Epoxyeicosatrienoic Acids and Fibrosis: Recent Insights for the Novel Therapeutic Strategies
Abstract
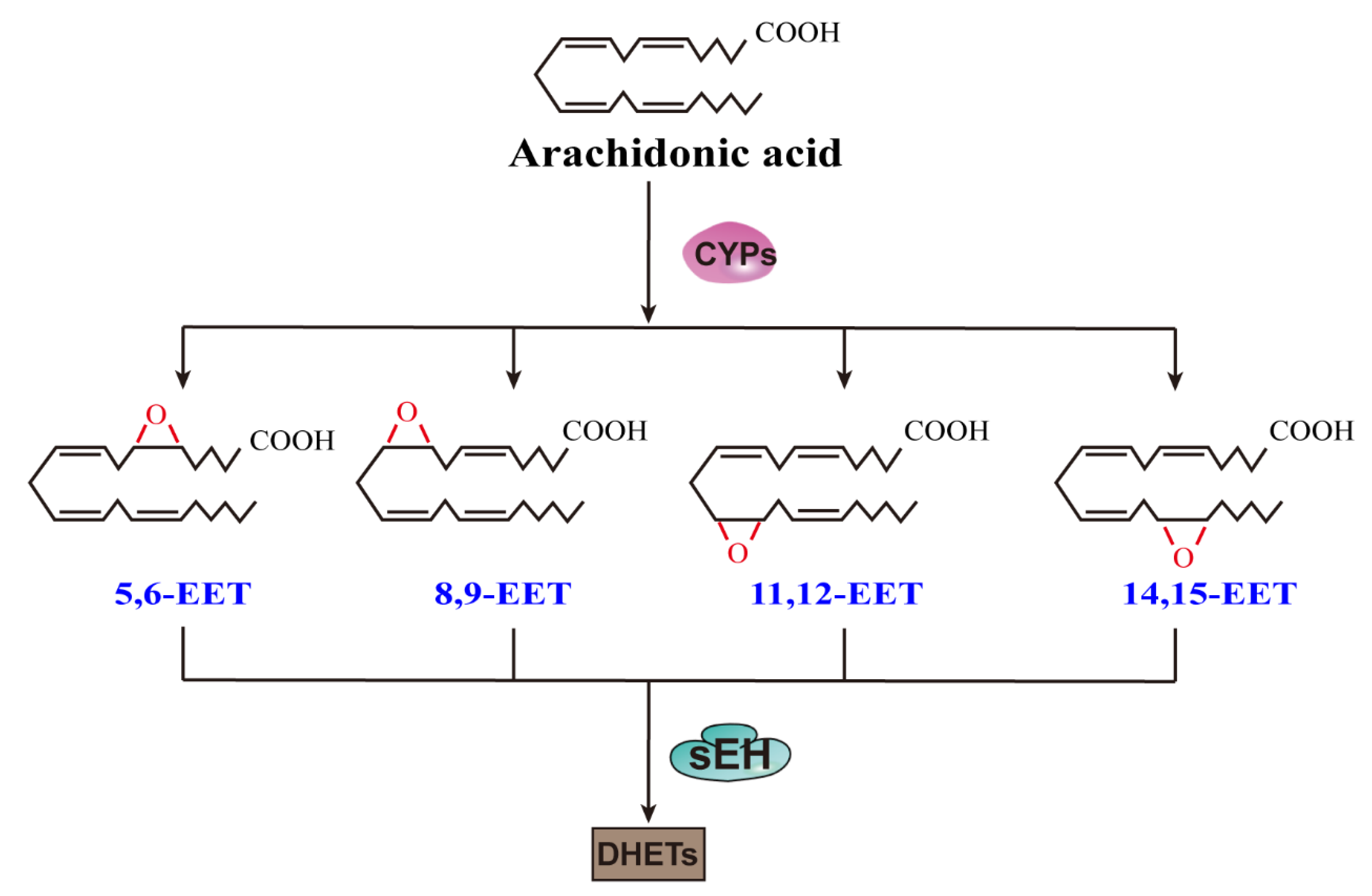
1. Introduction
2. EETs and Fibrosis
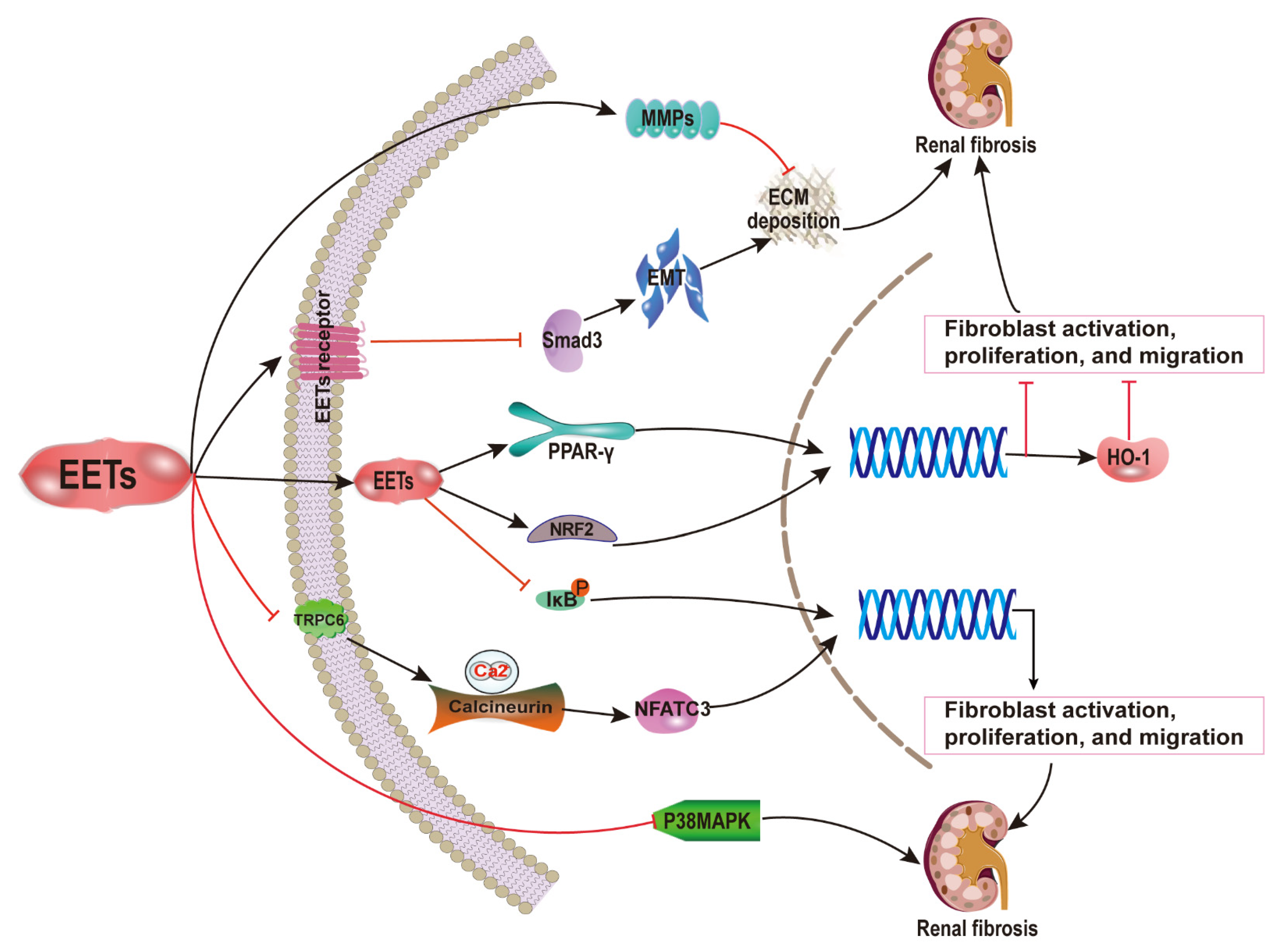
2.1. EETs and Renal Fibrosis
2.2. EETs and Cardiac Fibrosis
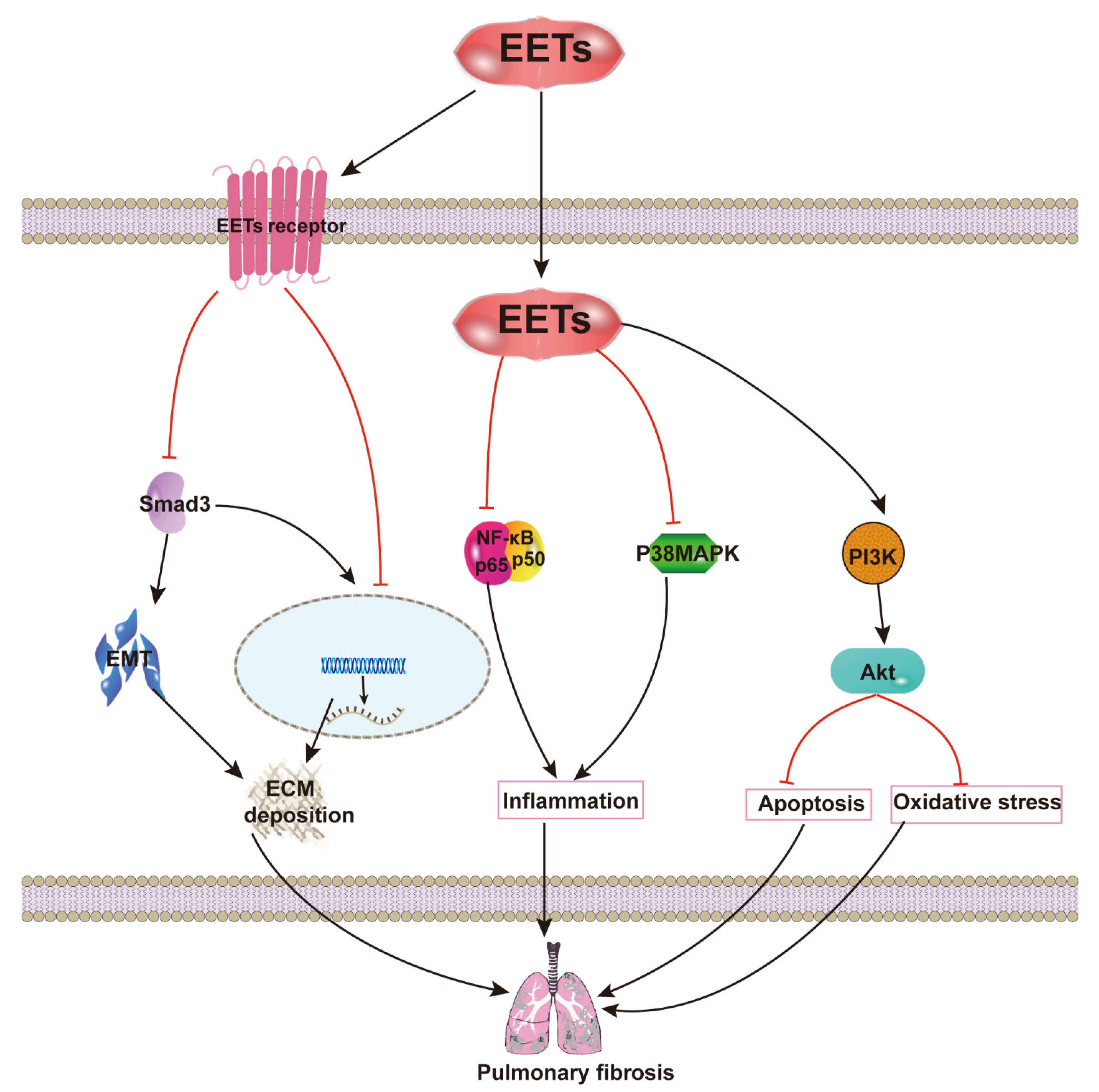
2.3. EETs and Pulmonary Fibrosis
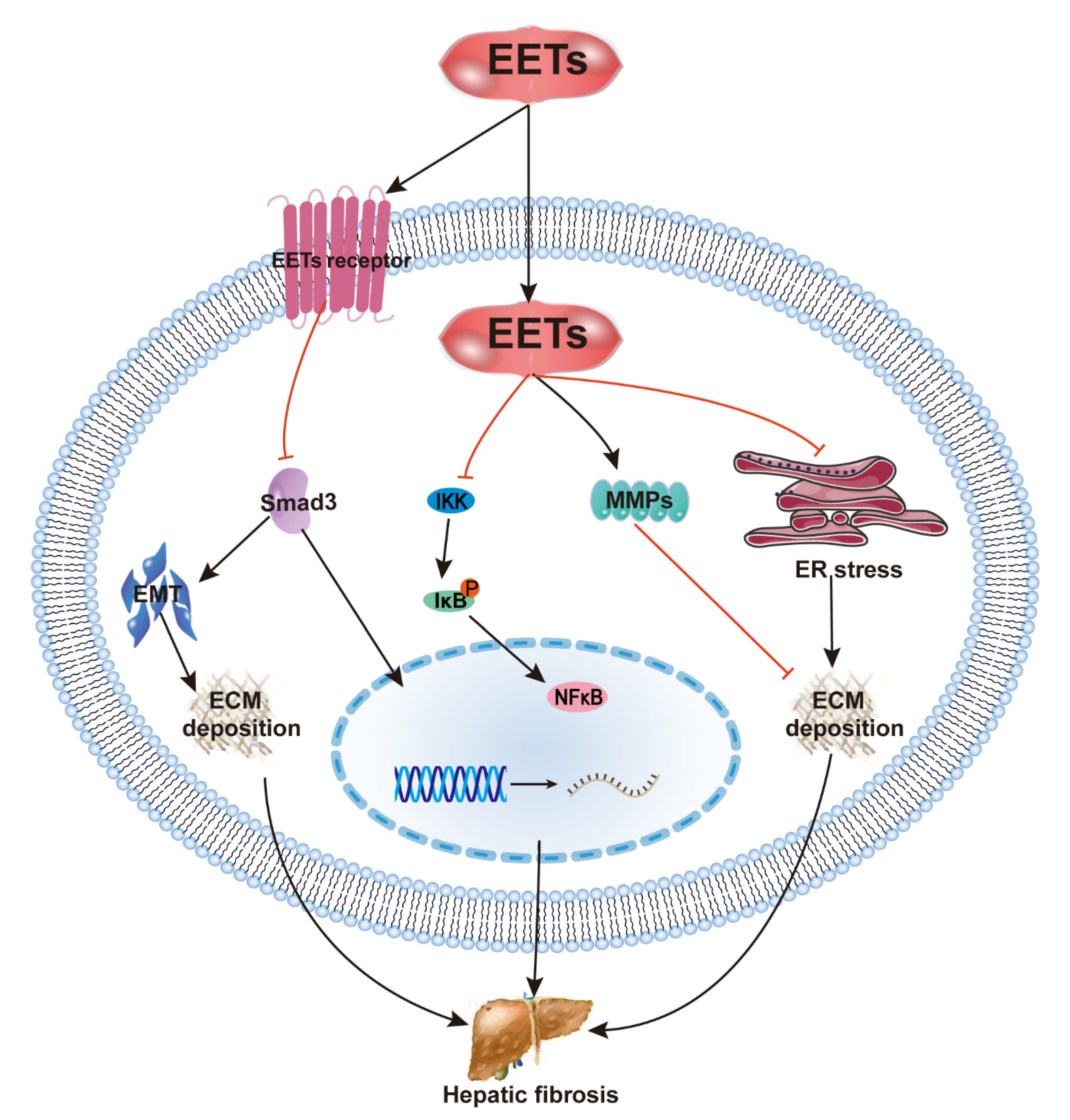
2.4. EETs and Hepatic Fibrosis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| AECs | alveolar epithelial cells |
| Ang II | angiotensin II |
| ARA | arachidonic acid |
| CaN | calcineurin |
| COX | cyclooxygenase |
| CYP | cytochrome P450 |
| DHETs | dihydroxyeicosatrienoic acid |
| DN | diabetic nephropathy |
| ECM | extracellular matrix |
| EETs | epoxyeicosatrienoic acids |
| EMT | epithelial–mesenchymal transition |
| ERS | endoplasmic reticulum stress |
| IPF | idiopathic pulmonary fibrosis |
| MAPK | mitogen-activated protein kinases |
| MMPs | matrix metalloproteinases |
| NF-κB | nuclear factor kappa B; |
| 20-HETE | 20-hydroxyeicosatetraenoic acid |
| HO-1 | heme oxygenase-1 |
| PTUPB | 4-(5-phenyl-3-{3-[3-(4-trifluoromethylphenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide |
| ROS | reactive oxygen species |
| sEH | soluble epoxide hydrolase |
| sEHI | sEH inhibitor |
| TGF-β | transforming growth factor beta |
| UUO | unilateral ureter obstruction |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
References
- Zhang, Y.-F.; Sun, C.-C.; Duan, J.-X.; Yang, H.-H.; Zhang, C.-Y.; Xiong, J.-B.; Zhong, W.-J.; Zu, C.; Guan, X.-X.; Jiang, H.-L.; et al. A COX-2/sEH dual inhibitor PTUPB ameliorates cecal ligation and puncture-induced sepsis in mice via anti-inflammation and anti-oxidative stress. Biomed. Pharmacother. 2020, 126, 109907. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Lee, D.-H.; Kim, Y. Biosynthesis and immunity of epoxyeicosatrienoic acids in a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2020, 107, 103643. [Google Scholar] [CrossRef]
- Shahabi, P.; Siest, G.; Meyer, U.A.; Visvikis-Siest, S. Human cytochrome P450 epoxygenases: Variability in expression and role in inflammation-related disorders. Pharmacol. Ther. 2014, 144, 134–161. [Google Scholar] [CrossRef] [PubMed]
- Biliktu, M.; Senol, S.P.; Temiz-Resitoglu, M.; Guden, D.S.; Horat, M.F.; Sahan-Firat, S.; Sevim, S.; Tunctan, B. Pharmacological inhibition of soluble epoxide hydrolase attenuates chronic experimental autoimmune encephalomyelitis by modulating inflammatory and anti-inflammatory pathways in an inflammasome-dependent and -independent manner. Inflammopharmacology 2020, 28, 1509–1524. [Google Scholar] [CrossRef]
- Fu, M.; Yu, J.; Chen, Z.; Tang, Y.; Dong, R.; Yang, Y.; Luo, J.; Hu, S.; Tu, L.; Xu, X. Epoxyeicosatrienoic acids improve glucose homeostasis by preventing NF-κB-mediated transcription of SGLT2 in renal tubular epithelial cells. Mol. Cell. Endocrinol. 2021, 523, 111149. [Google Scholar] [CrossRef] [PubMed]
