Simulated Microgravity Inhibits Rodent Dermal Fibroblastic Differentiation of Mesenchymal Stem Cells by Suppressing ERK/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Results
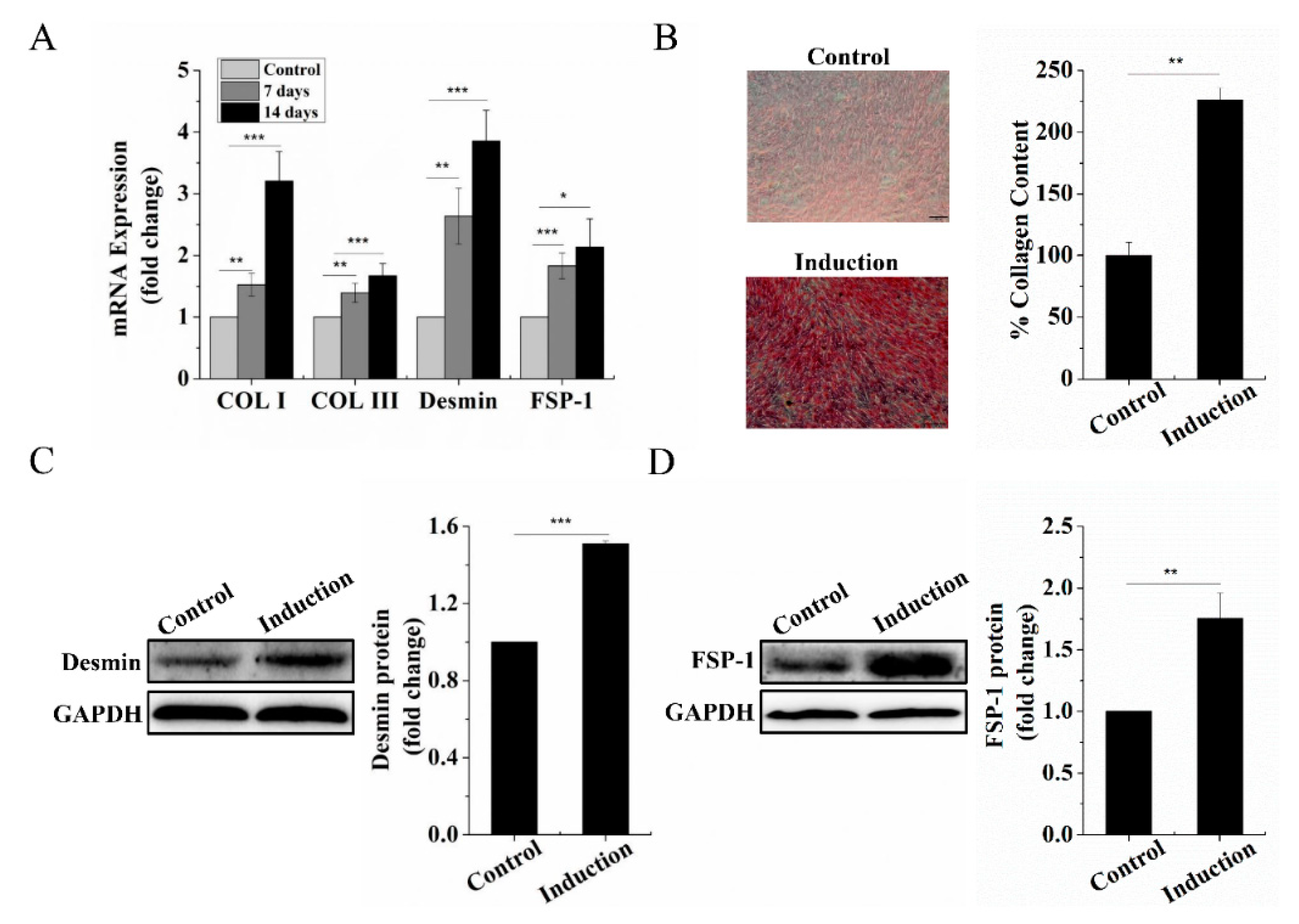
2.1. Dermal Fibroblastic Differentiation of BMSCs
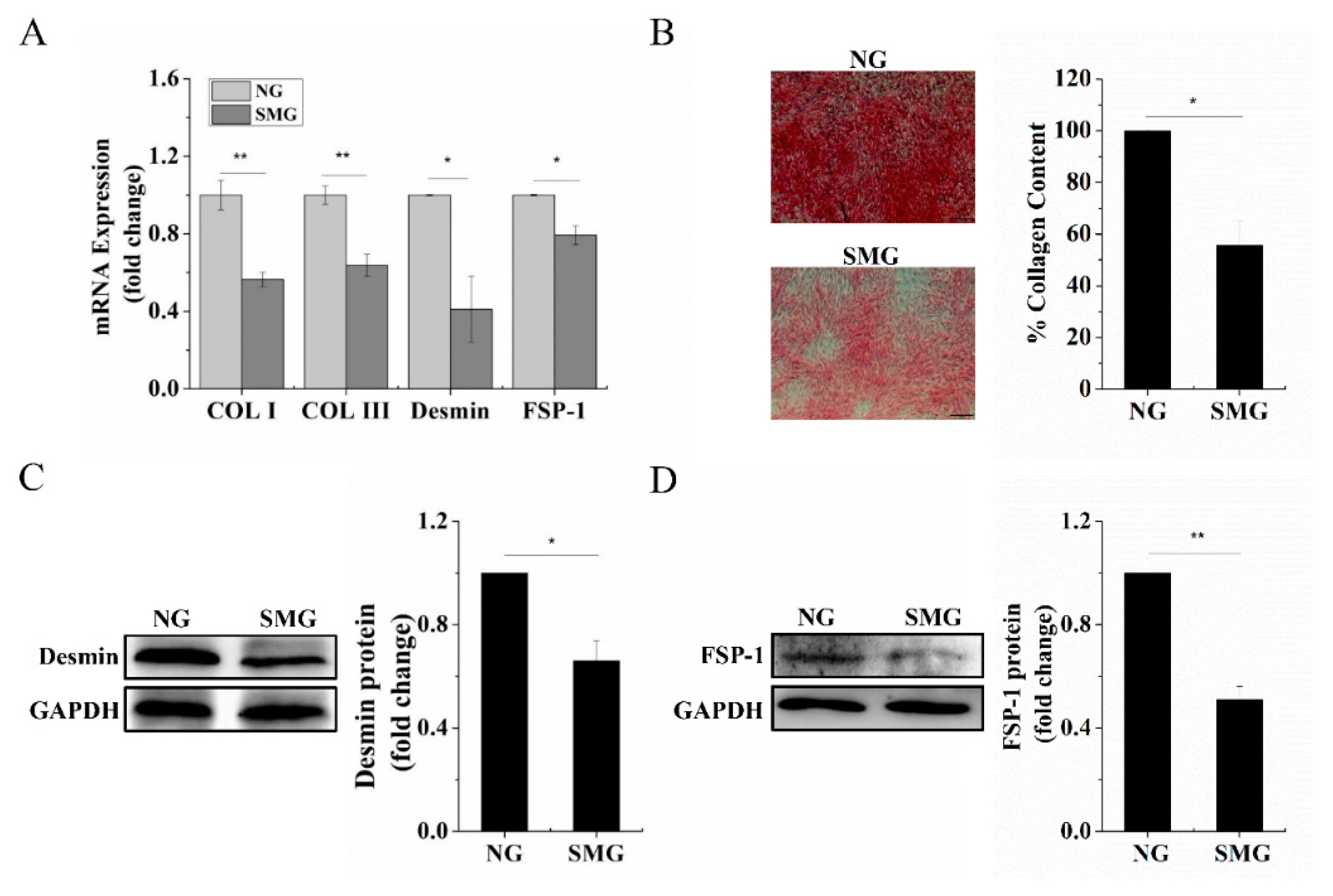
2.2. SMG Inhibited Dermal Fibroblastic Differentiation of BMSCs
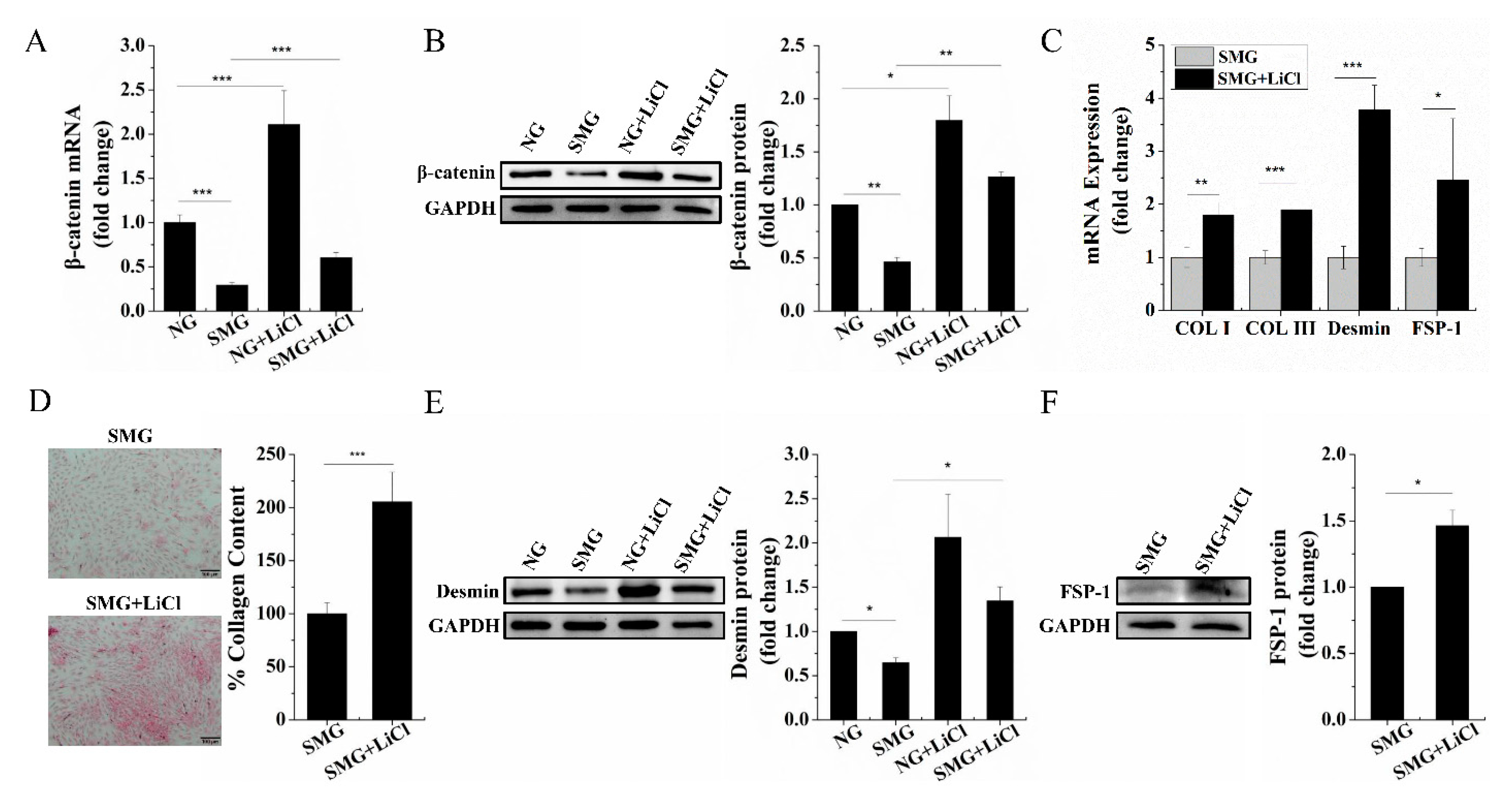
2.3. SMG Repressed the Wnt/β-Catenin Signaling and Upregulation of Wnt/β-Catenin Signaling Rescued Dermal Fibroblastic Differentiation of BMSCs
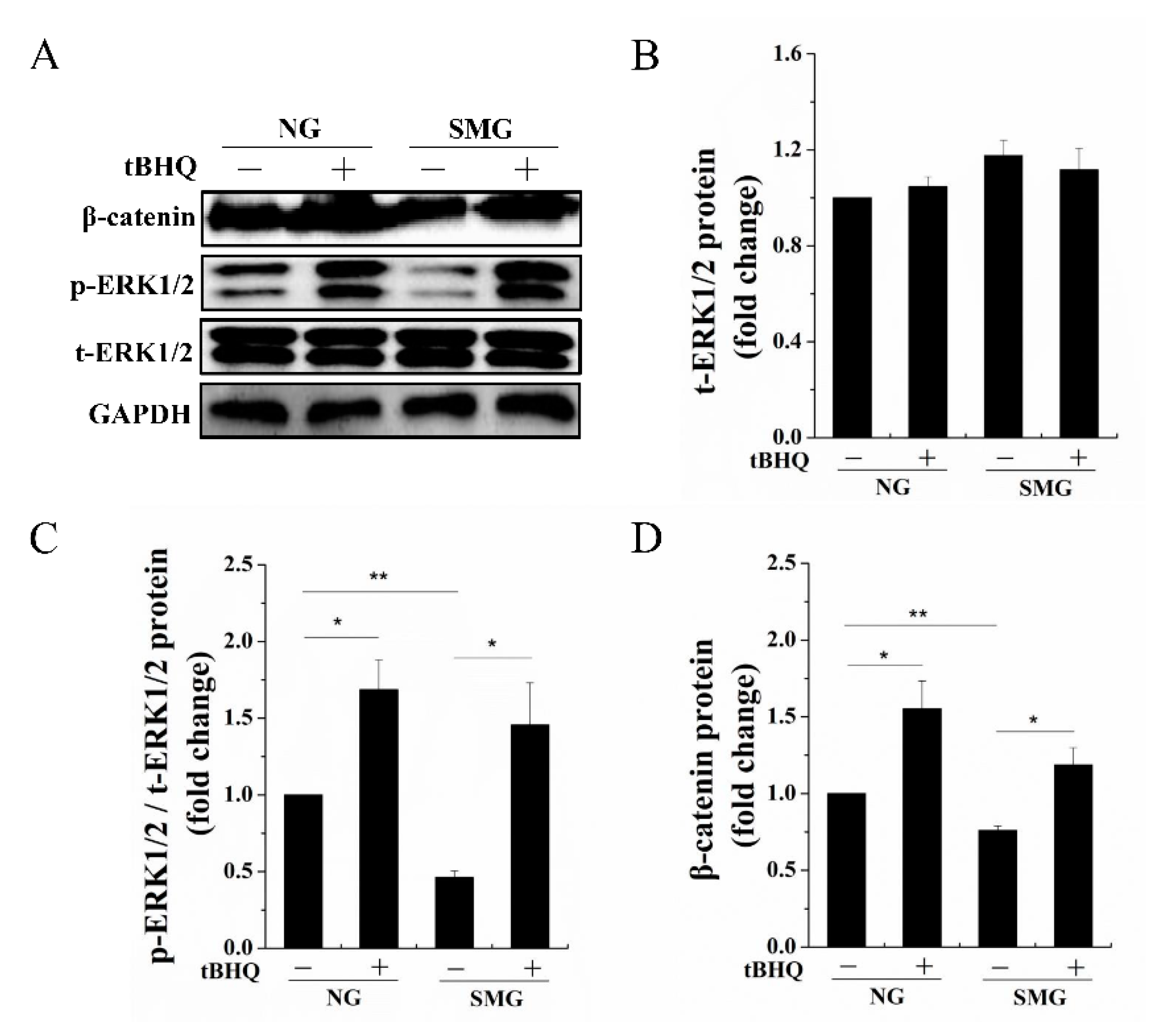
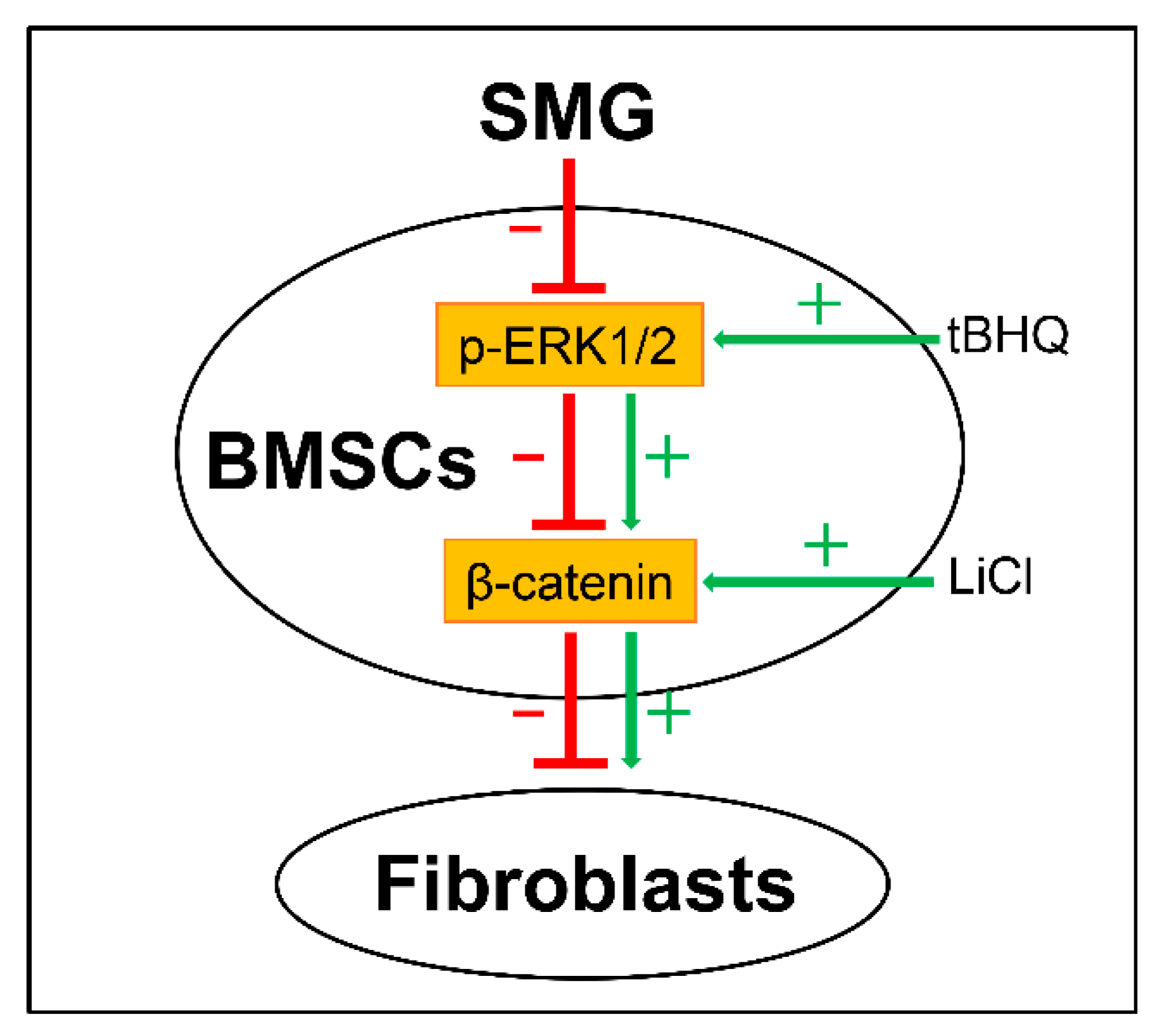
2.4. SMG Inhibited Phosphorylation of ERK1/2 then Enhancing Phosphorylation of ERK1/2 Improved the Expression of β-Catenin
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Dermal Fibroblastic Differentiation of BMSCs
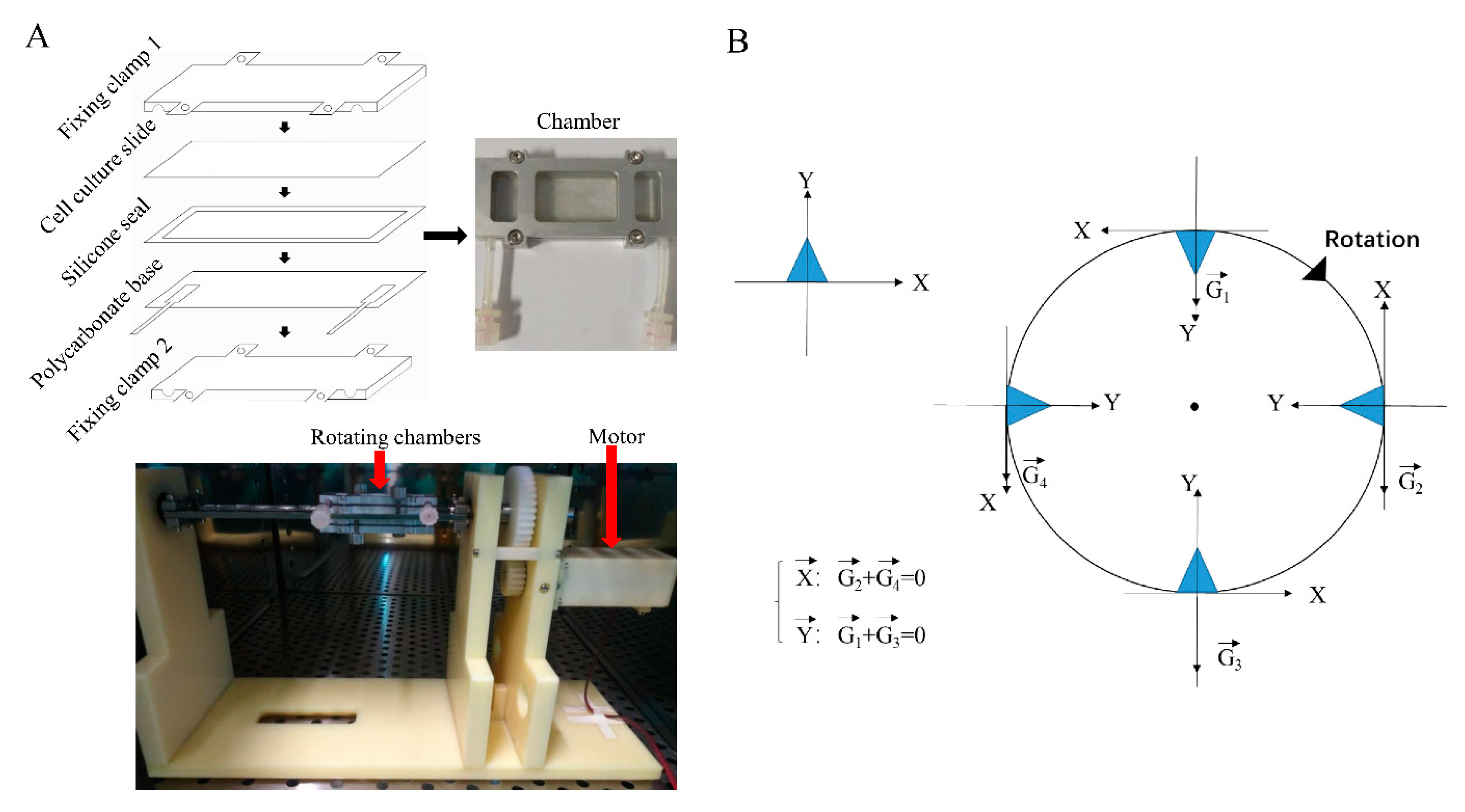
4.3. Microgravity Simulation
4.4. Quantitative Real-Time PCR
4.5. Western Blot
4.6. Sirius Red Staining
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buken, C.; Sahana, J.; Corydon, T.J.; Melnik, D.; Bauer, J.; Wehland, M.; Krüger, M.; Balk, S.; Abuagela, N.; Infanger, M.; et al. Morphological and Molecular Changes in Juvenile Normal Human Fibroblasts Exposed to Simulated Microgravity. Sci. Rep. 2019, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tronnier, H.; Wiebusch, M.; Heinrich, U. Change in Skin Physiological Parameters in Space—Report on and Results of the First Study on Man. Ski. Pharmacol. Physiol. 