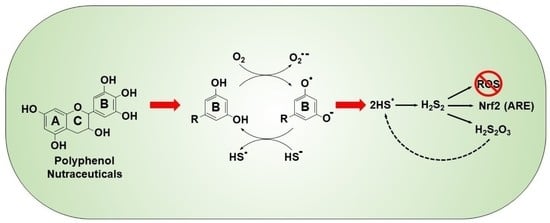
Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects
Abstract
1. Introduction
2. Results
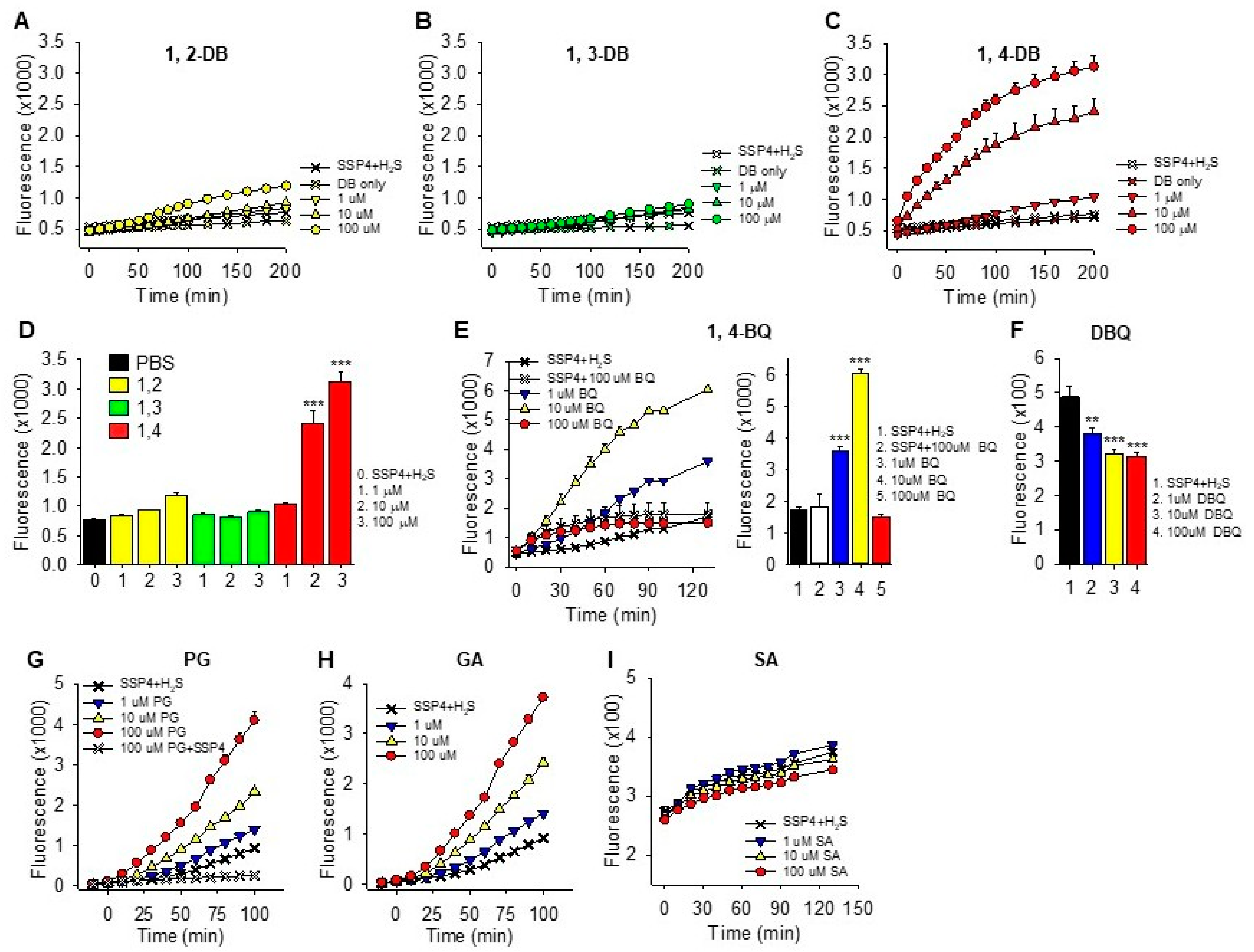
2.1. H2S Oxidation by Quinones and Hydroquinones
2.1.1. Polysulfide Production
Mechanism of Quinone-Catalyzed Polysulfide Production
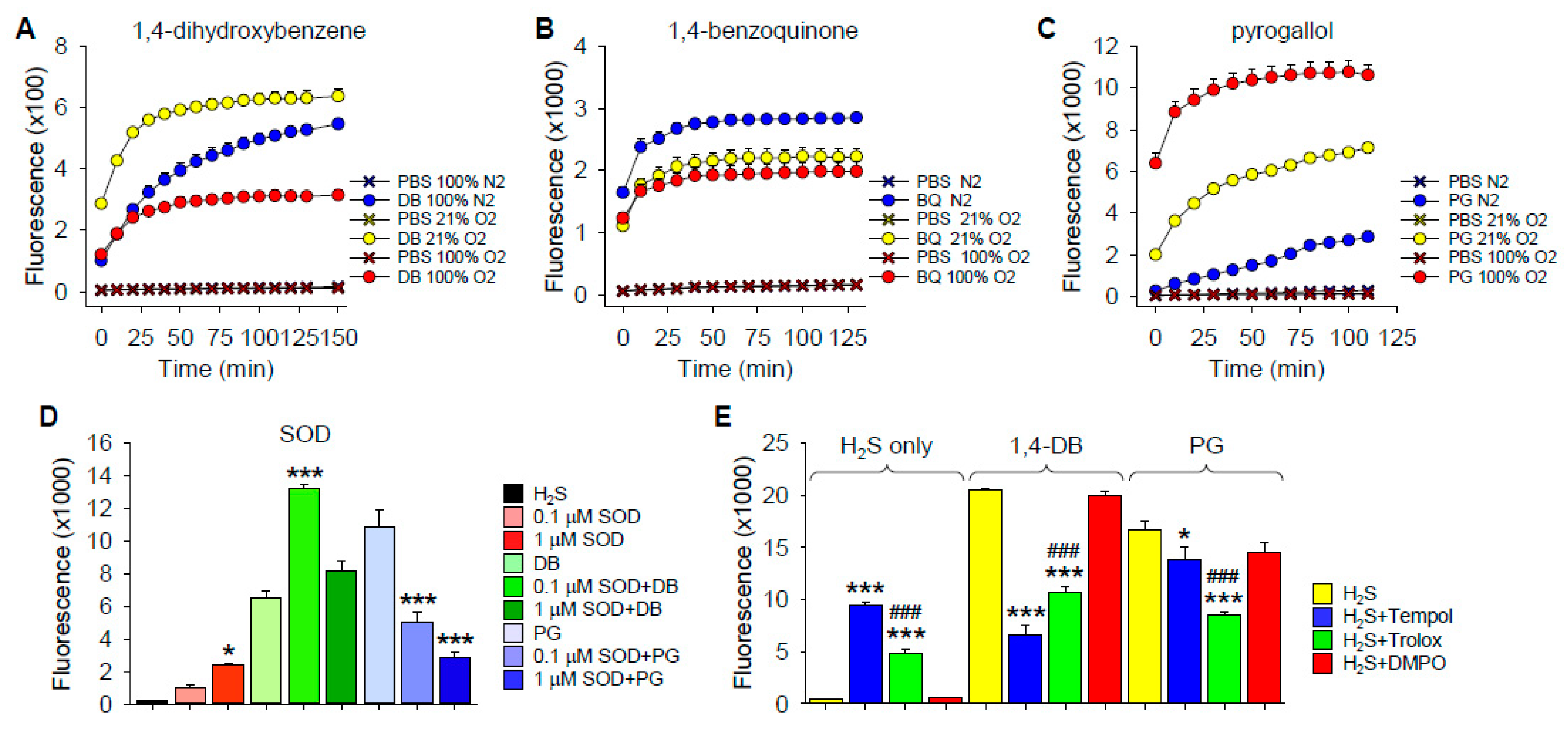
O2 Dependency of H2S Oxidation
Effects of Superoxide Dismutase
Effects of ROS/Redox Scavengers on H2S Oxidation by 1,4-DB and Pyrogallol
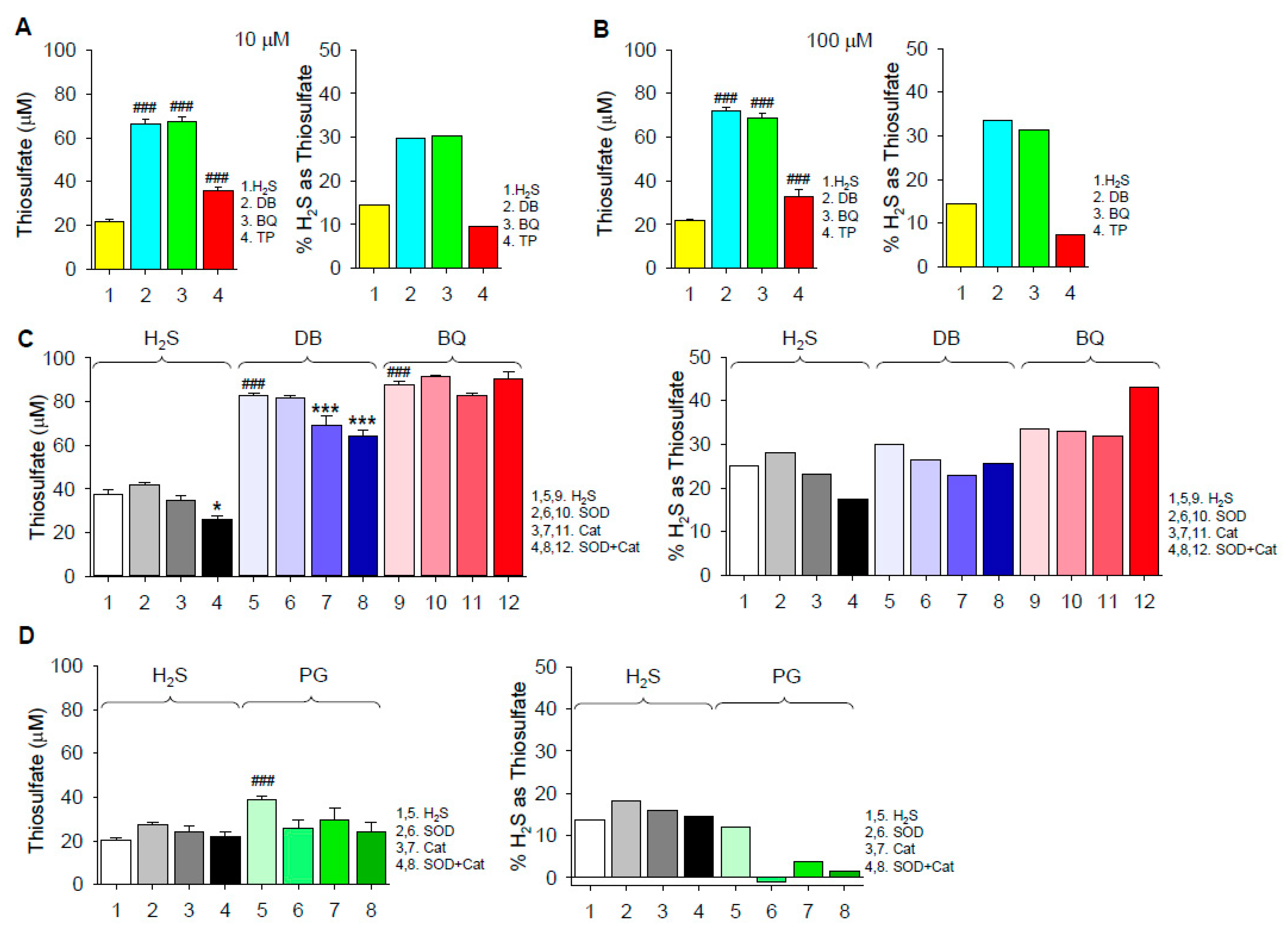
2.1.2. Thiosulfate Production
2.1.3. Absorbance Spectra of Hydroxybenzene/Benzoquinone Redox Reactions
2.1.4. Hydroxybenzene and Benzoquinone Comproportionation, Semiquinone Formation and Polysulfide Production
2.1.5. Hydroxybenzenes and Benzoquinone as Polysulfide Reductants
2.1.6. Effects of Dihydroxybenzene and p-Benzoquinone on Cellular Sulfur Metabolism
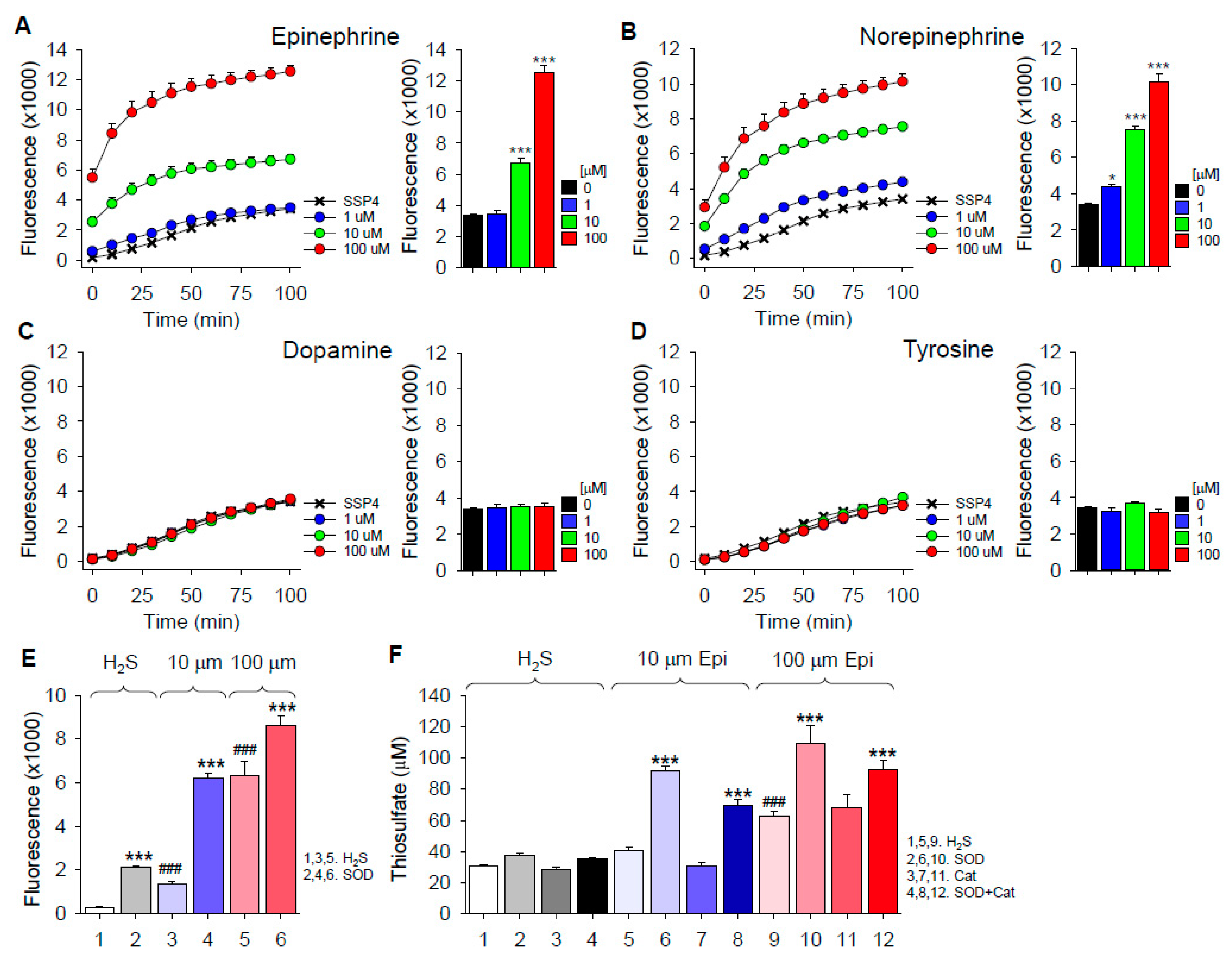
2.2. H2S Oxidation by Catecholamines
2.3. H2S Oxidation by Hydroxybenzophenones
3. Discussion
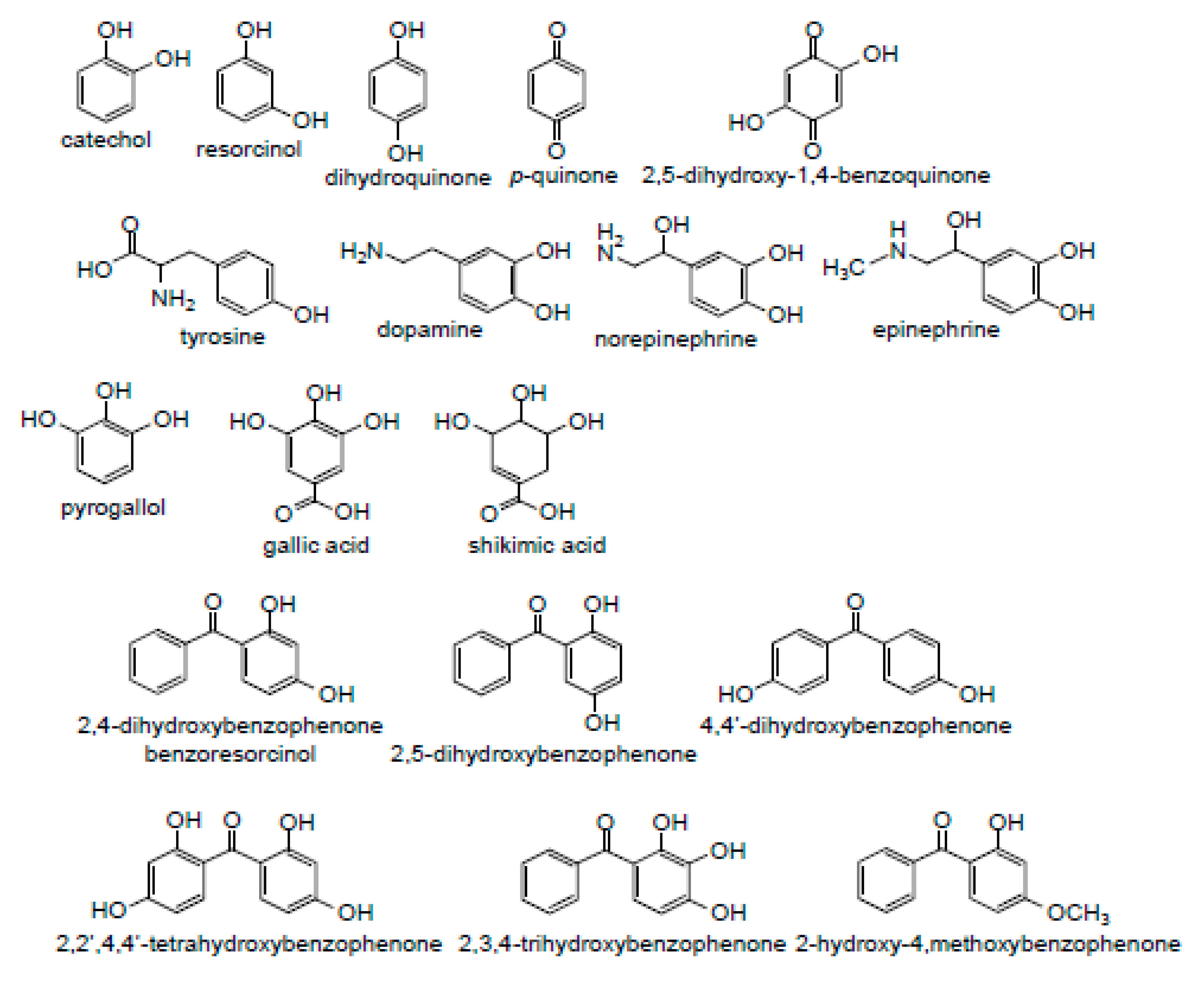
3.1. Specificity of Hydroxyl Groups and Their Positioning
3.1.1. Dihydroxybenzenes
3.1.2. Trihydroxybenzenes
3.1.3. Catecholamines
3.1.4. Hydroxybenzophenones
3.2. Mechanism of Polysulfide Formation
3.2.1. Polysulfide Production by 1,4-Dihydroxybenzene
3.2.2. Polysulfide Production by 1,4-Benzoquinone
3.3. Mechanism of Thiosulfate Formation
3.4. Extent of H2S Oxidation by 1,4-Dihydroxybenzene, 1,4-Benzoquinone, Pyrogallol and Epinephrine
3.5. Other Possible Cytoprotective Effects
4. Methods
4.1. H2S and Polysulfide Measurements in Buffer
4.2. Polyphenol Absorbance Spectrum
4.3. Thiosulfate Measurement in Buffer
4.4. Cells
4.5. Oxygen Dependency of Quinone Reactions with H2S in Buffer