- You, W.-T.; Zhou, T.; Ma, Z.-C.; Liang, Q.-D.; Xiao, C.-R.; Tang, X.-L.; Tan, H.-L.; Zhang, B.-L.; Wang, Y.-G.; Gao, Y. Author Correction: Ophiopogonin D maintains Ca2+ homeostasis in rat cardiomyocytes in vitro by upregulating CYP2J3/ EETs and suppressing ER stress. Acta Pharmacol. Sin. 2020, 41, 1622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, J.; Li, Q.; Zhan, C.; Xu, X.; Zhang, X.; Ai, D.; Zhu, Y.; Wen, Z.; Wang, D.W. CYP2J2-derived EETs attenuated ethanol-induced myocardial dysfunction through inducing autophagy and reducing apoptosis. Free. Radic. Biol. Med. 2018, 117, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, O.; Husková, Z.; Baranowska, I.; Walkowska, A.; Sadowski, J.; Kikerlová, S.; Vaňourková, Z.; Honetschlägerová, Z.; Škaroupková, P.; Červenka, L.; et al. Combined treatment with epoxyeicosatrienoic acid analog and 20-hydroxyeicosatetraenoic acid antagonist provides substantial hypotensive effect in spontaneously hypertensive rats. J. Hypertens. 2020, 38, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tang, Y.; Yu, J.; Dong, R.; Yang, Y.; Fu, M.; Luo, J.; Hu, S.; Wang, D.W.; Tu, L.; et al. sEH Inhibitor Tppu Ameliorates Cecal Ligation and Puncture-Induced Sepsis by Regulating Macrophage Functions. Shock 2020, 53, 761–771. [Google Scholar] [CrossRef]
- Liu, L.-P.; Li, B.; Shuai, T.-K.; Zhu, L.; Li, Y.-M. Deletion of soluble epoxide hydrolase attenuates mice Hyperoxic acute lung injury. BMC Anesthesiol. 2018, 18, 48. [Google Scholar] [CrossRef]
- Li, L.; Li, N.; Pang, W.; Zhang, X.; Hammock, B.D.; Ai, D.; Zhu, Y. Opposite Effects of Gene Deficiency and Pharmacological Inhibition of Soluble Epoxide Hydrolase on Cardiac Fibrosis. PLoS ONE 2014, 9, e94092. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ding, A.; Yang, D.; Cui, T.; Yang, H.; Zhang, H.; Wang, C. CYP2J2-produced epoxyeicosatrienoic acids attenuate ischemia/reperfusion-induced acute kidney injury by activating the SIRT1-FoxO3a pathway. Life Sci. 2020, 246, 117327. [Google Scholar] [CrossRef]
- Westphal, C.; Spallek, B.; Konkel, A.; Markó, L.; Qadri, F.; DeGraff, L.M.; Schubert, C.; Bradbury, J.A.; Regitz-Zagrosek, V.; Falck, J.; et al. CYP2J2 Overexpression Protects against Arrhythmia Susceptibility in Cardiac Hypertrophy. PLoS ONE 2013, 8, e73490. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Zhang, B.; Hu, R.; Li, J.; Feng, M.; Yao, W.; Zhang, C.; Wan, L.; Zhang, Y. Alleviation of Mechanical Allodynia by 14,15-Epoxyeicosatrienoic Acid in a Central Poststroke Pain Model: Possible Role of Allopregnanolone and δ-Subunit-Containing Gamma-Aminobutyric Acid A Receptors. J. Pain 2019, 20, 577–591. [Google Scholar] [CrossRef]
- Harris, T.R.; Hammock, B.D. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene 2013, 526, 61–74. [Google Scholar] [CrossRef]
- Rani, A.; Chin, C.; Bremner, R.; Mohanakumar, T.; Angara, S. Targeting chromatin dysregulation in organ fibrosis. Cytokine Growth Factor Rev. 2021, 57, 64–72. [Google Scholar] [CrossRef]
