Pathological ATX3 Expression Induces Cell Perturbations in E. coli as Revealed by Biochemical and Biophysical Investigations
Abstract
1. Introduction
2. Results
2.1. Toxic Effect of the Pathogenic Ataxin-3 Variant on E. coli Growth
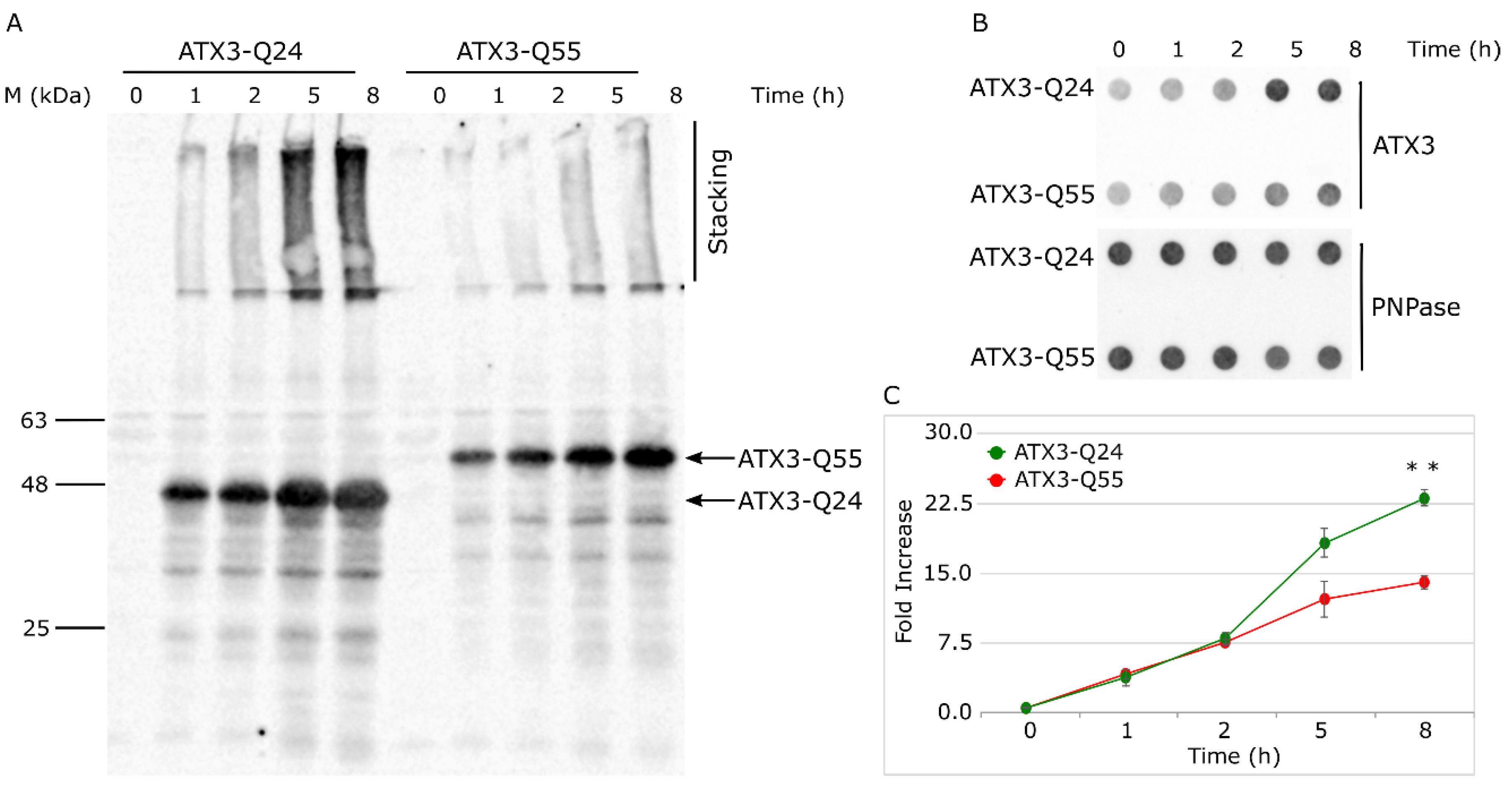
2.2. ATX3-Q55 Toxicity Is Not Correlated to a Higher Protein Expression or Increase of the Soluble Fraction
2.3. FTIR Analysis of E. coli Cell Modifications Induced by the Expression of ATX3-Q24 and ATX3-Q55
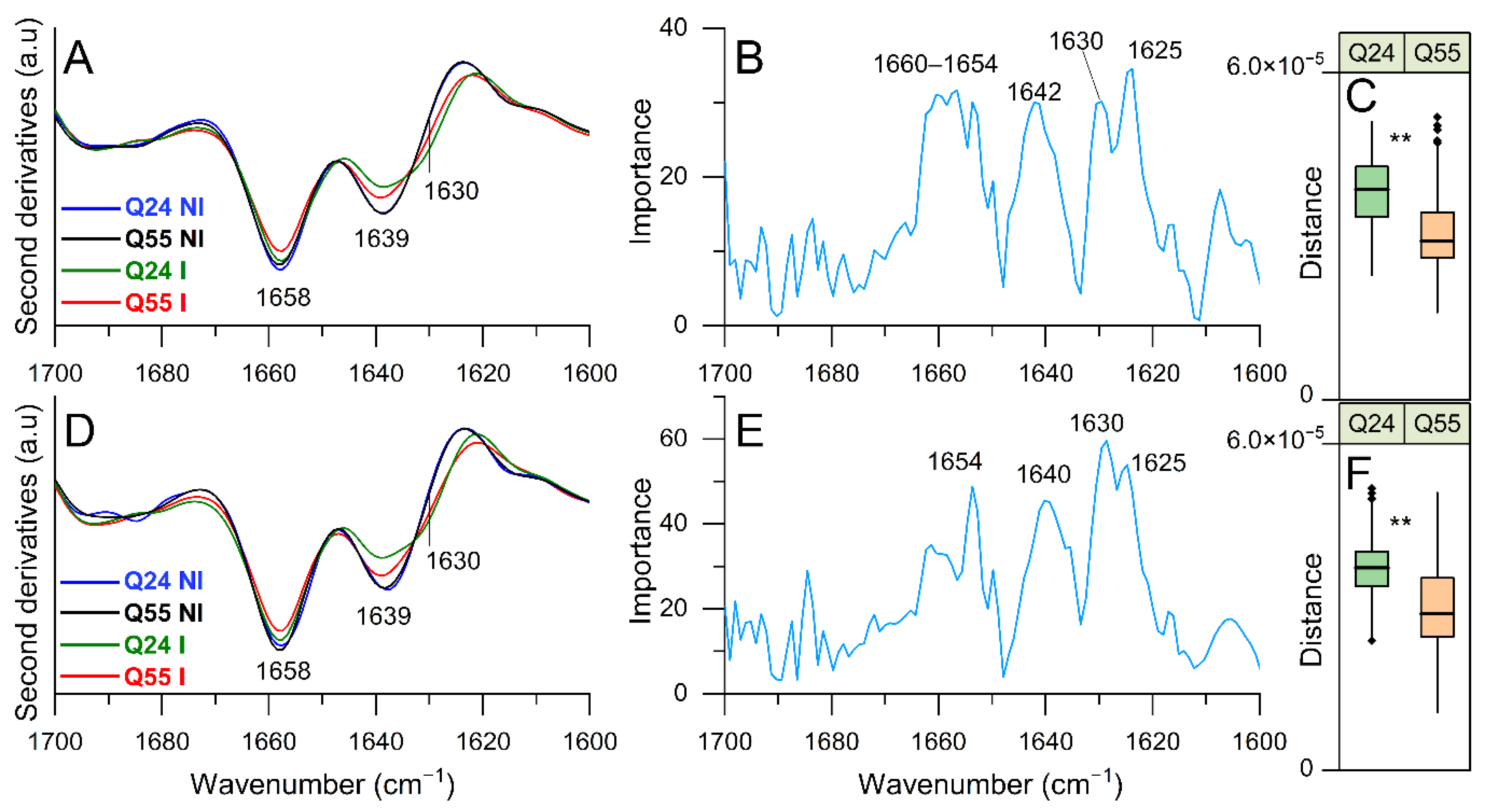
2.3.1. FTIR Study of Protein Secondary Structure Modifications: Analysis of Amide I Band
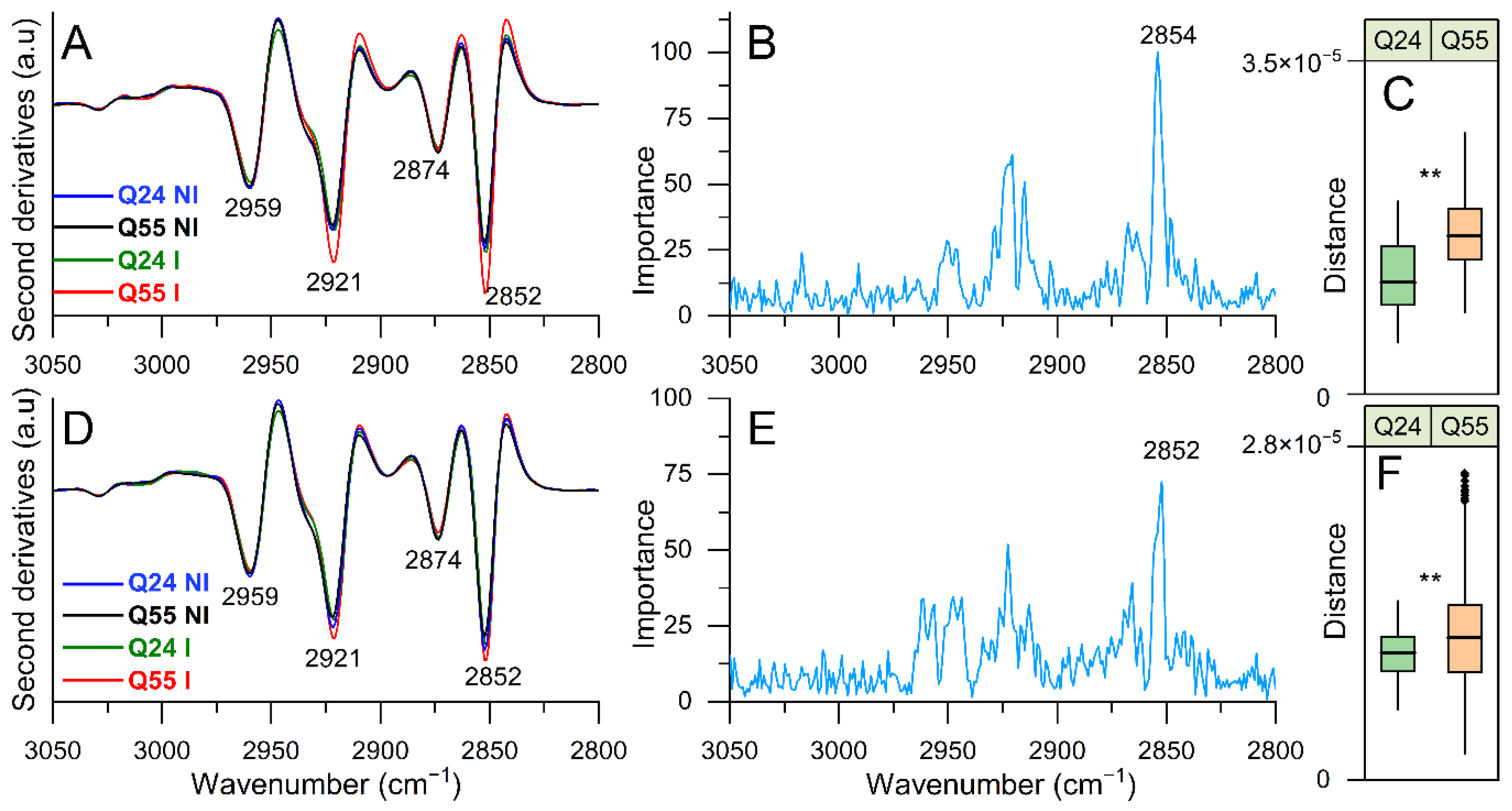
2.3.2. FTIR Study of Lipid Modifications: Analysis of the 3050–2800 cm−1 Spectral Range
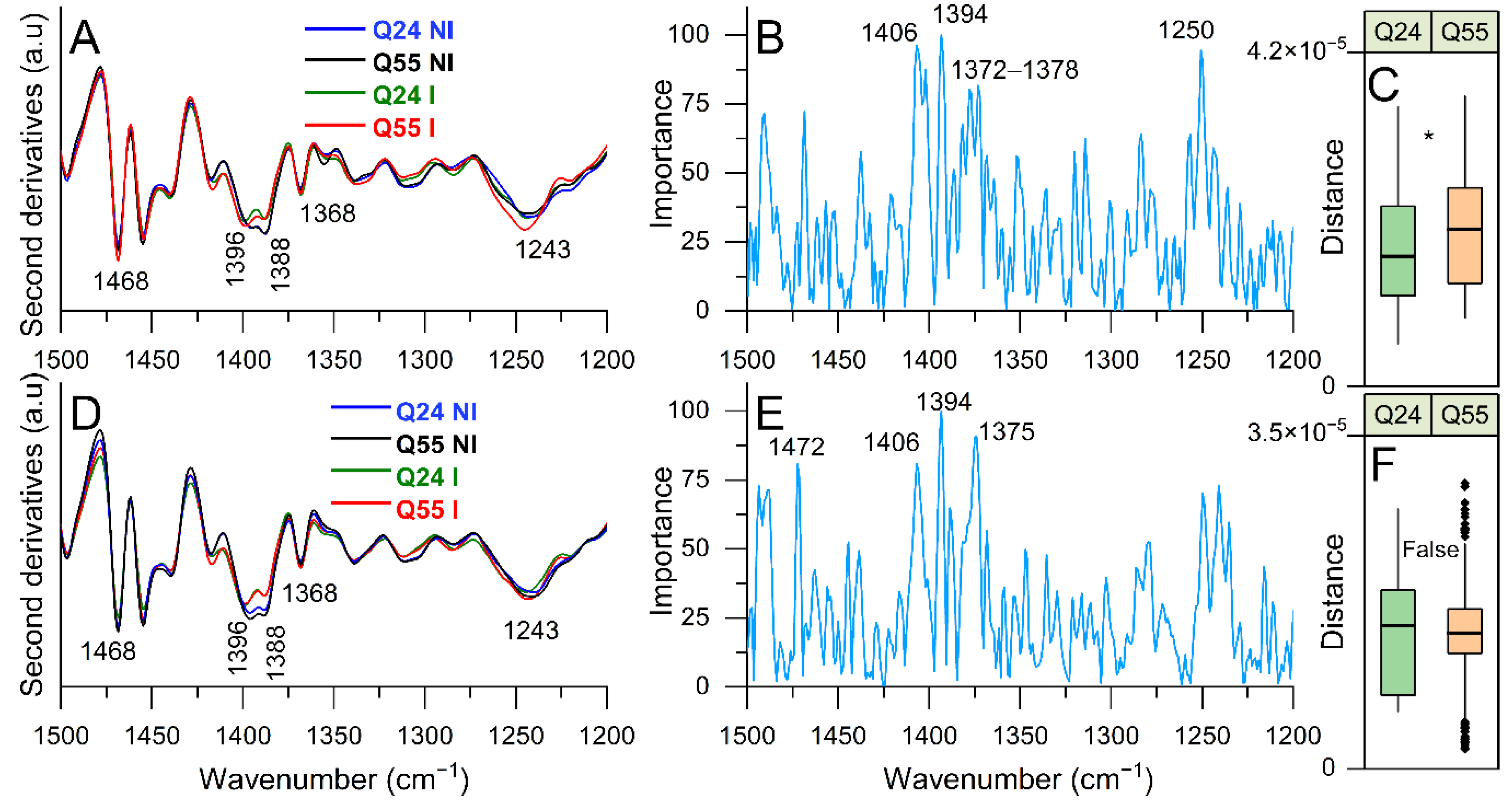
2.3.3. FTIR Study of Lipid and Peptidoglycan Modifications: Analysis of the 1500–1200 cm−1 Spectral Range
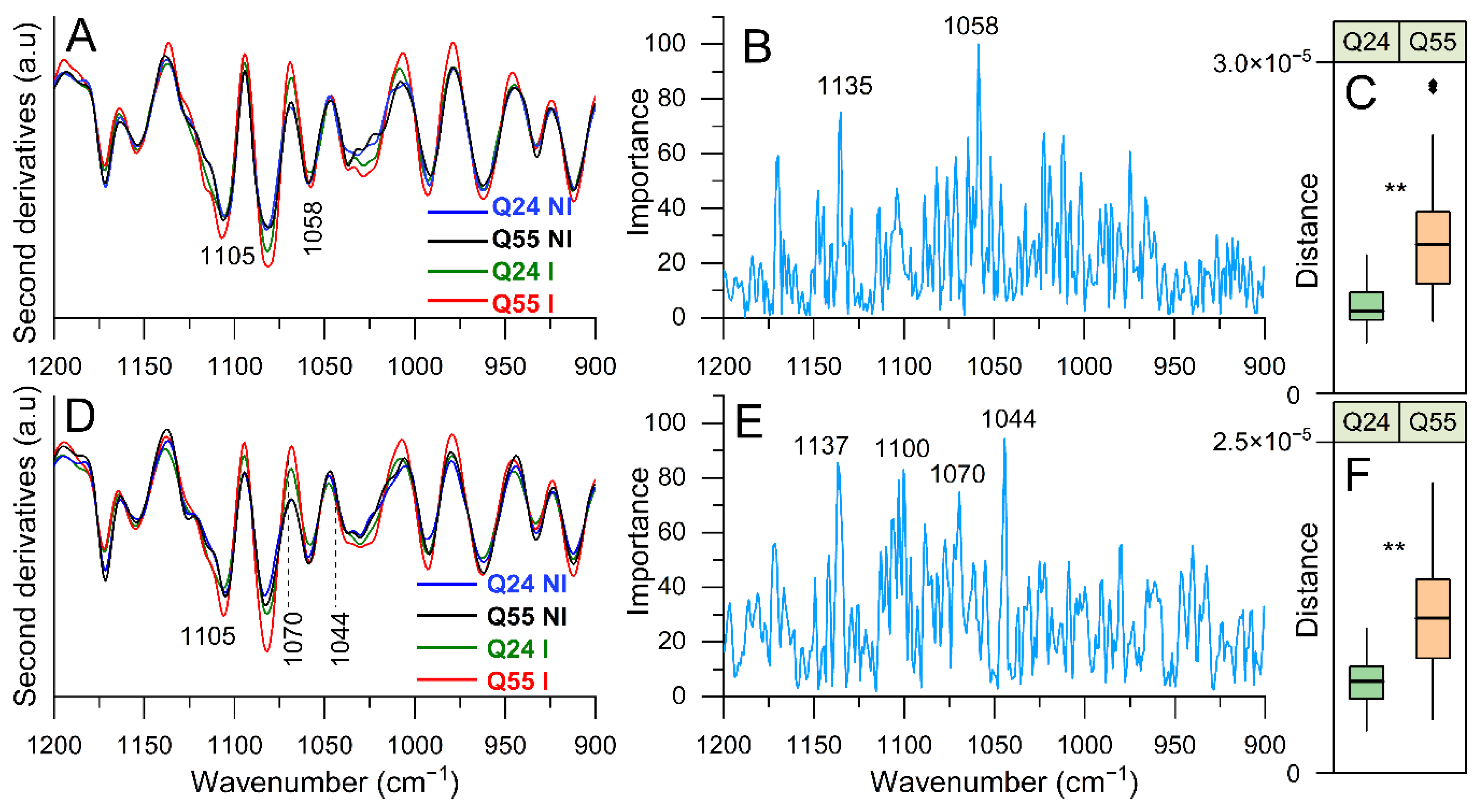
2.3.4. FTIR Study of Lipopolysaccharide and Peptidoglycans: Analysis of the 1200–900 cm−1 Spectral Range
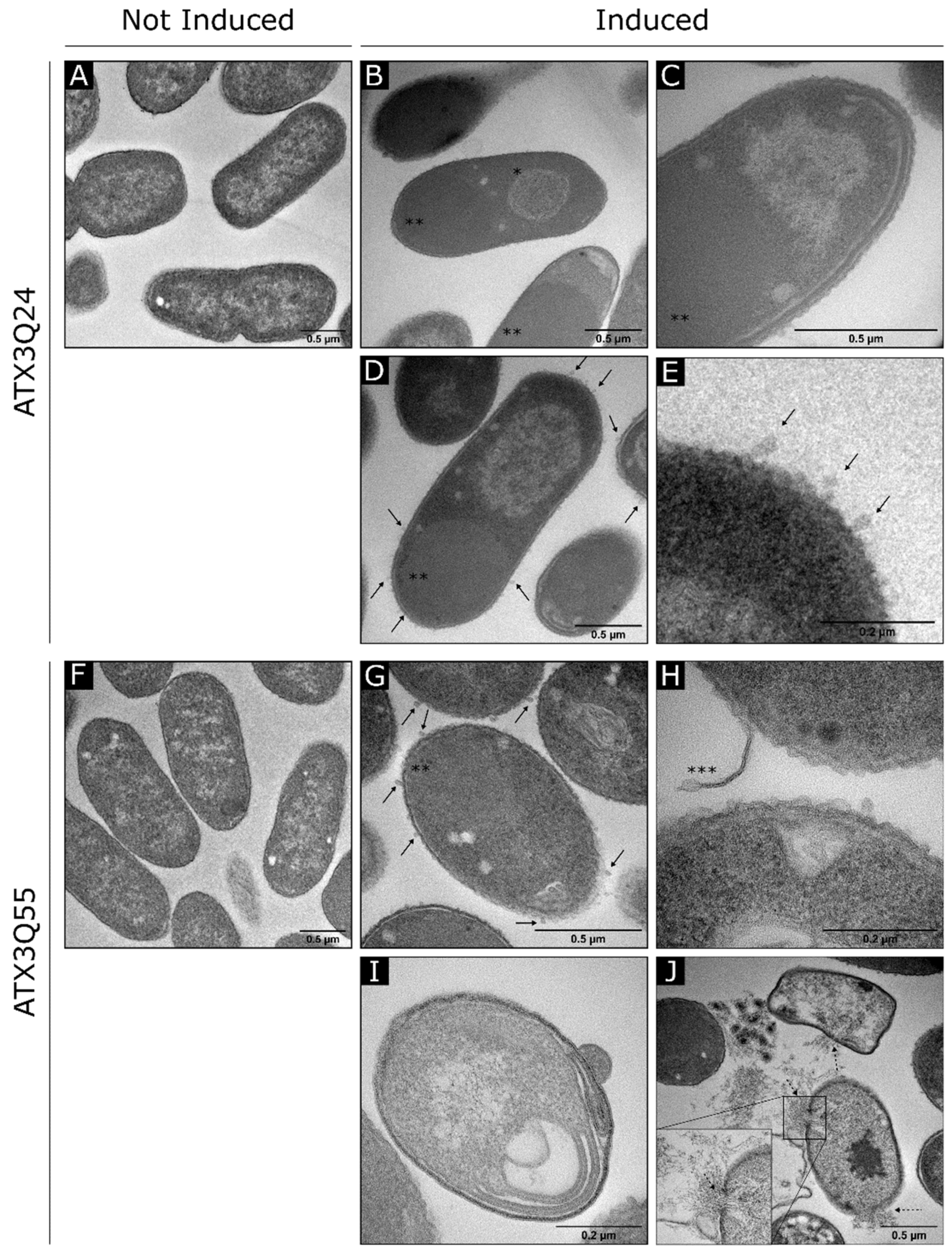
2.4. Electron Microscopy Analysis of ATX3 Expressing Strains
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Plasmid
4.2. Growth Curves Analysis
4.3. Colony-Formation Unit (CFU) Count
4.4. Western Blot Analysis of Total Extract and Soluble Protein Fractions
4.5. Dot-Blot Analysis of Total Extract Samples
4.6. FTIR Microspectroscopy Analysis
4.7. Multivariate Analysis of the FTIR Data
4.8. Transmission Electron Microscopy Analysis
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol 2009, 5, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kodali, R.; Wetzel, R. Polymorphism in the intermediates and products of amyloid assembly. Curr. Opin. Struct. Biol. 2007, 17, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.S.; Andersen, C.B.; Otzen, D.E. Amyloid structure--one but not the same: The many levels of fibrillar polymorphism. FEBS J. 2010, 277, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Bemporad, F.; Chiti, F. “Native-like aggregation” of