T6SS Mediated Stress Responses for Bacterial Environmental Survival and Host Adaptation
Abstract
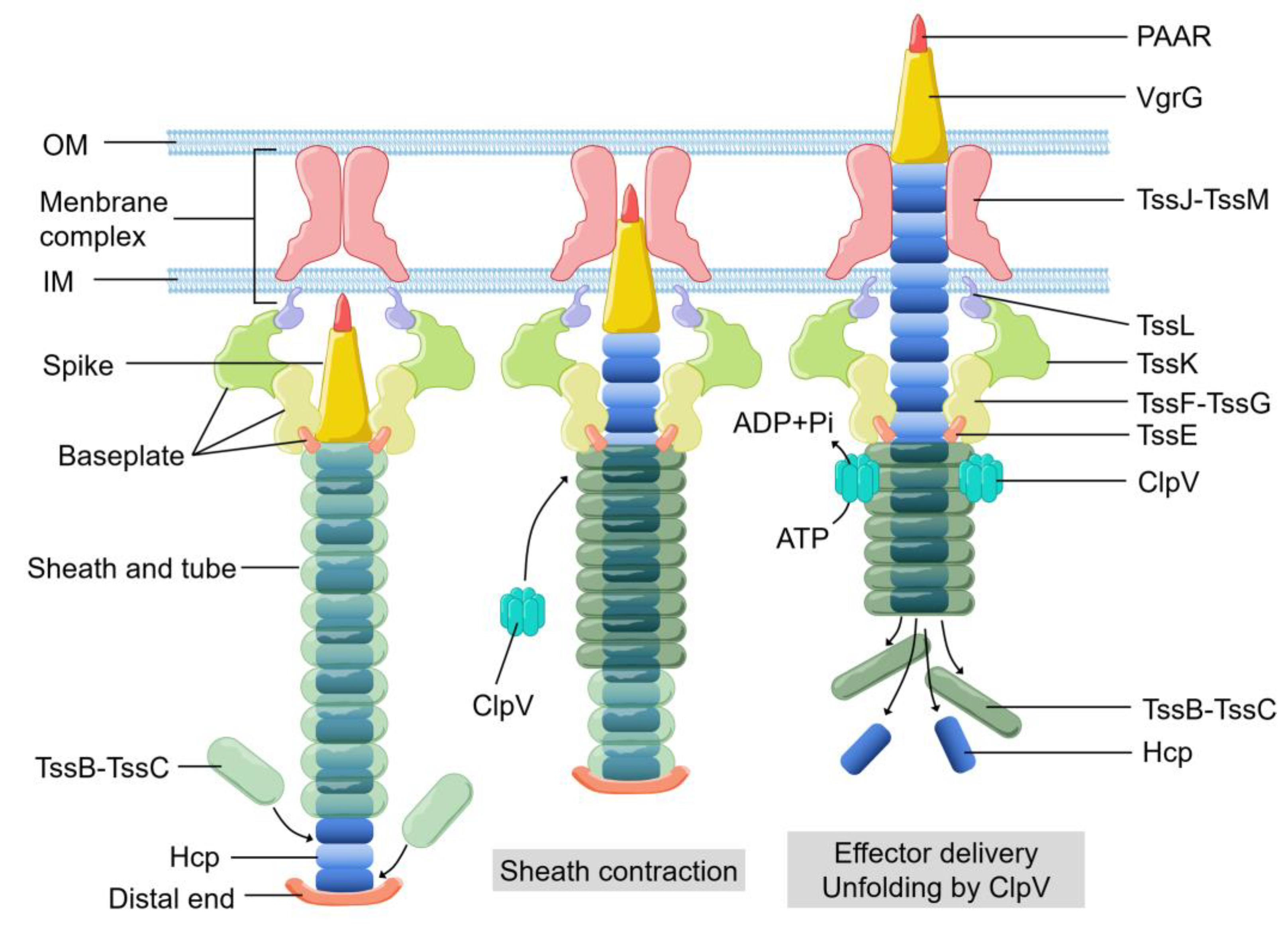
1. Introduction
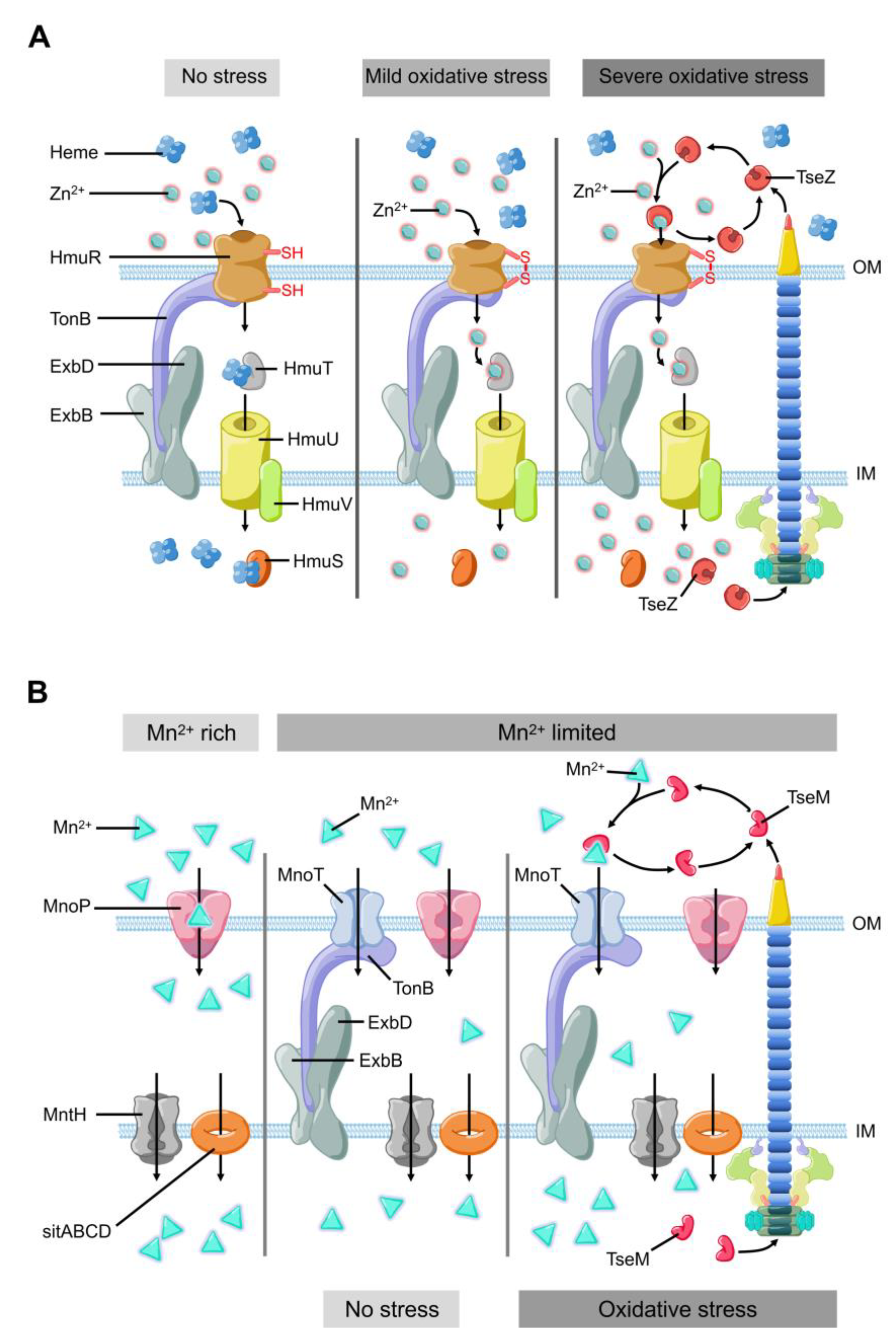
2. Metal Ion Uptake for ROS Stress Response
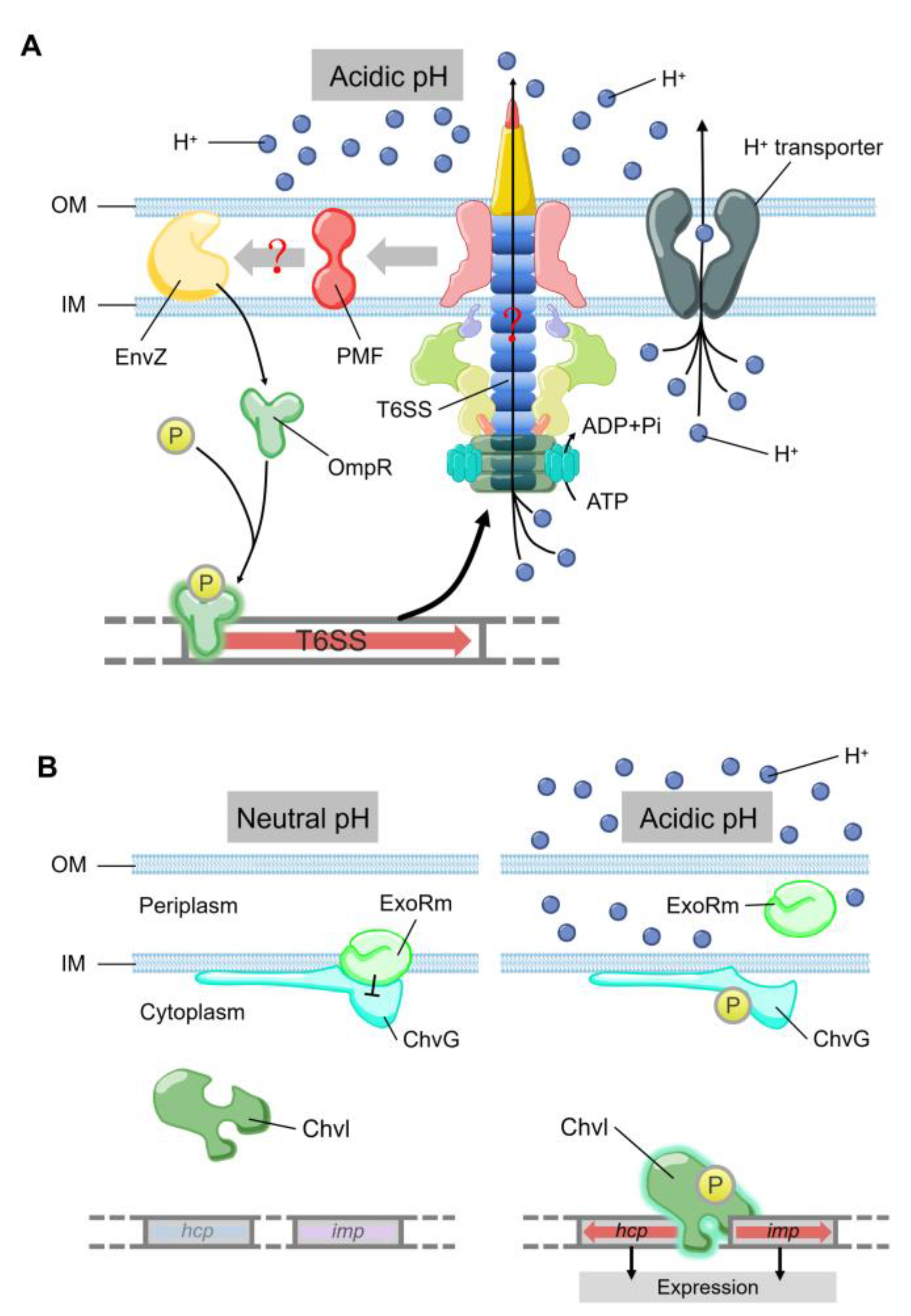
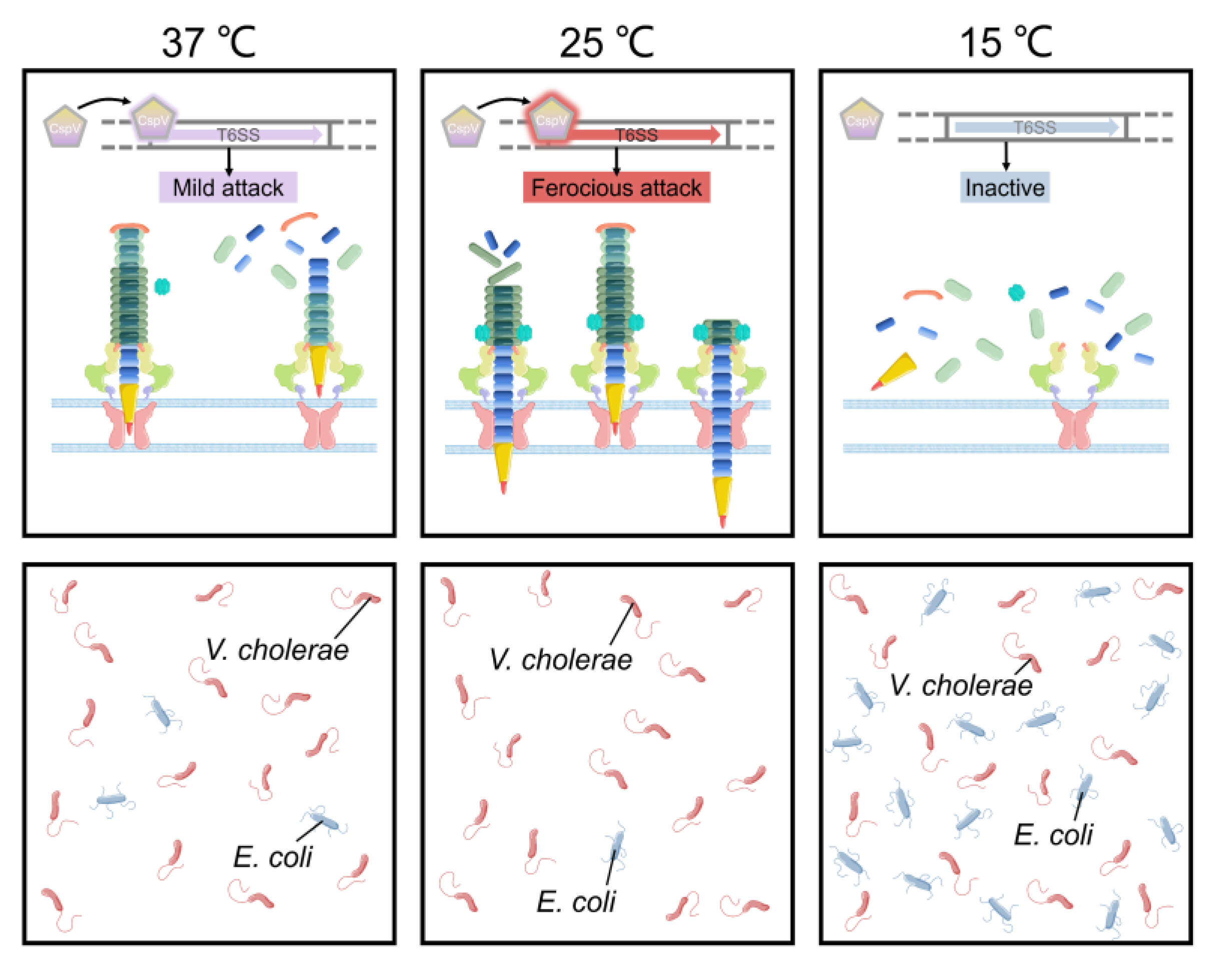
3. Adaptation to Changes in Temperature and pH
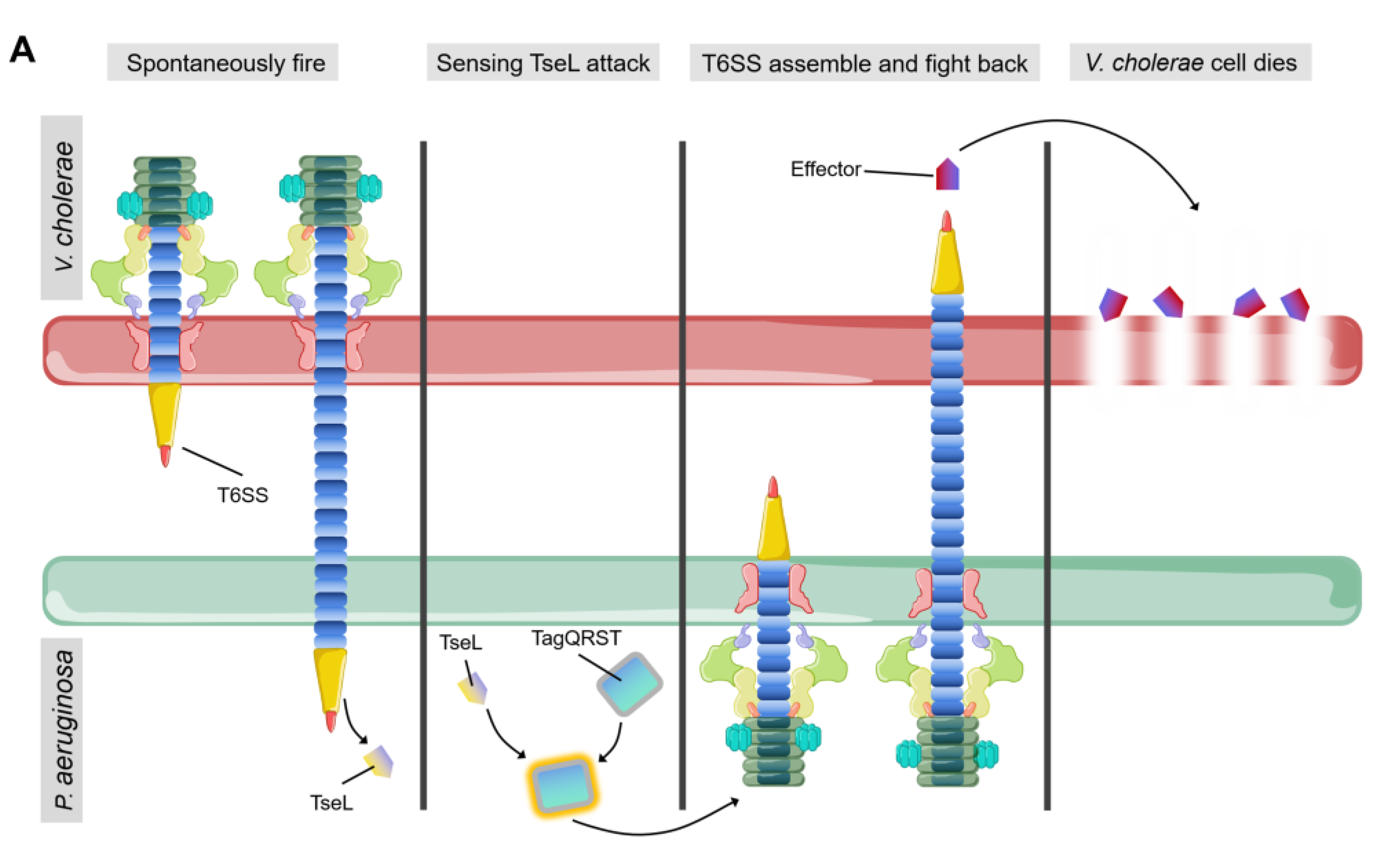
4. Interspecies and Intraspecies Competition
5. Involvement of T6SS in the Regulation of Host Immune Signaling Pathways
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Economou, A.; Christie, P.J.; Fernandez, R.C.; Palmer, T.; Plano, G.V.; Pugsley, A.P. Secretion by numbers: Protein traffic in prokaryotes. Mol. Microbiol. 2006, 62, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, M.; Feldman, M.F.; García Véscovi, E. A Transcriptional Regulatory Mechanism Finely Tunes the Firing of Type VI Secretion System in Response to Bacterial Enemies. mBio 2017, 8, e00559-17. [Google Scholar] [CrossRef]
- Gorasia, D.G.; Hanssen, E.; Veith, P.D.; Reynolds, E.C. Structural Characterization of the Type IX Secretion System in Porphyromonas gingivalis. Methods Mol. Biol. 2021, 2210, 113–121. [Google Scholar] [PubMed]
- Leiman, P.G.; Basler, M.; Ramagopal, U.A.; Bonanno, J.B.; Sauder, J.M.; Pukatzki, S.; Burley, S.K.; Almo, S.C.; Mekalanos, J.J. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. USA 2009, 106, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Bönemann, G.; Pietrosiuk, A.; Mogk, A. Tubules and donuts: A type VI secretion story. Mol. Microbiol. 2010, 76, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Cascales, E. Microbiology: And Amoebophilus Invented the Machine Gun. Curr. Biol. 2017, 27, R1170–R1173. [Google Scholar] [CrossRef]
- Cherrak, Y.; Flaugnatti, N.; Durand, E.; Journet, L.; Cascales, E. Structure and Activity of the Type VI Secretion System. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Bönemann, G.; Pietrosiuk, A.; Diemand, A.; Zentgraf, H.; Mogk, A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. Embo J. 2009, 28, 315–325. [Google Scholar] [CrossRef]
- Pietrosiuk, A.; Lenherr, E.D.; Falk, S.; Bönemann, G.; Kopp, J.; Zentgraf, H.; Sinning, I.; Mogk, A. Molecular basis for the unique role of the AAA+ chaperone ClpV in type VI protein secretion. J. Biol. Chem. 2011, 286, 30010–30021. [Google Scholar] [CrossRef]
- Basler, M.; Mekalanos, J.J. Type 6 secretion dynamics within and between bacterial cells. Science 2012, 337, 815. [Google Scholar] [CrossRef]