- Miao, H.; Wu, X.-Q.; Zhang, D.-D.; Wang, Y.-N.; Guo, Y.; Li, P.; Xiong, Q.; Zhao, Y.-Y. Deciphering the cellular mechanisms underlying fibrosis-associated diseases and therapeutic avenues. Pharmacol. Res. 2021, 163, 105316. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wan, Y.; Chen, R.; Zhang, C.; Li, X.; Meng, F.; Glaser, S.; Wu, N.; Zhou, T.; Li, S.; et al. The emerging role of cellular senescence in renal diseases. J. Cell. Mol. Med. 2020, 24, 2087–2097. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, H.; Qiu, T.; Dai, J.; Zhang, Y.; Chen, J.; Cai, H. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-κB activation. Aging Cell 2018, 18, e12858. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; La Russo, N.F. Cellular senescence, neuropeptides and hepatic fibrosis: Additional insights into increasing complexity. Hepatology 2017, 66, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Ribatti, D.; Lisi, S. Organ Fibrosis and Autoimmunity: The Role of Inflammation in TGFβ-Dependent EMT. Biomolecules 2021, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Skibba, M.; Khan, A.H.; Kolb, L.L.; Yeboah, M.M.; Falck, J.R.; Amaradhi, R.; Imig, J.D. Epoxyeicosatrienoic Acid Analog Decreases Renal Fibrosis by Reducing Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Neckář, J.; Khan, A.H.; Gross, G.J.; Cyprová, M.; Hrdlička, J.; Kvasilová, A.; Falck, J.R.; Campbell, W.B.; Sedláková, L.; Škutová, Š.; et al. Epoxyeicosatrienoic acid analog EET-B attenuates post-myocardial infarction remodeling in spontaneously hypertensive rats. Clin. Sci. 2019, 133, 939–951. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, G.-Y.; Liu, T.; Duan, J.-X.; Zhou, H.-F.; Lee, K.S.S.; Hammock, B.D.; Fang, X.; Jiang, J.-X.; Guan, C.-X. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell Tissue Res. 2016, 363, 399–409. [Google Scholar] [CrossRef]
- Dong, X.-W.; Jia, Y.-L.; Ge, L.-T.; Jiang, B.; Jiang, J.-X.; Shen, J.; Jin, Y.-C.; Guan, Y.; Sun, Y.; Xie, Q.-M. Soluble epoxide hydrolase inhibitor AUDA decreases bleomycin-induced pulmonary toxicity in mice by inhibiting the p38/Smad3 pathways. Toxicology 2017, 389, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.R.; Bettaieb, A.; Kodani, S.; Dong, H.; Myers, R.; Chiamvimonvat, N.; Haj, F.G.; Hammock, B.D. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol. Appl. Pharmacol. 2015, 286, 102–111. [Google Scholar] [CrossRef]
- Deng, W.; Zhu, Y.; Lin, J.; Zheng, L.; Zhang, C.; Luo, M. Inhibition of soluble epoxide hydrolase lowers portal hypertension in cirrhotic rats by ameliorating endothelial dysfunction and liver fibrosis. Prostaglandins Other Lipid Mediat. 2017, 131, 67–74. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, Y.C.; An, J.N.; Kim, J.H.; Lee, J.; Lee, H.-Y.; Cho, J.-Y.; Paik, J.H.; Oh, Y.K.; Lim, C.S.; et al. Active maintenance of endothelial cells prevents kidney fibrosis. Kidney Res. Clin. Pract. 2017, 36, 329–341. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, S.P.; Toews, M.L.; Imig, J.; Hwang, S.H.; Hammock, B.D.; Padanilam, B.J. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am. J. Physiol. Physiol. 2015, 308, F131–F139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Tu, L.; Li, X.; Yang, S.; Chen, C.; Xu, X.; Wang, P.; Wang, D.W. Delivery of AAV2-CYP2J2 Protects Remnant Kidney in the 5/6-Nephrectomized Rat via Inhibition of Apoptosis and Fibrosis. Hum. Gene Ther. 2012, 23, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Faulkner, J.; Pye, C.; Rouch, K.; Alhashim, A.; Maddipati, K.R.; Baban, B. Role of haem oxygenase in the renoprotective effects of soluble epoxide hydrolase inhibition in diabetic spontaneously hypertensive rats. Clin. Sci. 2013, 125, 349–359. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.; Jiali, N.; Nie, J.; Xu, X.; Wang, D.W. Soluble Epoxide Hydrolase Inhibition Protected against Angiotensin II-induced Adventitial Remodeling. Sci. Rep. 2017, 7, 6926. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, Y.; Wen, Z.; Chen, C.; Xu, X.; Zhu, Y.; Wang, Y.; Wang, D.W. CYP2J2 metabolites, epoxyeicosatrienoic acids, attenuate Ang II-induced cardiac fibrotic response by targeting Gα12/13. J. Lipid Res. 2017, 58, 1338–1353. [Google Scholar] [CrossRef]
- Yang, L.; Ni, L.; Duan, Q.; Wang, X.; Chen, C.; Chen, S.; Chaugai, S.; Zeldin, D.; Tang, J.R.; Wang, D.W. CYP epoxygenase 2J2 prevents cardiac fibrosis by suppression of transmission of pro-inflammation from cardiomyocytes to macrophages. Prostaglandins Other Lipid Mediat. 2015, 116, 64–75. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, H.; Wen, Z.; Chen, C.; Wang, D.W. CYP 2J2 and its metabolites (epoxyeicosatrienoic acids) attenuate cardiac hypertrophy by activating AMPK α2 and enhancing nuclear translocation of Akt1. Aging Cell 2016, 15, 940–952. [Google Scholar] [CrossRef]
- Kompa, A.R.; Wang, B.H.; Xu, G.; Zhang, Y.; Ho, P.-Y.; Eisennagel, S.; Thalji, R.K.; Marino, J.P.; Kelly, D.J.; Behm, D.J.; et al. Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int. J. Cardiol. 2013, 167, 210–219. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.; Li, Q.; Zhan, C.; He, Y.; Liu, J.; Wen, Z.; Wang, D.W. Pharmacological Inhibition of Soluble Epoxide Hydrolase Ameliorates Chronic Ethanol-Induced Cardiac Fibrosis by Restoring Autophagic Flux. Alcohol. Clin. Exp. Res. 2018, 42, 1970–1978. [Google Scholar] [CrossRef]
- Harris, T.R.; Kodani, S.; Yang, J.; Imai, D.M.; Hammock, B.D. An ω-3-enriched diet alone does not attenuate CCl4-induced hepatic fibrosis. J. Nutr. Biochem. 2016, 38, 93–101. [Google Scholar] [CrossRef]
