Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy?
Abstract
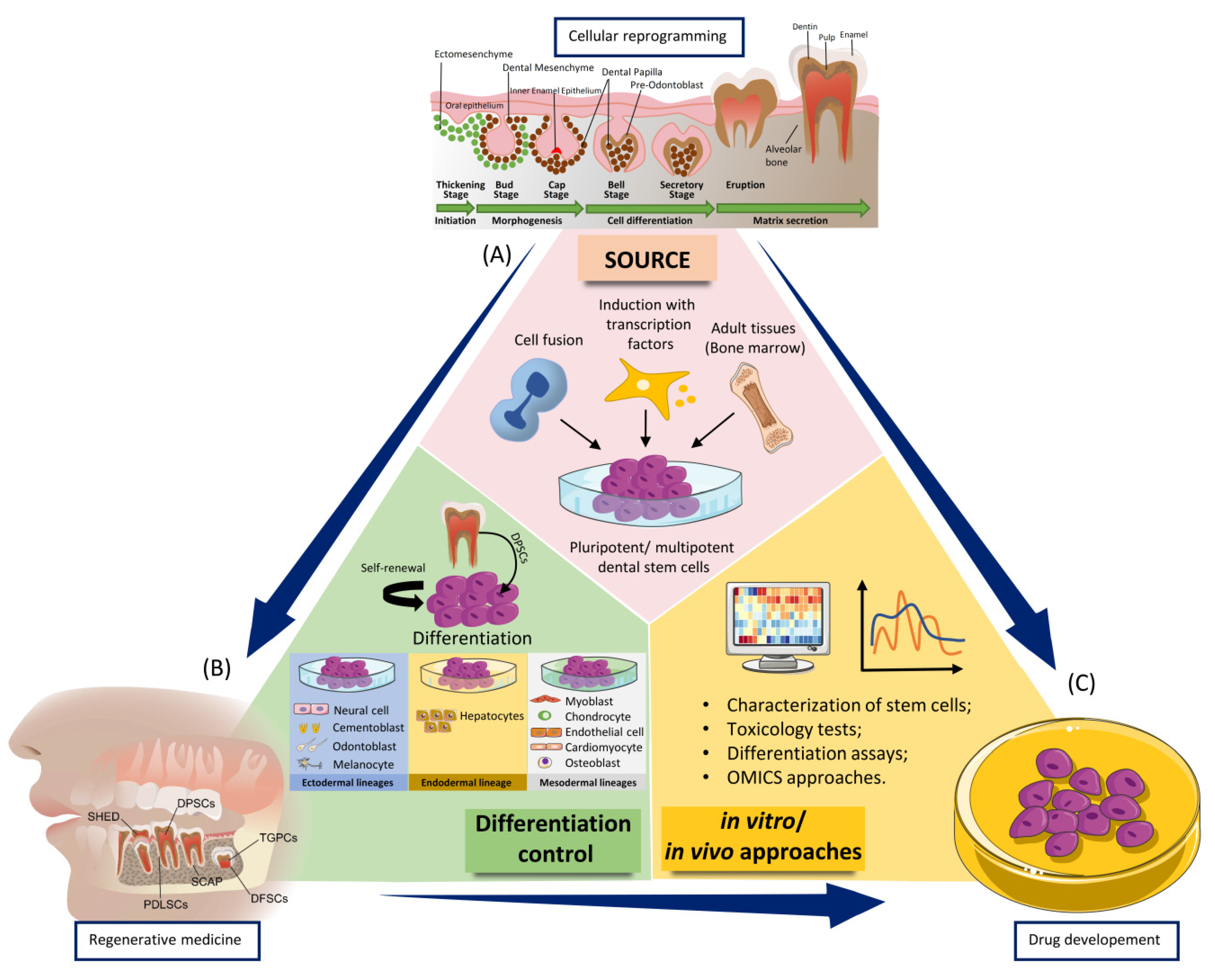
1. Introduction
2. Dental Stem Cells—Their Proangiogenic Ability and Subsequent Regenerative Capacity
2.1. General Considerations
2.2. Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
2.3. Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs)
Potential Clinical Approaches of HPcy-MSCs
2.4. Human Dental Pulp Stem Cells (DPSCs)
2.5. Human Exfoliated Deciduous Teeth (SHED)
2.6. Periodontal Ligament Stem Cells (PDLSCs)
2.7. Stem Cells from Apical Papilla (SCAP)
2.8. Gingival-Mesenchymal Derived Stem Cells (GMDSC)
2.9. Dental Follicle Stem Cells DFSCs
Potential Clinical Approaches of the DFSCs
3. Experimental Therapeutic Applications of Oral MSCs in Oral Diseases
3.1. Endodontic Diseases
3.2. Periodontal Disease
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watt, S.M.; Gullo, F.; van der Garde, M.; Markeson, D.; Camicia, R.; Khoo, C.P.; Zwaginga, J.J. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull. 2013, 108, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part II: The effect of bone-grafting and barrier membrane materials on angiogenesis. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e526–e537. [Google Scholar] [CrossRef] [PubMed]
- Alkharsah, K.R. VEGF Upregulation in Viral Infections and Its Possible Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 1642. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, B.; Palmieri, F.; Amantea, M.; Nuzzolese, M.; Valletta, R.; Zavan, B.; Vito, D. Promising Scaffold-Free Approaches in Translational Dentistry. Int. J. Environ. Res. Public Health 2020, 17, 3001. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Sonea, L.; Zimta, A.A.; Cojocneanu-Petric, R.; Tonchev, K.; Mehterov, N.; Diudea, D.; Buduru, S.; Berindan-Neagoe, I. A Looking-Glass of Non-coding RNAs in oral cancer. Int. J. Mol. Sci. 2017, 18, 2620. [Google Scholar] [CrossRef]
- Gupta, M.K.; Qin, R.-Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144–1155. [Google Scholar] [CrossRef]
- Zimta, A.A.; Baru, O.; Badea, M.; Buduru, S.D.; Berindan-Neagoe, I. The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. Int. J. Mol. Sci. 2019, 20, 406. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp. Physiol. 2005, 90, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Mu, X.; Shi, L.; Pan, S.; He, L.; Niu, Y.; Wang, X. A Customized Self-Assembling Peptide Hydrogel-Wrapped Stem Cell Factor Targeting Pulp Regeneration Rich in Vascular-Like Structures. ACS Omega 2020, 5, 16568–16574. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Roudnicky, F.; Burcin, M.; Patsch, C. Monolayer Generation of Vascular Endothelial Cells from Human Pluripotent Stem Cells. Methods Mol. Biol. 2019, 1994, 17–29. [Google Scholar] [PubMed]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Wolff, T.; Valente, P.; Di Maggio, N.; Pellegrino, M.; Gürke, L.; Banfi, A.; Gianni-Barrera, R. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss Med. Wkly. 2019, 149, w20011. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef]
