Advances in Growth Factor Delivery for Bone Tissue Engineering
Abstract
1. Introduction
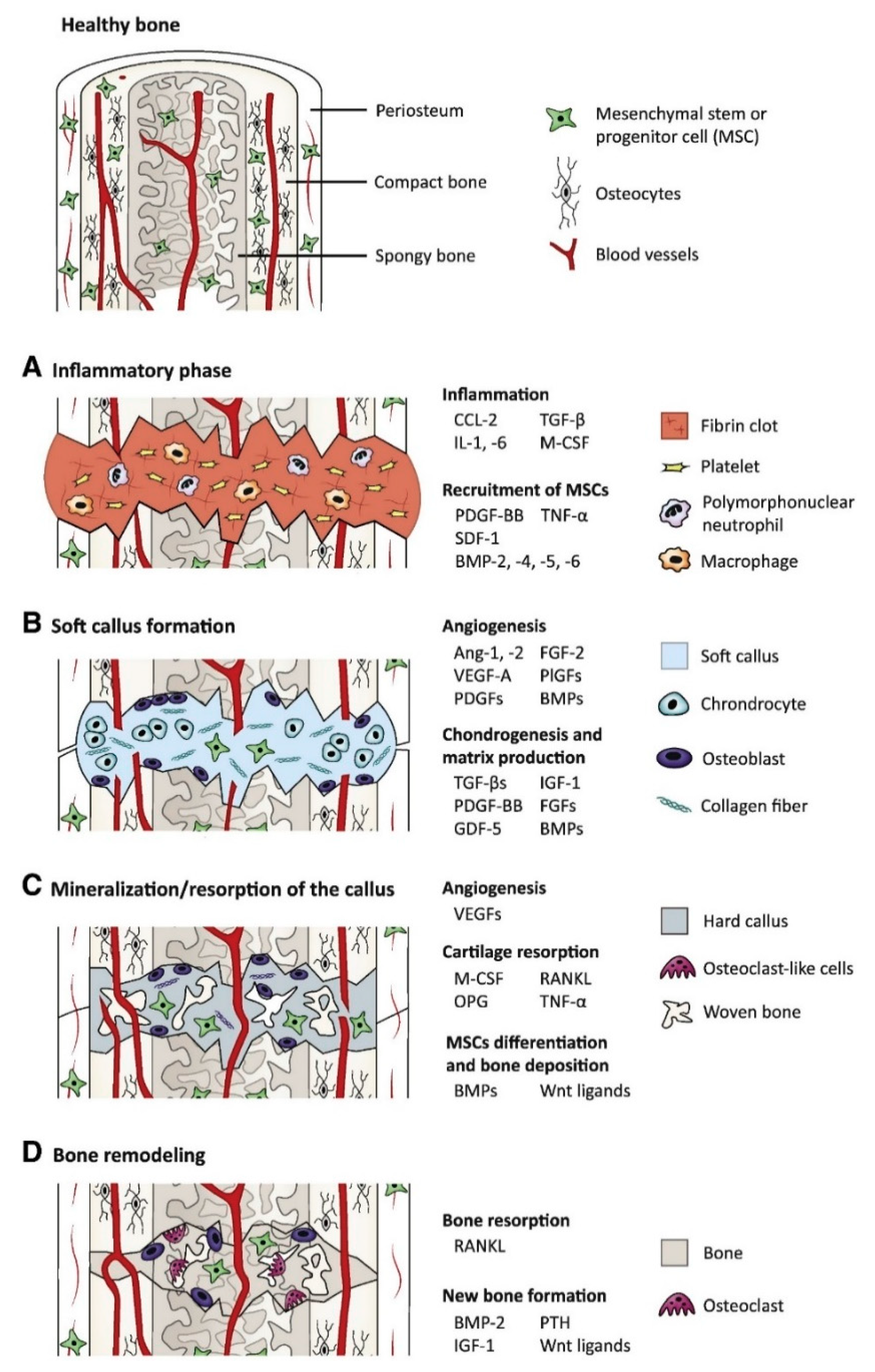
1.1. Growth Factors Roles in Bone Tissue Engineering
1.2. Scaffold Properties for Bone Tissue Engineering
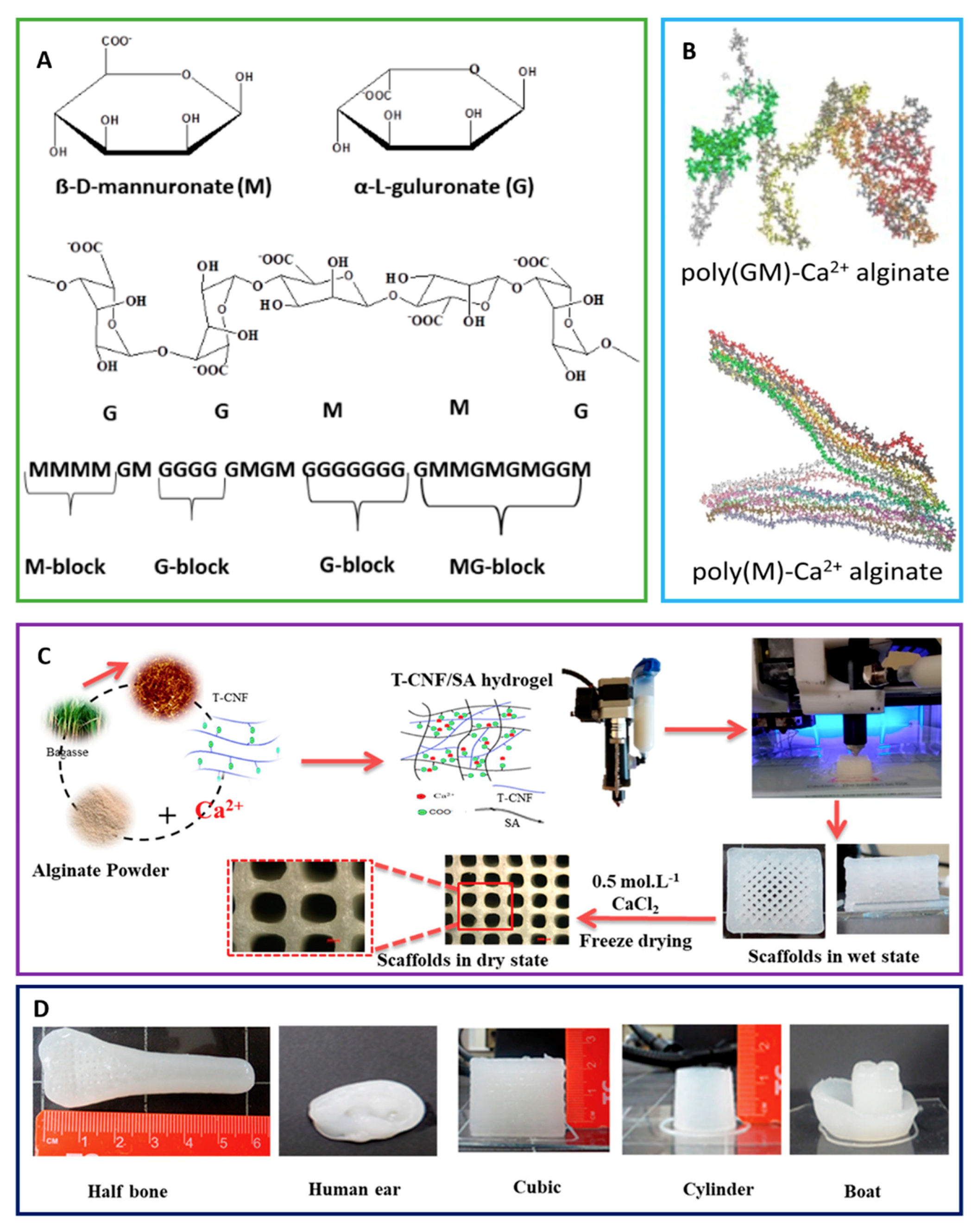
2. Polymer Scaffolds for GF Delivery
3. Encapsulation, Incorporation, and Related Delivery Strategies
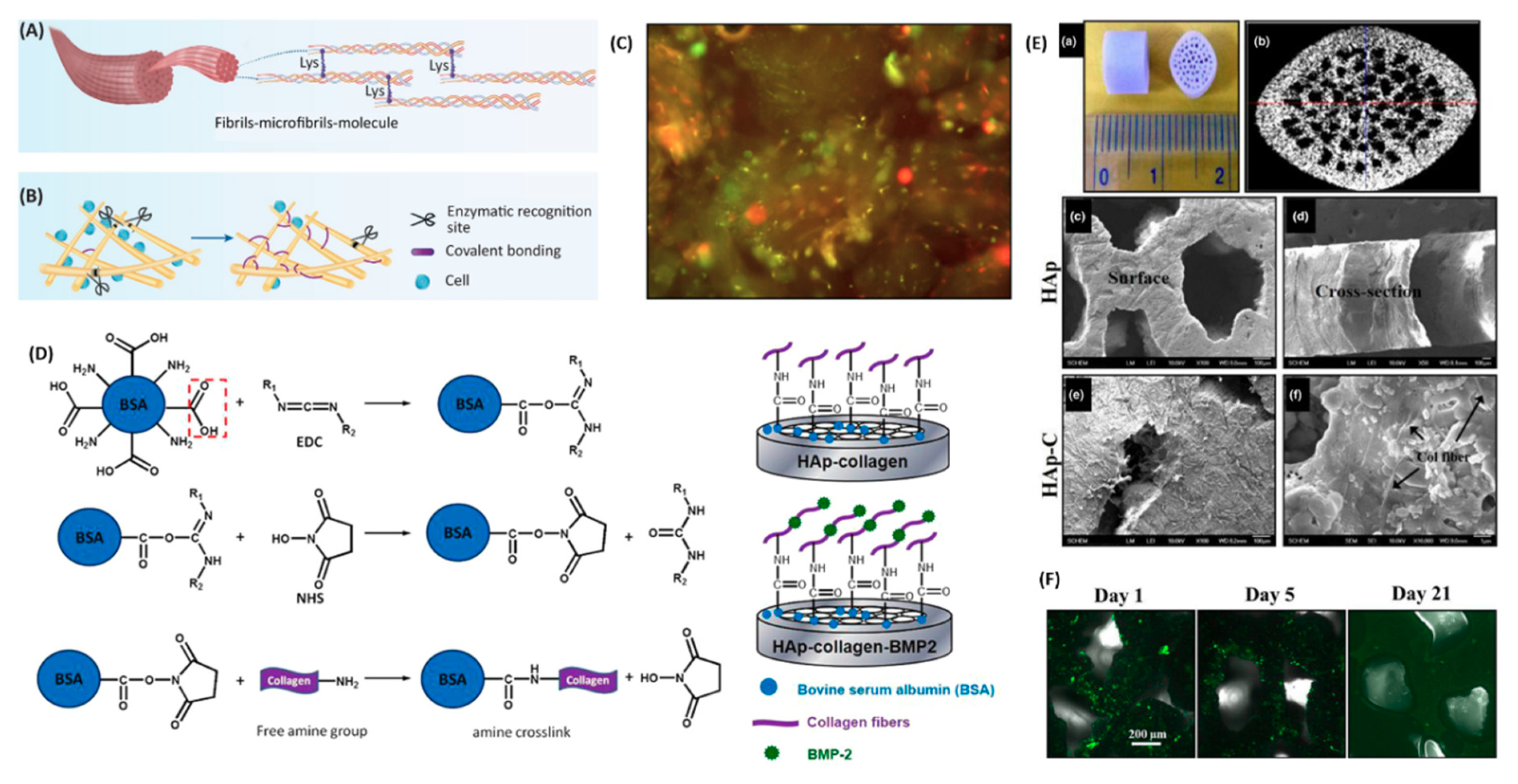
3.1. Physical Adsorption
3.2. Chemical Conjugation
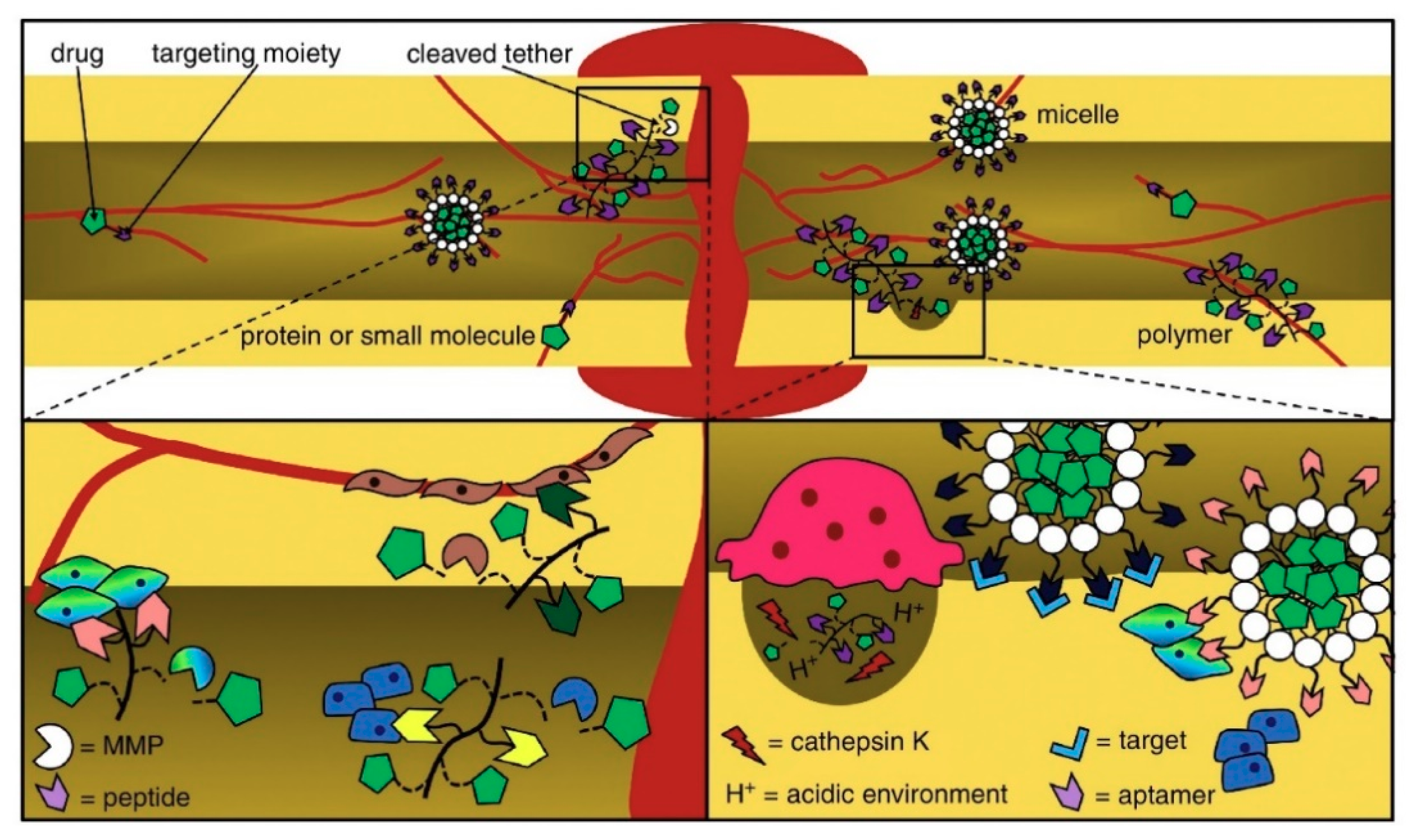
3.3. Spatiotemporally Controlled Delivery of GFs
4. Considerations for using GFs in Bone Tissue Engineering
4.1. Toxicity
4.2. Cost
5. Current Strategies and Future Trends
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neves, M.I.; Araújo, M.; Moroni, L.; Da Silva, R.M.P.; Barrias, C.C. Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks. Molecules 2020, 25, 978. [Google Scholar]
- Wang, L.; Fang, M.; Xia, Y.; Hou, J.; Nan, X.; Zhao, B.; Wang, X. Preparation and biological properties of silk fibroin/nano-hydroxyapatite/graphene oxide scaffolds with an oriented channel-like structure. RSC Adv. 2020, 10, 10118–10128. [Google Scholar] [CrossRef]
- Nie, L.; Deng, Y.; Li, P.; Hou, R.; Shavandi, A.; Yang, S. Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration. ACS Omega 2020, 5, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, M.A.; Sun, Z. Bio-mimetic composite scaffold from mussel shells, squid pen and crab chitosan for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 445–454. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.; Ali, M.A. Injectable gel from squid pen chitosan for bone tissue engineering applications. J. Sol-Gel Sci. Technol. 2016, 77, 675–687. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Khojasteh, A.; Behnia, H.; Naghdi, N.; Esmaeelinejad, M.; Alikhassy, Z.; Stevens, M. Effects of different growth factors and carriers on bone regeneration: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e405–e423. [Google Scholar]
- Termaat, M.F.; Boer, D.; Bakker, F.C.; Patka, P.; Haarman, H.J. Bone morphogenetic proteins: Development and clinical efficacy in the treatment of fractures and bone defects. J. Bone Jt. Surg. Am. 2005, 87, 1367–1378. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jones, A.A.; Lilly, L.C.; Fiorellini, J.P.; Howell, H. Evaluation of Recombinant Human Bone Morphogenetic Protein-2 in Oral Applications Including the Use of Endosseous Implants: 3-Year Results of a Pilot Study in Humans. J. Periodontol. 2000, 71, 1241–1257. [Google Scholar] [CrossRef]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Klosterhoff, B.S.; Stevens, H.Y.; Tran, L.; Guldberg, R.E. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017, 49, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006, 12, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Kubo, T.; Doi, K.; Morita, K.; Takeshita, R.; Katoh, S.; Shiba, T.; Gong, P.; Akagawa, Y. Effect of combined application of bFGF and inorganic polyphosphate on bioactivities of osteoblasts and initial bone regeneration. Acta Biomater. 2009, 5, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Saulacic, N.; Pippenger, B.E.; Zhang, Y.; Miron, R.J. Absorbable collagen sponges loaded with recombinant bone morphogenetic protein 9 induces greater osteoblast differentiation when compared to bone morphogenetic protein 2. Clin. Exp. Dent. Res. 2017, 3, 32–40. [Google Scholar] [CrossRef]
- Hertweck, J.; Ritz, U.; Götz, H.; Schottel, P.C.; Rommens, P.M.; Hofmann, A. CD34+ cells seeded in collagen scaffolds promote bone formation in a mouse calvarial defect model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1505–1516. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.T.; Vuong, J.T.; Anez-Bustillos, L.; Pan, A.; Mitchell, P.D.; Fell, G.L.; Baker, M.A.; Bielenberg, D.R.; Puder, M. Intranasal delivery of VEGF enhances compensatory lung growth in mice. PLoS ONE 2018, 13, e0198700. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Maruyama, K.; Hubbell, J.A. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 41–52. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Vascular Endothelial Growth Factor Control Mechanisms in Skeletal Growth and Repair. Dev. Dyn. 2017, 246, 227–234. [Google Scholar] [CrossRef]
- Onishi, T.; Shimizu, T.; Akahane, M.; Omokawa, S.; Okuda, A.; Kira, T.; Inagaki, Y.; Tanaka, Y. Osteogenic extracellular matrix sheet for bone tissue regeneration. Eur. Cells Mater. 2018, 36, 69–80. [Google Scholar] [CrossRef]
- Yan, H.J.; Casalini, T.; Hulsart-Billström, G.; Wang, S.; Oommen, O.P.; Salvalaglio, M.; Larsson, S.; Hilborn, J.; Varghese, O.P. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 2018, 161, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Vunjak-Novakovic, G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv. Transl. Res. 2015, 5, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional scaffolds with improved antimicrobial properties and osteogenicity based on piezoelectric electrospun fibers decorated with bioactive composite microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef] [PubMed]