4.6. Chemicals
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Araki, K.; Nakamura, Y.; Sagara, Y.; Ohba, K.; Sakai, H. Anticancer Effects of Green Tea and the Underlying Molecular Mechanisms in Bladder Cancer. Medicines 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xiao, X.; Chen, D.; Yu, B.; He, J. Tea and Its Components Prevent Cancer: A Review of the Redox-Related Mechanism. Int. J. Mol. Sci. 2019, 20, 5249. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M. Possible Mechanisms of Green Tea and Its Constituents against Cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef]
- Mangels, D.R.; Mohler, E.R., 3rd. Catechins as Potential Mediators of Cardiovascular Health. Arter. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Aghamohammadi, V.; Choghakhori, R.; Abbasnezhad, A. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 46, 210–216. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Maeta, K.; Nomura, W.; Takatsume, Y.; Izawa, S.; Inoue, Y. Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl. Environ. Microbiol. 2007, 73, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mori, T.; Tanaka, T.; Mizuno, D.; Yamaji, R.; Kumazawa, S.; Nakayama, T.; Akagawa, M. Covalent modification of proteins by green tea polyphenol (-)-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 2008, 45, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Hong, J.; Tian, S.; Lee, M.J.; Stark, R.E.; Ho, C.T.; Yang, C.S. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 2005, 18, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Hayakawa, S.; Nakano, S.; Ito, S.; Oishi, Y.; Suzuki, Y.; Isemura, M. In Vitro and In Silico Studies of the Molecular Interactions of Epigallocatechin-3-O-gallate (EGCG) with Proteins That Explain the Health Benefits of Green Tea. Molecules 2018, 23, 1295. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Skora, N.C.; Whelan, J.; Villa, B.P.; Yuan, X.; Mannam, V.; Howard, S.; et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: A possible new mechanism underpinning their biological action. Redox Biol. 2020, 37, 101731. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.; Straub, K.D. Fluorescence quenching by metal centered porphyrins and poryphyrin enzymes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R340–R346. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Rosenau, T. Degradation of 2,5-dihydroxy-1,4-benzoquinone by hydrogen peroxide: A combined kinetic and theoretical study. J. Org. Chem. 2013, 78, 3176–3182. [Google Scholar] [CrossRef]
- Radel, R.J.; Sullivan, J.M.; Hatfield, J.D. Catalytic oxidation of hydroquinone to quinone using molecular oxygen. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 566–570. [Google Scholar] [CrossRef]
- Rathore, D.S.; Chandel, C.S. Kinetics and Mechanism of the Aqueous Phase Oxidation of Hydrogen Sulfide by Oxygen: Catalyzed by Hydroquinone. Rasayan J. Chem. 2020, 13, 112–120. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The Unusual Reaction of Semiquinone Radicals with Molecular Oxygen. J. Org. Chem. 2008, 73, 1830–1841. [Google Scholar] [CrossRef]
- Weissberger, A.; Thomas, D.S.; Valle, J.E.L. Oxidation Processes. XV.1The Effect of Reducing Agents on the Autoxidation of Some Photographic Developing Agents. J. Am. Chem. Soc. 1943, 68, 1489–1495. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.R.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox Biol. 2017, 15, 74–85. [Google Scholar] [CrossRef]