- Renault, M.G.; Zamarreno Beas, J.; Douzi, B.; Chabalier, M.; Zoued, A.; Brunet, Y.R.; Cambillau, C.; Journet, L.; Cascales, E. The gp27-like Hub of VgrG Serves as Adaptor to Promote Hcp Tube Assembly. J. Mol. Biol. 2018, 430, 3143–3156. [Google Scholar] [CrossRef]
- Bladergroen, M.R.; Badelt, K.; Spaink, H.P. Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. Mol. Plant Microbe Interact. 2003, 16, 53–64. [Google Scholar] [CrossRef]
- Filloux, A.; Hachani, A.; Bleves, S. The bacterial type VI secretion machine: Yet another player for protein transport across membranes. Microbiology 2008, 154, 1570–1583. [Google Scholar] [CrossRef]
- Boyer, F.; Fichant, G.; Berthod, J.; Vandenbrouck, Y.; Attree, I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: What can be learned from available microbial genomic resources? BMC Genom. 2009, 10, 104. [Google Scholar] [CrossRef]
- Journet, L.; Cascales, E. The type VI secretion system in Escherichia coli and related species. EcoSal Plus 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Schell, M.A.; Ulrich, R.L.; Ribot, W.J.; Brueggemann, E.E.; Hines, H.B.; Chen, D.; Lipscomb, L.; Kim, H.S.; Mrázek, J.; Nierman, W.C.; et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 2007, 64, 1466–1485. [Google Scholar] [CrossRef]
- Nano, F.E.; Schmerk, C. The Francisella pathogenicity island. Ann. N. Y. Acad. Sci. 2007, 1105, 122–137. [Google Scholar] [CrossRef]
- Fu, Y.; Waldor, M.K.; Mekalanos, J.J. Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe 2013, 14, 652–663. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Xu, H.H.; Hao, L.; Deng, Z.; Rajakumar, K.; Ou, H.Y. SecReT6: A web-based resource for type VI secretion systems found in bacteria. Environ. Microbiol. 2015, 17, 2196–2202. [Google Scholar] [CrossRef]
- Abby, S.S.; Cury, J.; Guglielmini, J.; Néron, B.; Touchon, M.; Rocha, E.P. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 2016, 6, 23080. [Google Scholar] [CrossRef]
- Pukatzki, S.; Ma, A.T.; Revel, A.T.; Sturtevant, D.; Mekalanos, J.J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. USA 2007, 104, 15508–15513. [Google Scholar] [CrossRef]
- English, G.; Trunk, K.; Rao, V.A.; Srikannathasan, V.; Hunter, W.N.; Coulthurst, S.J. New secreted toxins and immunity proteins encoded within the Type VI secretion system gene cluster of Serratia marcescens. Mol. Microbiol. 2012, 86, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, S.L.; Trunk, K.; English, G.; Fritsch, M.J.; Pourkarimi, E.; Coulthurst, S.J. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J. Bacteriol. 2011, 193, 6057–6069. [Google Scholar] [CrossRef]
- Fu, Y.; Ho, B.T.; Mekalanos, J.J. Tracking Vibrio cholerae Cell-Cell Interactions during Infection Reveals Bacterial Population Dynamics within Intestinal Microenvironments. Cell Host Microbe 2018, 23, 274–281.e2. [Google Scholar] [CrossRef]