- Liu, F.; Zhuang, S. New Therapies for the Treatment of Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 625–659. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, C.; Ibrahim, M.M.; Kranz, J.; Zhang, X.; Ziegler, S.; Perales-Patón, J.; Jansen, J.; Reimer, K.C.; Smith, J.R.; Dobie, R.; et al. Decoding myofibroblast origins in human kidney fibrosis. Nat. Cell Biol. 2021, 589, 281–286. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Liu, X.; Chen, J.; Cai, Q.; Wang, J.; Huang, H. Apocynin improving cardiac remodeling in chronic renal failure disease is associated with up-regulation of epoxyeicosatrienoic acids. Oncotarget 2015, 6, 24699–24708. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Maalouf, R.; Jaffa, A.A.; Nassif, J.; Hamdy, A.; Rashid, A.; Ziyadeh, F.N.; Eid, A.A. 20-HETE and EETs in Diabetic Nephropathy: A Novel Mechanistic Pathway. PLoS ONE 2013, 8, e70029. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Khan, A.H.; Kolb, L.; Skibba, M.; Hartmann, M.; Blöcher, R.; Proschak, E.; Imig, J.D. A novel dual PPAR-γ agonist/sEH inhibitor treats diabetic complications in a rat model of type 2 diabetes. Diabetologia 2018, 61, 2235–2246. [Google Scholar] [CrossRef]
- Eli, J.; Stier, C.T.; Chander, P.N.; Manthati, V.L.; Falck, J.R.; Carroll, M.A. Pharmacological manipulation of arachidonic acid-epoxygenase results in divergent effects on renal damage. Front. Pharmacol. 2014, 5, 187. [Google Scholar] [CrossRef][Green Version]
- Campbell, W.B.; Imig, J.; Schmitz, J.M.; Falck, J.R. Orally Active Epoxyeicosatrienoic Acid Analogs. J. Cardiovasc. Pharmacol. 2017, 70, 211–224. [Google Scholar] [CrossRef]
- Khan, A.H.; Falck, J.R.; Manthati, V.L.; Campbell, W.B.; Imig, J.D. Epoxyeicosatrienoic acid analog attenuates angiotensin II hypertension and kidney injury. Front. Pharmacol. 2014, 5, 216. [Google Scholar] [CrossRef]
- Khan, A.H.; Neckar, J.; Manthati, V.; Errabelli, R.; Pavlov, T.S.; Staruschenko, A.; Falck, J.R.; Imig, J.D. Orally Active Epoxyeicosatrienoic Acid Analog Attenuates Kidney Injury in Hypertensive Dahl Salt–Sensitive Rat. Hypertension 2013, 62, 905–913. [Google Scholar] [CrossRef]
- Khan, A.H.; Fish, B.; Wahl, G.; Sharma, A.; Falck, J.R.; Paudyal, M.P.; Moulder, J.E.; Imig, J.; Cohen, E.P. Epoxyeicosatrienoic acid analogue mitigates kidney injury in a rat model of radiation nephropathy. Clin. Sci. 2016, 130, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Japp, A.G.; Raza, S.; Halliday, B.P.; Jones, D.A.; Newsome, S.; Ismail, N.A.; Morarji, K.; Khwaja, J.; Spath, N.; et al. Absence of Myocardial Fibrosis Predicts Favorable Long-Term Survival in New-Onset Heart Failure. Circ. Cardiovasc. Imaging 2018, 11, e007722. [Google Scholar] [CrossRef]
- Centurión, O.A.; Alderete, J.F.; Torales, J.M.; García, L.B.; Scavenius, K.E.; Miño, L.M. Myocardial Fibrosis as a Pathway of Prediction of Ventricular Arrhythmias and Sudden Cardiac Death in Patients with Nonischemic Dilated Cardiomyopathy. Crit. Pathways Cardiol. A J. Evid. Based Med. 2019, 18, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological Ventricular Remodeling. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, G.; Zhu, F.; Zheng, Z.; Wang, X.; Zhang, G.; Wang, F. Epoxyeicosatrienoic acid prevents maladaptive remodeling in pressure overload by targeting calcineurin/NFAT and Smad-7. Exp. Cell Res. 2020, 386, 111716. [Google Scholar] [CrossRef] [PubMed]
