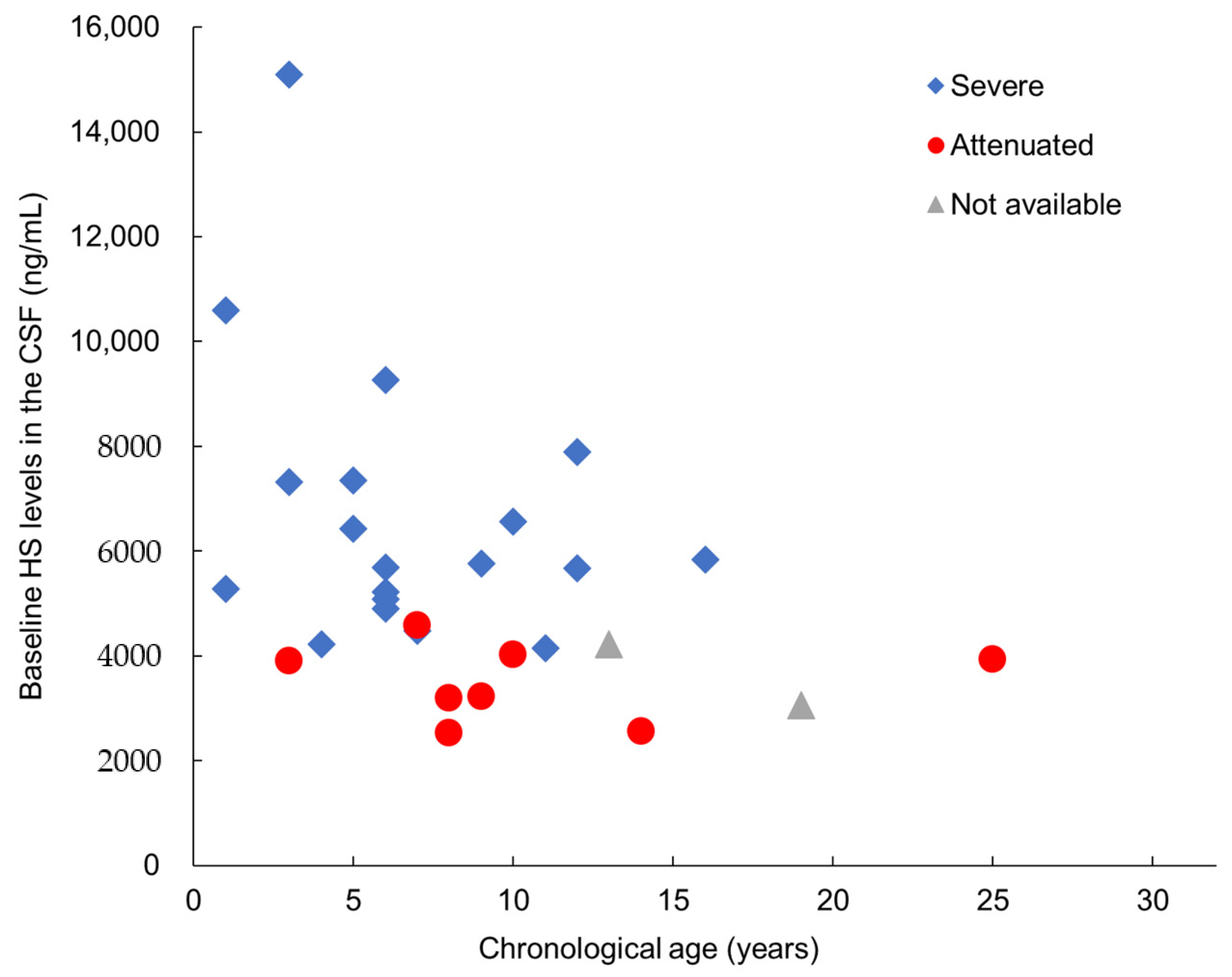
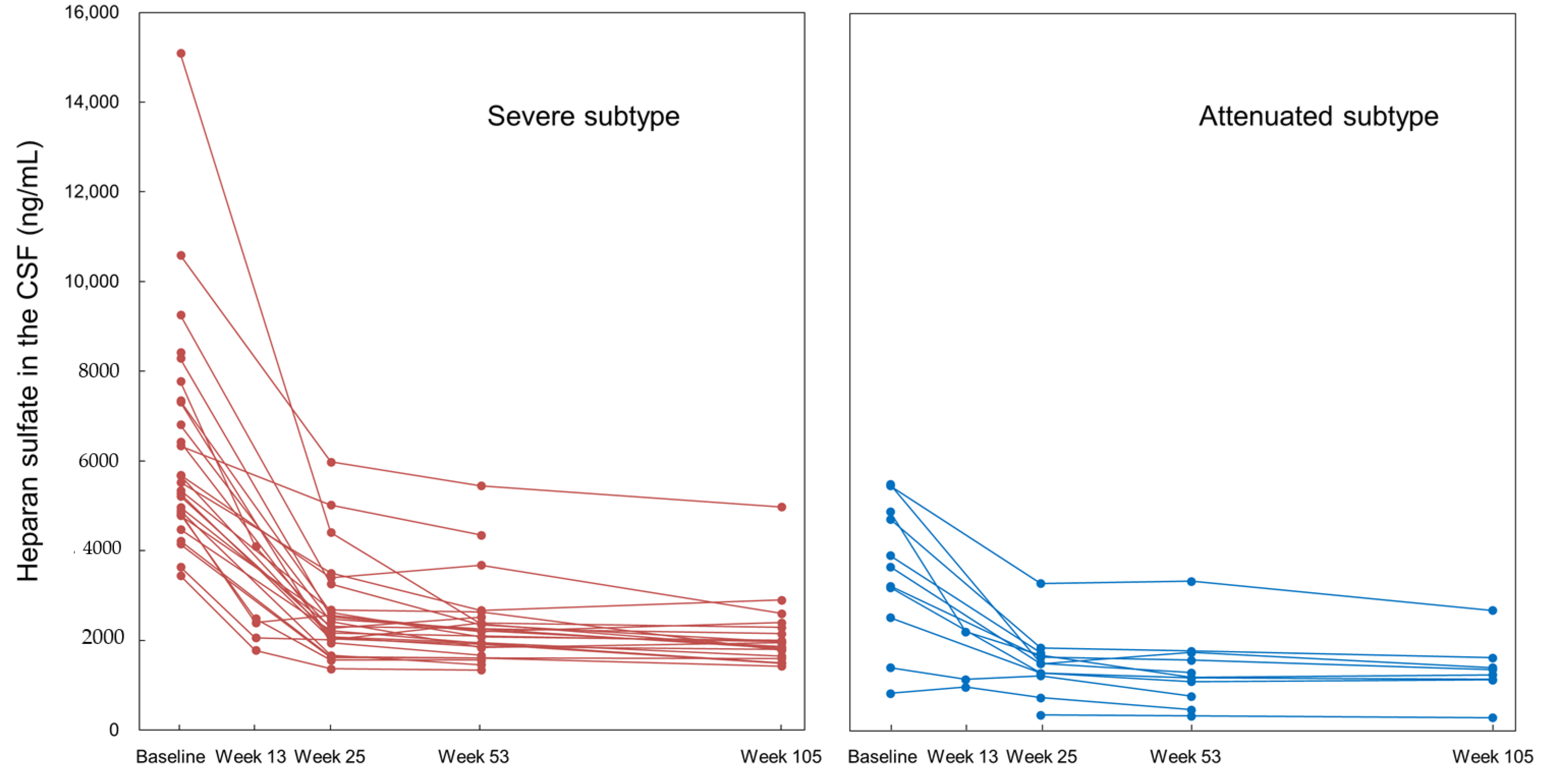
Substrate Reduction in the CSF
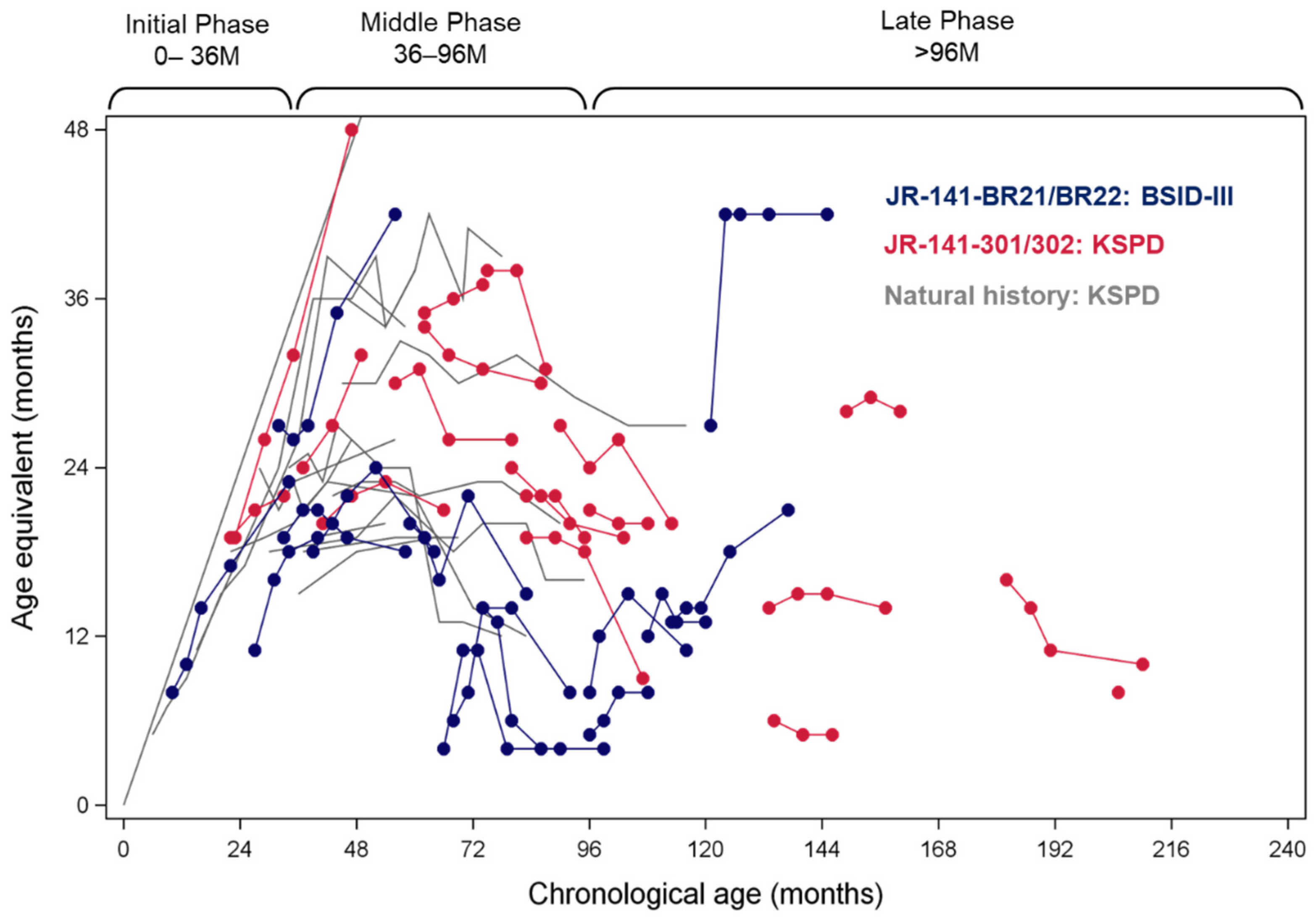
Neurocognitive Efficacy
Somatic Efficacy
2.3.2. Clinical Safety Data
3. Discussion
4. Materials and Methods
4.1. Preclinical Studies
4.1.1. Animals
4.1.2. BBB Penetration
4.1.3. Substrate Reduction
4.1.4. Evaluation of Neuroinflammation and Neurodegeneration
4.1.5. Evaluation of Neurocognitive Abnormalities
4.1.6. Safety
4.2. Clinial Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigger, B.W.; Begley, D.J.; Virgintino, D.; Pshezhetsky, A.V. Anatomical changes and pathophysiology of the brain in mucopolysaccharidosis disorders. Mol. Genet. Metab. 2018, 125, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Jakobkiewicz-Banecka, J.; Gabig-Ciminska, M.; Kloska, A.; Malinowska, M.; Piotrowska, E.; Banecka-Majkutewicz, Z.; Banecki, B.; Wegrzyn, A.; Wegrzyn, G. Glycosaminoglycans and mucopolysaccharidosis type III. Front. Biosci. 2016, 21, 1393–1409. [Google Scholar] [CrossRef]
- Sato, Y.; Okuyama, T. Novel Enzyme Replacement Therapies for Neuropathic Mucopolysaccharidoses. Int. J. Mol. Sci. 2020, 21, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, B.K.; Jego, V.; Mikl, J.; Jones, S.A. Survival in idursulfase-treated and untreated patients with mucopolysaccharidosis type II: Data from the Hunter Outcome Survey (HOS). J. Inherit. Metab. Dis. 2017, 40, 867–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muenzer, J.; Hendriksz, C.J.; Fan, Z.; Vijayaraghavan, S.; Perry, V.; Santra, S.; Solanki, G.A.; Mascelli, M.A.; Pan, L.; Wang, N.; et al. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med. 2016, 18, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Eisengart, J.B.; Pierpont, E.I.; Kaizer, A.M.; Rudser, K.D.; King, K.E.; Pasquali, M.; Polgree, L.E.; Dickson, P.I.; Le, S.Q.; Miller, W.P.; et al. Intrathecal enzyme replacement for Hurler syndrome: Biomarker association with neurocognitive outcomes. Genet. Med. 2019, 21, 2552–2560. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Kosuga, M.; Hamazaki, T.; Shintaku, H.; Okuyama, T. Impact of intracerebroventricular enzyme replacement therapy in patients with neuronopathic mucopolysaccharidosis type II. Mol. Ther. Methods Clin. Dev. 2021, 21, 67–75. [Google Scholar] [CrossRef]
- Boado, R.J.; Hui, E.K.-W.; Lu, J.Z.; Pardridge, W.M. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: Pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol. Bioeng. 2014, 111, 2317–2325. [Google Scholar] [CrossRef] [Green Version]
- Giugliani, R.; Giugliani, L.; de Oliveira Poswar, F.; Donis, K.C.; Corte, A.D.; Schmidt, M.; Boado, R.J.; Nestrasil, I.; Nguyen, C.; Chen, S.; et al. Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): An open label phase 1-2 trial. Orphanet. J. Rare Dis. 2018, 13, 110. [Google Scholar] [CrossRef]
- Couch, J.A.; Yu, Y.J.; Zhang, Y.; Tarrant, J.M.; Fuji, R.N.; Meilandt, W.J.; Solanoy, H.; Tong, R.K.; Hoyte, K.; Luk, W.; et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 2013, 5, 183ra57. [Google Scholar] [CrossRef]
- Sonoda, H.; Morimoto, H.; Yoden, E.; Koshimura, Y.; Kinoshita, M.; Golovina, G.; Takagi, H.; Yamamoto, R.; Minami, K.; Mizoguchi, A.; et al. A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018, 26, 1366–1374. [Google Scholar] [CrossRef]