- Basler, M.; Ho, B.T.; Mekalanos, J.J. Tit-for-tat: Type VI secretion system counterattack during bacterial cell-cell interactions. Cell 2013, 152, 884–894. [Google Scholar] [CrossRef]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530. [Google Scholar] [CrossRef]
- Kashyap, D.R.; Rompca, A.; Gaballa, A.; Helmann, J.D.; Chan, J.; Chang, C.J.; Hozo, I.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathog. 2014, 10, e1004280. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Collins, J.J.; Walker, G.C. Unraveling the physiological complexities of antibiotic lethality. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.G.; Dong, S.; Catalano, C.; Moore, R.; Liang, X.; Mekalanos, J.J. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc. Natl. Acad. Sci. USA 2015, 112, 2181–2186. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.M.; Schellhorn, H.E. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 2012, 525, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Kim, D.; Szubin, R.; Palsson, B.O. Genome-wide Reconstruction of OxyR and SoxRS Transcriptional Regulatory Networks under Oxidative Stress in Escherichia coli K-12 MG1655. Cell Rep. 2015, 12, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, K.; Gao, F.; Kang, Y.; Chaudhry, M.T.; Wang, Z.; Wang, Y.; Shen, X. ZntR positively regulates T6SS4 expression in Yersinia pseudotuberculosis. J. Microbiol. 2017, 55, 448–456. [Google Scholar] [CrossRef]
- Si, M.; Zhang, L.; Chaudhry, M.T.; Ding, W.; Xu, Y.; Chen, C.; Akbar, A.; Shen, X.; Liu, S.J. Corynebacterium glutamicum methionine sulfoxide reductase A uses both mycoredoxin and thioredoxin for regeneration and oxidative stress resistance. Appl. Environ. Microbiol. 2015, 81, 2781–2796. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.P.; Fleury, M. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef]
- Weber, B.; Hasic, M.; Chen, C.; Wai, S.N.; Milton, D.L. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ. Microbiol. 2009, 11, 3018–3028. [Google Scholar] [CrossRef]
- Si, M.; Zhao, C.; Burkinshaw, B.; Zhang, B.; Wei, D.; Wang, Y.; Dong, T.G.; Shen, X. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. USA 2017, 114, E2233–E2242. [Google Scholar] [CrossRef]