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv. Wound Care 2015, 4, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Sluzalska, K.D.; Slawski, J.; Sochacka, M.; Lampart, A.; Otlewski, J.; Zakrzewska, M. Intracellular Partners of Fibroblast Growth Factors 1 and 2—Implications for Functions. Cytokin Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone Morphogenetic Protein-2 and Tumor Growth: Diverse Effects and Possibilities for Therapy. Cytokin Growth Factor Rev. 2017, 34, 73–91. [Google Scholar] [CrossRef]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic Supramolecular Designs for the Controlled Release of Growth Factors in Bone Regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Tong, Z.; Guo, J.; Glen, R.C.; Morrell, N.W.; Li, W. A bone morphogenetic protein (BMP)-derived peptide based on the type I receptor-binding site modifies cell-type dependent BMP signalling. Sci. Rep. 2019, 9, 13446. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Willie, B.M.; Schwabe, P.; Seemann, P.; Duda, G.N. BMPs in Bone Regeneration: Less is More Effective, a Paradigm-Shift. Cytokin Growth Factor Rev. 2016, 27, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Botega, I.I.; Zamarioli, A.; Guedes, P.M.S.G.; da Silva, R.A.B.; Issa, J.P.M.; Butezloff, M.M.; Sousa, Y.T.C.S.; Ximenez, J.P.B.; Volpon, J.B. Bone callus formation is highly disrupted by dietary restriction in growing rats sustaining a femoral fracture. Acta Cir. Bras. 2019, 34. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Courchesne, N.M.D.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. USA 2014, 111, 12847–12852. [Google Scholar] [CrossRef] [PubMed]
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.-H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311. [Google Scholar] [CrossRef]
- Scarfì, S. Use of Bone Morphogenetic Proteins in Mesenchymal Stem Cell Stimulation of Cartilage and Bone Repair. World J. Stem Cells 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Sun, N.; Chen, Y.; Yu, F.; Zhixin, F.; Lin, J.; Sun, B.; Yu, B.; Cheng, X.; Zheng, X.; Wu, B. Monocrotaline pyrrole enhanced bone morphogenetic protein 7 signaling transduced by alternative activin A receptor type 2A in pulmonary arterial smooth muscle cells. Eur. J. Pharmacol. 2019, 863. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Liu, C. Biomaterial-guided immobilization and osteoactivity of bone morphogenetic protein-2. Appl. Mater. Today 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, B.; Yin, Y.; Chen, B.; Zhao, Y.; Xiao, Z.; Yang, B.; Shi, Y.; Fang, Y.; Ma, X.; et al. Collagen particles with collagen-binding bone morphogenetic protein-2 promote vertebral laminar regeneration in infant rabbits. Biomed. Mater. 2020, 15, 055008. [Google Scholar] [CrossRef]
- Subbiah, R.; Guldberg, R.E. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2019, 8, 1801000. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration; Springer: New York, NY, USA, 2017; Volume 34, pp. 1152–1170. [Google Scholar]
- Chen, D.; Zhang, C.; Huo, H.; Ji, C.; Sun, M.; Nie, L. Injectable temperature-sensitive hydrogel with VEGF loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018, 229, 138–141. [Google Scholar] [CrossRef]
- Nie, L.; Chen, D.; Zhong, S.; Shi, Q.; Sun, Y.; Politis, C.; Shavandi, A. Injectable cell-laden poly(N-isopropylacrylamide)/chitosan hydrogel reinforced via graphene oxide and incorporated with dual-growth factors. Mater. Lett. 2020, 280, 128572. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Kitasato, S.; Tanaka, T.; Chazono, M.; Komaki, H.; Kakuta, A.; Inagaki, N.; Akiyama, S.; Marumo, K. Local application of alendronate controls bone formation and β-tricalcium phosphate resorption induced by recombinant human bone morphogenetic protein-2. J. Biomed. Mater. Res. Part A 2020, 108, 528–536. [Google Scholar] [CrossRef]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for Bone Tissue Engineering. J. Control. Release 2016, 244, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Bien, N.D.; Miura, K.-I.; Sumita, Y.; Nakatani, Y.; Shido, R.; Kajii, F.; Kamakura, S.; Asahina, I. Bone regeneration by low-dose recombinant human bone morphogenetic protein-2 carried on octacalcium phosphate collagen composite. J. Hard Tissue Biol. 2020, 29, 123–130. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Chou, C.; Servies, N.; Smeekens, J.M.; Cheng, A.; Esancy, C.; Wu, R.; McDevitt, T.C.; Guldberg, R.E.; Krishnan, L. Competitive protein binding influences heparin-based modulation of spatial growth factor delivery for bone regeneration. Tissue Eng. Part A 2017, 23, 683–695. [Google Scholar] [CrossRef]
- Newman, M.R.; Benoit, D.S.W. Local and Targeted Drug Delivery for Bone Regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Gan, Q.; Zhu, J.; Yuan, Y.; Liu, H.; Qian, J.; Li, Y.; Liu, C. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J. Mater. Chem. B 2015, 3, 2056–2066. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Biocompatibility Evaluation of Tissue-Engineered Decellularized Scaffolds for Biomedical Application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Sefcik, R.S.; Bishop, T.J.; Montelone, S.M.; Crouser, N.; Welter, J.F.; Caplan, A.I.; Dean, D. Growth factor dose tuning for bone progenitor cell proliferation and differentiation on Resorbable Poly(propylene fumarate) Scaffolds. Tissue Eng. Part C Methods 2016, 22, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioprinting 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.F.; Ali, A. A Review of Synthesis Methods, Properties and Use of Hydroxyapatite as a Substitute of Bone. J. Biomim. Biomater. Biomed. Eng. 2015, 25, 98–117. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Venkataraman, N.; Bansal, S.; Bansal, P.; Narayan, S. Dynamics of bone graft healing around implants. J. Int. Clin. Dent. Res. Organ. 2015, 7, 40. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent Adv. biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Srivastava, R. Osteoinductive and Osteoconductive Biomaterials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–395. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 1–33. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef]