- Tanaka, N.; Kida, S.; Kinoshita, M.; Morimoto, H.; Shibasaki, T.; Tachibana, K.; Yamamoto, R. Evaluation of cerebrospinal fluid heparan sulfate as a biomarker of neuropathology in a murine model of mucopolysaccharidosis type II using high-sensitivity LC/MS/MS. Mol. Genet. Metab. 2018, 125, 53–58. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Minami, K.; Yamamoto, T.; Sonoda, H.; Yamaoka, M.; Tachibana, K.; Hirato, T.; Sato, Y. Iduronate-2-Sulfatase with Antihuman Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: A Phase 1/2 Trial. Mol. Ther. 2019, 27, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Giugliani, R.; Martins, A.M.; So, S.; Yamamoto, T.; Yamaoka, M.; Ikeda, T.; Tanizawa, K.; Sonoda, H.; Schmidt, M.; Sato, Y. Iduronate-2-sulfatase fused with anti-hTfR antibody, pabinafusp alfa, for MPS-II: A phase 2 trial in Brazil. Mol. Ther. 2021, 29, 2378–2386. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Nakamura, K.; Yamamoto, T.; Yamaoka, M.; Ikeda, T.; So, S.; Tanizawa, K.; Sonoda, H.; et al. A phase 2/3 trial of pabinafusp alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther. 2021, 29, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Receptor-mediated peptide transport through the bloodbrain barrier. Endocr. Rev. 1986, 7, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Eisenberg, J.; Yang, J. Human blood-brain barrier transferrin receptor. Metabolism 1987, 36, 892–895. [Google Scholar] [CrossRef]