- Wan, B.; Zhang, Q.; Ni, J.; Li, S.; Wen, D.; Li, J.; Xiao, H.; He, P.; Ou, H.Y.; Tao, J.; et al. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017, 13, e1006246. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Si, M.; Song, Y.; Zhu, W.; Gao, F.; Wang, Y.; Zhang, L.; Zhang, W.; Wei, G.; Luo, Z.Q.; et al. Type VI Secretion System Transports Zn2+ to Combat Multiple Stresses and Host Immunity. PLoS Pathog. 2015, 11, e1005020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, T.; Cui, R.; Zhang, Z.; Chen, K.; Li, M.; Hua, Y.; Gu, H.; Xu, L.; Wang, Y.; et al. HpaR, the Repressor of Aromatic Compound Metabolism, Positively Regulates the Expression of T6SS4 to Resist Oxidative Stress in Yersinia pseudotuberculosis. Front. Microbiol. 2020, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.D.; Culotta, V.C. Battles with iron: Manganese in oxidative stress protection. J. Biol. Chem. 2012, 287, 13541–13548. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef]
- Lisher, J.P.; Giedroc, D.P. Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell. Infect. Microbiol. 2013, 3, 91. [Google Scholar] [CrossRef]
- Vanaporn, M.; Wand, M.; Michell, S.L.; Sarkar-Tyson, M.; Ireland, P.; Goldman, S.; Kewcharoenwong, C.; Rinchai, D.; Lertmemongkolchai, G.; Titball, R.W. Superoxide dismutase C is required for intracellular survival and virulence of Burkholderia pseudomallei. Microbiology 2011, 157, 2392–2400. [Google Scholar] [CrossRef]
- Si, M.; Wang, Y.; Zhang, B.; Zhao, C.; Kang, Y.; Bai, H.; Wei, D.; Zhu, L.; Zhang, L.; Dong, T.G.; et al. The Type VI Secretion System Engages a Redox-Regulated Dual-Functional Heme Transporter for Zinc Acquisition. Cell Rep. 2017, 20, 949–959. [Google Scholar] [CrossRef]
- DeShazer, D. A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex. Microbiol. Res. 2019, 226, 48–54. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B.; Mealy, T.R.; Seaton, B.A.; Head, J.F. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 A resolution. Nat. Struct. Biol. 2001, 8, 710–714. [Google Scholar] [CrossRef]
- Grove, A. MarR family transcription factors. Curr. Biol. 2013, 23, R142–R143. [Google Scholar] [CrossRef] [PubMed]
- Barnese, K.; Gralla, E.B.; Valentine, J.S.; Cabelli, D.E. Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc. Natl. Acad. Sci. USA 2012, 109, 6892–6897. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Hohle, T.H.; O’Brian, M.R. Control of bacterial iron homeostasis by manganese. Proc. Natl. Acad. Sci. USA 2010, 107, 10691–10695. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Diaz-Ochoa, V.E.; Lam, D.; Lee, C.S.; Klaus, S.; Behnsen, J.; Liu, J.Z.; Chim, N.; Nuccio, S.P.; Rathi, S.G.; Mastroianni, J.R.; et al. Salmonella Mitigates Oxidative Stress and Thrives in the Inflamed Gut by Evading Calprotectin-Mediated Manganese Sequestration. Cell Host Microbe 2016, 19, 814–825. [Google Scholar] [CrossRef]
