Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves
Abstract
1. Introduction
2. Results
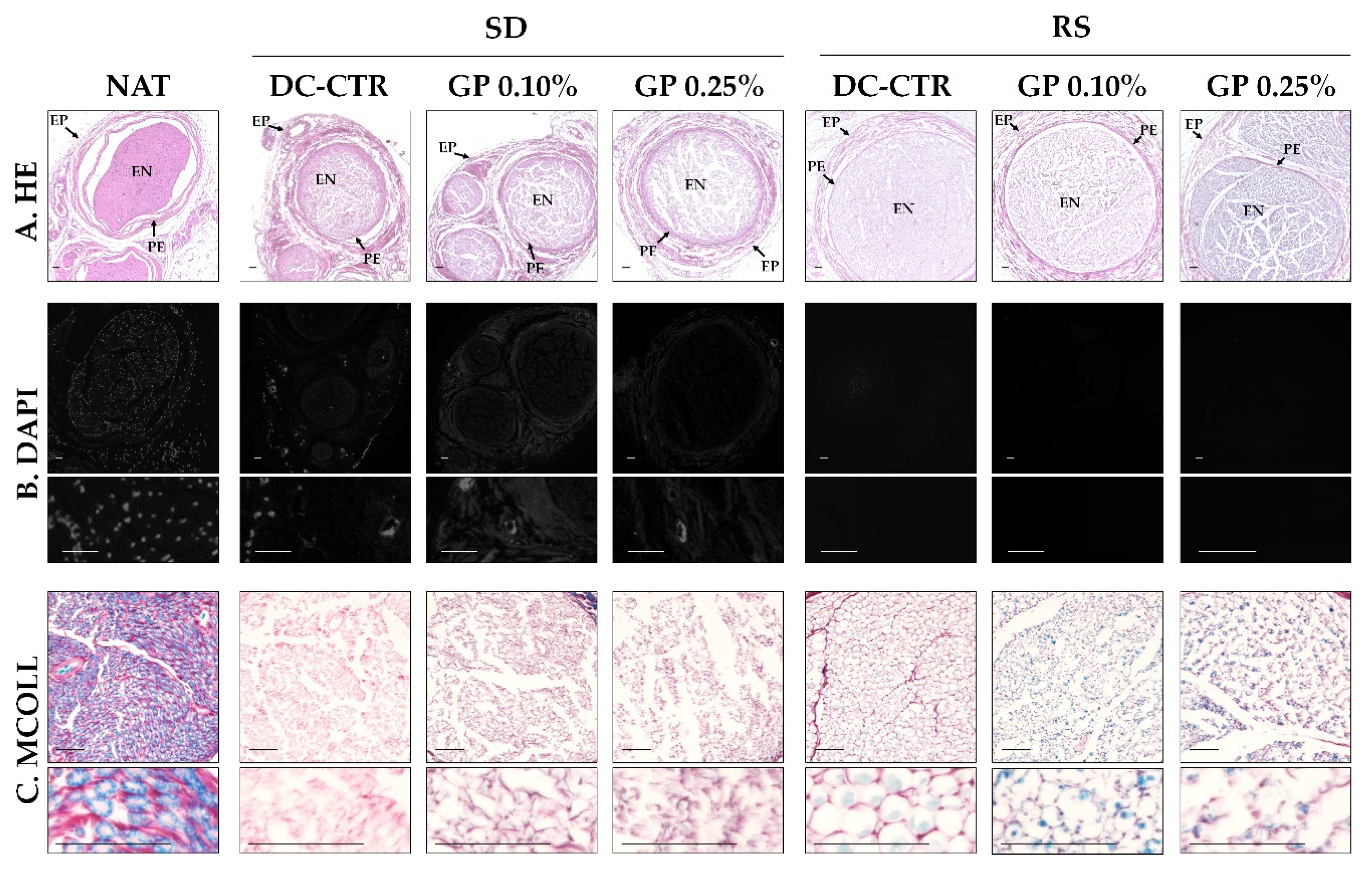
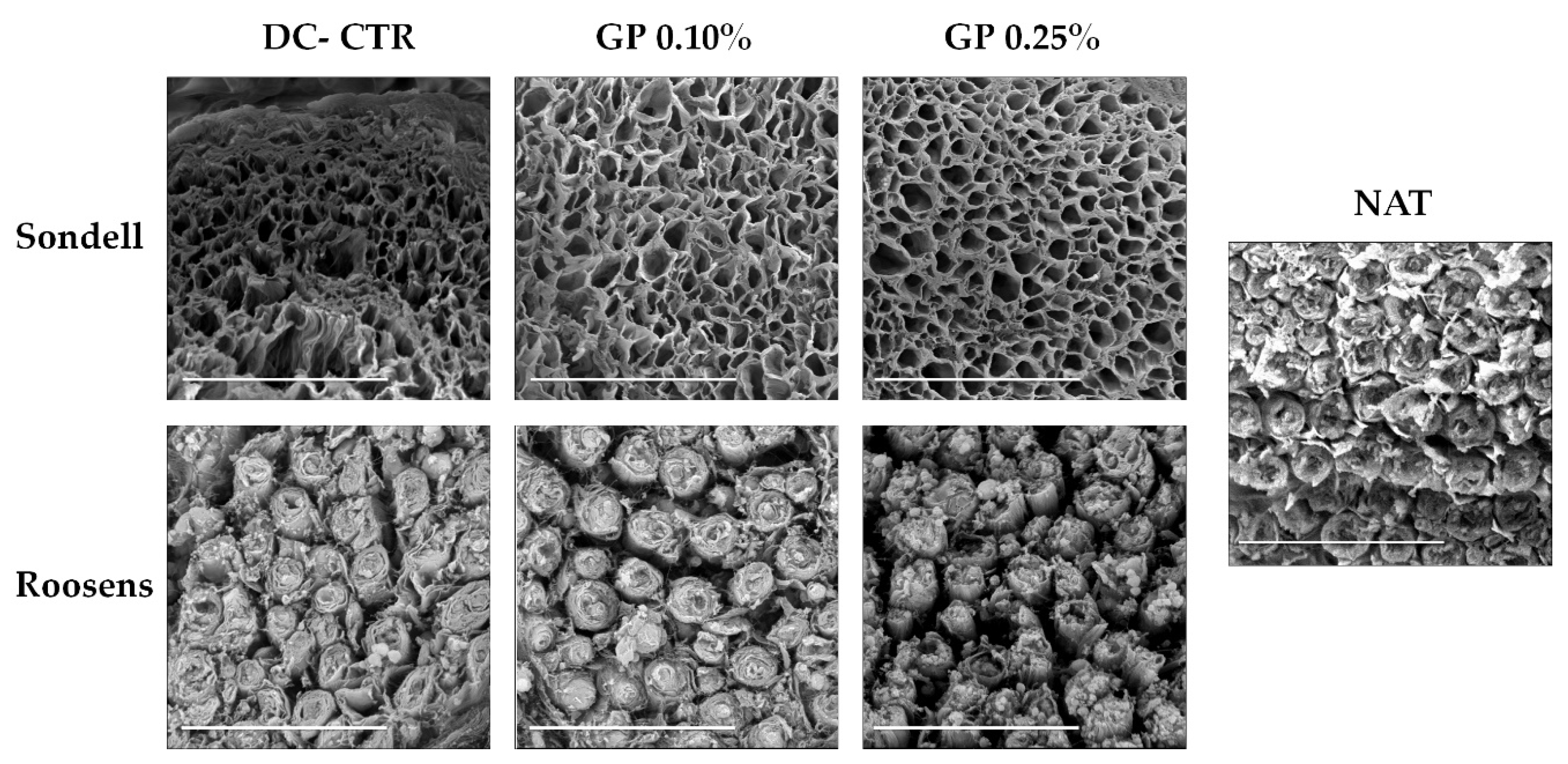
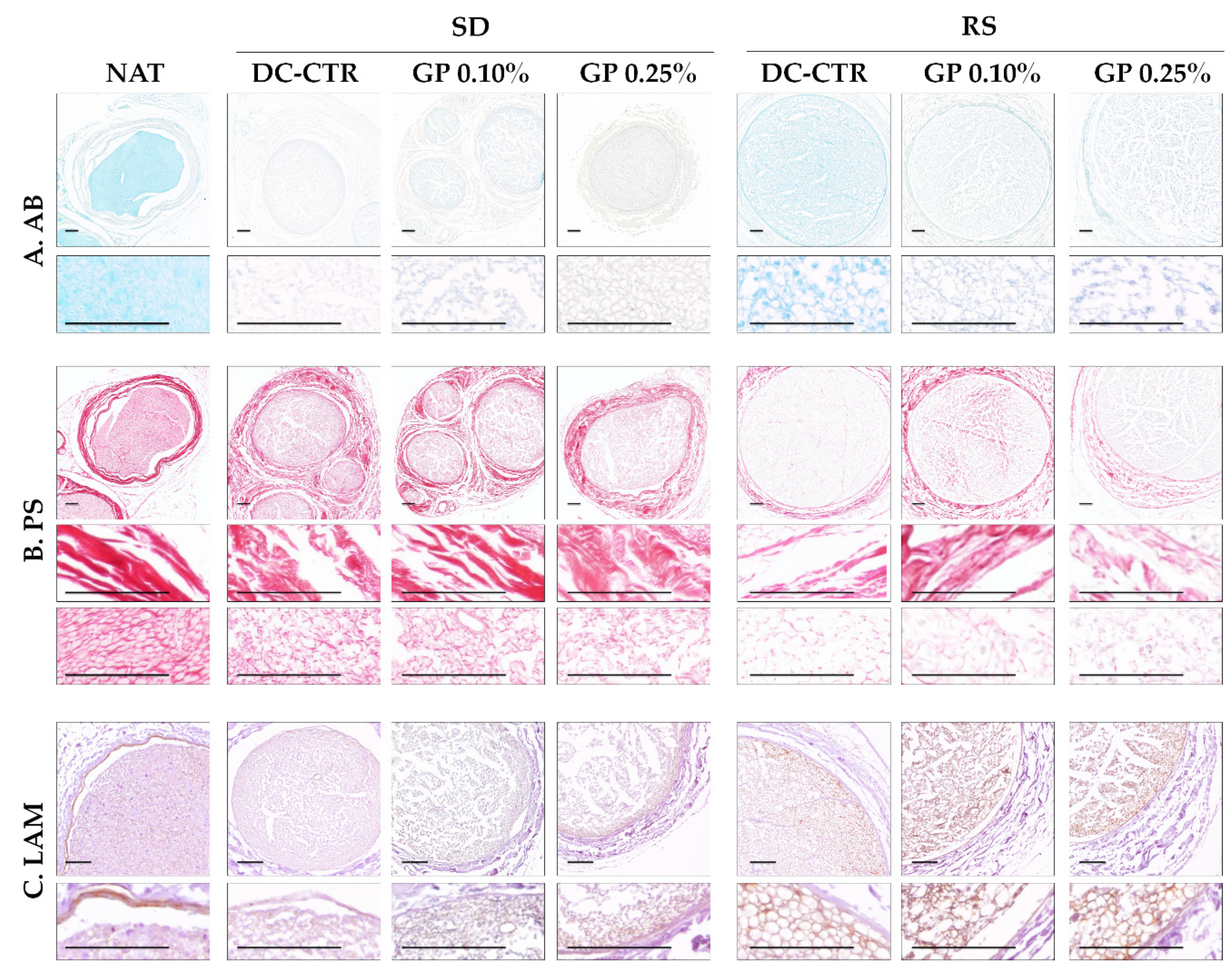
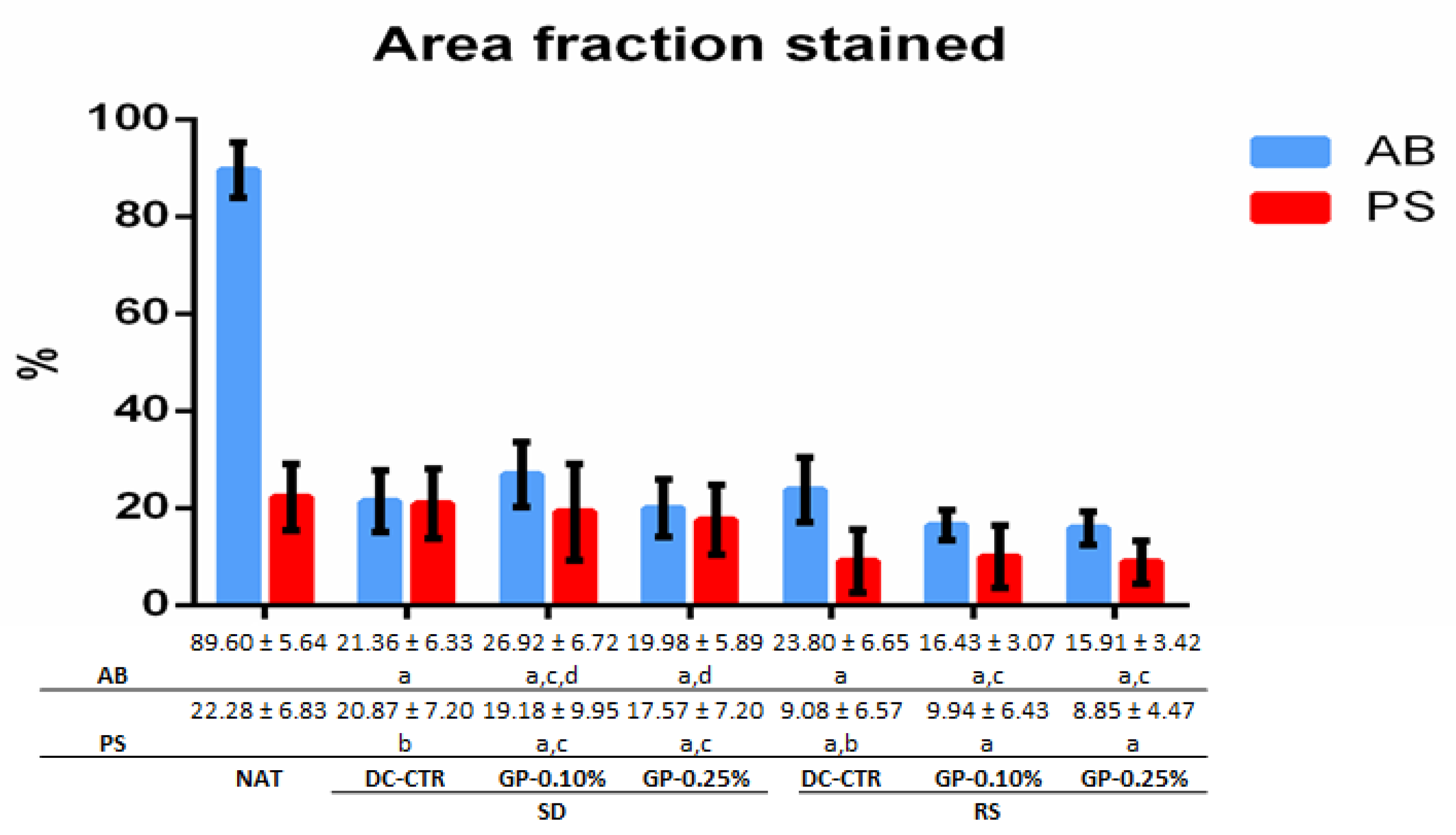
2.1. Histology and Quantitative Histochemistry
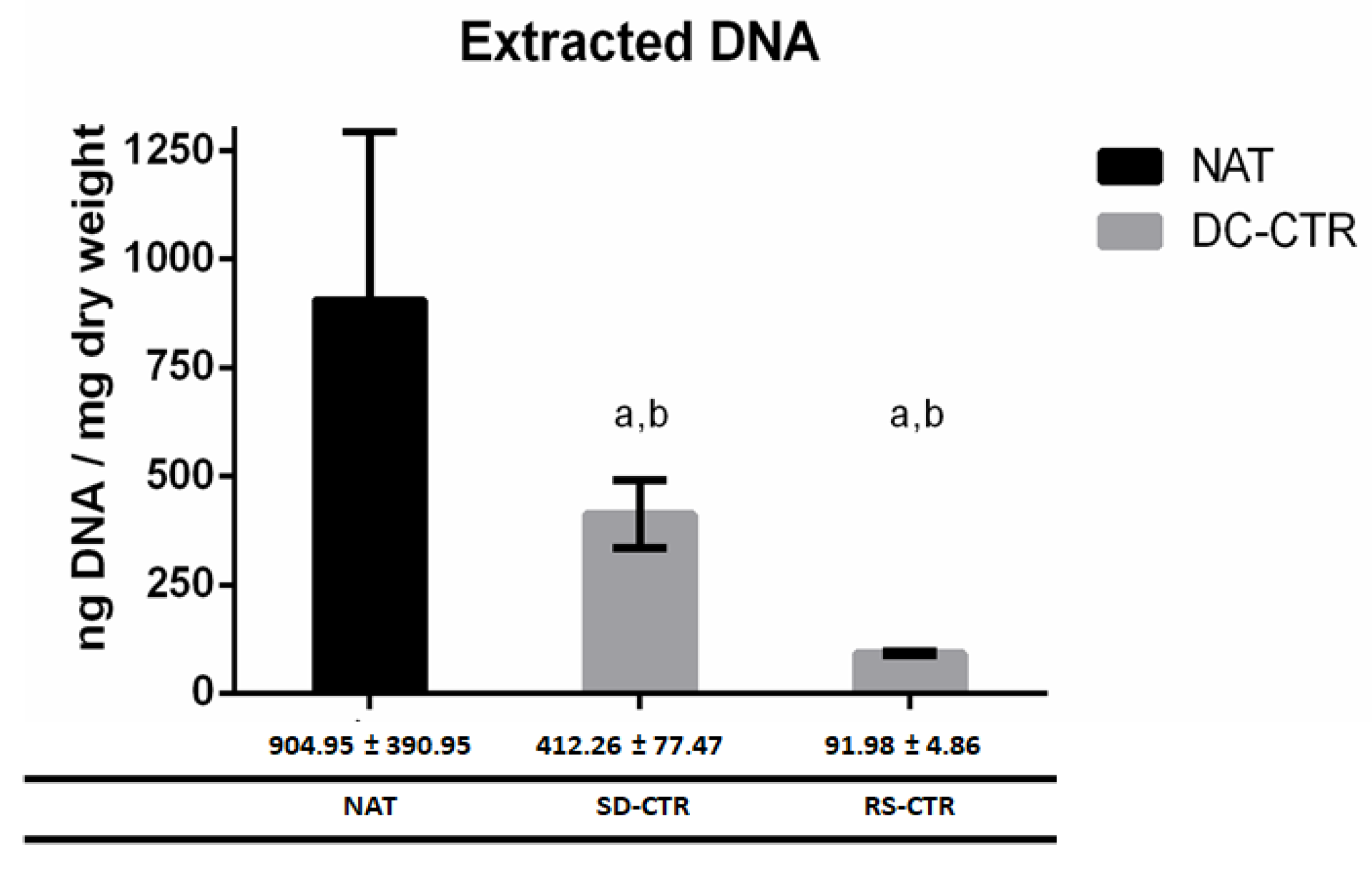
2.2. Quantification of DNA Content
2.3. Mechanical Characterization of Nerve Substitutes
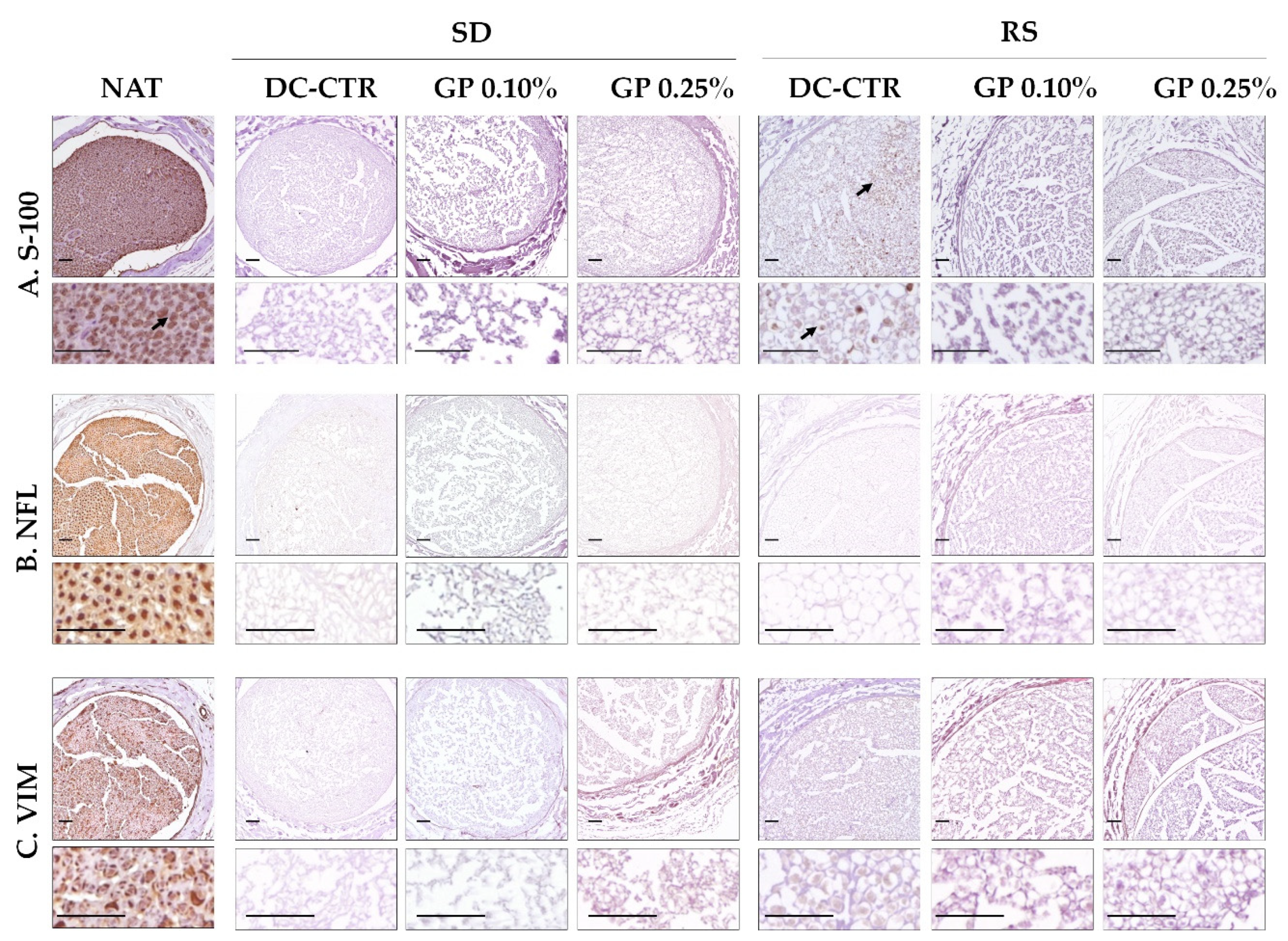
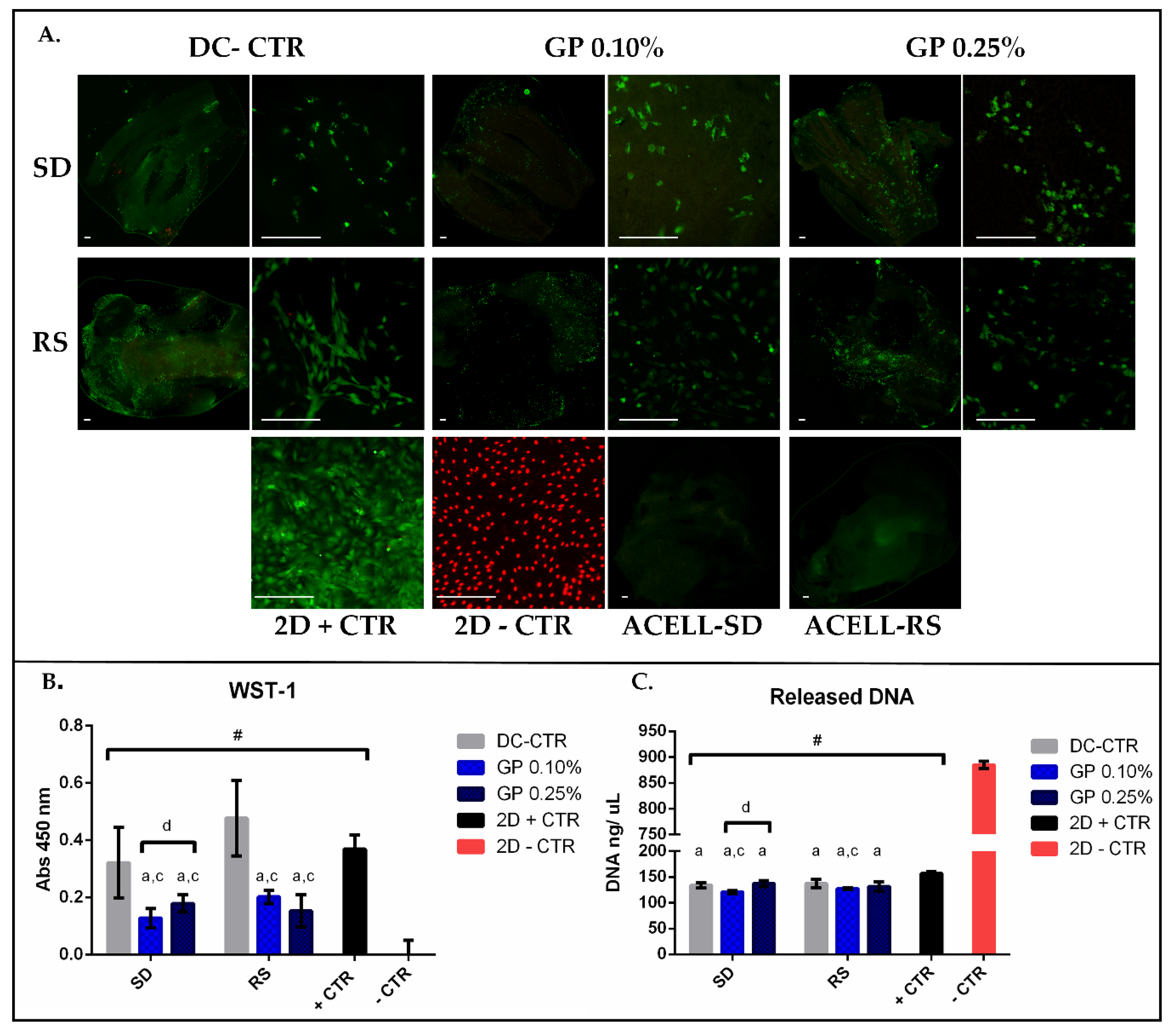
2.4. Ex-Vivo Biocompatibility
3. Discussion
4. Materials and Methods
4.1. Nerve Isolation and Decellularization Process
4.2. Genipin Crosslinking and Experimental Groups
4.3. Histological Analyses
4.4. Scanning Electron Microscopy (SEM)
4.5. Quantitative Histochemical Analysis of the ECM
4.6. Quantification of DNA Remnants
4.7. Tensile Tests
4.8. Ex-Vivo Biocompatibility (Cell-Biomaterial Interaction)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNs | Peripheral nerves |
| ECM | Extra cellular matrix |
| ANGs | Acellular nerve grafts |
| GP | Genipin |
| SD | Sondell |
| RS | Roosens |
| HD | Hudson-based |
| RT | Room temperature |
| PBS | Phosphate buffer saline |
| HBSS | Hank´s balance salt solution |
| SD-ANGs | Acellular nerve allografts decellularized by Sondell method |
| RS-ANGs | Acellular nerve allografts decellularized by Roosens method |
| SD-CTR | Sondell non-crosslinked decellularized control group |
| SD-GP 0.10% | Sondell 0.10% genipin crosslinked decellularized group |
| SD-GP 0.25% | Sondell 0.25% genipin crosslinked decellularized group |
| RS-CTR | Roosens non-crosslinked decellularized control group |
| RS-GP 0.10% | Roosens 0.10% genipin crosslinked decellularized group |
| RS-GP 0.25% | Roosens 0.25% genipin crosslinked decellularized group |
| NAT | Native nerve control group |
| HE | Hematoxylin & eosin |
| DAPI | 4´,6-diamidino-2-phenylindole |
| AB | Alcian blue |
| PS | Picrosirius |
| SEM | Surface electron microscopy |
| rAMSC | Rat adipose-derived mesenchymal stem cells |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | Fetal bovine serum |
| WST-1 | Water soluble tetrazolium-1 |
| L/D | Live/Dead® cell viability assay |
| NFL | Neurofilament |
| VIM | Vimentin |
| LAM | Laminin |
| DC-CTR | non-crosslinked decellularized control group |
| GP-ANGs | Genipin crosslinked acellular nerve grafts |
| 2D + CTR | 2 dimensional positive control |
| 2D − CTR | 2 dimensional negative control |
| ACELL-SD | Technical control of non-cellular seeding on Sondell acellular scaffold. |