- Neufeld, E.F.; Muenzer, J. The Mucopolysaccharidoses. In The Metabolic & Molecular Bases of Inherited Disease; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Tylki-Szymanska, A. Mucopolysaccharidosis type II, Hunter’s syndrome. Pediatr. Endocrinol. Rev. 2014, 12 (Suppl. 1), 107–113. [Google Scholar] [PubMed]
- Morimoto, H.; Kida, S.; Yoden, E.; Kinoshita, M.; Tanaka, N.; Yamamoto, R.; Koshimura, Y.; Takagi, H.; Takahashi, K.; Hirato, T.; et al. Clearance of heparan sulfate in the brain prevents neurodegeneration and neurocognitive impairment in MPS II mice. Mol. Ther. 2021, 29, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Fusar Poli, E.; Zalfa, C.; D’Avanzo, F.; Tomanin, R.; Carlessi, L.; Bossi, M.; Nodari, L.R.; Binda, E.; Marmiroli, P.; Scarpa, M.; et al. Murine neural stem cells model Hunter disease in vitro: Glial cell-mediated neurodegeneration as a possible mechanism involved. Cell Death Dis. 2013, 4, e906. [Google Scholar] [CrossRef] [Green Version]
- Zalfa, C.; Verpelli, C.; D’Avanzo, F.; Tomanin, R.; Vicidomini, C.; Cajola, L.; Manara, R.; Sala, C.; Scarpa, M.; Vescovi, A.L.; et al. Glial degeneration with oxidative damage drives neuronal demise in MPSII disease. Cell Death Dis. 2016, 7, e2331. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, R.; De Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- Jiménez, A.J.; Domínguez-Pinos, M.D.; Guerra, M.M.; Fernández-Llebrez, P.; Pérez-Fígares, J.M. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2014, 2, e28426. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [Green Version]
