Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Results
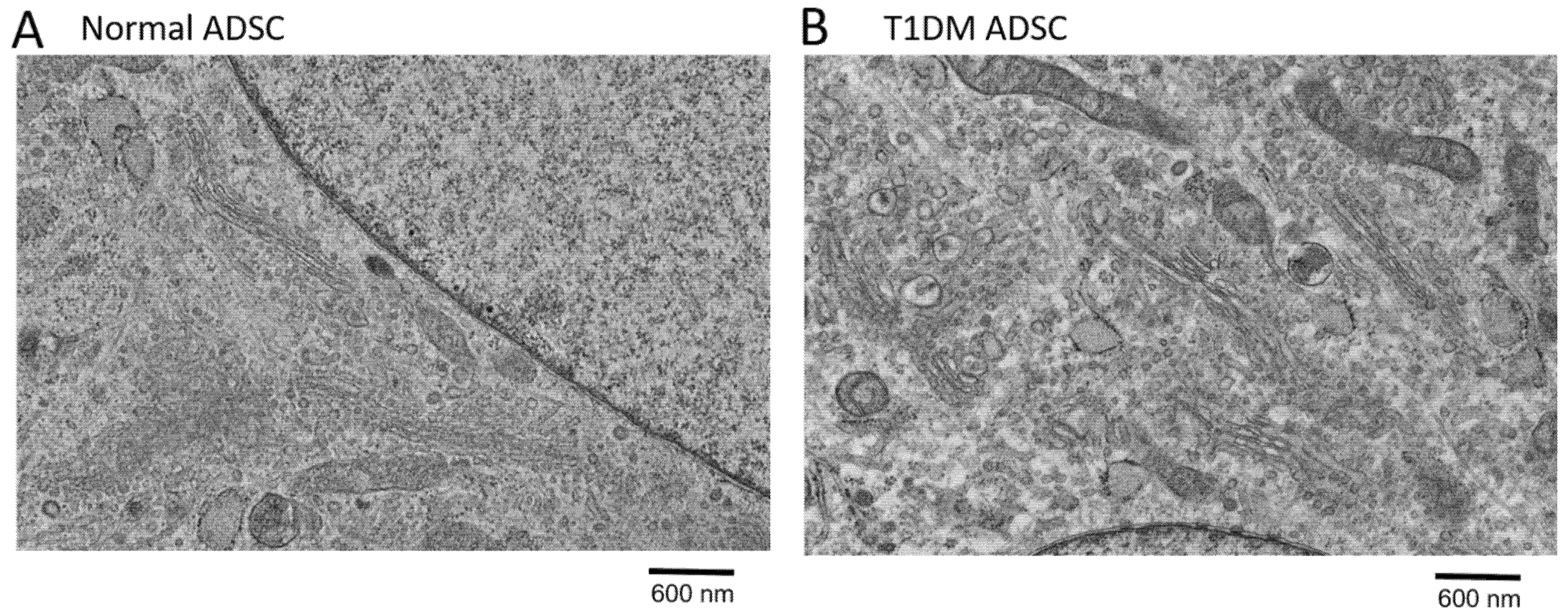
2.1. Degeneration of Organelles in Stem Cells Derived from Patients with Type 1 Diabetes Mellitus
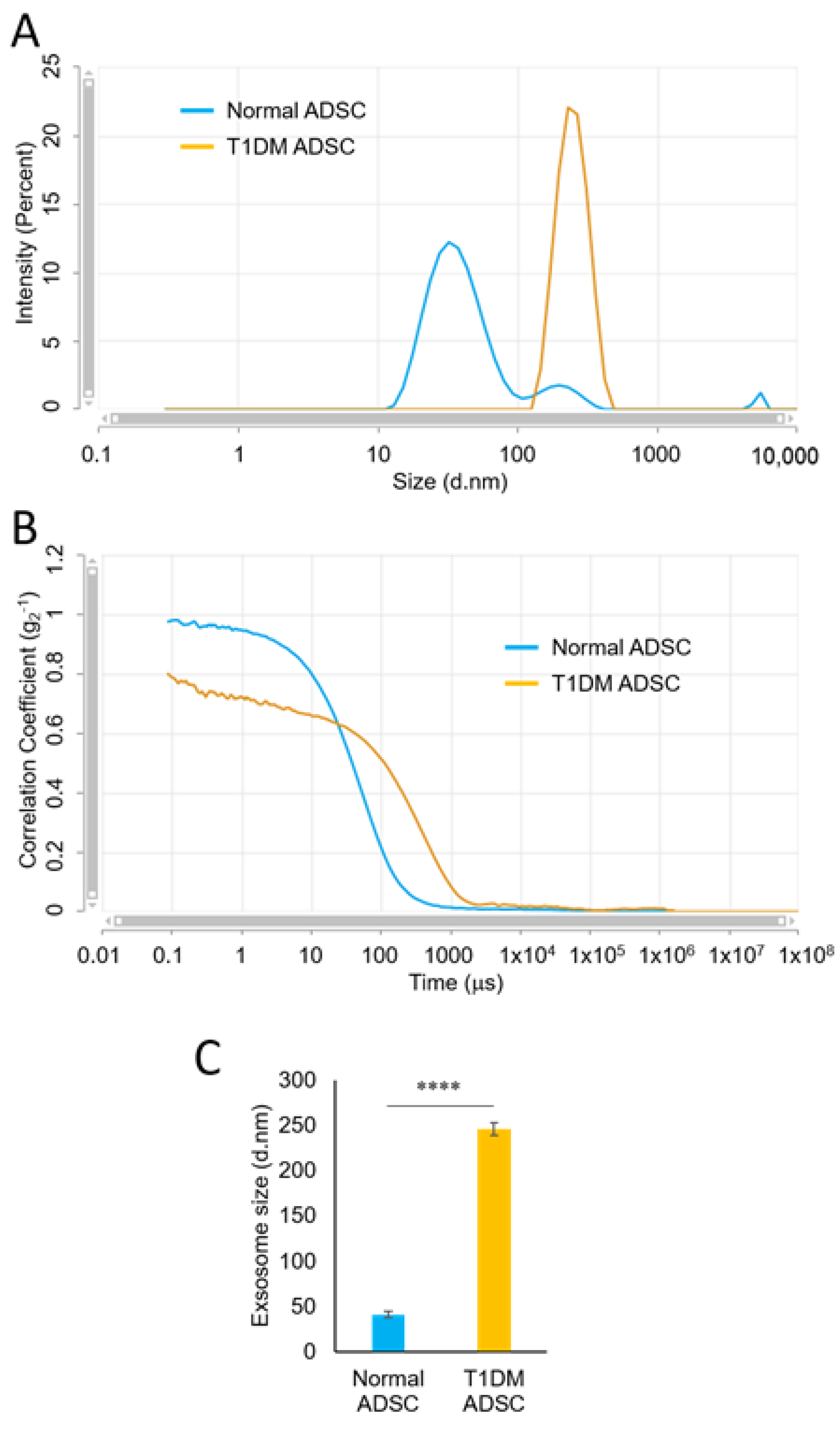
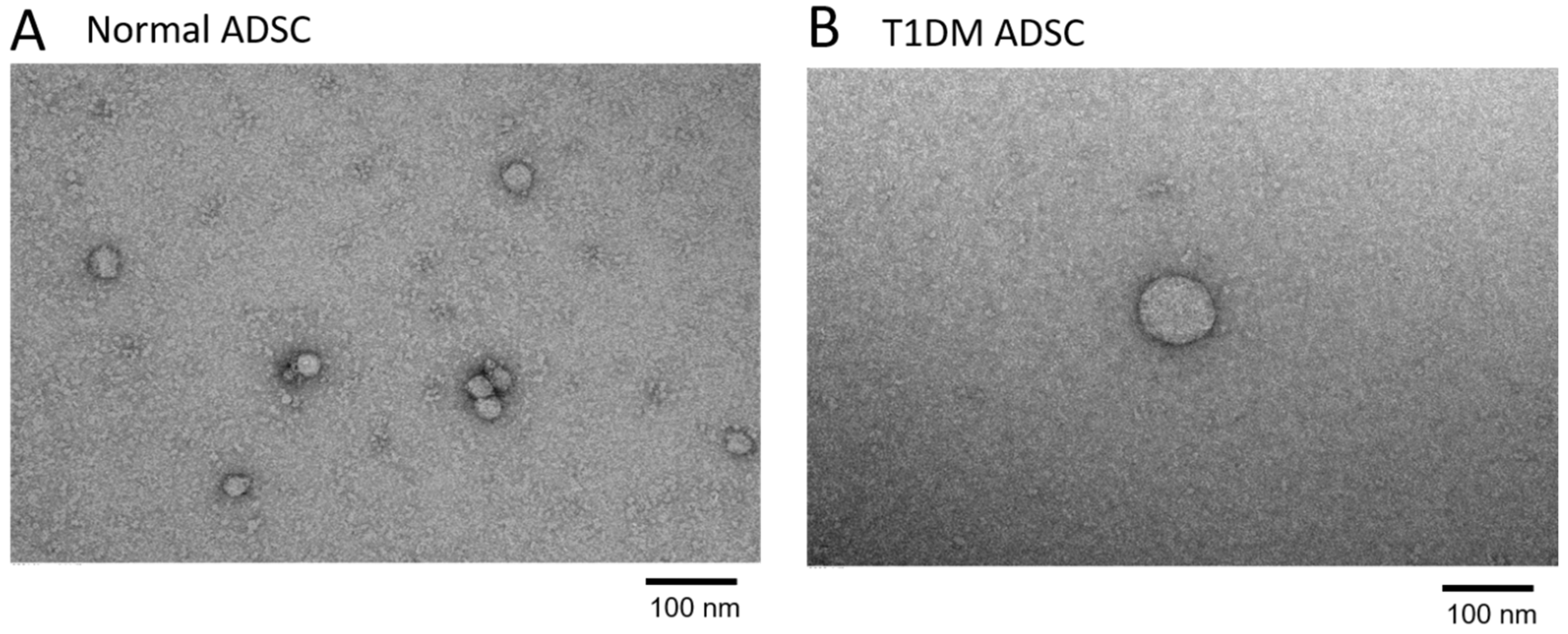
2.2. Enlargement of Exosomes in Stem Cells Derived from Patients with Type 1 Diabetes Mellitus
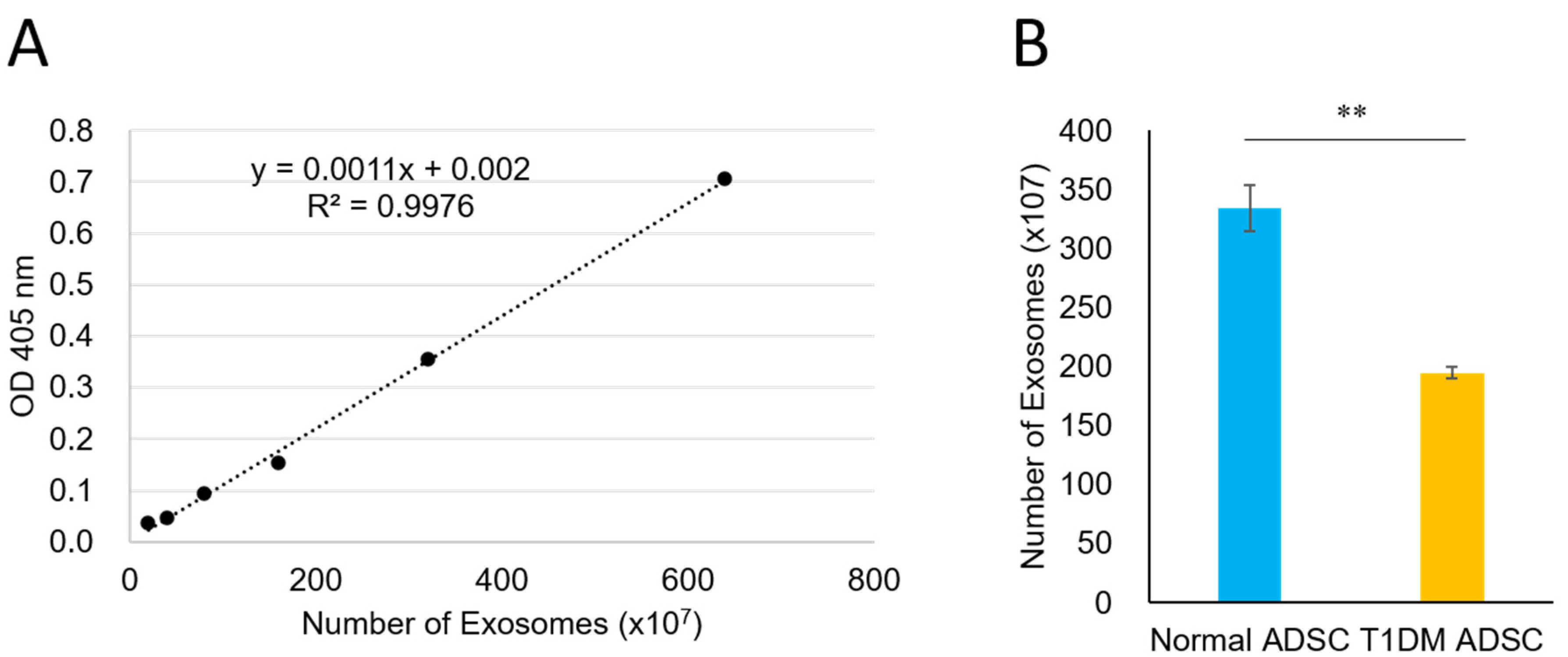
2.3. A Reduced Quantity of Exosomes in Stem Cells Derived from Patients with Type 1 Diabetes Mellitus
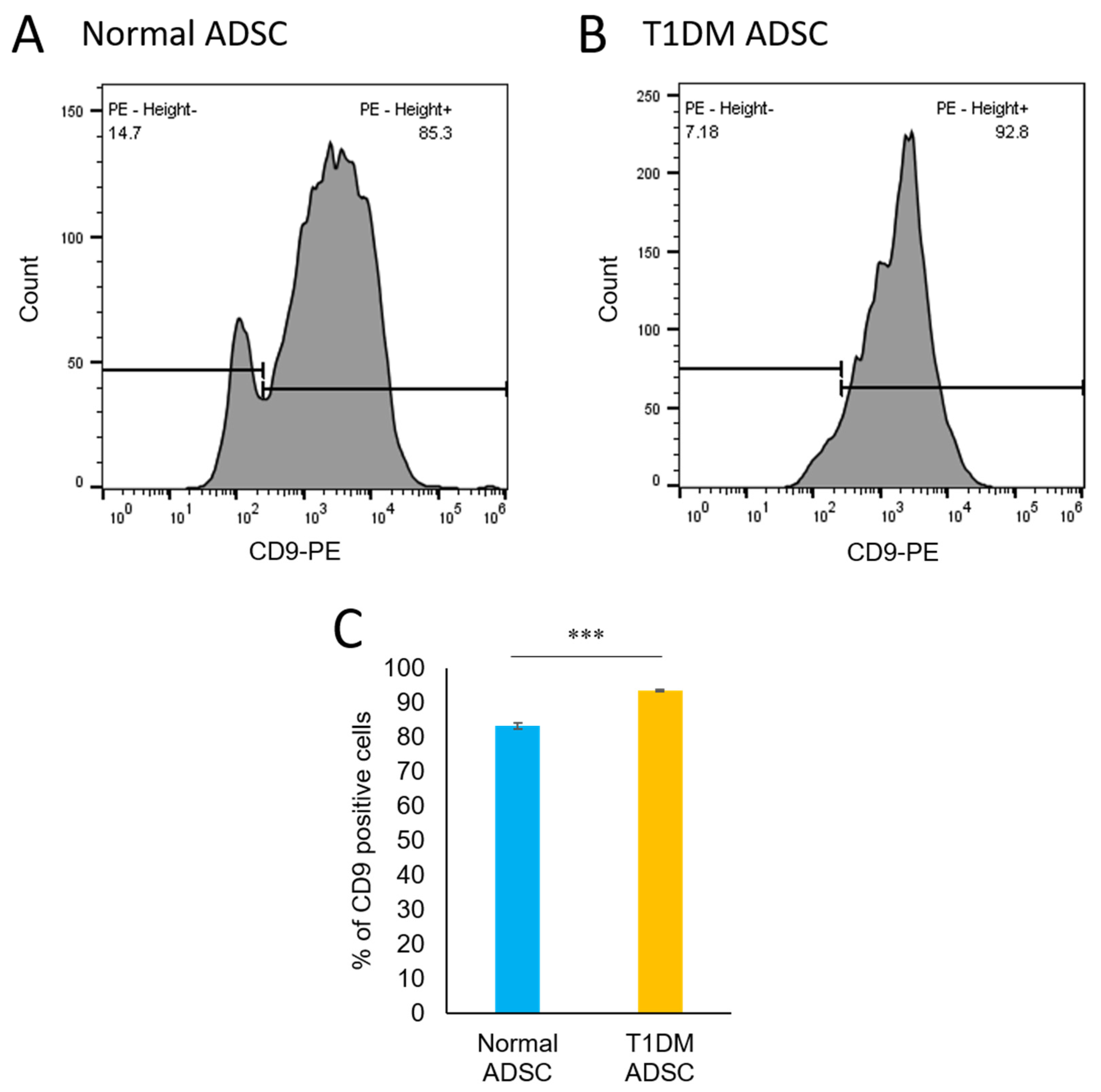
2.4. An Increased Number of CD9-Positive Exosomes in Stem Cells Derived from Patients with Type 1 Diabetes Mellitus
3. Discussion
4. Materials and Methods
4.1. Cell Culture for ADSCs
4.2. Electron Microscopic Observation
4.3. Exosome Extraction
4.4. Particle Size Measurement of the Exosomes
4.5. Quantification of the Exosomes Based on Acetylcholinesterase (AChE) Activity
4.6. Evaluation of the Exosome Markers CD63 and CD9