- Su, C.; Liu, Z.; Wang, Y.; Wang, Y.; Song, E.; Song, Y. The electrophilic character of quinones is essential for the suppression of Bach1. Toxicology 2017, 387, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Albarran, G.; Boggess, W.; Rassolov, V.; Schuler, R.H. Absorption spectrum, mass spectrometric properties, and electronic structure of 1,2-benzoquinone. J. Phys. Chem. A 2010, 114, 7470–7478. [Google Scholar] [CrossRef]
- Abrash, H.I.; Shih, D.; Elias, W.; Malekmehr, F. A Kinetic Study of the Air Oxidation of Pyrogallol and Purpurogallin. Int. J. Chem. Kinet. 1989, 21, 465–466. [Google Scholar] [CrossRef]
- Siegel, S.M.; Siegel, B.Z. Autoxidation of pyrogallol: General characteristics and inhibition by catalase. Nature 1958, 181, 1153–1154. [Google Scholar] [CrossRef]
- Tauber, H. Oxidation of pyrogallol to purpurogallin by crystallin catalase. J. Biol. Chem. 1953, 205, 395–400. [Google Scholar] [CrossRef]
- Kobayashi, H.; Oikawa, S.; Hirakawa, K.; Kawanishi, S. Metal-mediated oxidative damage to cellular and isolated DNA by gallic acid, a metabolite of antioxidant propyl gallate. Mutat. Res. 2004, 558, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Veselinović, A.M.a.G.M.N. Influence of Zn(II) Ion on the Autoxidation of Pyrogallol and Gallic Acid in Weakly Acidic Aqueous Solutions/Uticaj Zn(II) jona na autooksidaciju pirogalola i galne kiseline u slabo kiselim vodenim rastvorima. Acta Fac. Med. Naissensis 2015, 32, 127–135. [Google Scholar] [CrossRef][Green Version]
- Eyer, P. Effects of superoxide dismutase on the autoxidation of 1,4-hydroquinone. Chem. Biol. Interact. 1991, 80, 159–176. [Google Scholar] [CrossRef]