- Yamashita, Y.M. Cell biology of stem cells: Studying stem cells at the level of cell biology and studying cell biology using stem cells. Mol. Biol. Cell 2018, 29, 2912. [Google Scholar] [CrossRef] [PubMed]
- Behr, B.; Ko, S.H.; Wong, V.W.; Gurtner, G.C.; Longaker, M.T. Stem cells. Plast. Reconstr. Surg. 2010, 126, 1163–1171. [Google Scholar] [CrossRef]
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Med. Iran. 2017, 55, 6–23. [Google Scholar]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cell Mater. 2010, 20, 121–133. [Google Scholar] [CrossRef]
- Bruder, S.P.; Jaiswal, N.; Haynesworth, S.E. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 1997, 64, 278–294. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J. Stem cells from the Mammalian blastocyst. Stem Cells 2001, 19, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Majo, F.; Rochat, A.; Nicolas, M.; Jaoudé, G.A.; Barrandon, Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008, 456, 250–254. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Zhang, F.; Citra, F.; Wang, D.A. Prospects of induced pluripotent stem cell technology in regenerative medicine. Tissue Eng. Part B Rev. 2011, 17, 115–124. [Google Scholar] [CrossRef]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef]
- Bilousova, G.; Jun du, H.; King, K.B.; De Langhe, S.; Chick, W.S.; Torchia, E.C.; Chow, K.S.; Klemm, D.J.; Roop, D.R.; Majka, S.M. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells 2011, 29, 206–216. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Soleimani, M.; Hosseinkhani, S.; Parivar, K.; Yaghmaei, P. A comparative study of osteogenic differentiation human induced pluripotent stem cells and adipose tissue derived mesenchymal stem cells. Cell J. 2014, 16, 235–244. [Google Scholar]
- Rana, D.; Kumar, S.; Webster, T.J.; Ramalingam, M. Impact of Induced Pluripotent Stem Cells in Bone Repair and Regeneration. Curr. Osteoporos. Rep. 2019, 17, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Filip, S.; Mokry, J.; Horacek, J.; English, D. Stem cells and the phenomena of plasticity and diversity: A limiting property of carcinogenesis. Stem Cells Dev. 2008, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Taheri, B.; Soleimani, M.; Fekri Aval, S.; Esmaeili, E.; Bazi, Z.; Zarghami, N. Induced pluripotent stem cell-derived extracellular vesicles: A novel approach for cell-free regenerative medicine. J. Cell. Physiol. 2019, 234, 8455–8464. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- El-Sayed, M.; El-Feky, M.A.; El-Amir, M.I.; Hasan, A.S.; Tag-Adeen, M.; Urata, Y.; Goto, S.; Luo, L.; Yan, C.; Li, T.S. Immunomodulatory effect of mesenchymal stem cells: Cell origin and cell quality variations. Mol. Biol. Rep. 2019, 46, 1157–1165. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Monroy-García, A.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal Stem Cells of Dental Origin for Inducing Tissue Regeneration in Periodontitis: A Mini-Review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.J.; Liu, K.J.; Chao, Y.Y.; Sytwu, H.K.; Yen, B.L. Assessment of the Immunomodulatory Properties of Human Mesenchymal Stem Cells (MSCs). J. Vis. Exp. 2015, 106, e53265. [Google Scholar] [CrossRef]
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Sato, Y.; Akita, D.; Toriumi, T.; Namaki, S.; Matsuzaki, Y.; Yonehara, Y.; Honda, M. Bone marrow-derived mesenchymal stem cells enhance bone marrow regeneration in dental extraction sockets. J. Oral Sci. 2019, 61, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Nuti, N.; Corallo, C.; Chan, B.M.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Dörfer, C.; Fändrich, F.; Gieseler, F.; Moustafa, M.H.; Ungefroren, H. Adult mesenchymal stem cells explored in the dental field. Adv. Biochem. Eng. Biotechnol. 2013, 130, 89–103. [Google Scholar] [PubMed]
- Lee, D.K.; Song, S.U. Immunomodulatory mechanisms of mesenchymal stem cells and their therapeutic applications. Cell. Immunol. 2018, 326, 68–76. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef]
- Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef]
- Marrelli, M.; Paduano, F.; Tatullo, M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int. J. Biol. Sci. 2013, 9, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Codispoti, B.; Spagnuolo, G.; Zavan, B. Human Periapical Cyst-Derived Stem Cells Can Be A Smart “Lab-on-A-Cell” to Investigate Neurodegenerative Diseases and the Related Alteration of the Exosomes’ Content. Brain Sci. 2019, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Paduano, F.; Tatullo, M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J. Dent. Res. 2015, 94, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; Palmieri, F.; Tatullo, M. CD146 Expression Influences Periapical Cyst Mesenchymal Stem Cell Properties. Stem Cell Rev. Rep. 2016, 12, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.; Berbéri, A.; Fayyad-Kazan, M. An update on human periapical cyst-mesenchymal stem cells and their potential applications in regenerative medicine. Mol. Biol. Rep. 2020, 47, 2381–2389. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, B.; Zullo, M.J.; Codispoti, B.; Paduano, F.; Benincasa, C.; Fortunato, F.; Scacco, S.; Zavan, B.; Cocco, T. Exosomes from Human Periapical Cyst-MSCs: Theranostic Application in Parkinson’s Disease. Int. J. Med. Sci. 2020, 17, 657–663. [Google Scholar] [CrossRef]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Wake, H.; Ito, M.; Nabekura, J.; Wakita, H.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem Cells 2008, 26, 2408–2418. [Google Scholar] [CrossRef]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 156. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D.; Carnevale, G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381. [Google Scholar]