2008, 21, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; Thomas, S.; Tronnier, H.; Heinrich, U. Self-Reported Skin Changes by a Selected Number of Astronauts after Long-Duration Mission on ISS as Part of the Skin B Project. Ski. Pharmacol. Physiol. 2018, 32, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Neutelings, T.; Nusgens, B.V.; Liu, Y.; Tavella, S.; Ruggiu, A.; Cancedda, R.; Gabriel, M.; Colige, A.; Lambert, C. Skin physiology in microgravity: A 3-month stay aboard ISS induces dermal atrophy and affects cutaneous muscle and hair follicles cycling in mice. npj Microgravity 2015, 1, 15002. [Google Scholar] [CrossRef] [Green Version]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef]
- Li, H.; Fu, X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012, 348, 371–377. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Opalenik, S.R.; Davidson, J.M. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005, 19, 1561–1563. [Google Scholar] [CrossRef]
- Dees, C.; Distler, J.H.W. Canonical Wnt signalling as a key regulator of fibrogenesis—Implications for targeted therapies? Exp. Dermatol. 2013, 22, 710–713. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Wang, C.; Shi, C.; Sun, F.; Xu, X.; Qian, W.; Nie, S.; Han, X. Activated Wnt signaling induces myofibroblast differentiation of mesenchymal stem cells, contributing to pulmonary fibrosis. Int. J. Mol. Med. 2014, 33, 1097–1109. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Luo, X.; Yang, D.; Wang, C.; Cheng, Q.; Xiang, T.; Ren, G. Phospholipase Cδ1 suppresses cell migration and invasion of breast cancer cells by modulating KIF3A-mediated ERK1/2/β- catenin/MMP7 signalling. Oncotarget 2017, 8, 29056–29066. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Xia, W.; Liu, J.-C.; Yang, J.-Y.; Lee, D.-F.; Xia, J.; Bartholomeusz, G.; Li, Y.; Pan, Y.; Li, Z.; et al. Erk Associates with and Primes GSK-3β for Its Inactivation Resulting in Upregulation of β-Catenin. Mol. Cell 2005, 19, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Miao, H.-C.; Li, W.-J.; Sun, Y.; Huang, S.-L.; Li, Z.-Y.; Guo, Q.-L. LW-213 induces G2/M cell cycle arrest through AKT/GSK3β/β-catenin signaling pathway in human breast cancer cells. Mol. Carcinog. 2015, 55, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.Q.; Wang, R.; Ling, S.K.; Wan, Y.M.; Li, Y.H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 2007, 40, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Yoon, D.; Sim, H.; Hwang, I.; Lee, J.-S.; Chun, W. Accelerated Wound Healing by Fibroblasts Differentiated from Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells in a Pressure Ulcer Animal Model. Stem Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Shah, B.; Moioli, E.K.; Mao, J.J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Investig. 2010, 120, 3340–3349. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.; Sant, S.; Khademhosseini, A.; Jia, X. Controlling the Fibroblastic Differentiation of Mesenchymal Stem Cells Via the Combination of Fibrous Scaffolds and Connective Tissue Growth Factor. Tissue Eng. Part A 2011, 17, 2773–2785. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Myers, C.T.; Appleby, S.C.; Krieg, P.A. Use of small molecule inhibitors of the Wnt and Notch signaling pathways during Xenopus development. Methods 2014, 66, 380–389. [Google Scholar] [CrossRef]
- Gao, P.; Yang, J.; Gao, X.; Xu, D.; Niu, D.; Li, J.; Wen, Q. Salvianolic acid B improves bone marrow-derived mesenchymal stem cell differentiation into alveolar epithelial cells type I via Wnt signaling. Mol. Med. Rep. 2015, 12, 1971–1976. [Google Scholar] [CrossRef]
- Yu, R.; Tan, T.-H.; Kong, A.-N.T. Butylated Hydroxyanisole and Its Metabolitetert-Butylhydroquinone Differentially Regulate Mitogen-activated Protein Kinases. The role of oxidative stress in the activation of mitogen-activated protein kinases by phenolic antioxidants. J. Biol. Chem. 1997, 272, 28962–28970. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.S.; Choi, Y.; Lee, J.W. Cellular localization of NRF2 determines the self-renewal and osteogenic differentiation potential of human MSCs via the P53–SIRT1 axis. Cell Death Dis. 2016, 7, e2093. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.J.; Woo, K.-J.; Cho, D.; Bang, S.I. Lipo-PGE1 suppresses collagen production in human dermal fibroblasts via the ERK/Ets-1 signaling pathway. PLoS ONE 2017, 12, e0179614. [Google Scholar] [CrossRef] [Green Version]