- Song, Y.; Wagner, B.A.; Lehmler, H.J.; Buettner, G.R. Semiquinone radicals from oxygenated polychlorinated biphenyls: Electron paramagnetic resonance studies. Chem. Res. Toxicol. 2008, 21, 1359–1367. [Google Scholar] [CrossRef]
- Polewski, K. Spectroscopic detection of adrenaline-quinone formation in micelles. Biochim. Biophys. Acta 2000, 1523, 56–64. [Google Scholar] [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal. 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Wedmann, R.; Bertlein, S.; Macinkovic, I.; Boltz, S.; Miljkovic, J.; Munoz, L.E.; Herrmann, M.; Filipovic, M.R. Working with “H2S”: Facts and apparent artifacts. Nitric Oxide 2014, 41, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liang, F.; Shah, M.W.; Yan, X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014, 725, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Ogasawara, Y.; Shibuya, N.; Kimura, H.; Ishii, K. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013, 587, 3548–3555. [Google Scholar] [CrossRef]
- Meng, W.; Pei, Z.; Feng, Y.; Zhao, J.; Chen, Y.; Shi, W.; Xu, Q.; Lin, F.; Sun, M.; Xiao, K. Neglected role of hydrogen sulfide in sulfur mustard poisoning: Keap1 S-sulfhydration and subsequent Nrf2 pathway activation. Sci. Rep. 2017, 7, 9433. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, L.; Mao, J.; Huang, J.; Chen, J. Antioxidant effects of hydrogen sulfide on left ventricular remodeling in smoking rats are mediated via PI3K/Akt-dependent activation of Nrf2. Toxicol. Sci. 2015, 144, 197–203. [Google Scholar] [CrossRef]
- Roginsky, V.; Alegria, A.E. Oxidation of tea extracts and tea catechins by molecular oxygen. J. Agric. Food Chem. 2005, 53, 4529–4535. [Google Scholar] [CrossRef]
- Conant, J.B.; Fieser, L.F. Reduction Potentials of Quinones. II. The Potentials of Certain Derivatives of Benzoquinone, Naphthoquinone and Anthraquinone. J. Am. Chem. Soc. 1924, 46, 1858–1881. [Google Scholar] [CrossRef]