the acylphosphatase from Sulfolobus solfataricus and its biological implications. FEBS Lett. 2009, 583, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Masino, L.; Nicastro, G.; De Simone, A.; Calder, L.; Molloy, J.; Pastore, A. The Josephin domain determines the morphological and mechanical properties of ataxin-3 fibrils. Biophys. J. 2011, 100, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Longo, G.; Faggiano, S.; Lipiec, E.; Pastore, A.; Dietler, G. Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nat. Commun. 2015, 6, 7831. [Google Scholar] [CrossRef]
- Paulson, H.L. Dominantly inherited ataxias: Lessons learned from Machado-Joseph disease/spinocerebellar ataxia type 3. Semin. Neurol. 2007, 27, 133–142. [Google Scholar] [CrossRef]
- Cummings, C.J.; Zoghbi, H.Y. Trinucleotide repeats: Mechanisms and pathophysiology. Annu. Rev. Genom. Hum. Genet. 2000, 1, 281–328. [Google Scholar] [CrossRef]
- Masino, L.; Musi, V.; Menon, R.P.; Fusi, P.; Kelly, G.; Frenkiel, T.A.; Trottier, Y.; Pastore, A. Domain architecture of the polyglutamine protein ataxin-3: A globular domain followed by a flexible tail. FEBS Lett. 2003, 549, 21–25. [Google Scholar] [CrossRef]
- Masino, L.; Nicastro, G.; Menon, R.P.; Dal Piaz, F.; Calder, L.; Pastore, A. Characterization of the structure and the amyloidogenic properties of the Josephin domain of the polyglutamine-containing protein ataxin-3. J. Mol. Biol. 2004, 344, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Ellisdon, A.M.; Thomas, B.; Bottomley, S.P. The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J. Biol. Chem. 2006, 281, 16888–16896. [Google Scholar] [CrossRef] [PubMed]
- Ellisdon, A.M.; Pearce, M.C.; Bottomley, S.P. Mechanisms of ataxin-3 misfolding and fibril formation: Kinetic analysis of a disease-associated polyglutamine protein. J. Mol. Biol. 2007, 368, 595–605. [Google Scholar] [CrossRef]
