Trained Immunity as an Adaptive Branch of Innate Immunity
Abstract
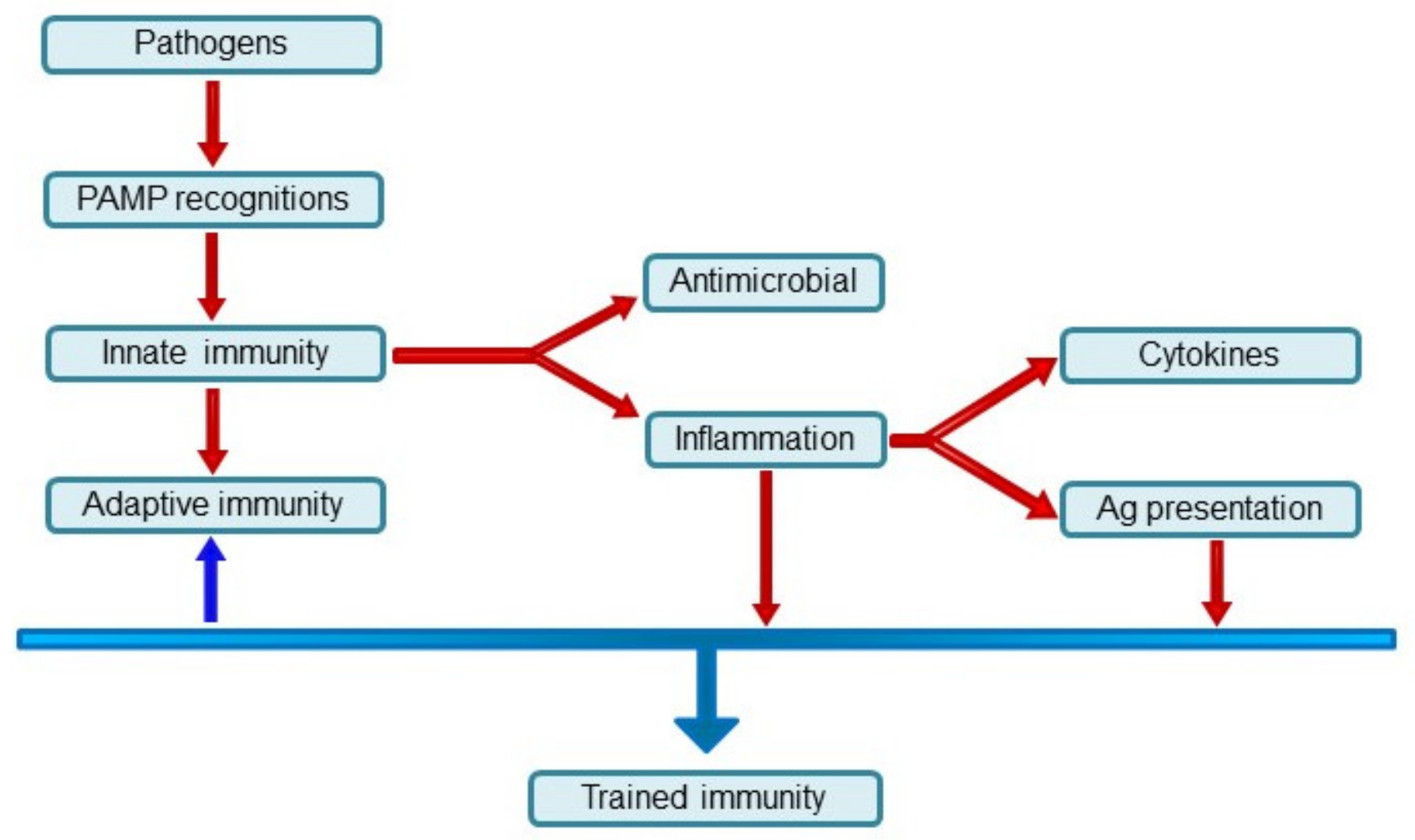
:1. Types of Immunity of Multicellular Organisms
2. Cells Mediating the Trained Immunity
2.1. Basophils
2.2. Neutrophils
2.3. Mononuclear Phagocytes
2.4. Innate Lymphoid Cells
2.5. NK Cells
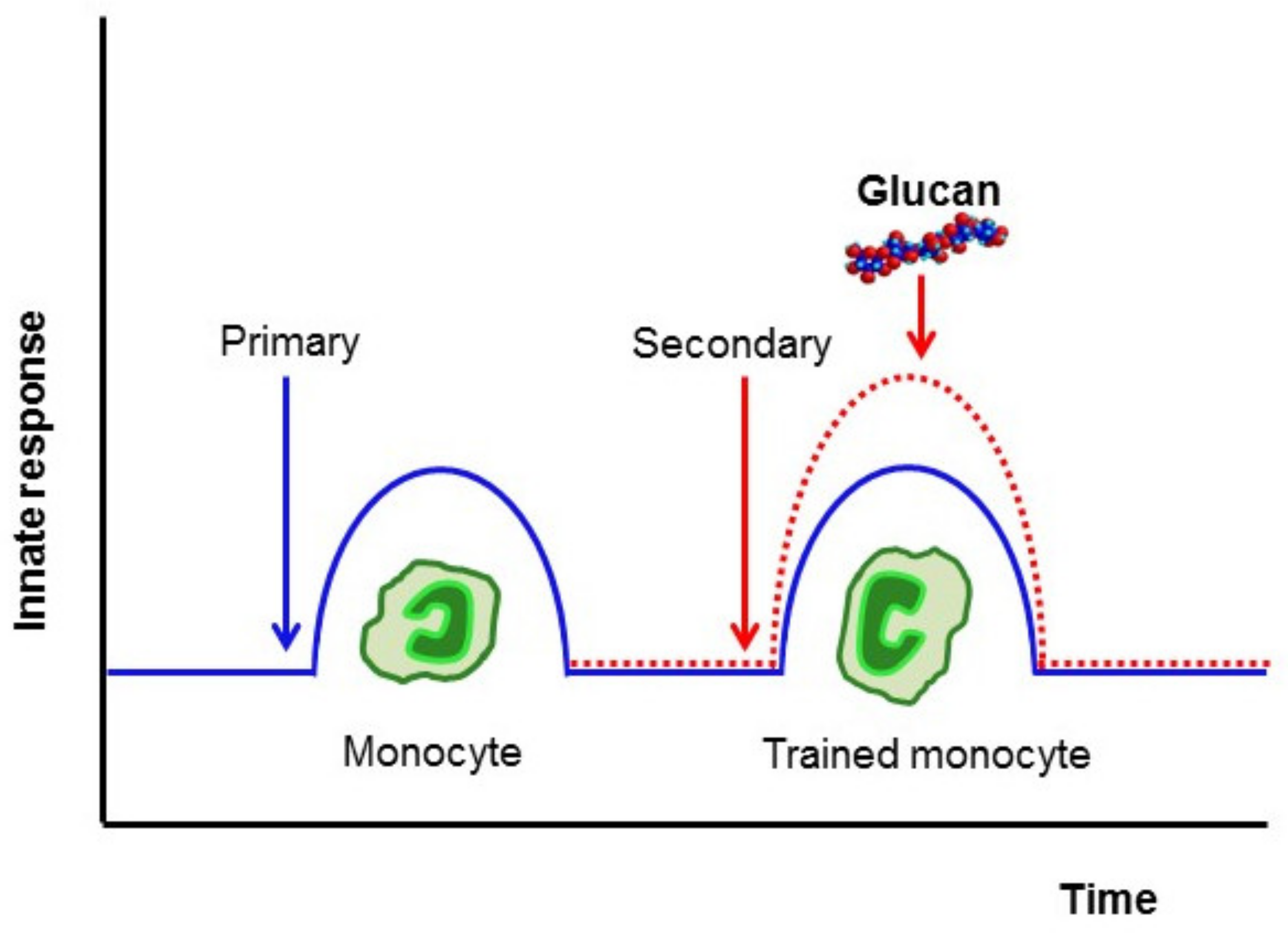
3. Induction of Trained Immunity
4. β-Glucan
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Santoni, G.; Cardinali, C.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Amantini, C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflammation 2015, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Matzinger, P. The evolution of the danger theory. Interview by Lauren Constable, Commissioning Editor. Expert Rev. Clin. Immunol. 2012, 8, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.; Eleftherianos, I. Memory and specificity in the insect immune system: Current perspectives and future challenges. Front. Immunol. 2017, 8, 539. [Google Scholar] [CrossRef]
- Coustau, C.; Kurtz, J.; Moret, Y. A novel mechanism of immune memory unveiled at the invertebrate-parasite interface. Trends Parasitol. 2016, 32, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 2005, 26, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.; Franz, K. Innate defence: Evidence for memory in invertebrate immunity. Nature 2003, 425, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Robin, G.P. Systemic signaling during plant defense. Curr. Opin. Plant Biol. 2013, 16, 527–533. [Google Scholar] [CrossRef]
