Substrate Stiffness Mediates Formation of Novel Cytoskeletal Structures in Fibroblasts during Cell–Microspheres Interaction
Abstract
1. Introduction
2. Results
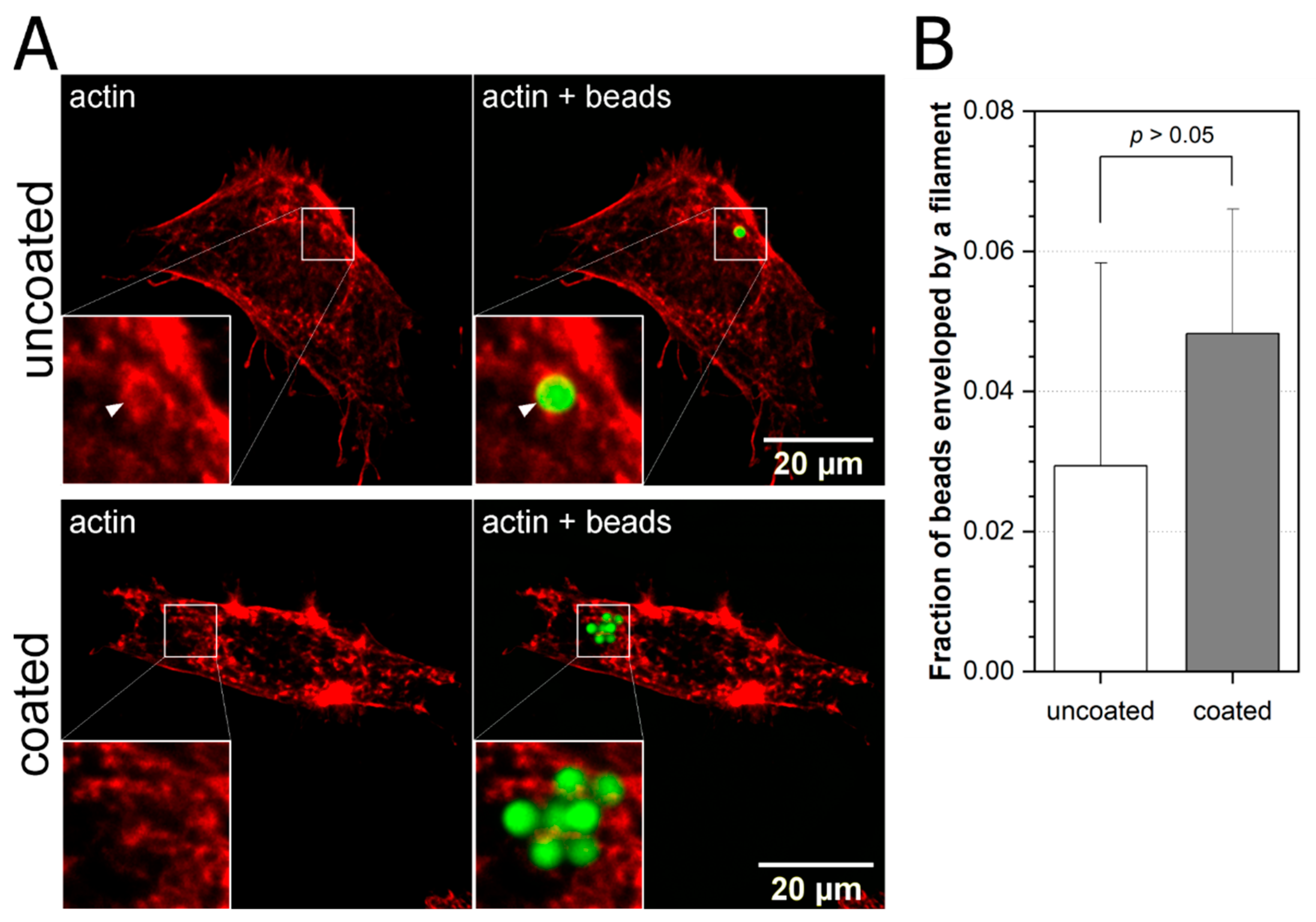
2.1. Cytoskeleton Remodeling Due to External Stimuli
2.2. Influence of Cytoskeletal Toxins on Cell–Bead Interaction
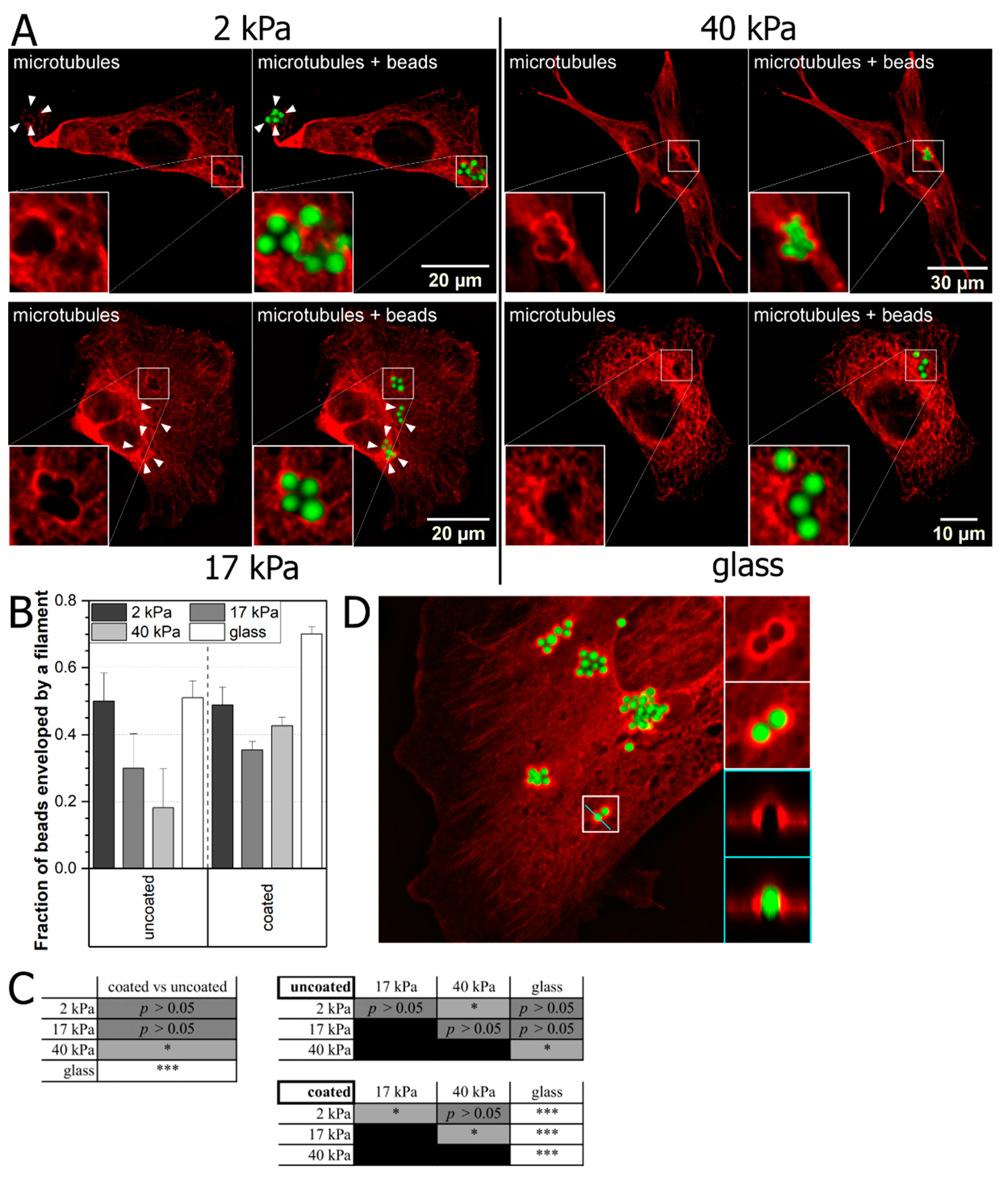
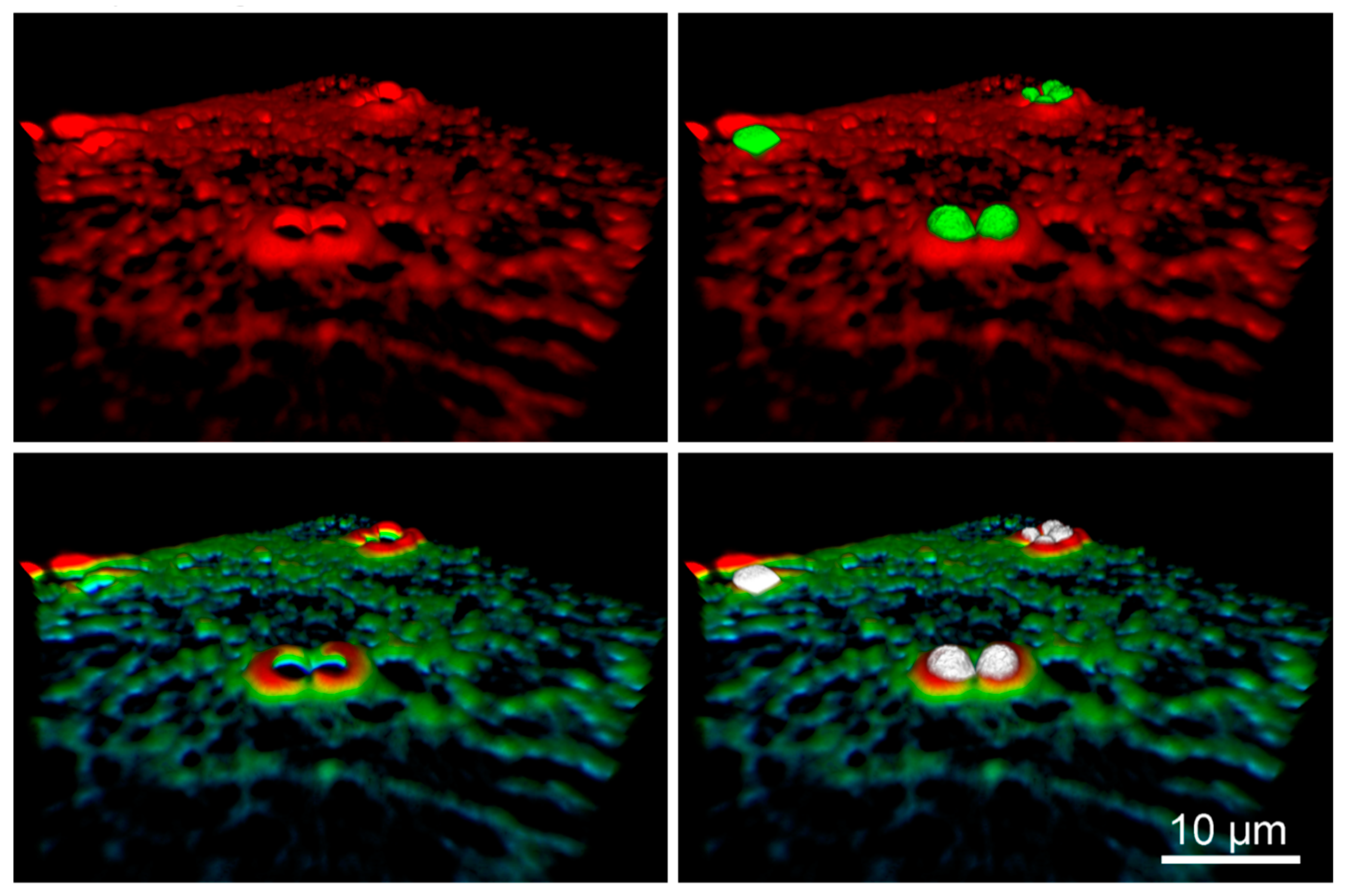
2.3. Role of Substrate Elasticity in Mechanical Stimulus-Mediated Cytoskeleton Remodeling
3. Discussion
3.1. Cytoskeletal Rearrangement
- more fibronectin coated beads are engulfed by the respective cytoskeleton after 3 h incubation, though the percentage varies between them from 45% in case of actin and vimentin to 28% for microtubules;
- for uncoated beads, the results are mixed—the highest percentage of engulfed beads is for vimentin (~50%) followed by actin (~30%) and then microtubules (~10%);
- in the case of uncoated beads and microtubules, we see that beads are more efficiently engulfed after 15 min. than after 3 h of incubation. This counter intuitive result may point to the different mechanisms of bead engulfment by microtubules in comparison to actin and vimentin.