- Gandia, C.; Armiñan, A.; García-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int. J. Mol. Sci. 2019, 20, 1132. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Omi, M.; Kobayashi, Y.; Nakamura, N.; Tosaki, T.; Miyabe, M.; Kojima, N.; Kubo, K.; Ozawa, S.; Maeda, H.; et al. Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: Therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells. Stem Cell Res. Ther. 2015, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Lambrichts, I.; Driesen, R.B.; Dillen, Y.; Gervois, P.; Ratajczak, J.; Vangansewinkel, T.; Wolfs, E.; Bronckaers, A.; Hilkens, P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J. Endod. 2017, 43, S12–S16. [Google Scholar] [CrossRef]
- Ma, L.; Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Maintained Properties of Aged Dental Pulp Stem Cells for Superior Periodontal Tissue Regeneration. Aging Dis. 2019, 10, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef]
- Li, Y.; Nan, X.; Zhong, T.Y.; Li, T.; Li, A. Treatment of Periodontal Bone Defects with Stem Cells from Inflammatory Dental Pulp Tissues in Miniature Swine. Tissue Eng. Regen. Med. 2019, 16, 191–200. [Google Scholar] [CrossRef]
- Song, D.; Xu, P.; Liu, S.; Wu, S. Dental pulp stem cells expressing SIRT1 improve new bone formation during distraction osteogenesis. Am. J. Transl. Res. 2019, 11, 832–843. [Google Scholar]
- Tsutsui, T.W. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning 2020, 13, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Ma, L.; Makino, Y.; Yamaza, H.; Akiyama, K.; Hoshino, Y.; Song, G.; Kukita, T.; Nonaka, K.; Shi, S.; Yamaza, T. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS ONE 2012, 7, e51777. [Google Scholar] [CrossRef]
- Du, Z.H.; Ding, C.; Zhang, Q.; Zhang, Y.; Ge, X.Y.; Li, S.L.; Yu, G.Y. Stem cells from exfoliated deciduous teeth alleviate hyposalivation caused by Sjögren syndrome. Oral Dis. 2019, 25, 1530–1544. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef]
- Bartold, P.M.; McCulloch, C.A.; Narayanan, A.S.; Pitaru, S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 2000 2000, 24, 253–269. [Google Scholar] [CrossRef]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.M.; Jung, I.H.; Kim, J.C.; Choi, S.H.; Cho, K.S.; Kim, C.S. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, Y.; Gao, L.N.; Zhang, Y.J.; Jin, Y.; Chen, F.M. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012, 33, 6974–6986. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, H.; Huang, Y.; Liu, C. Comparative analysis of in vitro periodontal characteristics of stem cells from apical papilla (SCAP) and periodontal ligament stem cells (PDLSCs). Arch. Oral Biol. 2013, 58, 997–1006. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Lei, G.; Yan, M.; Wang, Z.; Yu, Y.; Tang, C.; Wang, Z.; Yu, J.; Zhang, G. Dentinogenic capacity: Immature root papilla stem cells versus mature root pulp stem cells. Biol. Cell 2011, 103, 185–196. [Google Scholar] [CrossRef]
- Ikeda, E.; Hirose, M.; Kotobuki, N.; Shimaoka, H.; Tadokoro, M.; Maeda, M.; Hayashi, Y.; Kirita, T.; Ohgushi, H. Osteogenic differentiation of human dental papilla mesenchymal cells. Biochem. Biophys. Res. Commun. 2006, 342, 1257–1262. [Google Scholar] [CrossRef]
- Yang, C.; Sun, L.; Li, X.; Xie, L.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. The potential of dental stem cells differentiating into neurogenic cell lineage after cultivation in different modes in vitro. Cell. Reprogram. 2014, 16, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Simonovic, J.; Toljic, B.; Nikolic, N.; Peric, M.; Vujin, J.; Panajotovic, R.; Gajic, R.; Bekyarova, E.; Cataldi, A.; Parpura, V.; et al. Differentiation of stem cells from apical papilla into neural lineage using graphene dispersion and single walled carbon nanotubes. J. Biomed. Mater. Res. A 2018, 106, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Kumar, B.M.; Lee, W.J.; Jeon, R.H.; Jang, S.J.; Lee, Y.M.; Park, B.W.; Byun, J.H.; Ahn, C.S.; Kim, J.W.; et al. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp. Cell Res. 2014, 320, 92–107. [Google Scholar] [CrossRef]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. BioMed Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, P.; Zhu, L.; Dissanayaka, W.L.; Green, D.W.; Tong, E.H.; Jin, L.; Zhang, C. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng. Part A 2015, 21, 1163–1172. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Kritis, A.; Andreadis, D.; Papachristou, E.; Leyhausen, G.; Koidis, P.; Geurtsen, W.; Tsiftsoglou, A. Angiogenic Potential and Secretome of Human Apical Papilla Mesenchymal Stem Cells in Various Stress Microenvironments. Stem Cells Dev. 2015, 24, 2496–2512. [Google Scholar] [CrossRef]
- Hilkens, P.; Fanton, Y.; Martens, W.; Gervois, P.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 2014, 12, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, J.; Viswanath, A.; De Berdt, P.; Everard, A.; Cani, P.D.; Bouzin, C.; Feron, O.; Diogenes, A.; Leprince, J.G.; des Rieux, A. Hypoxia modulates the differentiation potential of stem cells of the apical papilla. J. Endod. 2014, 40, 1410–1418. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized Human Dental Pulp as a Scaffold for Regenerative Endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016, 10, 261–270. [Google Scholar] [CrossRef]