- Červenka, L.; Husková, Z.; Kopkan, L.; Kikerlová, S.; Sedláková, L.; Vaňourková, Z.; Alanova, P.; Kolar, F.; Hammock, B.D.; Hwang, S.H.; et al. Two pharmacological epoxyeicosatrienoic acid-enhancing therapies are effectively antihypertensive and reduce the severity of ischemic arrhythmias in rats with angiotensin II-dependent hypertension. J. Hypertens. 2018, 36, 1326–1341. [Google Scholar] [CrossRef]
- Seubert, J.M.; Sinal, C.J.; Graves, J.; DeGraff, L.M.; Bradbury, J.A.; Lee, C.R.; Goralski, K.; Carey, M.A.; Luria, A.; Newman, J.W.; et al. Role of Soluble Epoxide Hydrolase in Postischemic Recovery of Heart Contractile Function. Circ. Res. 2006, 99, 442–450. [Google Scholar] [CrossRef]
- Althurwi, H.N.; Tse, M.M.Y.; Abdelhamid, G.; Zordoky, B.; Hammock, B.D.; El-Kadi, A.O.S. Soluble epoxide hydrolase inhibitor, TUPS, protects against isoprenaline-induced cardiac hypertrophy. Br. J. Pharmacol. 2012, 168, 1794–1807. [Google Scholar] [CrossRef]
- Aliwarga, T.; Guo, X.; Evangelista, E.A.; Lemaitre, R.N.; Sotoodehnia, N.; Gharib, S.A.; Zeldin, D.C.; Liu, Q.; Totah, R.A. Higher Epoxyeicosatrienoic Acids in Cardiomyocytes-Specific CYP2J2 Transgenic Mice Are Associated with Improved Myocardial Remodeling. Biomedicines 2020, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Chen, C. The Role of Epoxyeicosatrienoic Acids in Cardiac Remodeling. Front. Physiol. 2021, 12, 117. [Google Scholar] [CrossRef]
- Qu, Y.-Y.; Yuan, M.-Y.; Liu, Y.; Xiao, X.-J.; Zhu, Y.-L. The Protective Effect of Epoxyeicosatrienoic Acids on Cerebral Ischemia/Reperfusion Injury is Associated with PI3K/Akt Pathway and ATP-Sensitive Potassium Channels. Neurochem. Res. 2014, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.A.; Olzinski, A.R.; Upson, J.J.; Zhao, S.; Wang, T.; Eisennagel, S.H.; Hoang, B.; Tunstead, J.R.; Marino, J.P.; Willette, R.N.; et al. Soluble Epoxide Hydrolase Inhibition Does Not Prevent Cardiac Remodeling and Dysfunction After Aortic Constriction in Rats and Mice. J. Cardiovasc. Pharmacol. 2013, 61, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Edin, M.L.; Lee, C.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Investig. 2012, 122, 178–191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wu, G.-R.; Zhou, Q.; Yue, H.; Rao, L.-Z.; Yuan, T.; Mo, B.; Wang, F.-X.; Chen, L.-M.; et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci. Adv. 2021, 7, eabb6075. [Google Scholar] [CrossRef]
- Nureki, S.-I.; Tomer, Y.; Venosa, A.; Katzen, J.; Russo, S.J.; Jamil, S.; Barrett, M.; Nguyen, V.; Kopp, M.; Mulugeta, S.; et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J. Clin. Investig. 2018, 128, 4008–4024. [Google Scholar] [CrossRef]
- Heukels, P.; Moor, C.; von der Thusen, J.; Wijsenbeek, M.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71, 112–127. [Google Scholar] [CrossRef]