- Dawson, C.R.; Nelson, J.M. The Influence of Catechol on the Stability of o-Benzoquinone in Aqueous Solutions. J. Am. Chem. Soc. 1938, 60, 245–249. [Google Scholar] [CrossRef]
- Mason, H.S. The chemistry of melanin; mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [CrossRef]
- Nagakura, S.; Kuboyama, A. Dipole Moments and Absorption Spectra of o-Benzoquinone and its Related Substances. J. Am. Chem. Soc. 1954, 76, 1003–1005. [Google Scholar] [CrossRef]
- Aebisher, D.; Brzostowska, E.M.; Mahendran, A.; Greer, A. Regioselective (biomimetic) synthesis of a pentasulfane from ortho-benzoquinone. J. Org. Chem. 2007, 72, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.C.; Sealy, R.C. o-Benzosemiquinone and its metal chelates. Electron spin resonance investigation of radicals from the photolysis of catechol in the presence of complexing metal ions. J. Am. Chem. Soc. 1982, 104, 1555–1560. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Stegmann, H.B.; Bergler, H.U.; Scheffler, K. “Spin-Stabilization” via Complex Formation; ESR Investigation of some Catecholamine-Semiquinones. Angew. Chem. Int. Ed. Engl. 1981, 20, 389–390. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Felix, C.C.; Sealy, R.C. Peroxidatic oxidation of catecholamines. A kinetic electron spin resonance investigation using the spin stabilization approach. J. Biol. Chem. 1984, 259, 7584–7589. [Google Scholar] [CrossRef]