| ACELL-RS | Technical control of non-cellular seeding on Roosens acellular scaffold. |
| MCOLL | Histochemical method for simultaneous staining of myelin and collagen fibers |
References
- Carriel, V.; Alaminos, M.; Garzon, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Geuna, S.; Raimondo, S.; Ronchi, G.; Di Scipio, F.; Tos, P.; Czaja, K.; Fornaro, M. Histology of the peripheral nerve and changes occurring during nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 27–46. [Google Scholar]
- Dahlin, L. Techniques of peripheral nerve repair. Scand. J. Surg. 2008, 97, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–873. [Google Scholar] [CrossRef]
- Kingham, P.J.; Terenghi, G. Bioengineered nerve regeneration and muscle reinnervation. J. Anat. 2006, 209, 511–526. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Lovati, A.B.; D’Arrigo, D.; Odella, S.; Tos, P.; Geuna, S.; Raimondo, S. Nerve Repair Using Decellularized Nerve Grafts in Rat Models. A Review of the Literature. Front. Cell. Neurosci. 2018, 12, 427. [Google Scholar] [CrossRef]
- Bain, J.; Mackinnon, S.; Hudson, A.; Falk, R.; Falk, J.; Hunter, D. The peripheral nerve allograft: An assessment of regeneration across nerve allografts in rats immunosuppressed with cyclosporin A. Plast. Reconstr. Surg. 1988, 82, 1052–1064. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Doolabh, V.B.; Novak, C.B.; Trulock, E.P. Clinical outcome following nerve allograft transplantation. Plast. Reconstr. Surg. 2001, 107, 1419–1429. [Google Scholar] [CrossRef]
- Gu, X. Progress and perspectives of neural tissue engineering. Front. Med. 2015, 9, 401–411. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; MacEwan, M.; Santosa, K.B.; Chenard, K.E.; Ray, W.Z.; Hunter, D.A.; Mackinnon, S.E.; Johnson, P.J. Acellular nerve allografts in peripheral nerve regeneration: A comparative study. Muscle Nerve 2011, 44, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, K.J.; Kalliainen, L.K. Collagen tube conduits in peripheral nerve repair: A retrospective analysis. Hand 2010, 5, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.L.; Tuffaha, S.H.; Luciano, J.P.; Yan, Y.; Hunter, D.A.; Magill, C.K.; Moore, A.M.; Tong, A.Y.; Mackinnon, S.E.; Borschel, G.H. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009, 39, 787–799. [Google Scholar] [CrossRef]