- Cui, Y.; Xin, H.; Tao, Y.; Mei, L.; Wang, Z. Arenaria kansuensis attenuates pulmonary fibrosis in mice via the activation of Nrf2 pathway and the inhibition of NF-kB/TGF -beta1/Smad2/3 pathway. Phytother. Res. 2021, 35, 974–986. [Google Scholar] [CrossRef]
- Chleilat, E.; Pethe, A.; Pfeifer, D.; Krieglstein, K.; Roussa, E. TGF-β Signaling Regulates SLC8A3 Expression and Prevents Oxidative Stress in Developing Midbrain Dopaminergic and Dorsal Raphe Serotonergic Neurons. Int. J. Mol. Sci. 2020, 21, 2735. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, J.; Yang, H.; Sun, C.; Zhong, W.; Tao, J.; Guan, X.; Jiang, H.; Hammock, B.D.; Hwang, S.H.; et al. COX-2/sEH dual inhibitor PTUPB alleviates bleomycin-induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J. 2020, 287, 1666–1680. [Google Scholar] [CrossRef]
- Feng, W.; Xu, X.; Zhao, G.; Li, G.; Liu, T.; Zhao, J.; Dong, R.; Wang, D.W.; Tu, L. EETs and CYP2J2 inhibit TNF-α-induced apoptosis in pulmonary artery endothelial cells and TGF-β1-induced migration in pulmonary artery smooth muscle cells. Int. J. Mol. Med. 2013, 32, 685–693. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, G.; Yang, S.; Ping, W.; Fu, X.; Zhang, N.; Wang, D.W.; Wang, J. CYP2J2 and EETs Protect against Oxidative Stress and Apoptosisin Vivoandin VitroFollowing Lung Ischemia/Reperfusion. Cell. Physiol. Biochem. 2014, 33, 1663–1680. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Lu, W.; Ding, L.; Bao, Y.; Hong, F.; Chen, Y.; Gao, H.; Xu, X.; Wang, G.; Wang, W.; et al. PEDF promotes the repair of bone marrow endothelial cell injury and accelerates hematopoietic reconstruction after bone marrow transplantation. J. Biomed. Sci. 2020, 27, 91. [Google Scholar] [CrossRef]
- Cheranov, S.Y.; Karpurapu, M.; Wang, N.; Zhang, B.; Venema, R.C.; Rao, G.N. An essential role for SRC-activated STAT-3 in 14,15-EET–induced VEGF expression and angiogenesis. Blood 2008, 111, 5581–5591. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, X.; Liang, J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp. Cell Res. 2017, 352, 420–426. [Google Scholar] [CrossRef]
- Liao, X.; Zhan, W.; Tian, T.; Yu, L.; Li, R.; Yang, Q. MicroRNA-326 attenuates hepatic stellate cell activation and liver fibrosis by inhibiting TLR4 signaling. J. Cell. Biochem. 2019, 121, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Deutsch, M.; Alothman, S.; Alqunaibit, D.; Werba, G.; Pansari, M.; Pergamo, M.; Ochi, A.; Torres-Hernandez, A.; Levie, E.; et al. Dectin-1 Regulates Hepatic Fibrosis and Hepatocarcinogenesis by Suppressing TLR4 Signaling Pathways. Cell Rep. 2015, 13, 1909–1921. [Google Scholar] [CrossRef]