3.2. Cytoskeletal Toxins
3.3. Elastic Polyacrylamide (PA) Substrates
- the effect of bead envelopment occurs, especially in the cell lamella,
- the bead envelopment depends on the elasticity of the cellular substrates,
- microtubules in cells cultured on soft cellular substrates, form cup-like structures surrounding beads placed on their dorsal surface.
4. Materials and Methods
4.1. Cell Culture
4.2. Beads and Bead Coating
4.3. Cell Labeling and Immunoflurescence
4.4. Cytoskeletal Drugs
4.5. Preparation of Polyacrylamide (PA) Gel Substrates with Different Mechanical Properties
4.6. Confocal Microscopy and Data Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desjardins, M.; Griffiths, G. Phagocytosis: Latex leads the way. Curr. Opin. Cell Biol. 2003, 15, 498–503. [Google Scholar] [CrossRef]
- Resnick, A. Use of optical tweezers to probe epithelial mechanosensation. J. Biomed. Opt. 2010, 15, 015005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sako, Y.; Nagafuchi, A.; Tsukita, S.; Takeichi, M.; Kusumi, A. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: Corralling and tethering by the membrane skeleton. J. Cell Biol. 1998, 140, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Tavano, F.; Bonin, S.; Pinato, G.; Stanta, G.; Cojoc, D. Custom-Built Optical Tweezers for Locally Probing the Viscoelastic Properties of Cancer Cells. Int. J. Optomechatronics 2011, 5, 234–248. [Google Scholar] [CrossRef]
- Pontes, B.; Viana, N.B.; Salgado, L.T.; Farina, M.; Neto, V.M.; Nussenzveig, H.M. Cell Cytoskeleton and Tether Extraction. Biophys. J. 2011, 101, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Gu, J.G. Membrane Mechanics of Primary Afferent Neurons in the Dorsal Root Ganglia of Rats. Biophys. J. 2017, 112, 1654–1662. [Google Scholar] [CrossRef][Green Version]
- Pontes, B.; Monzo, P.; Gauthier, N.C. Membrane tension: A challenging but universal physical parameter in cell biology. Semin. Cell Dev. Biol. 2017, 71, 30–41. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Internal cell manipulation using infrared laser traps. Proc. Natl. Acad. Sci. USA 1989, 86, 7914–7918. [Google Scholar] [CrossRef]
- Dai, J.; Sheetz, M.P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys. J. 1995, 68, 988–996. [Google Scholar] [CrossRef]
- Datar, A.; Bornschlögl, T.; Bassereau, P.; Prost, J.; Pullarkat, P.A. Dynamics of membrane tethers reveal novel aspects of cytoskeleton-membrane interactions in axons. Biophys. J. 2015, 108, 489–497. [Google Scholar] [CrossRef]
- Pascoal, P.; Kosanic, D.; Gjoni, M.; Vogel, H. Membrane nanotubes drawn by optical tweezers transmit electrical signals between mammalian cells over long distances. Lab Chip 2010, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Ayala, Y.A.; Pontes, B.; Ether, D.S.; Pires, L.B.; Araujo, G.R.; Frases, S.; Romão, L.F.; Farina, M.; Moura-Neto, V.; Viana, N.B.; et al. Rheological properties of cells measured by optical tweezers. BMC Biophys. 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.; Block, S.M. Biological Applications of Optical Forces. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 247–285. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Yamane, T. Optical trapping and manipulation of single cells using infrared laser beams. Nature 1987, 330, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Nussenzveig, H.M. Cell membrane biophysics with optical tweezers. Eur. Biophys. J. 2018, 47, 499–514. [Google Scholar] [CrossRef]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Bandmann, V.; Müller, J.D.; Köhler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef]