- Kneib, C.; von Glehn, C.; Costa, F.; Costa, M.; Susin, M. Evaluation of humoral immune response to donor HLA after implantation of cellularized versus decellularized human heart valve allografts. HLA 2012, 80, 165–174. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Buckenmeyer, M.J.; Meder, T.J.; Prest, T.A.; Brown, B.N. Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods 2020, 171, 41–61. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef]
- Philips, C.; Cornelissen, M.; Carriel, V. Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts. J. Neural Eng. 2018, 15, 021003. [Google Scholar] [CrossRef]
- Roosens, A.; Somers, P.; De Somer, F.; Carriel, V.; Van Nooten, G.; Cornelissen, R. Impact of Detergent-Based Decellularization Methods on Porcine Tissues for Heart Valve Engineering. Ann. Biomed. Eng. 2016, 44, 2827–2839. [Google Scholar] [CrossRef]
- Philips, C.; Campos, F.; Roosens, A.; Sanchez-Quevedo, M.D.C.; Declercq, H.; Carriel, V. Qualitative and Quantitative Evaluation of a Novel Detergent-Based Method for Decellularization of Peripheral Nerves. Ann. Biomed. Eng. 2018, 46, 1921–1937. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Philips, C.; Campos, F.; Durand-Herrera, D.; Garcia-Garcia, O.D.; Roosens, A.; Alaminos, M.; Campos, A.; Carriel, V. Detergent-based decellularized peripheral nerve allografts: An in vivo preclinical study in the rat sciatic nerve injury model. J. Tissue Eng. Regen. Med. 2020, 14, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Arora, B.; Tandon, R.; Attri, P.; Bhatia, R. Chemical Crosslinking: Role in Protein and Peptide Science. Curr. Protein Pept. Sci. 2017, 18, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Courtman, D.W.; Errett, B.F.; Wilson, G.J. The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices. J. Biomed. Mater. Res. Part A 2001, 55, 576–586. [Google Scholar] [CrossRef]
- Simon, P.; Kasimir, M.; Seebacher, G.; Weigel, G.; Ullrich, R.; Salzer-Muhar, U.; Rieder, E.; Wolner, E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT® in pediatric patients. Eur. J. Cardio-Thorac. Surg. 2003, 23, 1002–1006. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Garcia-Martinez, L.; Duran, J.D.; Lopez-Lopez, M.T.; Alaminos, M.; Sanchez-Quevedo, M.C.; Carriel, V. Ex vivo characterization of a novel tissue-like cross-linked fibrin-agarose hydrogel for tissue engineering applications. Biomed. Mater. 2016, 11, 055004. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C.; Chiu, C.T. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J. Biomed. Mater. Res. 1998, 42, 560–567. [Google Scholar] [CrossRef]