- Fan, B.; Guo, Z.; Li, X.; Li, S.; Gao, P.; Xiao, X.; Wu, J.; Shen, C.; Jiao, Y.; Hou, W. Electroactive barium titanate coated titanium scaffold improves osteogenesis and osseointegration with low-intensity pulsed ultrasound for large segmental bone defects. Bioact. Mater. 2020, 5, 1087–1101. [Google Scholar] [CrossRef]
- Lv, J.; Xiu, P.; Tan, J.; Jia, Z.; Cai, H.; Liu, Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: Implantation of electron beam melting-fabricated porous Ti 6 Al 4 V scaffolds incorporating growth factor-doped fibrin glue. Biomed. Mate. 2015, 10, 035013. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.W.; Haus, M.T.; de Ruijter, A.E.; Spauwen, P.H.; Jansen, J.A. Bone formation in transforming growth factor beta-I-loaded titanium fiber mesh implants. Clin. Oral Implants Res. 2002, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Comesaña, R.; Lusquiños, F.; Del Val, J.; Quintero, F.; Riveiro, A.; Boutinguiza, M.; Jones, J.R.; Hill, R.G.; Pou, J. Toward smart implant synthesis: Bonding bioceramics of different resorbability to match bone growth rates. Sci. Rep. 2015, 5, 10677. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, K.A.; Bock, N.; Dargaville, T.R.; Ann Woodruff, M. Scaffolds for growth factor delivery as applied to bone tissue engineering. Int. J. Polym. Sci. 2012, 2012, 174942. [Google Scholar] [CrossRef]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shan, T.; Ma, Y.x.; Tay, F.R.; Niu, L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Luo, X.; Ren, X.; Tan, T.C.; Smith, R.A.A.; Swaminathan, K.; Sekar, S.; Bhakoo, K.; Nurcombe, V.; Hui, J.H.; et al. A Heparan Sulfate Device for the Regeneration of Osteochondral Defects. Tissue Eng. Part A 2019, 25, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.T.B.; Abueva, C.D.G.; Jang, D.W.; Lee, B.T. Collagen and bone morphogenetic protein-2 functionalized hydroxyapatite scaffolds induce osteogenic differentiation in human adipose-derived stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1363–1371. [Google Scholar] [CrossRef]
- Walsh, D.P.; Raftery, R.M.; Chen, G.; Heise, A.; O’Brien, F.J.; Cryan, S.A. Rapid healing of a critical-sized bone defect using a collagen-hydroxyapatite scaffold to facilitate low dose, combinatorial growth factor delivery. J. Tissue Eng. Regen. Med. 2019, 13, 1843–1853. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering; Springer: New York, NY, USA, 2018; Volume 1077, pp. 371–387. [Google Scholar]
- Mejía-Suaza, M.L.; Moncada, M.E.; Ossa-Orozco, C.P.; Mejía-Suaza, M.L.; Moncada, M.E.; Ossa-Orozco, C.P. Characterization of electrospun silk fibroin scaffolds for bone tissue engineering: A review. TecnoLógicas 2020, 23, 33–51. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216. [Google Scholar] [CrossRef]
- Naskar, D.; Ghosh, A.K.; Mandal, M.; Das, P.; Nandi, S.K.; Kundu, S.C. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 2017, 136, 67–85. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Investigating the potential of combined growth factors delivery, from non-mulberry silk fibroin grafted poly(ε-caprolactone)/hydroxyapatite nanofibrous scaffold, in bone tissue engineering. Appl. Mater. Today 2016, 5, 52–67. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Progr. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Nataraj, D.; Narendra, R. Chemical modifications of alginate and its derivatives. Int. J. Chem. Res. 2019, 1–17. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Beneventi, D.; Dufresne, A. Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering. Biomacromolecules 2018, 19, 4442–4452. [Google Scholar] [CrossRef]
- Bayer, E.A.; Jordan, J.; Roy, A.; Gottardi, R.; Fedorchak, M.V.; Kumta, P.N.; Little, S.R. Programmed platelet-derived growth factor-BB and bone morphogenetic protein-2 delivery from a hybrid calcium phosphate/alginate scaffold. Tissue Eng. Part A 2017, 23, 1382–1393. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, Z.; Ye, Z.; Zhu, H.; Aparicio, C. Bioinspired mineralization with hydroxyapatite and hierarchical naturally aligned nanofibrillar cellulose. ACS Appl. Mater. Interfaces 2019, 11, 27598–27604. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional Cellulose-Based Hydrogels as Extracellular Matrices for Tissue Engineering. J. Biol. Eng. 2019, 13, 1–19. [Google Scholar] [CrossRef]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; Penteado, L.D.A.; Medeiros, A.I.D.; Ribeiro, S.J.D.L.; Capote, T.S.D.O. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Liao, M.; Dai, L.; Tang, Y.; Zhang, H.; Coates, P.; Sefat, F.; Zheng, L.; Song, J.; et al. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019, 213. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable chitosan-based thermosensitive hydrogel/nanoparticle-loaded system for local delivery of vancomycin in the treatment of osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Gohil, S.V.; Padmanabhan, A.; Deschamps, J.; Nair, L.S. Chitosan-Based Scaffolds for Growth Factor Delivery. Tissue Eng. Ther. 2017, 2, 175–207. [Google Scholar]