- Larjava, H.; Wiebe, C.; Gallant-Behm, C.; Hart, D.A.; Heino, J.; Häkkinen, L. Exploring scarless healing of oral soft tissues. J. Can. Dent. Assoc. 2011, 77, b18. [Google Scholar] [PubMed]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef] [PubMed]
- Grawish, M.E. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J. Stem Cells 2018, 10, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Lee, J.E.; Yun, J.H.; Kim, I.; Ko, Y.; Park, J.B. Isolation and characterization of human mesenchymal stem cells from gingival connective tissue. J. Periodontal Res. 2015, 50, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.P.; Ferre, F.C.; Couty, L.; Lataillade, J.J.; Gourven, M.; Naveau, A.; Coulomb, B.; Lafont, A.; Gogly, B. Multipotent progenitor cells in gingival connective tissue. Tissue Eng. Part A 2010, 16, 2891–2899. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef]
- Jin, S.; Yang, C.; Huang, J.; Liu, L.; Zhang, Y.; Li, S.; Zhang, L.; Sun, Q.; Yang, P. Conditioned medium derived from FGF-2-modified GMSCs enhances migration and angiogenesis of human umbilical vein endothelial cells. Stem Cell Res. Ther. 2020, 11, 68. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef]
- Honda, M.J.; Imaizumi, M.; Tsuchiya, S.; Morsczeck, C. Dental follicle stem cells and tissue engineering. J. Oral Sci. 2010, 52, 541–552. [Google Scholar] [CrossRef]
- Wise, G.E. Cellular and molecular basis of tooth eruption. Orthod. Craniofac. Res. 2009, 12, 67–73. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.F.; Souza, M.M. Human dental follicle cells express embryonic, mesenchymal and neural stem cells markers. Arch. Oral Biol. 2017, 73, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation 2009, 77, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Ekat, K.; Brosemann, A.; Köntges, A.; David, R.; Lang, H. Isolation, Characterization and MicroRNA-based Genetic Modification of Human Dental Follicle Stem Cells. J. Vis. Exp. 2018, 141, e58089. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C. Effects of Cellular Senescence on Dental Follicle Cells. Pharmacology 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Reichert, T.E. Dental stem cells in tooth regeneration and repair in the future. Expert Opin. Biol. Ther. 2018, 18, 187–196. [Google Scholar] [CrossRef]
- Mori, G.; Ballini, A.; Carbone, C.; Oranger, A.; Brunetti, G.; Di Benedetto, A.; Rapone, B.; Cantore, S.; Di Comite, M.; Colucci, S.; et al. Osteogenic differentiation of dental follicle stem cells. Int. J. Med. Sci. 2012, 9, 480–487. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Buergers, R.; Morsczeck, C.; Gosau, M. β-Tricalcium phosphate induces apoptosis on dental follicle cells. Calcif. Tissue Int. 2013, 92, 412–417. [Google Scholar] [CrossRef]