- Musilkova, J.; Filova, E.; Pala, J.; Matejka, R.; Hadraba, D.; Vondrasek, D.; Kaplan, O.; Riedel, T.; Brynda, E.; Kucerova, J.; et al. Human decellularized and crosslinked pericardium coated with bioactive molecular assemblies. Biomed. Mater. 2019. [Google Scholar] [CrossRef]
- Tang, Y.; Song, W.; Qiao, J.; Rong, B.; Wu, Y.; Yan, X. A study of corneal structure and biomechanical properties after collagen crosslinking with genipin in rabbit corneas. Mol. Vis. 2019, 25, 574–582. [Google Scholar]
- Gu, Y.; Wang, F.; Wang, R.; Li, J.; Wang, C.; Li, L.; Xu, Z.; Zhang, J. Preparation and evaluation of decellularized porcine carotid arteries cross-linked by genipin: The preliminary results. Cell Tissue Bank. 2018, 19, 311–321. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Vizcaino, G.; Rodriguez, I.A.; Duran-Herrera, D.; Lopez-Lopez, M.T.; Sanchez-Montesinos, I.; Alaminos, M.; Sanchez-Quevedo, M.C.; Carriel, V. Generation of genipin cross-linked fibrin-agarose hydrogel tissue-like models for tissue engineering applications. Biomed. Mater. 2018, 13, 025021. [Google Scholar] [CrossRef]
- Gorczyca, G.; Tylingo, R.; Szweda, P.; Augustin, E.; Sadowska, M.; Milewski, S. Preparation and characterization of genipin cross-linked porous chitosan-collagen-gelatin scaffolds using chitosan-CO2 solution. Carbohydr. Polym. 2014, 102, 901–911. [Google Scholar] [CrossRef]
- Vyborny, K.; Vallova, J.; Koci, Z.; Kekulova, K.; Jirakova, K.; Jendelova, P.; Hodan, J.; Kubinova, S. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Jiang, T.; Ren, X.J.; Tang, J.L.; Yin, H.; Wang, K.J.; Zhou, C.L. Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3514–3521. [Google Scholar] [CrossRef]
- Koo, H.J.; Song, Y.S.; Kim, H.J.; Lee, Y.H.; Hong, S.M.; Kim, S.J.; Kim, B.C.; Jin, C.; Lim, C.J.; Park, E.H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. 2004, 495, 201–208. [Google Scholar] [CrossRef]
- Zhong, Y.; Jiang, A.; Sun, F.; Xiao, Y.; Gu, Y.; Wu, L.; Zhang, Y.; Shi, H. A Comparative Study of the Effects of Different Decellularization Methods and Genipin-Cross-Linking on the Properties of Tracheal Matrices. Tissue Eng. Regen. Med. 2019, 16, 39–50. [Google Scholar] [CrossRef]
- Koci, Z.; Sridharan, R.; Hibbitts, A.J.; Kneafsey, S.L.; Kearney, C.J.; O’Brien, F.J. The Use of Genipin as an Effective, Biocompatible, Anti-Inflammatory Cross-Linking Method for Nerve Guidance Conduits. Adv. Biosyst. 2020, 4, e1900212. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Cok, M.; Asaro, F.; Scognamiglio, F.; Marsich, E.; Lopez, F.; Donati, I.; Sacco, P. On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH. Int. J. Mol. Sci. 2020, 21, 6831. [Google Scholar] [CrossRef]