- Natalello, A.; Frana, A.M.; Relini, A.; Apicella, A.; Invernizzi, G.; Casari, C.; Gliozzi, A.; Doglia, S.M.; Tortora, P.; Regonesi, M.E. A major role for side-chain polyglutamine hydrogen bonding in irreversible ataxin-3 aggregation. PLoS ONE 2011, 6, e18789. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Calloni, G.; Chiti, F.; Formigli, L.; Nosi, D.; Dobson, C.M.; Stefani, M. Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J. Biol. Chem. 2004, 279, 31374–31382. [Google Scholar] [CrossRef]
- Glabe, C.G. Structural classification of toxic amyloid oligomers. J. Biol. Chem. 2008, 283, 29639–29643. [Google Scholar] [CrossRef] [PubMed]
- Pellistri, F.; Bucciantini, M.; Invernizzi, G.; Gatta, E.; Penco, A.; Frana, A.M.; Nosi, D.; Relini, A.; Regonesi, M.E.; Gliozzi, A.; et al. Different ataxin-3 amyloid aggregates induce intracellular Ca 2+ deregulation by different mechanisms in cerebellar granule cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 3155–3165. [Google Scholar] [CrossRef][Green Version]
- Kourie, J.I.; Shorthouse, A.A. Properties of cytotoxic peptide-formed ion channels. Am. J. Physiol. Cell Physiol. 2000, 278, C1063–C1087. [Google Scholar] [CrossRef]
- Sethuraman, A.; Belfort, G. Protein structural perturbation and aggregation on homogeneous surfaces. Biophys. J. 2005, 88, 1322–1333. [Google Scholar] [CrossRef]
- Chou, A.-H.; Chen, Y.-L.; Hu, S.-H.; Chang, Y.-M.; Wang, H.-L. Polyglutamine-expanded ataxin-3 impairs long-term depression in Purkinje neurons of SCA3 transgenic mouse by inhibiting HAT and impairing histone acetylation. Brain Res. 2014, 1583. [Google Scholar] [CrossRef]
- Chen, X.; Tang, T.-S.; Tu, H.; Nelson, O.; Pook, M.; Hammer, R.; Nukina, N.; Bezprozvanny, I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J. Neurosci. 2008, 28, 12713–12724. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-C.; Kuo, C.-L.; Cheng, W.-L.; Liu, C.-S.; Hsieh, M. Decreased antioxidant enzyme activity and increased mitochondrial DNA damage in cellular models of Machado-Joseph disease. J. Neurosci. Res. 2009, 87, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-Y.; Jhang, Y.-L.; Cheng, P.-H.; Chang, Y.-F.; Mao, S.-H.; Yang, H.-I.; Lin, C.