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Gillard, P.; Benhalima, K. Insulin analogues in type 1 diabetes mellitus: Getting better all the time. Nat. Rev. Endocrinol. 2017, 13, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Howard-Thompson, A.; Khan, M.; Jones, M.; George, C.M. Type 2 Diabetes Mellitus: Outpatient Insulin Management. Am. Fam. Physician 2018, 97, 29–37. [Google Scholar]
- Orliaguet, L.; Ejlalmanesh, T.; Alzaid, F. Metabolic and Molecular Mechanisms of Macrophage Polarisation and Adipose Tissue Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 5731. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Kondo, Y.; Toyoda, T.; Inagaki, N.; Osafune, K. iPSC technology-based regenerative therapy for diabetes. J. Diabetes Investig. 2018, 9, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gürtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Zhao, Z.; Gao, H. Effect of hTIMP-1 overexpression in human umbilical cord mesenchymal stem cells on the repair of pancreatic islets in type-1 diabetic mice. Cell Biol. Int. 2021, 45, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Ohgushi, H.; Caplan, A.I. Stem cell technology and bioceramics: From cell to gene engineering. J. Biomed. Mater. Res. 1999, 48, 913–927. [Google Scholar] [CrossRef]
- Iwashima, S.; Ozaki, T.; Maruyama, S.; Saka, Y.; Kobori, M.; Omae, K.; Yamaguchi, K.; Niimi, T.; Toriyama, K.; Kamei, Y.; et al. Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cells Dev. 2009, 18, 533–543. [Google Scholar] [CrossRef]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, M.; Hata, S.; Tsurudome, Y.; Ushijima, K. Characterizing the degeneration of nuclear membrane and mitochondria of adipose-derived mesenchymal stem cells from patients with type II diabetes. J. Cell. Mol. Med. 2021, 25, 4298–4306. [Google Scholar] [CrossRef]
- Horiguchi, M.; Turudome, Y.; Ushijima, K. The Transplantation Resistance of Type II Diabetes Mellitus Adipose-Derived Stem Cells Is Due to G6PC and IGF1 Genes Related to the FoxO Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 6595. [Google Scholar] [CrossRef]
- Kholafazad Kordasht, H.; Hasanzadeh, M. Biomedical analysis of exosomes using biosensing methods: Recent progress. Anal. Methods 2020, 12, 2795–2811. [Google Scholar] [CrossRef] [PubMed]