- Lloyd, Y.M.; Ngati, E.P.; Salanti, A.; Leke, R.G.F.; Taylor, D.W. A versatile, high through-put, bead-based phagocytosis assay for Plasmodium falciparum. Sci. Rep. 2017, 7, 14705. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Dupuy, A.G.; Caron, E. Integrin-dependent phagocytosis: Spreading from microadhesion to new concepts. J. Cell Sci. 2008, 121, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, P.J.; Nevanlinna, H.A.; Gorman, P.; Sheer, D.; Lam, G.; Goodfellow, P.N. Assignment of the gene encoding the beta-subunit of the human fibronectin receptor (β-FNR) to chromosome 10p11.2. Ann. Hum. Genet. 1989. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.V.; Dolin, C.E.; Poole, L.G.; Massey, V.L.; Wilkey, D.; Beier, J.I.; Merchant, M.L.; Frieboes, H.B.; Arteel, G.E. Modeling the Kinetics of Integrin Receptor Binding to Hepatic Extracellular Matrix Proteins. Sci. Rep. 2017, 7, 12444. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Choi, C.K.; Horwitz, A.R. Integrins in cell migration--the actin connection. J. Cell Sci. 2009, 122, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F. Fibroblast spreading and phagocytosis: Similar cell responses to different-sized substrata. J. Cell. Physiol. 1984, 119, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L.; Shin, J.H.; MacKintosh, F.C.; Mahadevan, L.; Matsudaira, P.A.; Weitz, D.A. Scaling of F-Actin Network Rheology to Probe Single Filament Elasticity and Dynamics. Phys. Rev. Lett. 2004, 93, 188102. [Google Scholar] [CrossRef]
- Fabry, B.; Maksym, G.N.; Butler, J.P.; Glogauer, M.; Navajas, D.; Taback, N.A.; Millet, E.J.; Fredberg, J.J. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E 2003, 68, 041914. [Google Scholar] [CrossRef]
- Bursac, P.; Lenormand, G.; Fabry, B.; Oliver, M.; Weitz, D.A.; Viasnoff, V.; Butler, J.P.; Fredberg, J.J. Cytoskeletal remodelling and slow dynamics in the living cell. Nat. Mater. 2005, 4, 557–561. [Google Scholar] [CrossRef]
- Jaumouillé, V.; Cartagena-Rivera, A.X.; Waterman, C.M. Coupling of β2 integrins to actin by a mechanosensitive molecular clutch drives complement receptor-mediated phagocytosis. Nat. Cell Biol. 2019, 21, 1357–1369. [Google Scholar] [CrossRef]
- Vorselen, D.; Labitigan, R.L.D.; Theriot, J.A. A mechanical perspective on phagocytic cup formation. Curr. Opin. Cell Biol. 2020, 66, 112–122. [Google Scholar] [CrossRef]
- Nobezawa, D.; Ikeda, S.; Wada, E.; Nagano, T.; Miyata, H. Directional Transport of a Bead Bound to Lamellipodial Surface Is Driven by Actin Polymerization. Biomed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cramer, L.P. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr. Biol. 1999, 9, 1095–1105. [Google Scholar] [CrossRef]
- Schiff, P.B.; Horwitz, S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 1980, 77, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987, 105, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Khandani, A.; Eng, E.; Jongstra-Bilen, J.; Schreiber, A.D.; Douda, D.; Samavarchi-Tehrani, P.; Harrison, R.E. Microtubules regulate PI-3K activity and recruitment to the phagocytic cup during Fcγ receptor-mediated phagocytosis in nonelicited macrophages. J. Leukoc. Biol. 2007, 82, 417–428. [Google Scholar] [CrossRef]