-W.; Chen, C.-M.; Yang, S.-H. The Truncated C-terminal Fragment of Mutant ATXN3 Disrupts Mitochondria Dynamics in Spinocerebellar Ataxia Type 3 Models. Front. Mol. Neurosci. 2017, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Ami, D.; Natalello, A.; Lotti, M.; Doglia, S.M. Why and how protein aggregation has to be studied in vivo. Microb. Cell Fact. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, Z.; Thakur, A.K.; Wetzel, R.; Gierasch, L.M. In-cell aggregation of a polyglutamine-containing chimera is a multistep process initiated by the flanking sequence. J. Biol. Chem. 2007, 282, 36736–36743. [Google Scholar] [CrossRef]
- Caballero, A.B.; Espargaró, A.; Pont, C.; Busquets, M.A.; Estelrich, J.; Muñoz-Torrero, D.; Gamez, P.; Sabate, R. Bacterial Inclusion Bodies for Anti-Amyloid Drug Discovery: Current and Future Screening Methods. Curr. Protein Pept. Sci. 2019, 20, 563–576. [Google Scholar] [CrossRef]
- Delivoria, D.C.; Chia, S.; Habchi, J.; Perni, M.; Matis, I.; Papaevgeniou, N.; Reczko, M.; Chondrogianni, N.; Dobson, C.M.; Vendruscolo, M.; et al. Bacterial production and direct functional screening of expanded molecular libraries for discovering inhibitors of protein aggregation. Sci. Adv. 2019, 5, eaax5108. [Google Scholar] [CrossRef]
- Invernizzi, G.; Aprile, F.A.; Natalello, A.; Ghisleni, A.; Penco, A.; Relini, A.; Doglia, S.M.; Tortora, P.; Regonesi, M.E. The relationship between aggregation and toxicity of polyglutamine-containing ataxin-3 in the intracellular environment of Escherichia coli. PLoS ONE 2012, 7, e51890. [Google Scholar] [CrossRef]
- Ami, D.; Natalello, A.; Doglia, S.M. Fourier transform infrared microspectroscopy of complex biological systems: From intact cells to whole organisms. Methods Mol. Biol. 2012, 895, 85–100. [Google Scholar] [CrossRef]
- Ami, D.; Mereghetti, P.; Doglia, S.M. Multivariate Analysis for Fourier Transform Infrared Spectra of Complex Biological Systems and Processes. Multivar. Anal. Manag. Eng. Sci. 2013. [Google Scholar] [CrossRef]