- Yamanoto, R.; Yoden, E.; Tanaka, N.; Kinoshita, M.; Imakiire, A.; Hirato, T.; Minami, K. Nonclinical safety evaluation of pabinafusp alfa, an anti-human transferrin receptor antibody and iduronate-2-sulfatase fusion protein, for the treatment of neuronopathic mucopolysaccharidosis type II. Mol. Genet. Metab. Rep. 2021, 27, 100758. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Okamoto, S.; Seto, T.; Hamazaki, T.; So, S.; Yamamoto, T.; Tanizawa, K.; Sonoda, H.; Sato, Y. Divergent developmental trajectories in two siblings with neuropathic mucopolysaccharidosis type II (Hunter syndrome) receiving conventional and novel enzyme replacement therapies: A case report. JIMD Rep. 2021, in press. [Google Scholar] [CrossRef]
- Seo, J.-H.; Okuyama, T.; Shapiro, E.; Fukuhara, Y.; Kosuga, M. Natural history of cognitive development in neuronopathic mucopolysaccharidosis type II (Hunter syndrome): Contribution of genotype to cognitive developmental course. Mol. Gen. Metab. Rep. 2020, 24, 100630. [Google Scholar] [CrossRef] [PubMed]
- Bowlby, J. Attachment, 2nd ed.; Basic Books: New York, NY, USA, 1969. [Google Scholar]

| Week | Changes in AE scores | Japan | Brazil | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Severe | Attenuated | Total | Severe | Attenuated | Total | |||
| Week 52 | Improvement | 2 (11%) | 8 (100%) | 10 (37%) | 8 (57%) | 5 (100%) | 13 (68%) | 23 (50%) |
| Stabilization | 13 (68%) | 0 | 13 (48%) | 5 (36%) | 0 | 5 (26%) | 18 (39%) | |
| Deterioration | 4 (22%) | 0 | 4 (15%) | 1 (7%) | 0 | 1 (5%) | 5 (11%) | |
| Week 104 | Improvement | 4 (27%) | 1 (100%) | 5 (31%) | 6 (50%) | 5 (100%) | 11 (65%) | 16 (48%) |
| Stabilization | 5 (33%) | 0 | 5 (31%) | 4 (33%) | 0 | 4 (24%) | 9 (27%) | |