- Bastami, F.; Paknejad, Z.; Jafari, M.; Salehi, M.; Rezai Rad, M.; Khojasteh, A. Fabrication of a three-dimensional β-tricalcium-phosphate/gelatin containing chitosan-based nanoparticles for sustained release of bone morphogenetic protein-2: Implication for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 481–491. [Google Scholar] [CrossRef]
- Xia, Y.J.; Xia, H.; Chen, L.; Ying, Q.S.; Yu, X.; Li, L.H.; Wang, J.H.; Zhang, Y. Efficient delivery of recombinant human bone morphogenetic protein (rhBMP-2) with dextran sulfate-chitosan microspheres. Exp. Ther. Med. 2018, 15, 3265–3272. [Google Scholar] [CrossRef]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally Derived Proteins and Glycosaminoglycan Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef]
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J. Control. Release 2019, 313, 131–147. [Google Scholar] [CrossRef]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated Polysaccharide-Based Scaffolds for Orthopaedic Tissue Engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef]
- Thanyaphoo, S.; Kaewsrichan, J. A new biocompatible delivery scaffold containing heparin and bone morphogenetic protein 2. Acta Pharm. 2016, 66, 373–385. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jing, Y.; Sun, H.; Liu, X. Hierarchical nanofibrous microspheres with controlled growth factor delivery for bone regeneration. Adv. Healthc. Mater. 2015, 4, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, J.; Fan, D. Fabrication of high-strength and porous hybrid scaffolds based on nano-hydroxyapatite and human-like collagen for bone tissue regeneration. Polymers 2020, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; Cheng, A.; Stevens, H.; Logun, M.T.; Webb, R.; Jordan, E.; Xia, B.; Karumbaiah, L.; Guldberg, R.E.; Stice, S. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem Cells Transl. Med. 2019, 8, 575–585. [Google Scholar] [CrossRef]
- Fenbo, M.; Sijing, L.; Ruiz-Ortega, L.I.; Yuanjun, Z.; Lei, X.; Kui, W.; Lijun, L.; Bin, T. Effects of alginate/chondroitin sulfate-based hydrogels on bone defects healing. Mater. Sci. Eng. C 2020, 116, 111217. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, J. Keratan Sulfate, a Complex Glycosaminoglycan with Unique Functional Capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Jia, W.; Wang, Q.; Liu, Y.; Wang, X.; Wang, C.; Jiang, J.; Gu, G.; Guo, Z.; et al. Improving in vitro biocompatibility on biomimetic mineralized collagen bone materials modified with hyaluronic acid oligosaccharide. Mater. Sci. Eng. C 2019, 104, 110008. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Zimmerman, G.; Marcucio, R. Biological perspectives of delayed fracture healing. Injury 2014, 45. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.E.B.; Edlund, K.; Zelikin, A.N. Micro-structured, spontaneously eroding hydrogels accelerate endothelialization through presentation of conjugated growth factors. Biomaterials 2015, 49, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Grazia Cascone, M.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef]
- Marquetti, I.; Desai, S. Molecular modeling the adsorption behavior of bone morphogenetic protein-2 on hydrophobic and hydrophilic substrates. Chem. Phys. Lett. 2018, 706, 285–294. [Google Scholar] [CrossRef]
- Mücksch, C.; Urbassek, H.M. Accelerated molecular dynamics study of the effects of surface hydrophilicity on protein adsorption. Langmuir 2016, 32, 9156–9162. [Google Scholar] [CrossRef]
- Utesch, T.; Daminelli, G.; Mroginski, M.A. Molecular dynamics simulations of the adsorption of bone morphogenetic protein-2 on surfaces with medical relevance. Langmuir 2011, 27, 13144–13153. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.L.; Toriki, E.S.; Tucker, N.D.J.; Nilsson, B.L. Electrostatic interactions regulate the release of small molecules from supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 6366–6377. [Google Scholar] [CrossRef]
- Huang, B.; Lou, Y.; Li, T.; Lin, Z.; Sun, S.; Yuan, Y.; Liu, C.; Gu, Y. Molecular dynamics simulations of adsorption and desorption of bone morphogenetic protein-2 on textured hydroxyapatite surfaces. Acta Biomater. 2018, 80, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Riahi, N.; Murschel, F.; Lerouge, S.; Durocher, Y.; Henry, O.; De Crescenzo, G. Bioavailability of immobilized epidermal growth factor: Covalent versus noncovalent grafting. Biointerphases 2017, 12, 010501. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, Y.; Wang, J.; Werkmeister, J.A.; McLean, K.M.; Liu, C. 2-N, 6-O-sulfated chitosan-assisted BMP-2 immobilization of PCL scaffolds for enhanced osteoinduction. Mater. Sci. Eng. C 2017, 74, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Schuster, L.; Ardjomandi, N.; Munz, M.; Umrath, F.; Klein, C.; Rupp, F.; Reinert, S.; Alexander, D. Establishment of collagen: Hydroxyapatite/BMP-2 mimetic peptide composites. Materials 2020, 13, 1203. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.Z.; Sun, H.P.; Xu, J.Y.; Li, Y.M.; Xie, X.; Zhang, L.M.; Deng, F.L. Sustained dual release of placental growth factor-2 and bone morphogenic protein-2 from heparin-based nanocomplexes for direct osteogenesis. Int. J. Nanomed. 2016, 11, 1147–1158. [Google Scholar] [CrossRef]