- Morsczeck, C.; Völlner, F.; Saugspier, M.; Brandl, C.; Reichert, T.E.; Driemel, O.; Schmalz, G. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin. Oral Investig. 2010, 14, 433–440. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Liu, W.; Li, Q.; Jin, Z.; Jin, Y. Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS ONE 2014, 9, e108752. [Google Scholar] [CrossRef]
- Martinez Saez, D.; Sasaki, R.T.; Neves, A.D.; da Silva, M.C. Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature. Cells Tissues Organs 2016, 202, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp Stem Cell-Mediated Functional Pulp Regeneration. J. Dent. Res. 2019, 98, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, H.; Gao, B. Potential Therapeutic Effects of Exosomes in Regenerative Endodontics. Arch Oral Biol 2020, 120, 104946. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Zhang, C. Scaffold-based and Scaffold-free Strategies in Dental Pulp Regeneration. J. Endod. 2020, 46, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T. Pulp and dentin tissue engineering and regeneration: Current progress. Regen. Med. 2009, 4, 697–707. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, F.; Zhang, X.; Wang, S.; Jin, Y.; Zhang, W.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Human Dental Pulp Stem Cells Mediated Dentin-Pulp Complex Regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Yin, Y.; He, X.T.; An, Y.; Tian, B.M.; Hong, Y.L.; Wu, L.A.; Chen, F.M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020, 11, 110. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Braicu, C.; Irimie, A.; Craciun, L.; Berindan-Neagoe, I. Nanopharmacology in translational hematology and oncology. Int. J. Nanomed. 2014, 9, 3465–3479. [Google Scholar]
- Nagata, M.; Iwasaki, K.; Akazawa, K.; Komaki, M.; Yokoyama, N.; Izumi, Y.; Morita, I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng. Part A 2017, 23, 367–377. [Google Scholar] [CrossRef]
- Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The Angiogenic Potential of DPSCs and SCAPs in an In Vivo Model of Dental Pulp Regeneration. Stem Cells Int. 2017, 2017, 2582080. [Google Scholar] [CrossRef]
- Rodas-Junco, B.A.; Canul-Chan, M.; Rojas-Herrera, R.A.; De-la-Peña, C.; Nic-Can, G.I. Stem Cells from Dental Pulp: What Epigenetics Can Do with Your Tooth. Front. Physiol. 2017, 8, 999. [Google Scholar] [CrossRef]
| Expression | Lack of Expression | |
|---|---|---|
| Minimal criteria of MSCs as defined by ISCT | CD73/5′-Nucleotidase | CD34 |
| CD90/Thy1 | CD45 | |
| CD105/Endoglin | CD11b or CD14 | |
| CD79 alpha or CD19 alpha | ||
| HLA-II | ||
| Other common surface markers of dental MSCs | STRO-1 | |
| SSEA-4 | ||
| CD349 | ||
| 3G5 |
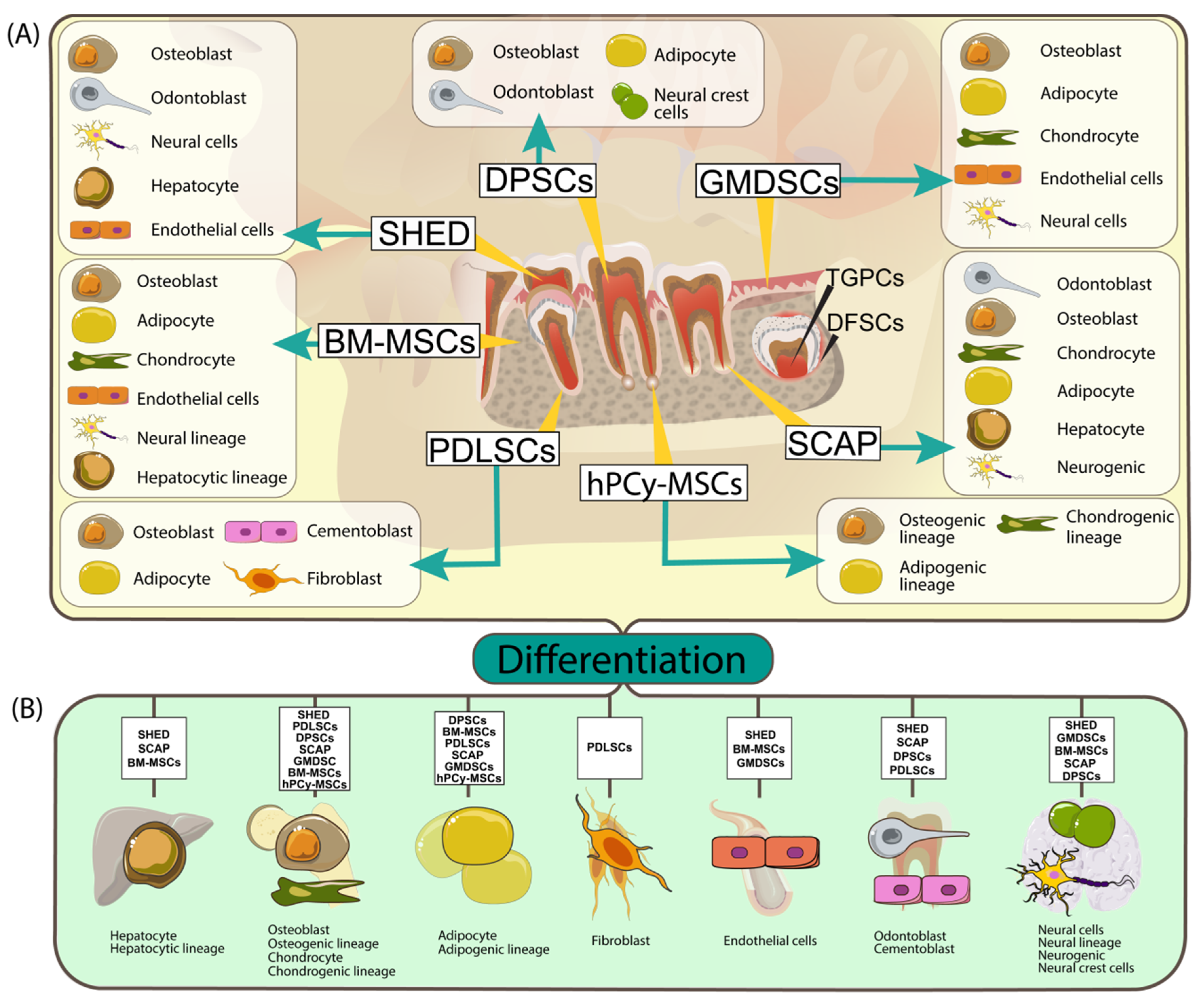