- Valon, L.; Marín-Llauradó, A.; Wyatt, T.; Charras, G.; Trepat, X. Optogenetic control of cellular forces and mechanotransduction. Nat. Commun. 2017, 8, 14396. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Preparation of Hydrogel Substrates with Tunable Mechanical Properties. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 10, pp. 10.16.1–10.16.16. [Google Scholar]
- Kirshner, H.; Aguet, F.; Sage, D.; Unser, M. 3-D PSF fitting for fluorescence microscopy: Implementation and localization application. J. Microsc. 2013, 249, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Griffa, A.; Garin, N.; Sage, D. Comparison of Deconvolution Software in 3D Microscopy: A User Point of View—Part 1. G.I.T. Imaging Microsc. 2010, 12, 43–45. [Google Scholar]
- Sage, D.; Donati, L.L.; Soulez, F.F.; Fortun, D.; Schmit, G.; Seitz, A.; Guiet, R.; Vonesch, C.C.; Unser, M. DeconvolutionLab2: An open-source software for deconvolution microscopy. Methods 2017, 115, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Baster, Z.; Rajfur, Z. BatchDeconvolution: A Fiji plugin for increasing deconvolution workflow. Bio-Algorithms Med.-Syst. 2020, 16. [Google Scholar] [CrossRef]
- Richardson, W.H. Bayesian-Based Iterative Method of Image Restoration*. J. Opt. Soc. Am. 1972, 62, 55. [Google Scholar] [CrossRef]
- Lucy, L.B. An iterative technique for the rectification of observed distributions. Astron. J. 1974, 79, 745. [Google Scholar] [CrossRef]
- Gibson, S.F.; Lanni, F. Diffraction by a circular aperture as a model for three-dimensional optical microscopy. J. Opt. Soc. Am. A 1989, 6, 1357. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Wan, Y.; Otsuna, H.; Chien, C.-B.; Hansen, C. FluoRender: An Application of 2D Image Space Methods for 3D and 4D Confocal Microscopy Data Visualization in Neurobiology Research. IEEE Pac. Vis. Symp. 2012, 201–208. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, O.; Baster, Z.; Szczypior, M.; Rajfur, Z. Substrate Stiffness Mediates Formation of Novel Cytoskeletal Structures in Fibroblasts during Cell–Microspheres Interaction. Int. J. Mol. Sci. 2021, 22, 960. https://doi.org/10.3390/ijms22020960
Adamczyk O, Baster Z, Szczypior M, Rajfur Z. Substrate Stiffness Mediates Formation of Novel Cytoskeletal Structures in Fibroblasts during Cell–Microspheres Interaction. International Journal of Molecular Sciences. 2021; 22(2):960. https://doi.org/10.3390/ijms22020960
Chicago/Turabian StyleAdamczyk, Olga, Zbigniew Baster, Maksymilian Szczypior, and Zenon Rajfur. 2021. "Substrate Stiffness Mediates Formation of Novel Cytoskeletal Structures in Fibroblasts during Cell–Microspheres Interaction" International Journal of Molecular Sciences 22, no. 2: 960. https://doi.org/10.3390/ijms22020960
APA StyleAdamczyk, O., Baster, Z., Szczypior, M., & Rajfur, Z. (2021). Substrate Stiffness Mediates Formation of Novel Cytoskeletal Structures in Fibroblasts during Cell–Microspheres Interaction. International Journal of Molecular Sciences, 22(2), 960. https://doi.org/10.3390/ijms22020960