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, P.S.P.; Migliaresi, C.; Motta, A.; Balmayor, E.R.; van Griensven, M. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Deegan, A.; Musa, F.; Xu, T.; Yang, Y. The effects of biomimetically conjugated VEGF on osteogenesis and angiogenesis of MSCs (human and rat) and HUVECs co-culture models. Colloids Surf. B Biointerfaces 2018, 167, 550–559. [Google Scholar] [CrossRef]
- Aksel, H.; Öztürk, Ş.; Serper, A.; Ulubayram, K. VEGF/BMP-2 loaded three-dimensional model for enhanced angiogenic and odontogenic potential of dental pulp stem cells. Int. Endod. J. 2018, 51, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- King, W.J.; Krebsbach, P.H. Growth factor delivery: How surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012, 64, 1239–1256. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. C 2020, 117, 111245. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF within Multilayered Polydopamine-Coated Scaffolds and the Resulting Osteogenic and Angiogenic Synergy of Co-Cultured Human Mesenchymal Stem Cells and Human Endothelial Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef]
- Reyes, R.; De la Riva, B.; Delgado, A.; Hernández, A.; Sánchez, E.; Évora, C. Effect of triple growth factor controlled delivery by a brushite-PLGA system on a bone defect. Injury 2012, 43, 334–342. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Zhang, Z.; Gupte, M.J.; Jin, X.; Ma, P.X. Injectable Peptide Decorated Functional Nanofibrous Hollow Microspheres to Direct Stem Cell Differentiation and Tissue Regeneration. Adv. Funct. Mater. 2015, 25, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem Rev 2018, 118, 7702–7743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cui, Z.-K.; Kim, P.J.; Jung, L.Y.; Lee, M. Design of hydrogels to stabilize and enhance bone morphogenetic protein activity by heparin mimetics. Acta Biomater. 2018, 72, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Piard, C.; Lin, J.; Nan, K.; Guo, T.; Caccamese, J.; Fisher, J.; Chen, Y. Imaging stem cell distribution, growth, migration, and differentiation in 3-D scaffolds for bone tissue engineering using mesoscopic fluorescence tomography. Biotechnol. Bioeng. 2018, 115, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, K.F.; Williams, R.J.; Nisbet, D.R. Dynamic and Responsive Growth Factor Delivery from Electrospun and Hydrogel Tissue Engineering Materials. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, J.; Deng, F.; Liu, Y.; Zhuang, X.; Xu, J.; Li, L. Co-delivery and controlled release of stromal cell-derived factor-1α chemically conjugated on collagen scaffolds enhances bone morphogenetic protein-2-driven osteogenesis in rats. Mol. Med. Rep. 2016, 14, 737–745. [Google Scholar] [CrossRef]
- Yu, X.; Sun, H.; Yang, J.; Liu, Y.; Zhang, Z.; Wang, J.; Deng, F. Evaluation of bone-regeneration effects and ectopic osteogenesis of collagen membrane chemically conjugated with stromal cell-derived factor-1 in vivo. Biomed. Mater. 2019, 15, 015009. [Google Scholar] [CrossRef]
- Lee, H.; Lim, S.; Birajdar, M.S.; Lee, S.H.; Park, H. Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1559–1566. [Google Scholar] [CrossRef]
- Rindone, A.N.; Kachniarz, B.; Achebe, C.C.; Riddle, R.C.; O’Sullivan, A.N.; Dorafshar, A.H.; Grayson, W.L. Heparin-Conjugated Decellularized Bone Particles Promote Enhanced Osteogenic Signaling of PDGF-BB to Adipose-Derived Stem Cells in Tissue Engineered Bone Grafts. Adv. Healthc. Mater. 2019, 8, e1801565. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Werengowska-Ciećwierz, K.; Wiśniewski, M.; Terzyk, A.P.; Furmaniak, S. The Chemistry of Bioconjugation in Nanoparticles-Based Drug Delivery System. Adv. Condens. Matter Phys. 2015, 2015, 198175. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Lee, J.H. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials 2011, 32, 8254–8260. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Elliott, C.G.; Li, Z.; Xu, Y.; Hamilton, D.W.; Guan, J. Creating 3D Angiogenic Growth Factor Gradients in Fibrous Constructs to Guide Fast Angiogenesis. Biomacromolecules 2012, 13, 3262–3271. [Google Scholar] [CrossRef]
- Wu, J.; Mao, Z.; Tan, H.; Han, L.; Ren, T.; Gao, C. Gradient biomaterials and their influences on cell migration. Interface Focus 2012, 2, 337–355. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, P.; Tuzlakoglu, K.; Reis, R.L.; Hasirci, N.; Hasirci, V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 2009, 30, 3551–3559. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Armstrong, J.P.K.; Pence, I.J.; Kit-Anan, W.; Puetzer, J.L.; Correia Carreira, S.; Moore, A.C.; Stevens, M.M. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials 2018, 176, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ouyang, L.; Armstrong, J.P.K.; Stevens, M.M. Advances in the Fabrication of Biomaterials for Gradient Tissue Engineering. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Freeman, F.E.; Pitacco, P.; van Dommelen, L.H.A.; Nulty, J.; Browe, D.C.; Shin, J.-Y.; Alsberg, E.; Kelly, D.J. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci. Adv. 2020, 6, eabb5093. [Google Scholar] [CrossRef]
- Kang, M.S.; Kim, J.-H.; Singh, R.K.; Jang, J.-H.; Kim, H.-W. Therapeutic-designed electrospun bone scaffolds: Mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015, 16, 103–116. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Liu, C. Biomaterials Act as Enhancers of Growth Factors in Bone Regeneration. Adv. Funct. Mater. 2016, 26, 8810–8823. [Google Scholar] [CrossRef]