| MSC | Origin | In Vitro Differentiation | Therapeutic Application in the Dental Field | References |
|---|---|---|---|---|
| BM-MSCs | Non-hematopoietic components of bone marrow | Osteoblast Adipocyte Chondrocyte Endothelial cells Neural lineage Hepatocytic lineage | Accelerate bone healing and increased bone quantity Angiogenesis by activating VEGF family factors, subsequently improving bone repair process | [44,45,108] |
| DPSCs | Dental pulp | Osteoblast Odontoblast Adipocyte Neural crest cells | Regenerative endodontics procedures Periodontal regeneration Osteogenic differentiation and subsequently, bone regeneration | [45,57,59,63] |
| SHED | Pulpal tissue of deciduous teeth | Osteoblast Odontoblast Neural cells Hepatocyte Endothelial cells | Dentin-pulp regeneration Bio-root and periodontal regeneration Accelerated angiogenesis Reduced inflammation and apoptosis Maintain normal salivary workflow (Sjögren syndrome) | [72,76,121] |
| PDLSCs | Mature periodontal ligament | Osteoblast Adipocyte Cementoblast Fibroblast | Periodontal tissue regeneration Alveolar bone regeneration | [80,81,83] |
| SCAP | Apical papilla from an immature tooth root | Odontoblast Osteoblast Neurogenic Adipocyte Chondrocyte Hepatocyte | Endodontics Bio-root engineering Periodontal regeneration Bone repair Pro-angiogenic effect | [90,91,92,94,96] |
| GMDSCs | Gingival connective tissue | Osteoblast Adipocyte Chondrocyte Endothelial cells Neural cells | Blood vessel formation | [95,108] |
| hPCy-MSCs | Human periapical cyst | osteogenic, adipogenic, and chondrogenic lineage | Regenerative medicine, brain regeneration | [49,51] |
| DFSCs | Dental follicle | Cementoblast Osteoblast Chondrocyte Adipocyte | Angiogenesis Periodontal/bone regeneration Immunomodulation | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baru, O.; Nutu, A.; Braicu, C.; Cismaru, C.A.; Berindan-Neagoe, I.; Buduru, S.; Badea, M. Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy? Int. J. Mol. Sci. 2021, 22, 929. https://doi.org/10.3390/ijms22020929
Baru O, Nutu A, Braicu C, Cismaru CA, Berindan-Neagoe I, Buduru S, Badea M. Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy? International Journal of Molecular Sciences. 2021; 22(2):929. https://doi.org/10.3390/ijms22020929
Chicago/Turabian StyleBaru, Oana, Andreea Nutu, Cornelia Braicu, Cosmin Andrei Cismaru, Ioana Berindan-Neagoe, Smaranda Buduru, and Mîndra Badea. 2021. "Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy?" International Journal of Molecular Sciences 22, no. 2: 929. https://doi.org/10.3390/ijms22020929
APA StyleBaru, O., Nutu, A., Braicu, C., Cismaru, C. A., Berindan-Neagoe, I., Buduru, S., & Badea, M. (2021). Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy? International Journal of Molecular Sciences, 22(2), 929. https://doi.org/10.3390/ijms22020929