- Invernizzi, G.; Annoni, E.; Natalello, A.; Doglia, S.M.; Lotti, M. In vivo aggregation of bovine beta-lactoglobulin is affected by Cys at position 121. Protein Expr. Purif. 2008, 62, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Nilsson, L.; Kurland, C.G. Gratuitous overexpression of genes in Escherichia coli leads to growth inhibition and ribosome destruction. J. Bacteriol. 1995, 177, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Lafranconi, P.; Natalello, A.; Ami, D.; Doglia, S.M.; Lotti, M. Concepts and tools to exploit the potential of bacterial inclusion bodies in protein science and biotechnology. FEBS J. 2011, 278, 2408–2418. [Google Scholar] [CrossRef]
- Jarrige, A.C.; Mathy, N.; Portier, C. PNPase autocontrols its expression by degrading a double-stranded structure in the pnp mRNA leader. EMBO J. 2001, 20, 6845–6855. [Google Scholar] [CrossRef] [PubMed]
- Briani, F.; Carzaniga, T.; Dehò, G. Regulation and functions of bacterial PNPase. Wiley Interdiscip. Rev. RNA 2016, 7, 241–258. [Google Scholar] [CrossRef]
- Shehi, E.; Fusi, P.; Secundo, F.; Pozzuolo, S.; Bairati, A.; Tortora, P. Temperature-dependent, irreversible formation of amyloid fibrils by a soluble human ataxin-3 carrying a moderately expanded polyglutamine stretch (Q36). Biochemistry 2003, 42, 14626–14632. [Google Scholar] [CrossRef]
- Fontanella, L.; Pozzuolo, S.; Costanzo, A.; Favaro, R.; Dehò, G.; Tortora, P. Photometric assay for polynucleotide phosphorylase. Anal. Biochem. 1999, 269, 353–358. [Google Scholar] [CrossRef]
- Lajoie, P.; Snapp, E.L. Formation and toxicity of soluble polyglutamine oligomers in living cells. PLoS ONE 2010, 5, e15245. [Google Scholar] [CrossRef]
- Bonanomi, M.; Natalello, A.; Visentin, C.; Pastori, V.; Penco, A.; Cornelli, G.; Colombo, G.; Malabarba, M.G.; Doglia, S.M.; Relini, A.; et al. Epigallocatechin-3-gallate and tetracycline differently affect ataxin-3 fibrillogenesis and reduce toxicity in spinocerebellar ataxia type 3 model. Hum. Mol. Genet. 2014, 23, 6542–6552. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Natalello, A.; Doglia, S.M. Insoluble protein assemblies characterized by fourier transform infrared spectroscopy. Methods Mol. Biol. 2015, 1258, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Casal, H.L.; Mantsch, H.H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta 1984, 779, 381–401. [Google Scholar] [CrossRef]
- Ami, D.; Natalello, A.; Schultz, T.; Gatti-Lafranconi, P.; Lotti, M.; Doglia, S.M.; de Marco, A. Effects of recombinant protein misfolding and aggregation on bacterial membranes. Biochim. Biophys. Acta 2009, 1794, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, B.; Zhang, X.; Yang, Y.; Hardwidge, P.R.; Ren, W.; Zhu, G. The role of bacterial cell envelope structures in acid stress resistance in E. coli. Appl. Microbiol. Biotechnol. 2020, 104, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Naumann, D.; Barnickel, G.; Bradaczek, H.; Labischinski, H.; Giesbrecht, P. Infrared Spectroscopy, a Tool for Probing Bacterial Peptidoglycan. Eur. J. Biochem. 1982, 125, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kochan, K.; Perez-Guaita, D.; Pissang, J.; Jiang, J.-H.; Peleg, A.Y.; McNaughton, D.; Heraud, P.; Wood, B.R. In vivo atomic force microscopy-infrared spectroscopy of bacteria. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef]
- Banyay, M.; Sarkar, M.; Gräslund, A. A library of IR bands of nucleic acids in solution. Biophys. Chem. 2003, 104, 477–488. [Google Scholar] [CrossRef]
- Needham, B.D.; Trent, M.S. Fortifying the barrier: The impact of lipid: A remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013, 11, 467–481. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Arechaga, I.; Miroux, B.; Karrasch, S.; Huijbregts, R.; de Kruijff, B.; Runswick, M.J.; Walker, J.E. Characterisation of new intracellular membranes in Escherichia coli accompanying large scale over-production of the b subunit of F(1)F(o) ATP synthase. FEBS Lett. 2000, 482, 215–219. [Google Scholar] [CrossRef]