- Sondell, M.; Lundborg, G.; Kanje, M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998, 795, 44–54. [Google Scholar] [CrossRef]
- Choudhury, D.; Yee, M.; Sheng, Z.L.J.; Amirul, A.; Naing, M.W. Decellularization systems and devices: State-of-the-art. Acta Biomater. 2020, 115, 51–59. [Google Scholar] [CrossRef]
- Gonzalez-Andrades, M.; Carriel, V.; Rivera-Izquierdo, M.; Garzon, I.; Gonzalez-Andrades, E.; Medialdea, S.; Alaminos, M.; Campos, A. Effects of Detergent-Based Protocols on Decellularization of Corneas with Sclerocorneal Limbus. Evaluation of Regional Differences. Transl. Vis. Sci. Technol. 2015, 4, 13. [Google Scholar] [CrossRef]
- Stocum, D.L. Regenerative Biology and Medicine; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2006; 448p. [Google Scholar]
- Yu, T.; Wen, L.; He, J.; Xu, Y.; Li, T.; Wang, W.; Ma, Y.; Ahmad, M.A.; Tian, X.; Fan, J.; et al. Fabrication and evaluation of an optimized acellular nerve allograft with multiple axial channels. Acta Biomater. 2020, 115, 235–249. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Yu, W.M.; Chen, Z.L.; Carey, D.J.; Strickland, S. Regulation of Schwann cell function by the extracellular matrix. Glia 2008, 56, 1498–1507. [Google Scholar] [CrossRef]
- Carriel, V.; Garrido-Gomez, J.; Hernandez-Cortes, P.; Garzon, I.; Garcia-Garcia, S.; Saez-Moreno, J.A.; Del Carmen Sanchez-Quevedo, M.; Campos, A.; Alaminos, M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural Eng. 2013, 10, 026022. [Google Scholar] [CrossRef]
- Chato-Astrain, J.; Campos, F.; Roda, O.; Miralles, E.; Durand-Herrera, D.; Saez-Moreno, J.A.; Garcia-Garcia, S.; Alaminos, M.; Campos, A.; Carriel, V. In vivo Evaluation of Nanostructured Fibrin-Agarose Hydrogels With Mesenchymal Stem Cells for Peripheral Nerve Repair. Front. Cell. Neurosci. 2018, 12, 501. [Google Scholar] [CrossRef]
- Xiao, W.; Li, S.; Wang, S.; Ho, C.T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2017, 25, 43–61. [Google Scholar] [CrossRef]
- Tambe, N.; Di, J.; Zhang, Z.; Bernacki, S.; El-Shafei, A.; King, M.W. Novel genipin-collagen immobilization of polylactic acid (PLA) fibers for use as tissue engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1188–1197. [Google Scholar] [CrossRef]
- Nair, M.; Johal, R.K.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: A comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials 2020, 254, 120109. [Google Scholar] [CrossRef]