- Thangapazham, R.L.; Darling, T.N.; Meyerle, J. Alteration of Skin Properties with Autologous Dermal Fibroblasts. Int. J. Mol. Sci. 2014, 15, 8407–8427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badiavas, E.V.; Abedi, M.; Butmarc, J.; Falanga, V.; Quesenberry, P. Participation of bone marrow derived cells in cutaneous wound healing. J. Cell. Physiol. 2003, 196, 245–250. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, K.; Gao, X.; Liu, Y.B.; Dulchavsky, D.S.; Kwon, D.; Arbab, A.S.; Bansal, M.; Li, Y.; Chopp, M.; Dulchavsky, S.A.; et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ebnerasuly, F.; Hajebrahimi, Z.; Tabaie, S.M.; Darbouy, M. Effect of Simulated Microgravity Conditions on Differentiation of Adipose Derived Stem Cells towards Fibroblasts Using Connective Tissue Growth Factor. Iran. J. Biotechnol. 2017, 15, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Chai, J.; Sun, T.; Li, N.; Tao, R. Differentiation of human umbilical cord mesenchymal stem cells into dermal fibroblasts in vitro. Biochem. Biophys. Res. Commun. 2011, 413, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Taskin, M.B.; Rubert, M.; Seliktar, O.; Besenbacher, F.; Chen, M. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci. Rep. 2015, 5, 8480. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Luo, Q.; Yuan, L.; Song, G. Microgravity directs stem cell differentiation. Histol. Histopathol. 2016, 32, 99–106. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Yang, Y.; Li, J.; Zhang, X.; Li, J.; Wang, Z.; Ma, J. The simulated microgravity enhances the differentiation of mesenchymal stem cells into neurons. Neurosci. Lett. 2011, 505, 171–175. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Chen, J.; Zhang, X.; Xie, J.; Li, Z.; Ma, J.; Wang, W.; Wang, Z. The simulated microgravity enhances multipotential differentiation capacity of bone marrow mesenchymal stem cells. Cytotechnology 2013, 66, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, S.; Mizumoto, T.; Nasu, A.; Horii, T.; Otomo, K.; Denno, H.; Takebayashi, T.; Miyamoto, K.; Horiuchi, T. Regulation of osteogenetic differentiation of mesenchymal stem cells by two axial rotational culture. J. Artif. Organs 2011, 14, 310–317. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, Q.; Lin, C.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem. Biophys. Res. Commun. 2015, 468, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Emond, M.R.; Chenoweth, K.P.; Jontes, J.D. δ-Protocadherins regulate neural progenitor cell division by antagonizing Ryk and Wnt/β-catenin signaling. iScience 2021, 24, 102932. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gong, X.; Zhu, H.; Wang, C.; Xu, X.; Cui, D.; Qian, W.; Han, X. Inhibition of Wnt/β-Catenin Signaling Promotes Engraftment of Mesenchymal Stem Cells to Repair Lung Injury. J. Cell. Physiol. 