| Deterioration | 6 (40%) | 0 | 6 (38%) | 2 (17%) | 0 | 2 (12%) | 8 (24%) | |
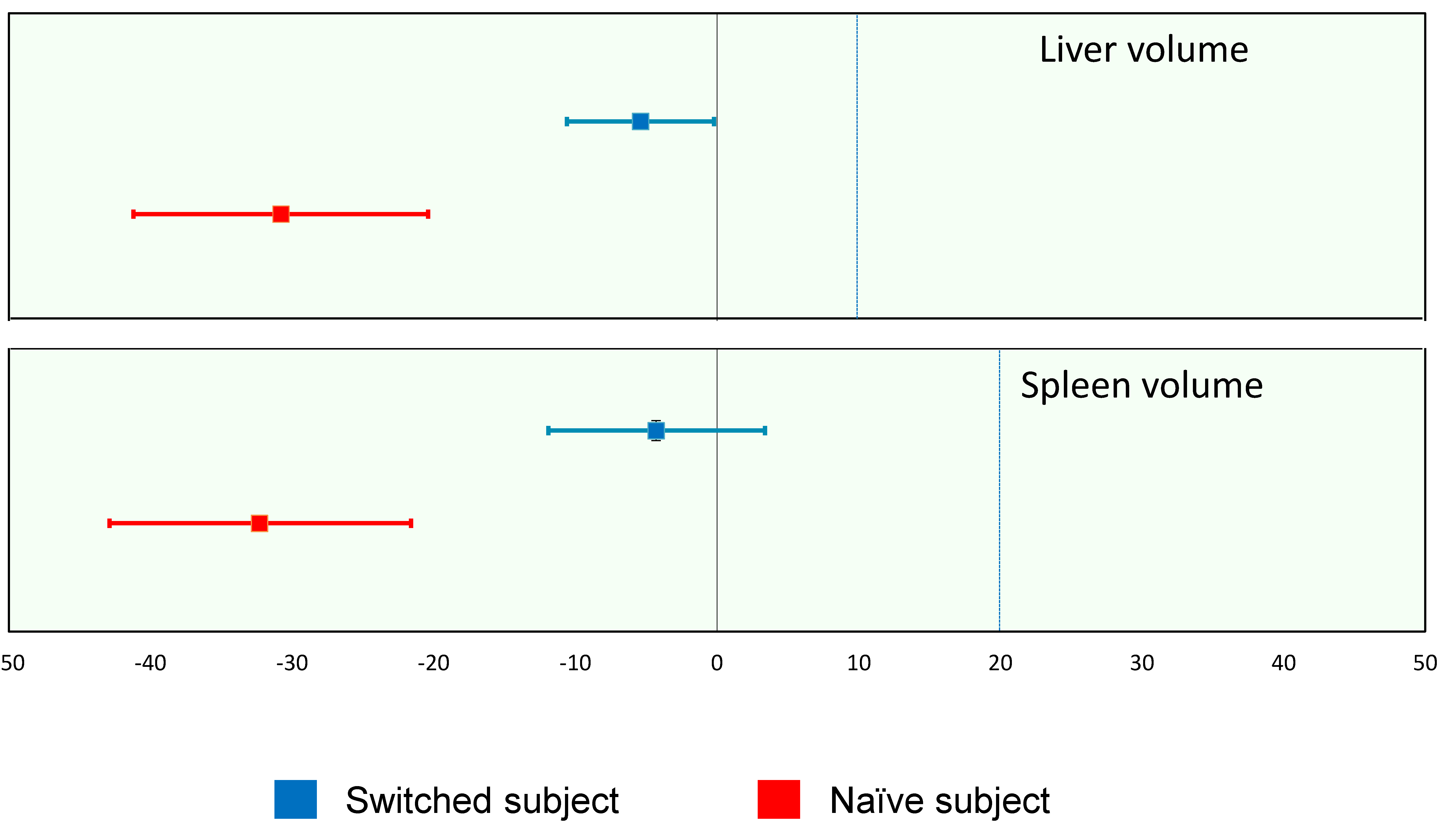
| Organs | ERT Status | n | Mean (SD) | Median [Min–Max] | 95% CI |
|---|---|---|---|---|---|
| Liver volume | Switched subjects | 28 | −5.4 (13.3) | −6.0 [−38.0–23.4] | −10.6–−0.2 |
| Naïve subjects | 4 | −30.8 (6.5) | −31.1 [−38.4–−22.6] | −41.2–−20.4 | |
| Spleen volume | Switched subjects | 28 | −4.3 (19.7) | −3.7 [−62.2–31.6] | −11.9–3.4 |
| Naïve subjects | 4 | −32.3 (6.7) | −33.3 [−38.7–−23.8] | −42.9–−21.6 |
| Phase I/II Study in Japan | Phase II/III Study in Japan | Phase II Study in Brazil | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Events/Reactions | n | Proportion (%) | Number of Events | n | Proportion (%) | Number of Events | n | Proportion (%) | Number of Events | n | Proportion (%) | Number of Events |
| Number of subjects | 14 | -- | -- | 28 | -- | -- | 20 | -- | -- | 62 | -- | -- |
| Adverse events | 9 | 64.3 | 20 | 28 | 100.0 | 340 | 20 | 100.0 | 202 | 57 | 91.9 | 562 |
| Serious adverse events | 1 | 7.1 | 1 | 5 | 17.9 | 10 | 7 | 35.0 | 7 | 13 | 21.0 | 18 |
| (Deaths) | 0 | 0.0 | 0 | 1 | 3.6 | 2 | 1 | 5.0 | 1 | 2 | 3.2 | 3 |
| Significant adverse events | 4 | 28.6 | 8 | 17 | 60.7 | 61 | 11 | 55.0 | 47 | 32 | 51.6 | 116 |
| (Infusion associated reaction) | 4 | 28.6 | 8 | 14 | 50.0 | 51 | 10 | 50.0 | 45 | 28 | 45.2 | 104 |
| Adverse drug reactions | 7 | 50.0 | 11 | 15 | 53.6 | 59 | 11 | 55.0 | 46 | 33 | 53.2 | 116 |
| Serious adverse drug reactions | 1 | 7.1 | 1 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 1 | 1.6 | 1 |