- Carriel, V.; Scionti, G.; Campos, F.; Roda, O.; Castro, B.; Cornelissen, M.; Garzon, I.; Alaminos, M. In vitro characterization of a nanostructured fibrin agarose bio-artificial nerve substitute. J. Tissue Eng. Regen. Med. 2017, 11, 1412–1426. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Chato-Astrain, J.; Sanchez-Porras, D.; Garcia-Garcia, O.D.; Carmona, R.; Lopez-Lopez, M.T.; Alaminos, M.; Carriel, V.; Rodriguez, I.A. Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quevedo, D.; Diaz-Ramos, M.; Chato-Astrain, J.; Sanchez-Porras, D.; Tamimi, I.; Campos, A.; Campos, F.; Carriel, V. Improving the regenerative microenvironment during tendon healing by using nanostructured fibrin/agarose-based hydrogels in a rat Achilles tendon injury model. Bone Jt. J. 2020, 102, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Zor, F.; Selek, F.N.; Orlando, G.; Williams, D.F. Biocompatibility in regenerative nanomedicine. Nanomedicine 2019, 14, 2763–2775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Yang, T.; Zhang, N.; Dong, L.; Ma, S.; Liu, X.; Zhou, M.; Li, B. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: An in vitro evaluation. Cell Tissue Bank. 2014, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Vizcaino-Lopez, G.; Chato-Astrain, J.; Durand-Herrera, D.; Alaminos, M.; Campos, A.; Sanchez-Montesinos, I.; Campos, F. Scleral surgical repair through the use of nanostructured fibrin/agarose-based films in rabbits. Exp. Eye Res. 2019, 186, 107717. [Google Scholar] [CrossRef] [PubMed]
- Gobinathan, S.; Zainol, S.S.; Azizi, S.F.; Iman, N.M.; Muniandy, R.; Hasmad, H.N.; Yusof, M.R.B.; Husain, S.; Abd Aziz, H.; Lokanathan, Y. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.; Pinheiro, A.; Young, C.; Smith, P.; Wright, E. Evaluation of genipin for stabilization of decellularized porcine cartilage. J. Orthop. Res. 2017, 35, 1949–1957. [Google Scholar] [CrossRef]
- Hernandez-Cortes, P.; Garrido, J.; Camara, M.; Ravassa, F.O. Failed digital nerve reconstruction by foreign body reaction to Neurolac nerve conduit. Microsurgery 2010, 30, 414–416. [Google Scholar] [CrossRef]
- Carriel, V.; Garzon, I.; Alaminos, M.; Campos, A. Evaluation of myelin sheath and collagen reorganization pattern in a model of peripheral nerve regeneration using an integrated histochemical approach. Histochem. Cell Biol. 2011, 136, 709–717. [Google Scholar] [CrossRef]