- Wolf, S.G.; Frenkiel, D.; Arad, T.; Finkel, S.E.; Kolter, R.; Minsky, A. DNA protection by stress-induced biocrystallization. Nature 1999, 400, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Minsky, A.; Shimoni, E.; Frenkiel-Krispin, D. Stress, order and survival. Nat. Rev. Mol. Cell Biol. 2002, 3, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Loiko, N.; Danilova, Y.; Moiseenko, A.; Kovalenko, V.; Tereshkina, K.; Tutukina, M.; El-Registan, G.; Sokolova, O.; Krupyanskii, Y. Morphological peculiarities of the DNA-protein complexes in starved Escherichia coli cells. PLoS ONE 2020, 15, e0231562. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Von Meyenburg, K.; Jørgensen, B.B.; van Deurs, B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984, 3, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Lesley, S.A.; Graziano, J.; Cho, C.Y.; Knuth, M.W.; Klock, H.E. Gene expression response to misfolded protein as a screen for soluble recombinant protein. Protein Eng. 2002, 15, 153–160. [Google Scholar] [CrossRef]
- Villa, R.; Lotti, M.; Gatti-Lafranconi, P. Components of the E. coli envelope are affected by and can react to protein over-production in the cytoplasm. Microb. Cell Fact. 2009, 8, 32. [Google Scholar] [CrossRef]
- Molina-García, L.; Moreno-Del Álamo, M.; Botias, P.; Martín-Moldes, Z.; Fernández, M.; Sánchez-Gorostiaga, A.; Alonso-Del Valle, A.; Nogales, J.; García-Cantalejo, J.; Giraldo, R. Outlining core pathways of amyloid toxicity in bacteria with the RepA-WH1 Prionoid. Front. Microbiol. 2017, 8, 539. [Google Scholar] [CrossRef]
- Delhaye, A.; Collet, J.-F.; Laloux, G. A Fly on the wall: How stress response systems can sense and respond to damage to Peptidoglycan. Front. Cell Infect. Microbiol. 2019, 9, 380. [Google Scholar] [CrossRef]
- Grabowicz, M.; Silhavy, T.J. Envelope stress responses: An interconnected safety Net. Trends Biochem. Sci. 2017, 42, 232–242. [Google Scholar] [CrossRef]
- Rowlett, V.W.; Mallampalli, V.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef] [PubMed]