- Sabri, M.; Caza, M.; Proulx, J.; Lymberopoulos, M.H.; Brée, A.; Moulin-Schouleur, M.; Curtiss, R., 3rd; Dozois, C.M. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain chi7122. Infect. Immun. 2008, 76, 601–611. [Google Scholar] [CrossRef]
- Hohle, T.H.; Franck, W.L.; Stacey, G.; O’Brian, M.R. Bacterial outer membrane channel for divalent metal ion acquisition. Proc. Natl. Acad. Sci. USA 2011, 108, 15390–15395. [Google Scholar] [CrossRef]
- Champion, O.L.; Karlyshev, A.; Cooper, I.; Ford, D.C.; Wren, B.W.; Duffield, M.; Oyston, P.; Titball, R.W. Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 2011, 157, 1115–1122. [Google Scholar] [CrossRef]
- Kehres, D.G.; Zaharik, M.L.; Finlay, B.B.; Maguire, M.E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 2000, 36, 1085–1100. [Google Scholar] [CrossRef]
- Storz, G.; Altuvia, S. (1994). OxyR regulon. Methods Enzymol. 1994, 234, 217–223. [Google Scholar]
- Altuvia, S.; Weinstein-Fischer, D.; Zhang, A.; Postow, L.; Storz, G. A small, stable RNA induced by oxidative stress: Role as a pleiotropic regulator and antimutator. Cell 1997, 90, 43–53. [Google Scholar] [CrossRef]
- Michán, C.; Manchado, M.; Dorado, G.; Pueyo, C. In vivo transcription of the Escherichia coli oxyR regulon as a function of growth phase and in response to oxidative stress. J. Bacteriol. 1999, 181, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, X.; Doan, B.; Lewis, K.A.; Schneider, T.D.; Storz, G. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 2001, 183, 4571–4579. [Google Scholar] [CrossRef] [PubMed]
- Khademian, M.; Imlay, J.A. Escherichia coli cytochrome c peroxidase is a respiratory oxidase that enables the use of hydrogen peroxide as a terminal electron acceptor. Proc. Natl. Acad. Sci. USA 2017, 114, E6922–E6931. [Google Scholar] [CrossRef] [PubMed]
- Gerken, H.; Charlson, E.S.; Cicirelli, E.M.; Kenney, L.J.; Misra, R. MzrA: A novel modulator of the EnvZ/OmpR two-component regulon. Mol. Microbiol. 2009, 72, 1408–1422. [Google Scholar] [CrossRef]
- Flamez, C.; Ricard, I.; Arafah, S.; Simonet, M.; Marceau, M. Phenotypic analysis of Yersinia pseudotuberculosis 32777 response regulator mutants: New insights into two-component system regulon plasticity in bacteria. Int. J. Med. Microbiol. 2008, 298, 193–207. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Song, Y.; Wang, T.; Xu, S.; Peng, Z.; Lin, X.; Zhang, L.; Shen, X. A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ. Microbiol. 2013, 15, 557–569. [Google Scholar] [CrossRef]
- Wu, C.F.; Lin, J.S.; Shaw, G.C.; Lai, E.M. Acid-induced type VI secretion system is regulated by ExoR-ChvG/ChvI signaling cascade in Agrobacterium tumefaciens. PLoS Pathog. 2012, 8, e1002938. [Google Scholar] [CrossRef]