- Roginsky, V.A.; Barsukova, T.K.; Bruchelt, G.; Stegmann, H.B. The Oxidation of Catecholamines and 6-Hydroxydopamine by Molecular Oxygen: Effect of Ascorbate. Z. Nat. 1997, 52, 389–390. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Patel, S.; Yuan, X.; Mannam, V.; Howard, S.; Batinic-Haberle, I.; Fukuto, J.; Minnion, M.; et al. Manganese Porphyrin-Based SOD Mimetics Produce Polysulfides from Hydrogen Sulfide. Antioxidants 2019, 8, 639. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Monks, T.J.; Jones, D.C. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Tu, T.; Giblin, D.; Gross, M.L. Structural determinant of chemical reactivity and potential health effects of quinones from natural products. Chem. Res. Toxicol. 2011, 24, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Terland, O.; Almas, B.; Flatmark, T.; Andersson, K.K.; Sorlie, M. One-electron oxidation of catecholamines generates free radicals with an in vitro toxicity correlating with their lifetime. Free Radic. Biol. Med. 2006, 41, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Jewett, S.L.; Eddy, L.J.; Hochstein, P. Is the autoxidation of catecholamines involved in ischemia-reperfusion injury? Free Radic. Biol. Med. 1989, 6, 185–188. [Google Scholar] [CrossRef]
- Hochstein, P.; Cohen, G. The cytotoxicity of melanin precursors. Ann. N. Y. Acad. Sci. 1963, 100, 876–886. [Google Scholar] [CrossRef]
- Rotman, A.; Daly, J.W.; Creveling, C.R. Oxygen-dependent reaction of 6-hydroxydopamine, 5,6-dihydroxytryptamine, and related compounds with proteins in vitro: A model for cytotoxicity. Mol. Pharmacol. 1976, 12, 887–899. [Google Scholar]
- Miller, J.W.; Selhub, J.; Joseph, J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: Protective effects of O-methylation and melatonin. Free Radic. Biol. Med. 1996, 21, 241–249. [Google Scholar] [CrossRef]