2013, 229, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Lee, Y.-C.; Lo, S.; Chung, Y.-N.; Hsieh, Y.-C.; Chiu, W.-C.; Yuan, S.-S.F. A positive feedback loop of IL-17B-IL-17RB activates ERK/β-catenin to promote lung cancer metastasis. Cancer Lett. 2018, 422, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Qian, L.; Yu, Y.; An, H.; Guo, Z.; Han, Y.; Chen, Y.; Bai, Y.; Wang, Q.; Cao, X. Fas Signal Promotes the Immunosuppressive Function of Regulatory Dendritic Cells via the ERK/β-Catenin Pathway. J. Biol. Chem. 2013, 288, 27825–27835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Cheng, Y.; Wang, J.; Ding, Z.; Halim, A.; Luo, Q.; Song, G. Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation. Int. J. Mol. Sci. 2020, 21, 9747. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.; Lebert, M.; et al. Ground-Based Facilities for Simulation of Microgravity: Organism-Specific Recommendations for Their Use, and Recommended Terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, N.; Zhang, C.; Sun, S.; Gao, Y.; Long, M. Effects of Simulated Microgravity on Functions of Neutrophil-like HL-60 Cells. Microgravity Sci. Technol. 2015, 27, 515–527. [Google Scholar] [CrossRef] [Green Version]
| Genes | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| COL I | GGAGAGAGTGCCAACTCCAG | GTGCTTTGGAAAATGGTGCT |
| COL III | TCCCAGAACATTACATACCACT | GCTATTTCCTTCAGCCTTGA |