- Poynton, A.R.; Lane, J.M. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 2002, 27, S40–S48. [Google Scholar] [CrossRef]
- Axelrad, T.W.; Steen, B.; Lowenberg, D.W.; Creevy, W.R.; Einhorn, T.A. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J. Bone Jt. Surg. Br. Vol. 2008, 90, 1617–1622. [Google Scholar] [CrossRef]
- Lee, K.B.; Taghavi, C.E.; Murray, S.S.; Song, K.J.; Keorochana, G.; Wang, J.C. BMP induced inflammation: A comparison of rhBMP-7 and rhBMP-2. J. Orthop. Res. 2012, 30, 1985–1994. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol. Lett. 2009, 31, 1817. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4, S343. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.; Cornett, C.A. Bone graft and bone graft substitutes in spine surgery: Current concepts and controversies. JAAOS J. Am. Acad. Orthop. Surg. 2013, 21, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Zoidis, E.; Zapf, J.; Hassa, P.; Hottiger, M.O.; Auer, J.A.; Schneider, R.; Gander, B.; Luginbuehl, V.; Bettschart-Wolfisberger, R. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone 2003, 33, 660–672. [Google Scholar] [CrossRef]
- Meinel, L.; Illi, O.E.; Zapf, J.; Malfanti, M.; Merkle, H.P.; Gander, B. Stabilizing insulin-like growth factor-I in poly (D, L-lactide-co-glycolide) microspheres. J. Control. Release 2001, 70, 193–202. [Google Scholar] [CrossRef]
- Aspenberg, P.; Jeppsson, C.; Wang, J.S.; Boström, M. Transforming growth factor beta and bone morphogenetic protein 2 for bone ingrowth: A comparison using bone chambers in rats. Bone 1996, 19, 499–503. [Google Scholar] [CrossRef]
- Luginbuehl, V.; Meinel, L.; Merkle, H.P.; Gander, B. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 2004, 58, 197–208. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Leong, K. Poly (α-hydroxy acids): Carriers for bone morphogenetic proteins. In The Biomaterials: Silver Jubilee Compendium; Elsevier: Amsterdam, The Netherlands, 1996; pp. 139–146. [Google Scholar]
- Hollinger, J.O.; Schmitt, J.M.; Buck, D.C.; Shannon, R.; Joh, S.P.; Zegzula, H.D.; Wozney, J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J. Biomed. Mater. Res. 1998, 43, 356–364. [Google Scholar] [CrossRef]
- Sciadini, M.F.; Dawson, J.M.; Berman, L.M.; Johnson, K.D. Dose-response characteristics of recombinant human bone morphogenetic protein-2 (rhBMP-2) in a canine segmental defect model. Trans. Orthop. Res. Soc. 1995, 20, 594. [Google Scholar]
- Gautschi, O.P.; Frey, S.P.; Zellweger, R. Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, Z.; Calori, G.M.; Kanakaris, N.K.; Nikolaou, V.S.; Giannoudis, P.V. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. Int. Orthop. 2009, 33, 1407. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.R.; Donell, S.; Ryder, J.; Shemilt, I.; Mugford, M.; Harvey, I.; Song, F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: A systematic review. In NIHR Health Technology Assessment Programme: Executive Summaries; NIHR Journals Library: Rockville, MD, USA, 2007. [Google Scholar]
- Gothard, D.; Smith, E.L.; Kanczler, J.M.; Rashidi, H.; Qutachi, O.; Henstock, J.; Rotherham, M.; El Haj, A.; Shakesheff, K.M.; Oreffo, R.O.C. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur. Cells Mater. 2014, 28, 166–208. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Lienemann, P.S.; Lutolf, M.P.; Ehrbar, M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1078–1089. [Google Scholar] [CrossRef]
- Draenert, F.G.; Nonnenmacher, A.L.; Kämmerer, P.W.; Goldschmitt, J.; Wagner, W. BMP-2 and bFGF release and in vitro effect on human osteoblasts after adsorption to bone grafts and biomaterials. Clin. Oral Implants Res. 2013, 24, 750–757. [Google Scholar] [CrossRef]
- Sahoo, S.; Ang, L.T.; Goh, J.C.-H.; Toh, S.-L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 93A, 1539–1550. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Ohe, J.-Y.; Lee, J.B.; Heo, D.N.; Byun, W.; Bae, H.; Kwon, Y.-D.; Kwon, I.K. Photo-cured hyaluronic acid-based hydrogels containing growth and differentiation factor 5 (GDF-5) for bone tissue regeneration. Bone 2014, 59, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Xing, Z.; Wu, X.; Xue, Y.; Pedersen, T.O.; Sun, Y.; Døskeland, A.P.; Nickel, J.; Waag, T.; Lygre, H. Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J. Control. Release 2015, 197, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Krell, E.S.; DiGiovanni, C.W. The Efficacy of Platelet-Derived Growth Factor as a Bone-Stimulating Agent. Foot Ankle Clin. 2016, 21, 763–770. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanni, C.W.; Lin, S.S.; Daniels, T.R.; Glazebrook, M.; Evangelista, P.; Donahue, R.; Beasley, W.; Baumhauer, J.F. The Importance of Sufficient Graft Material in Achieving Foot or Ankle Fusion. J. Bone Joint Surg. Am. 2016, 98, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, S.; Mathew, D.; Jo, J.I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef]
- Bouyer, M.; Guillot, R.; Lavaud, J.; Plettinx, C.; Olivier, C.; Curry, V.; Boutonnat, J.; Coll, J.L.; Peyrin, F.; Josserand, V.; et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016, 104, 168–181. [Google Scholar] [CrossRef]
- Kobayashi, N.; Hashimoto, Y.; Otaka, A.; Yamaoka, T.; Morita, S. Porous alpha-tricalcium phosphate with immobilized basic fibroblast growth factor enhances bone regeneration in a canine mandibular bone defect model. Materials 2016, 9, 853. [Google Scholar] [CrossRef]