- Townsley, L.; Sison Mangus, M.P.; Mehic, S.; Yildiz, F.H. Response of Vibrio cholerae to Low-Temperature Shifts: CspV Regulation of Type VI Secretion, Biofilm Formation, and Association with Zooplankton. Appl. Environ. Microbiol. 2016, 82, 4441–4452. [Google Scholar] [CrossRef]
- Basler, M.; Pilhofer, M.; Henderson, G.P.; Jensen, G.J.; Mekalanos, J.J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 2012, 483, 182–186. [Google Scholar] [CrossRef]
- Slamti, L.; Livny, J.; Waldor, M.K. Global gene expression and phenotypic analysis of a Vibrio cholerae rpoH deletion mutant. J. Bacteriol. 2007, 189, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.G.; Kortmann, J.; Narberhaus, F.; Klose, K.E. RNA thermometer controls temperature-dependent virulence factor expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2014, 111, 14241–14246. [Google Scholar] [CrossRef] [PubMed]
- Lories, B.; Roberfroid, S.; Dieltjens, L.; De Coster, D.; Foster, K.R.; Steenackers, H.P. Biofilm Bacteria Use Stress Responses to Detect and Respond to Competitors. Curr. Biol. 2020, 30, 1231–1244.e4. [Google Scholar] [CrossRef] [PubMed]
- Kamal, F.; Liang, X.; Manera, K.; Pei, T.T.; Kim, H.; Lam, L.G.; Pun, A.; Hersch, S.J.; Dong, T.G. Differential Cellular Response to Translocated Toxic Effectors and Physical Penetration by the Type VI Secretion System. Cell Rep. 2020, 31, 107766. [Google Scholar] [CrossRef] [PubMed]
- Casabona, M.G.; Silverman, J.M.; Sall, K.M.; Boyer, F.; Couté, Y.; Poirel, J.; Grunwald, D.; Mougous, J.D.; Elsen, S.; Attree, I. An ABC transporter and an outer membrane lipoprotein participate in posttranslational activation of type VI secretion in Pseudomonas aeruginosa. Environ. Microbiol. 2013, 15, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.; Schwarz, S.; Mougous, J.D. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol. Microbiol. 2009, 72, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Laubacher, M.E.; Ades, S.E. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 2008, 190, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.G.; Raivio, T.L. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 2002, 45, 1599–1611. [Google Scholar] [CrossRef]
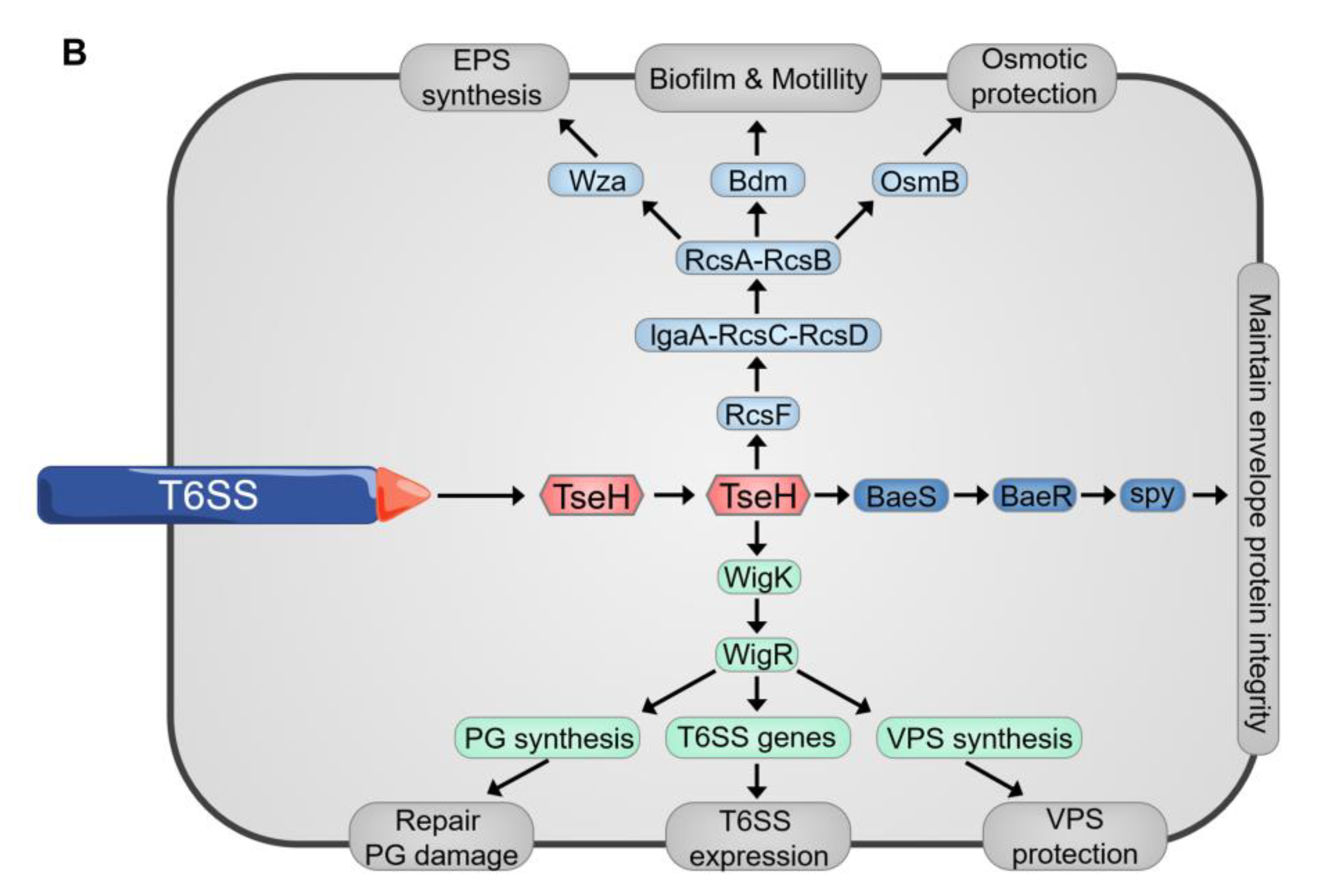
- Hersch, S.J.; Watanabe, N.; Stietz, M.S.; Manera, K.; Kamal, F.; Burkinshaw, B.; Lam, L.; Pun, A.; Li, M.; Savchenko, A.; et al. Envelope stress responses defend against type six secretion system attacks independently of immunity proteins. Nat. Microbiol. 2020, 5, 706–714. [Google Scholar] [CrossRef]
- Stout, V.; Torres-Cabassa, A.; Maurizi, M.R.; Gutnick, D.; Gottesman, S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 1991, 173, 1738–1747. [Google Scholar] [CrossRef]