| Desmin | CTCTACGAGGAGGAGATGCG | AGGTCAATTCGAGCCAGAGTG |
| FSP-1 | GCACTTCCTCTCTCTTGGTCTG | GTCTTCACTTCTTCCGGGGC |
| β-catenin | AACGGCTTTCGGTTGAGCTG | TGGCGATATCCAAGGGCTTC |
| β-actin | ACGGTCAGGTCATCACTATCG | GGCATAGAGGTCTTTACGGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhou, Y.; Lv, W.; Luo, Q.; Song, G. Simulated Microgravity Inhibits Rodent Dermal Fibroblastic Differentiation of Mesenchymal Stem Cells by Suppressing ERK/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 10702. https://doi.org/10.3390/ijms221910702
Cheng Y, Zhou Y, Lv W, Luo Q, Song G. Simulated Microgravity Inhibits Rodent Dermal Fibroblastic Differentiation of Mesenchymal Stem Cells by Suppressing ERK/β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(19):10702. https://doi.org/10.3390/ijms221910702
Chicago/Turabian StyleCheng, Yansiwei, Yuhao Zhou, Wenjun Lv, Qing Luo, and Guanbin Song. 2021. "Simulated Microgravity Inhibits Rodent Dermal Fibroblastic Differentiation of Mesenchymal Stem Cells by Suppressing ERK/β-Catenin Signaling Pathway" International Journal of Molecular Sciences 22, no. 19: 10702. https://doi.org/10.3390/ijms221910702
APA StyleCheng, Y., Zhou, Y., Lv, W., Luo, Q., & Song, G. (2021). Simulated Microgravity Inhibits Rodent Dermal Fibroblastic Differentiation of Mesenchymal Stem Cells by Suppressing ERK/β-Catenin Signaling Pathway. International Journal of Molecular Sciences, 22(19), 10702. https://doi.org/10.3390/ijms221910702