- Liodaki, E.; Bos, I.; Lohmeyer, J.A.; Senyaman, O.; Mauss, K.L.; Siemers, F.; Mailaender, P.; Stang, F. Removal of collagen nerve conduits (NeuraGen) after unsuccessful implantation: Focus on histological findings. J. Reconstr. Microsurg. 2013, 29, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Campos, F.; Aneiros-Fernandez, J.; Kiernan, J.A. Tissue Fixation and Processing for the Histological Identification of Lipids. Methods Mol. Biol. 2017, 1560, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Campos, A.; Alaminos, M.; Raimondo, S.; Geuna, S. Staining Methods for Normal and Regenerative Myelin in the Nervous System. Methods Mol. Biol. 2017, 1560, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Garzon, I.; Campos, A.; Cornelissen, M.; Alaminos, M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J. Tissue Eng. Regen. Med. 2017, 11, 553–563. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Durand-Herrera, D.; Campos, F.; Jaimes-Parra, B.D.; Sanchez-Lopez, J.D.; Fernandez-Valades, R.; Alaminos, M.; Campos, A.; Carriel, V. Wharton’s jelly-derived mesenchymal cells as a new source for the generation of microtissues for tissue engineering applications. Histochem. Cell Biol. 2018, 150, 379–393. [Google Scholar] [CrossRef]
- Rodriguez-Arco, L.; Rodriguez, I.A.; Carriel, V.; Bonhome-Espinosa, A.B.; Campos, F.; Kuzhir, P.; Duran, J.D.; Lopez-Lopez, M.T. Biocompatible magnetic core-shell nanocomposites for engineered magnetic tissues. Nanoscale 2016, 8, 8138–8150. [Google Scholar] [CrossRef]
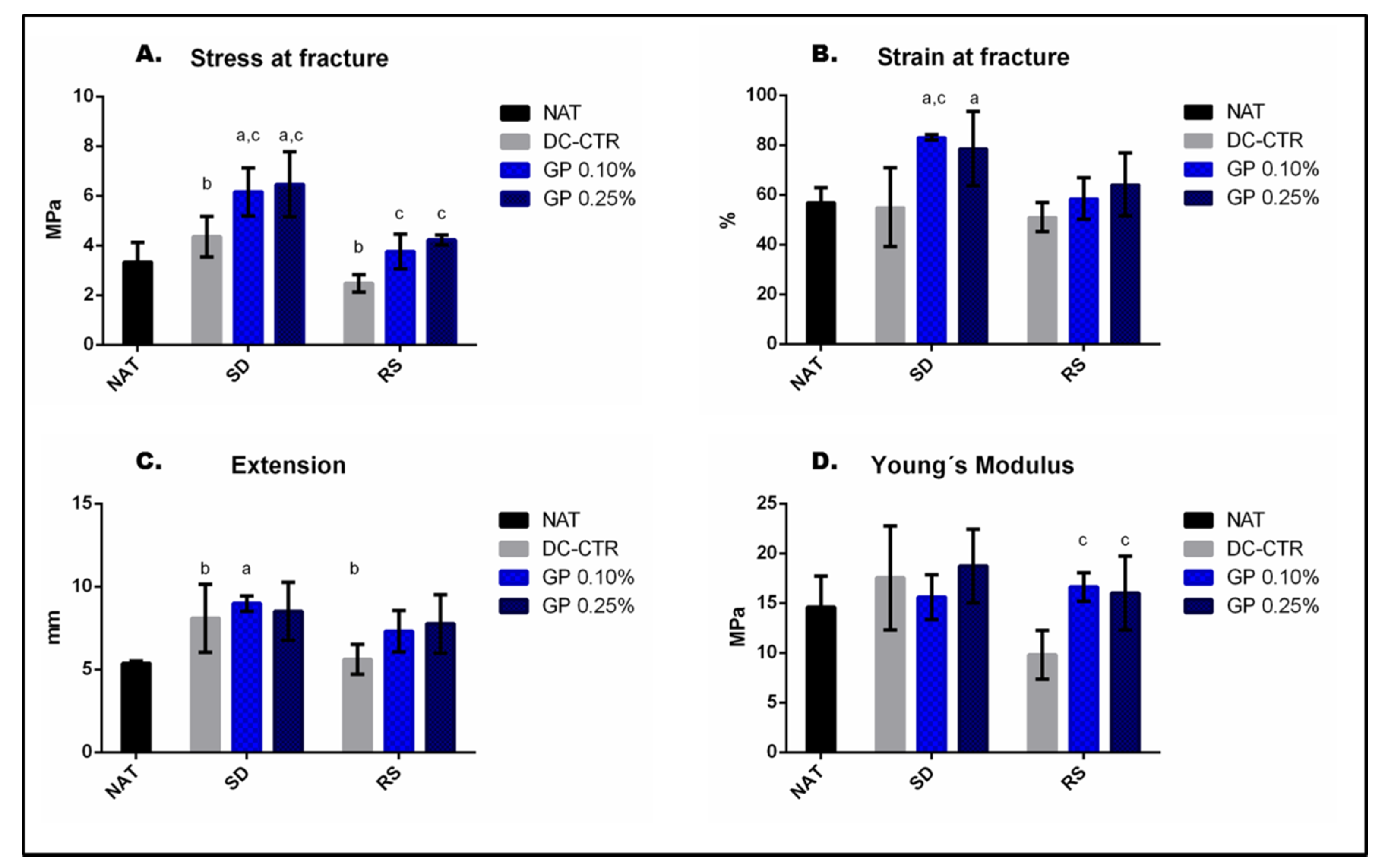
| Groups | Stress at Fracture | Strain at Fracture | Extension | Young´s Modulus | ||||
|---|---|---|---|---|---|---|---|---|
| (MPa) | (%) | (mm) | (MPa) | |||||
| NAT | 3.33 | ±0.79 | 56.97 | ±4.68 | 5.99 | ±0.36 | 13.40 | ±3.21 |
| SD-CTR | 4.36 | ±0.82 | 55.08 | ±15.89 | 8.17 | ±2.06 | 16.70 | ±5.23 |
| SD-GP 0.10% | 6.16 | ±0.96 | 83.16 | ±1.13 | 8.98 | ±0.46 | 15.61 | ±2.25 |
| SD-GP 0.25% | 6.46 | ±1.31 | 78.64 | ±14.86 | 8.51 | ±1.76 | 18.73 | ±3.70 |
| RS-CTR | 2.47 | ±0.35 | 51.02 | ±5.82 | 5.61 | ±0.90 | 9.81 | ±2.44 |
| RS-GP 0.10% | 3.76 | ±0.70 | 58.55 | ±8.35 | 7.31 | ±1.24 | 16.63 | ±1.44 |
| RS-GP 0.25% | 4.22 | ±0.20 | 64.24 | ±12.61 | 7.75 | ±1.76 | 16.02 | ±3.69 |
| WST-1 | Released DNA | |||||
|---|---|---|---|---|---|---|
| (Abs 450 nm) | (%) | (ng/uL) | (%) | |||
| Mean | ±SD | Normalized | Mean | ±SD | Normalized | |
| SD-CTR | 0.32 | ±0.12 | 87.35 | 133.94 | ±5.09 | 15.66 |
| SD-GP 0.1% | 0.13 | ±0.03 | 34.70 | 120.91 | ±2.87 | 14.14 |
| SD-GP 0.25% | 0.18 | ±0.03 | 48.62 | 137.28 | ±5.83 | 16.06 |
| RS-CTR | 0.48 | ±0.13 | 129.43 | 137.16 | ±7.88 | 16.04 |
| RS-GP 0.10% | 0.20 | ±0.02 | 54.81 | 127.41 | ±1.92 | 14.90 |