- Doglia, S.M.; Ami, D.; Natalello, A.; Gatti-Lafranconi, P.; Lotti, M. Fourier transform infrared spectroscopy analysis of the conformational quality of recombinant proteins within inclusion bodies. Biotechnol. J. 2008, 3, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Filzmoser, P.; Maronna, R.; Werner, M. Outlier identification in high dimensions. Comput. Stat. Data Anal. 2008, 52, 1694–1711. [Google Scholar] [CrossRef]
- Pérez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees. Available online: https://cds.cern.ch/record/2253780 (accessed on 17 November 2020).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ami, D.; Sciandrone, B.; Mereghetti, P.; Falvo, J.; Catelani, T.; Visentin, C.; Tortora, P.; Ventura, S.; Natalello, A.; Regonesi, M.E. Pathological ATX3 Expression Induces Cell Perturbations in E. coli as Revealed by Biochemical and Biophysical Investigations. Int. J. Mol. Sci. 2021, 22, 943. https://doi.org/10.3390/ijms22020943
Ami D, Sciandrone B, Mereghetti P, Falvo J, Catelani T, Visentin C, Tortora P, Ventura S, Natalello A, Regonesi ME. Pathological ATX3 Expression Induces Cell Perturbations in E. coli as Revealed by Biochemical and Biophysical Investigations. International Journal of Molecular Sciences. 2021; 22(2):943. https://doi.org/10.3390/ijms22020943
Chicago/Turabian StyleAmi, Diletta, Barbara Sciandrone, Paolo Mereghetti, Jacopo Falvo, Tiziano Catelani, Cristina Visentin, Paolo Tortora, Salvador Ventura, Antonino Natalello, and Maria Elena Regonesi. 2021. "Pathological ATX3 Expression Induces Cell Perturbations in E. coli as Revealed by Biochemical and Biophysical Investigations" International Journal of Molecular Sciences 22, no. 2: 943. https://doi.org/10.3390/ijms22020943
APA StyleAmi, D., Sciandrone, B., Mereghetti, P., Falvo, J., Catelani, T., Visentin, C., Tortora, P., Ventura, S., Natalello, A., & Regonesi, M. E. (2021). Pathological ATX3 Expression Induces Cell Perturbations in E. coli as Revealed by Biochemical and Biophysical Investigations. International Journal of Molecular Sciences, 22(2), 943. https://doi.org/10.3390/ijms22020943