- Harris, T.R.; Kodani, S.; Rand, A.A.; Yang, J.; Imai, D.M.; Hwang, S.H.; Hammock, B.D. Celecoxib Does Not Protect against Fibrosis and Inflammation in a Carbon Tetrachlorideȁ4Induced Model of Liver Injury. Mol. Pharmacol. 2018, 94, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Visentin, M.; Gui, T.; Zhao, L.; Thasler, W.E.; Häusler, S.; Hartling, I.; Cremonesi, A.; Hiller, C.; Kullak-Ublick, G.A. Effects of Farnesoid X Receptor Activation on Arachidonic Acid Metabolism, NF-kB Signaling, and Hepatic Inflammation. Mol. Pharmacol. 2018, 94, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Sacerdoti, D.; Jiang, H.; Gaiani, S.; McGiff, J.C.; Gatta, A.; Bolognesi, M. 11,12-EET increases porto-sinusoidal resistance and may play a role in endothelial dysfunction of portal hypertension. Prostaglandins Other Lipid Mediat. 2011, 96, 72–75. [Google Scholar] [CrossRef]
- Bueno, M.; Calyeca, J.; Rojas, M.; Mora, A.L. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 2020, 33, 101509. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, A.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The Role of Senescence in the Development of Nonalcoholic Fatty Liver Disease and Progression to Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef] [PubMed]

| Model/Cell | Method | Effect and Mechanism | |
|---|---|---|---|
| Kidney | UUO [24,30,31] |
| Reduce fibrotic response of fibroblasts, collagen deposition, inflammation, and oxidative stress, and prevent EndMT and EMT
|
| 5/6-Nx [32] |
| Reduce collagen deposition, apoptosis, and renal dysfunction
| |
| Diabetic SHR [33] |
| Reduce collagen deposition and inflammation
| |
| Heart | Ang II infusion hearts [11,34,35,36,37] |
| Reduce fibrotic response of fibroblasts, collagen deposition, hypertrophy, remodeling, and oxidative stress and inflammation
|
| Post-MI [38] |
| Reduce fibrotic response of fibroblasts, collagen deposition
| |
| Iso infusion hearts [36] |
| Reduce fibroblasts activation and macrophage infiltration
| |
| EtOH [39] |
| Reduce fibroblasts activation and restore autophagic flux
| |
| Lung | BLM [26,27] |
| Reduce fibrotic response of fibroblasts, collagen deposition, and inflammation and prevent EMT
|
| Liver | CCl4 [28,29,40] |
| Reduce collagen deposition
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.-X.; Rao, D.-N.; Liu, Y.-Z.; Zhou, Y.; Yang, H.-H. Epoxyeicosatrienoic Acids and Fibrosis: Recent Insights for the Novel Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 10714. https://doi.org/10.3390/ijms221910714
Guan X-X, Rao D-N, Liu Y-Z, Zhou Y, Yang H-H. Epoxyeicosatrienoic Acids and Fibrosis: Recent Insights for the Novel Therapeutic Strategies. International Journal of Molecular Sciences. 2021; 22(19):10714. https://doi.org/10.3390/ijms221910714
Chicago/Turabian StyleGuan, Xin-Xin, Dong-Ning Rao, Yan-Zhe Liu, Yong Zhou, and Hui-Hui Yang. 2021. "Epoxyeicosatrienoic Acids and Fibrosis: Recent Insights for the Novel Therapeutic Strategies" International Journal of Molecular Sciences 22, no. 19: 10714. https://doi.org/10.3390/ijms221910714
APA StyleGuan, X.-X., Rao, D.-N., Liu, Y.-Z., Zhou, Y., & Yang, H.-H. (2021). Epoxyeicosatrienoic Acids and Fibrosis: Recent Insights for the Novel Therapeutic Strategies. International Journal of Molecular Sciences, 22(19), 10714. https://doi.org/10.3390/ijms221910714