| RS-GP 0.25% | 0.15 | ±0.06 | 41.36 | 131.61 | ±9.12 | 15.39 |
| 2D + CTR | 0.37 | ±0.07 | 100.00 | 127.14 | ±2.26 | 18.33 |
| 2D − CTR | 0.00 | ±0.26 | 0.00 | 885.03 | ±7.11 | 100.00 |
| Antibody/Reagent | Dilution/Incubation | Pretreatment | Reference |
|---|---|---|---|
| Rabbit Policlonal anti- S100 antibody (Z0311) | 1: 400 Overnight at 4 °C | Citrate buffer pH = 630 min at 95 °C | DakoCytomation, Glostrup, Denmark (ref. Z0311) |
| Mouse Monoclonal Neurofilament | 1: 500 1 h at RT | EDTA buffer pH = 8 25 min at 95 °C | Sigma-Aldrich N2912, Steinheim, Germany (ref. RMdO20) |
| Mouse anti-vimentin monoclonal clone V9 | 1:200 1 h at RT | Citrate buffer pH = 6 25 min at 95 °C | Sigma-Aldrich. St. Louis, MO, USA (ref. V6630) |
| Rabbit anti-laminin polyclonal | 1:200 Overnight at 4 °C | Citrate buffer pH = 6 25 min at 95 °C | Abcam. Cambridge, UK (ref. ab11575) |
| ImmPRESS® HRP Anti-Mouse IgG (Peroxidase) | Ready to use 30 min at RT | - | Vector Laboratories. Burlingame, EEUU (ref. MP-7402) |
| ImmPRESS® HRP Anti-Rabbit IgG (Peroxidase) | Ready to use 30 min at RT | - | Vector Laboratories. Burlingame, EEUU (ref. MP-7401) |
| Chromogen: Diaminobenzidine ready to use kit | - | - | Vector Laboratories. Burlingame, EEUU (ref. SK-4100) |
| Contrast: Harris Hematoxylin | 30 s | - | Thermo Scientific. Runcorn, UK (ref. 6765004) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, Ó.D.; El Soury, M.; González-Quevedo, D.; Sánchez-Porras, D.; Chato-Astrain, J.; Campos, F.; Carriel, V. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. Int. J. Mol. Sci. 2021, 22, 674. https://doi.org/10.3390/ijms22020674
García-García ÓD, El Soury M, González-Quevedo D, Sánchez-Porras D, Chato-Astrain J, Campos F, Carriel V. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. International Journal of Molecular Sciences. 2021; 22(2):674. https://doi.org/10.3390/ijms22020674
Chicago/Turabian StyleGarcía-García, Óscar Darío, Marwa El Soury, David González-Quevedo, David Sánchez-Porras, Jesús Chato-Astrain, Fernando Campos, and Víctor Carriel. 2021. "Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves" International Journal of Molecular Sciences 22, no. 2: 674. https://doi.org/10.3390/ijms22020674
APA StyleGarcía-García, Ó. D., El Soury, M., González-Quevedo, D., Sánchez-Porras, D., Chato-Astrain, J., Campos, F., & Carriel, V. (2021). Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. International Journal of Molecular Sciences, 22(2), 674. https://doi.org/10.3390/ijms22020674