- Jo, D.W.; Cho, Y.D.; Seol, Y.J.; Lee, Y.M.; Lee, H.J.; Kim, Y.K. A randomized controlled clinical trial evaluating efficacy and adverse events of different types of recombinant human bone morphogenetic protein-2 delivery systems for alveolar ridge preservation. Clin. Oral Implants Res. 2019, 30, 396–409. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Ahmad, T.; Madhurakkat Perikamana, S.K.; Lee, J.; Kim, E.M.; Shin, H. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials 2020, 255, 120192. [Google Scholar] [CrossRef]
- Yang, D.H.; Moon, S.W.; Lee, D.W. Surface modification of titanium with BMP-2/GDF-5 by a heparin linker and its efficacy as a dental implant. Int. J. Mol. Sci. 2017, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Alvarez-Urena, P.; Ducheyne, P.; Srinivasan, A.; Baskin, J.; Waters, H.; Gruber, R. 6.2 Bone Tissue Engineering: Growth Factors and Cytokines. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 20–53. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Keceli, H.G.; Bayram, C.; Celik, E.; Ercan, N.; Demirbilek, M.; Nohutcu, R.M. Dual delivery of platelet-derived growth factor and bone morphogenetic factor-6 on titanium surface to enhance the early period of implant osseointegration. J. Periodontal Res. 2020, 55, 694–704. [Google Scholar] [CrossRef]

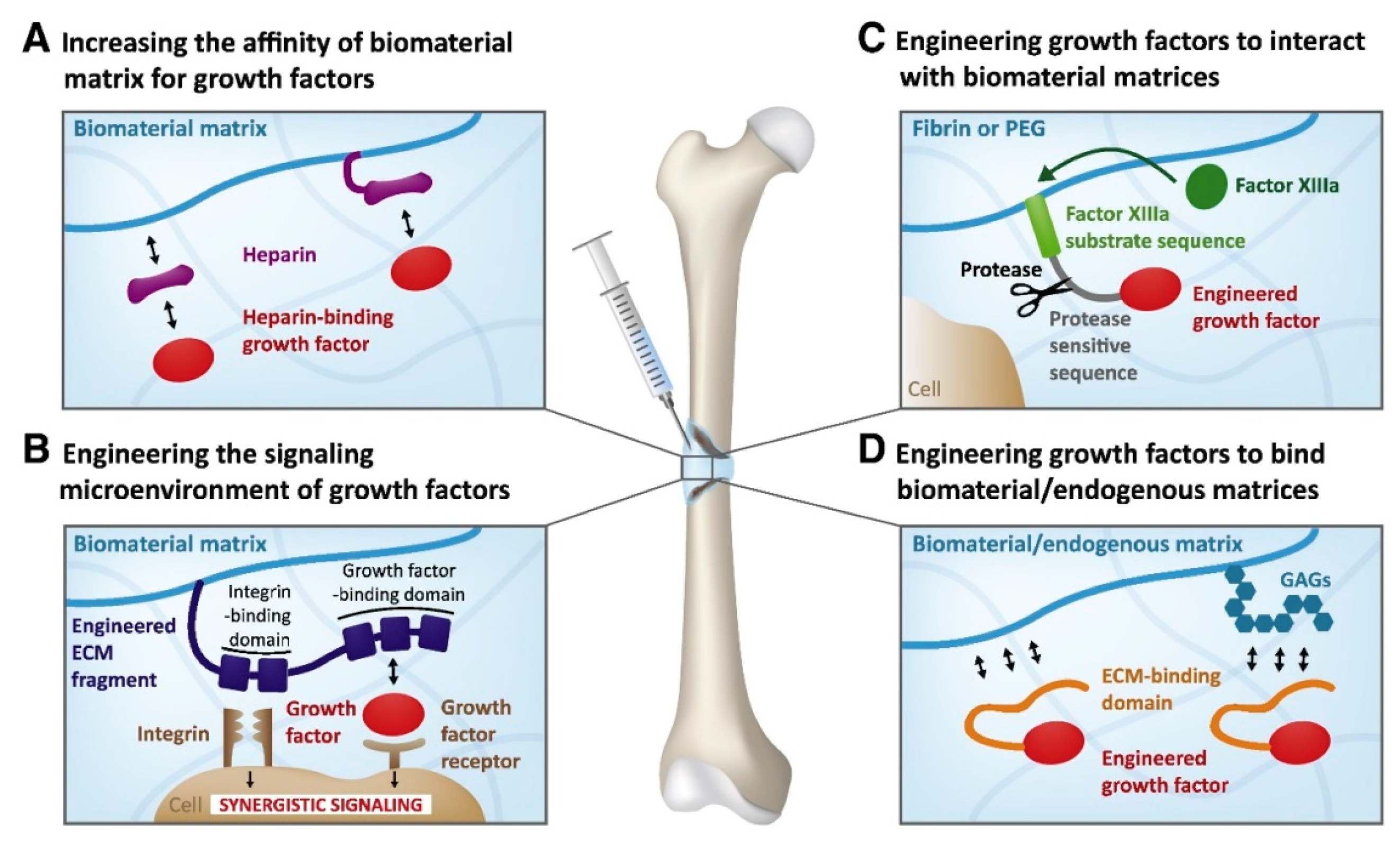
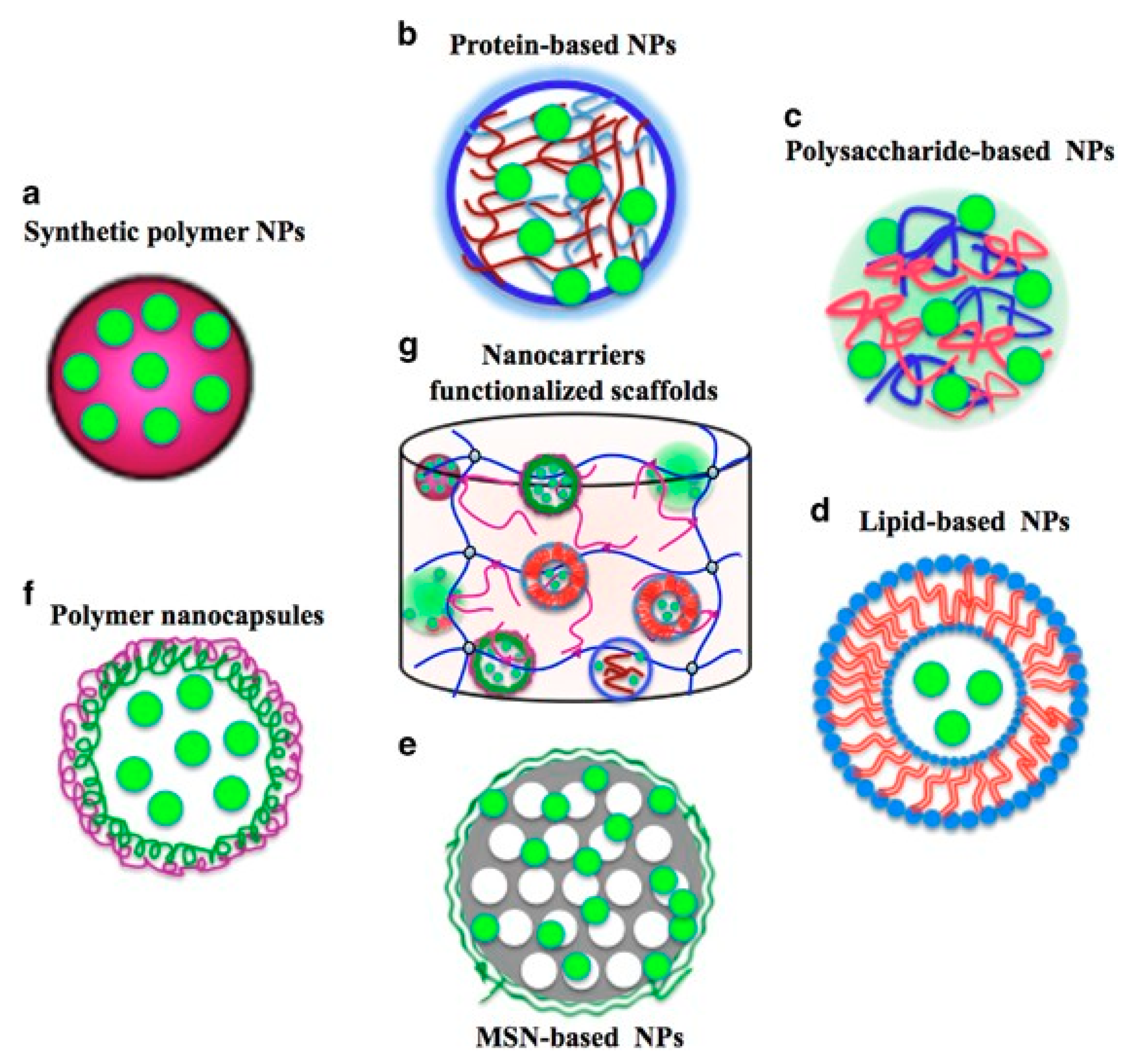
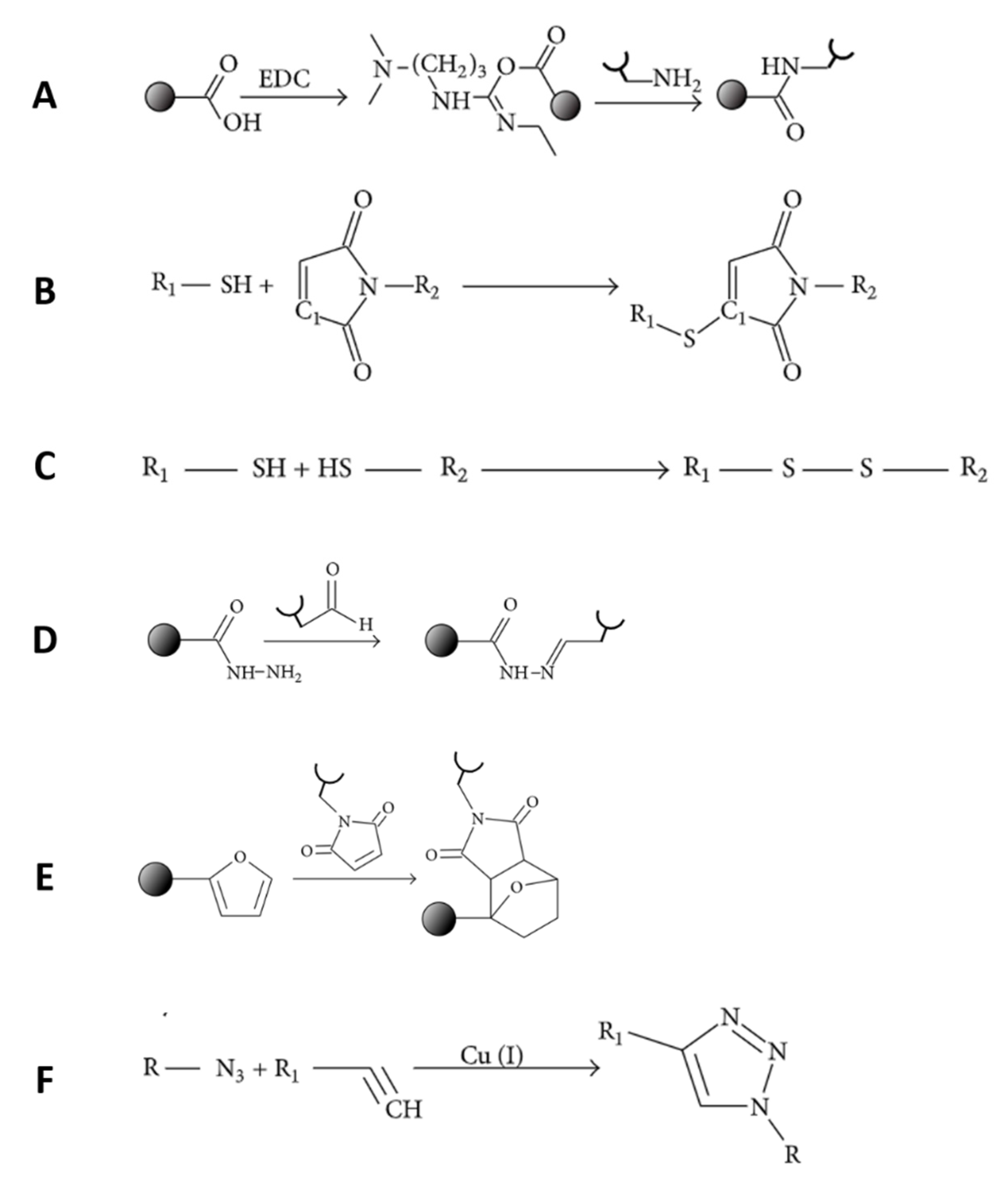
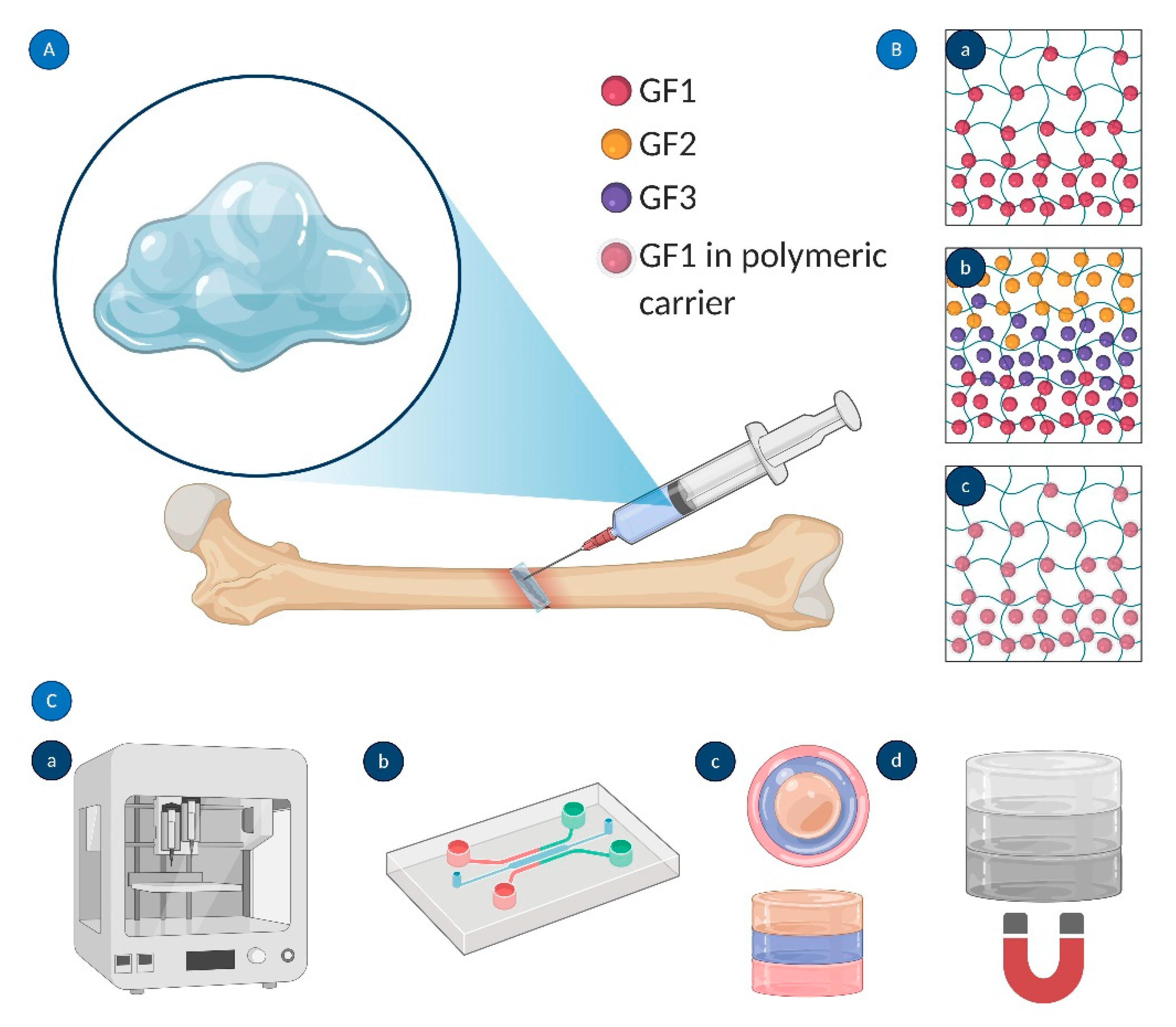
| Growth Factor | Material Carrier | Fabrication Method | Delivery Remarks or Mechanism of Action | Application | In Vivo or In Vitro Tests | References |
|---|---|---|---|---|---|---|
| PDGF-BB | β-tricalcium phosphate (TCP) particles | - | Interaction with PDGF receptors stimulates recruitment and proliferation of cells and promotes revascularization. | Distal radius fractures, hindfoot/ankle fusion; healing in hindfoot and ankle arthrodesis | In phase III randomized, controlled trial, 66.5% of PDGF-treated joints and 62.6% of autograft-treated joints showed fusion on computed tomography scanning at 24 weeks postoperatively. | [199,200] |
| FGF2 + BMP2 VEGF + BMP2 | Silica-coated nanohydroxyapatite-gelatin reinforced with poly (L-lactic acid) (PLLA) yarns | GFs dissolved in PBS and loaded onto the scaffolds | FGF2 mainly promoted cell migration, whereas VEGF augmented new blood vessel formation at the defect site. | Promotes vascularisation and bone regeneration in a critical-sized calvarial defect | In in vivo and in vitro tests, VEGF was released for 1 week whereas BMP2 and FGF2 were released for 3 weeks. In vitro studies have shown that the composite matrix degraded partially within 2–3 weeks in the presence of a collagenase enzyme. Release of growth factors was faster in vivo than in vitro. This disparity may be due to a complex in vivo environment containing multiple matrix-degrading enzymes (MMP2 and MMP9), cell types, etc. that are involved in the healing process. | [201] |
| BMP-2 | Polyelectrolyte (PEM) film coating | Polyelectrolyte film loaded with tunable doses of BMP-2 as the osteoinductive surface coating of a hollow PLGA tube | (a) Release owing to the swelling of the film (b) Release due to biodegradability of the film | Triggers fast volumetric bone regeneration via the surface of an implant | (a) Microcomputed tomography and quantitative analysis, and C2C12 cell culture and in vitro BMP-2 bioactivity assay (b) In vivo critical-size femoral defect in the rat: formation of vascularized cortical and cancellous bone (c) The formation of new bone dependent on the dose of BMP-2: higher doses lead to hematoma | [202] |
| BMP-2 and TGF-β1 | Silk protein fibroin reinforced with functionalized carbon nanofiber (CNF) | Facile green aqueous-based | Prolonged-release kinetics; timely growth, attachment, multiplication, and differentiation of mesenchymal and osteoblasts cells | Extracellular matrix for osseointegration | (a) Cytocompatibility of growth factor loaded matrices showed immunocompatibility due to low release of pro-inflammatory cytokines (TNF-α and IL-1β). (b) In vivo analysis of new bone formation within the implants (radiological, μ-CT, fluorochrome labeling, and histological analysis) demonstrated more efficient bone regeneration on loaded scaffolds. | [83] |
| bFGF (basic fibroblast growth factor) | Porous α-TCP particles | Immobilization on heparin-modified α-TCP by immersion | Stimulation of osteoblast proliferation and differentiation | Mandible cortical bone regeneration | In an in vivo test on a canine model, higher bone mineral content and formation of homogenous cortical bone with Haversian structure dependent on bFGF dosage (optimal dose of 4.2 μg) was seen. | [34,203] |
| rhBMP-2 | Absorbable collagen sponge (ACS) and β-TCP/hydroxyapatite particle (TCP/HAp) | Immobilization on the carrier by immersion | Stimulation of osteoblast proliferation and differentiation | Tooth alveolar ridge preservation | In an in vivo test on a human model, similar bone height and width with no associated deleterious effects were seen. | [204,205] |
| BMP-2 | Alginate and Collagen | Scaffold loading by droplet | (a) Collagen sponges showed initial burst release within a day. (b) Alginate showed a more controlled release. | Regeneration of femoral segmental defects | (a) BMP-2 release in vitro was accelerated from collagen sponge, and loaded alginate induced higher bioactivity. (b) In an in vivo test on a rat model, an alginate scaffold showed higher total bone volume at 12 weeks; heterotopic bone volume was similar for alginate and collagen. | [2,11] |