- Suárez, H.; Andreu, Z.; Mazzeo, C.; Toribio, V.; Pérez-Rivera, A.E.; López-Martín, S.; García-Silva, S.; Hurtado, B.; Morato, E.; Peláez, L.; et al. CD9 inhibition reveals a functional connection of extracellular vesicle secretion with mitophagy in melanoma cells. J. Extracell. Vesicles 2021, 10, e12082. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; Brayman, K.L. Brayman Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Transl. Med. 2013, 2, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Read, J.; Ingram, A.; Al Saleh, H.A.; Platko, K.; Gabriel, K.; Kapoor, A.; Pinthus, J.; Majeed, F.; Qureshi, T.; Al-Nedawi, K. Nuclear transportation of exogenous epidermal growth factor receptor and androgen receptor via extracellular vesicles. Eur. J. Cancer 2017, 70, 62–74. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.J.; Jin, H. Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annu. Rev. Plant Biol. 2021, 72, 497–524. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimaraes, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.B.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE 2010, 5, e11113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Feng, B.; Cao, H. The Clinical Efficacy and Safety of Stem Cell Therapy for Diabetes Mellitus: A Systematic Review and Meta-Analysis. Aging Dis. 2020, 11, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiguchi, M.; Okada, Y.; Turudome, Y.; Ushijima, K. Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 10906. https://doi.org/10.3390/ijms222010906
Horiguchi M, Okada Y, Turudome Y, Ushijima K. Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus. International Journal of Molecular Sciences. 2021; 22(20):10906. https://doi.org/10.3390/ijms222010906
Chicago/Turabian StyleHoriguchi, Michiko, Yuko Okada, Yuya Turudome, and Kentaro Ushijima. 2021. "Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus" International Journal of Molecular Sciences 22, no. 20: 10906. https://doi.org/10.3390/ijms222010906
APA StyleHoriguchi, M., Okada, Y., Turudome, Y., & Ushijima, K. (2021). Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus. International Journal of Molecular Sciences, 22(20), 10906. https://doi.org/10.3390/ijms222010906