- Weaver, A.I.; Murphy, S.G.; Umans, B.D.; Tallavajhala, S.; Onyekwere, I.; Wittels, S.; Shin, J.H.; VanNieuwenhze, M.; Waldor, M.K.; Dörr, T.; et al. Genetic Determinants of Penicillin Tolerance in Vibrio cholerae. Antimicrob. Agents Chemother. 2018, 62, e01326-18. [Google Scholar] [CrossRef] [PubMed]
- Dörr, T.; Alvarez, L.; Delgado, F.; Davis, B.M.; Cava, F.; Waldor, M.K. A cell wall damage response mediated by a sensor kinase/response regulator pair enables beta-lactam tolerance. Proc. Natl. Acad. Sci. USA 2016, 113, 404–409. [Google Scholar] [CrossRef]
- Teschler, J.K.; Cheng, A.T.; Yildiz, F.H. The Two-Component Signal Transduction System VxrAB Positively Regulates Vibrio cholerae Biofilm Formation. J. Bacteriol. 2017, 199, e00139-17. [Google Scholar] [CrossRef]
- Toska, J.; Ho, B.T.; Mekalanos, J.J. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl. Acad. Sci. USA 2018, 115, 7997–8002. [Google Scholar] [CrossRef]
- Hermans, K.; Nguyen, T.L.; Roberfroid, S.; Schoofs, G.; Verhoeven, T.; De Coster, D.; Vanderleyden, J.; De Keersmaecker, S.C. Gene expression analysis of monospecies Salmonella typhimurium biofilms using differential fluorescence induction. J. Microbiol. Methods 2011, 84, 467–478. [Google Scholar] [CrossRef] [PubMed]
- García, B.; Latasa, C.; Solano, C.; García-del Portillo, F.; Gamazo, C.; Lasa, I. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 2004, 54, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Horiyama, T.; Yamaguchi, A.; Nishino, K. TolC dependency of multidrug efflux systems in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2010, 65, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Rosner, J.L.; Martin, R.G. Transcriptional activation by MarA, SoxS and Rob of two tolC promoters using one binding site: A complex promoter configuration for tolC in Escherichia coli. Mol. Microbiol. 2008, 69, 1450–1455. [Google Scholar] [CrossRef]
- Thijs, I.M.; De Keersmaecker, S.C.; Fadda, A.; Engelen, K.; Zhao, H.; McClelland, M.; Marchal, K.; Vanderleyden, J. Delineation of the Salmonella enterica serovar Typhimurium HilA regulon through genome-wide location and transcript analysis. J. Bacteriol. 2007, 189, 4587–4596. [Google Scholar] [CrossRef]
- Unterweger, D.; Miyata, S.T.; Bachmann, V.; Brooks, T.M.; Mullins, T.; Kostiuk, B.; Provenzano, D.; Pukatzki, S. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat. Commun. 2014, 5, 3549. [Google Scholar] [CrossRef]
- Kostiuk, B.; Unterweger, D.; Provenzano, D.; Pukatzki, S. T6SS intraspecific competition orchestrates Vibrio cholerae genotypic diversity. Int. Microbiol. 2017, 20, 130–137. [Google Scholar] [PubMed]