| hBMP-2 and hGDF5 (human growth and differentiation factor) | Titanium (Ti) | Coated onto Ti with a smooth surface using heparin-binding interaction | Initial burst release at day 1 followed by controlled release for 30 days | Orthopedic and dental bone formation and osseointegration | (a) An in vitro test showed a high proliferation rate and alkaline phosphatase activity resulting in calcium deposition and gene expression. (b) An in vivo test on a rabbit model showed bone regeneration and osseointegration between the implants and host bone. Bone formation by osteoblasts and bone resorption by osteoclasts was observed through histological analysis | [205,206] |
| BMP-2 and FGF-2 | Gelatin nanofibers | Immobilization on nanofibers through avidin-biotin binding after HAp deposition | A synergism between multiple growth factor delivery and the HAp nanofiber coating stimulated the expression of osteogenic gene markers. | Promotes bone growth and mimics the natural extracellular matrix | Immobilization of FGF-2 and BMP-2 in administered ratios on the surfaces of gelatin fibers resulted in cell proliferation. | [2,34] |
| PDGF | Poly(ι-lactic acid) (PLLA) nanofibers | Immobilization on PLLA nanofibers coated with biominerals | Osteogenic and endothelial differentiation with gene expression | Vascularized bone regeneration | (a) In vitro. PDGF increased the proliferation of hADSCs (human adipose-derived stem cells). (b) In an in vivo mouse calvarial defect, bone regenerated 42.48% of an area and formed capillaries and arterioles. | [205] |
| VEGF and BMP-2 | nHAp/poly lactic-co-glycolic acid microspheres (PLGAs)/chitosan [207] hydrogel | Water-oil-water double emulsion solvent evaporation method (PLGA-loaded microspheres) and immersion (HAp and CS) | Sustained release with early burst release in the first 10 days followed by a steady release of BMP-2 (days 11 and 21) and VEFG (day 11 and 19), and bioactivity preservation | Ossification and vascularization in critical-sized mandibular bone defects | In an in vivo rabbit model, bone defect cavities gradually reduced with time and healed after 12 weeks with callus remodeling. | [2] |
| Name of Growth Factor | Abbreviation | Source | Biological Response | Mechanism of Action | Functions |
|---|---|---|---|---|---|
| Bone Morphognetic Proteins | BMP | Mesenchymal Osteoblast Endothelial Chondrocyte | Chondrogenic, osteogenic, and osteoinductive | Bone induction | BMPs are osteoinductive and induce bone formation by causing the migration of MSCs and their differentiation into osteoblast. BMPs do not initiate osteoclast activity. |
| Fibroblast Growth Factors | FGF | Mesenchymal Osteoblast Chondrocyte Inflammatory Cell Endothelia | Angiogenesis and connective tissue cell proliferation | Angiogenesis, proliferation, and osteogenic differentiation | FGFs induce angiogenesis by increasing osteoblast proliferation and a potent stimulant for wound healing. |
| Insulin-Like Growth Factors | IGF | Osteoblast Chondrocyte Hepatocyte Endothelial | Anabolic and catabolic effect on osteogenesis | Osteogenic differentiation | IGFs stimulate osteoblast proliferation and bone matrix synthesis. IGFs also stimulate osteoclasts. |
| Platelet-Derived Growth Factor | PDGF | Platelet Osteoblast Inflammatory Cells Endothelial | Osteoinductive, angiogenesis, and connective tissue cell proliferation | Cell proliferation and vascularization | PDGFs are a key regulator of wound healing/tissue repair and stimulate bone cell proliferation and angiogenesis |
| Transforming Growth Factor-Beta | TGF-β | Platelet Osteoblast Chondrocyte Endothelial Inflammatory Cells Fibroblast | Osteoinductive, immunosuppression, angiogenesis, andcell growth and differentiation | Osteogenic and chondrogenic differentiation | TGF-β induces proliferation and differentiation of bone by stimulating migration of osteoprogenitor cells and by regulating cell proliferation, cell differentiation, and extracellular matrix (ECM) synthesis and inhibits proliferation and differentiation of osteoclast progenitor cells. |
| Vascular Endothelial Growth Factor | VEGF | Platelet Osteoblast Chondrocyte | Osteoinductive, chemotactic, and angiogenesis | Angiogenesis | VEGF regulates migration, proliferation, and survival of endothelial cells through nutrient supply from newly formed blood vessels. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. https://doi.org/10.3390/ijms22020903
Oliveira ÉR, Nie L, Podstawczyk D, Allahbakhsh A, Ratnayake J, Brasil DL, Shavandi A. Advances in Growth Factor Delivery for Bone Tissue Engineering. International Journal of Molecular Sciences. 2021; 22(2):903. https://doi.org/10.3390/ijms22020903
Chicago/Turabian StyleOliveira, Érica Resende, Lei Nie, Daria Podstawczyk, Ahmad Allahbakhsh, Jithendra Ratnayake, Dandara Lima Brasil, and Amin Shavandi. 2021. "Advances in Growth Factor Delivery for Bone Tissue Engineering" International Journal of Molecular Sciences 22, no. 2: 903. https://doi.org/10.3390/ijms22020903
APA StyleOliveira, É. R., Nie, L., Podstawczyk, D., Allahbakhsh, A., Ratnayake, J., Brasil, D. L., & Shavandi, A. (2021). Advances in Growth Factor Delivery for Bone Tissue Engineering. International Journal of Molecular Sciences, 22(2), 903. https://doi.org/10.3390/ijms22020903