| (Deaths) | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 |
| Significant adverse drug reactions | 4 | 28.6 | 8 | 14 | 50.0 | 51 | 10 | 50.0 | 45 | 28 | 45.2 | 104 |
| (Infusion associated reaction) | 4 | 28.6 | 8 | 14 | 50.0 | 51 | 10 | 50.0 | 45 | 28 | 45.2 | 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giugliani, R.; Martins, A.M.; Okuyama, T.; Eto, Y.; Sakai, N.; Nakamura, K.; Morimoto, H.; Minami, K.; Yamamoto, T.; Yamaoka, M.; et al. Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data. Int. J. Mol. Sci. 2021, 22, 10938. https://doi.org/10.3390/ijms222010938
Giugliani R, Martins AM, Okuyama T, Eto Y, Sakai N, Nakamura K, Morimoto H, Minami K, Yamamoto T, Yamaoka M, et al. Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data. International Journal of Molecular Sciences. 2021; 22(20):10938. https://doi.org/10.3390/ijms222010938
Chicago/Turabian StyleGiugliani, Roberto, Ana Maria Martins, Torayuki Okuyama, Yoshikatsu Eto, Norio Sakai, Kimitoshi Nakamura, Hideto Morimoto, Kohtaro Minami, Tatsuyoshi Yamamoto, Mariko Yamaoka, and et al. 2021. "Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data" International Journal of Molecular Sciences 22, no. 20: 10938. https://doi.org/10.3390/ijms222010938
APA StyleGiugliani, R., Martins, A. M., Okuyama, T., Eto, Y., Sakai, N., Nakamura, K., Morimoto, H., Minami, K., Yamamoto, T., Yamaoka, M., Ikeda, T., So, S., Tanizawa, K., Sonoda, H., Schmidt, M., & Sato, Y. (2021). Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data. International Journal of Molecular Sciences, 22(20), 10938. https://doi.org/10.3390/ijms222010938