- Bibli, S.I.; Luck, B.; Zukunft, S.; Wittig, J.; Chen, W.; Xian, M.; Papapetropoulos, A.; Hu, J.; Fleming, I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018, 18, 295–304. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y. Effects of inhibiting antioxidant pathways on cellular hydrogen sulfide and polysulfide metabolism. Free Radic. Biol. Med. 2019, 135, 1–14. [Google Scholar] [CrossRef]
- Olson, K.R.; Briggs, A.; Devireddy, M.; Xian, M.; Gao, Y. Are the beneficial effects of ‘antioxidant’ lipoic acid mediated through metabolism of reactive sulfur species? Free Radic. Biol. Med. 2019, 146, 139–149. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Zhang, Y.; Ma, X.; Iqbal, M.Z.; Miao, L.; Zhou, Z.; Shen, Z.; Wu, A. High-Performance Colorimetric Detection of Thiosulfate by Using Silver Nanoparticles for Smartphone-Based Analysis. ACS Sens. 2017, 2, 1152–1159. [Google Scholar] [CrossRef]
- May, P.M.; Batka, D.; Hefter, G.; Konigsberger, E.; Rowland, D. Goodbye to S(2-) in aqueous solution. Chem. Commun. (Camb.) 2018, 54, 1980–1983. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, K.R.; Gao, Y.; Straub, K.D. Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects. Int. J. Mol. Sci. 2021, 22, 961. https://doi.org/10.3390/ijms22020961
Olson KR, Gao Y, Straub KD. Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects. International Journal of Molecular Sciences. 2021; 22(2):961. https://doi.org/10.3390/ijms22020961
Chicago/Turabian StyleOlson, Kenneth R., Yan Gao, and Karl D. Straub. 2021. "Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects" International Journal of Molecular Sciences 22, no. 2: 961. https://doi.org/10.3390/ijms22020961
APA StyleOlson, K. R., Gao, Y., & Straub, K. D. (2021). Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects. International Journal of Molecular Sciences, 22(2), 961. https://doi.org/10.3390/ijms22020961