- Chen, K.W.; Schroder, K. Antimicrobial functions of inflammasomes. Curr. Opin. Microbiol. 2013, 16, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Chen, H.; Yang, D.; Han, F.; Tan, J.; Zhang, L.; Xiao, J.; Zhang, Y.; Liu, Q. The Bacterial T6SS Effector EvpP Prevents NLRP3 Inflammasome Activation by Inhibiting the Ca2+-Dependent MAPK-Jnk Pathway. Cell Host Microbe 2017, 21, 47–58. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Shi, Y.Q.; Zhang, W.Q.; Wen, Z.L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012, 287, 25353–25360. [Google Scholar] [CrossRef]
- Tan, J.; Yang, D.; Wang, Z.; Zheng, X.; Zhang, Y.; Liu, Q. EvpP inhibits neutrophils recruitment via Jnk-caspy inflammasome signaling in vivo. Fish Shellfish Immunol. 2019, 92, 851–860. [Google Scholar] [CrossRef]
- Walz, A.; Peveri, P.; Aschauer, H.; Baggiolini, M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem. Biophys. Res. Commun. 1987, 149, 755–761. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.T.; Zhu, K.Y.; Jin, Y.; Deng, M.; Le, H.Y.; Fu, Y.F.; Chen, Y.; Zhu, J.; Look, A.T.; et al. In vivo interstitial migration of primitive macrophages mediated by JNK-matrix metalloproteinase 13 signaling in response to acute injury. J. Immunol. 2008, 181, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Boudinot, P.; Calado, Â.; Mulero, V. Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J. Immunol. 2015, 194, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordoñez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, S.; Ling, M.; Lv, Z.; Lin, S. Salmonella enteritidis Hcp distribute in the cytoplasm and regulate TNF signaling pathway in BHK-21 cells. 3 Biotech 2020, 10, 301. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.-W.; Xue, P.; Fu, Y.; Yang, L. T6SS Mediated Stress Responses for Bacterial Environmental Survival and Host Adaptation. Int. J. Mol. Sci. 2021, 22, 478. https://doi.org/10.3390/ijms22020478
Yu K-W, Xue P, Fu Y, Yang L. T6SS Mediated Stress Responses for Bacterial Environmental Survival and Host Adaptation. International Journal of Molecular Sciences. 2021; 22(2):478. https://doi.org/10.3390/ijms22020478
Chicago/Turabian StyleYu, Kai-Wei, Peng Xue, Yang Fu, and Liang Yang. 2021. "T6SS Mediated Stress Responses for Bacterial Environmental Survival and Host Adaptation" International Journal of Molecular Sciences 22, no. 2: 478. https://doi.org/10.3390/ijms22020478
APA StyleYu, K.-W., Xue, P., Fu, Y., & Yang, L. (2021). T6SS Mediated Stress Responses for Bacterial Environmental Survival and Host Adaptation. International Journal of Molecular Sciences, 22(2), 478. https://doi.org/10.3390/ijms22020478