- Palmieri, B.; Vadala, M.; Palmieri, L. Immune memory: An evolutionary perspective. Hum. Vaccines Immunother. 2021, 17, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Gourbal, B.; Pinaud, S.; Beckers, G.J.M.; Van Der Meer, J.W.M.; Conrath, U.; Netea, M.G. Innate immune memory: An evolutionary perspective. Immunol. Rev. 2018, 283, 21–40. [Google Scholar] [CrossRef]
- Purvis, A.; Hector, A. Getting the measure of biodiversity. Nature 2000, 405, 212–219. [Google Scholar] [CrossRef]
- Milutinovic, B.; Kurtz, J. Immune memory in invertebrates. Semin. Immunol. 2016, 28, 328–342. [Google Scholar] [CrossRef]
- Reimer-Michalski, E.M.; Conrath, U. Innate immune memory in plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; van der Meer, J.W. Trained immunity: An ancient way of remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menu, P.; Vince, J.E. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Hirano, M.; Das, S.; Guo, P.; Cooper, M.D. The evolution of adaptive immunity in vertebrates. Adv. Immunol. 2011, 109, 125–157. [Google Scholar] [CrossRef]
- Sima, P.; Vetvicka, V. Evolution of Immune Functions; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Větvicčka, V.; šíma, P. Evolutionary Mechanisms of Defense Reactions; Birkhauser Verlag: Basel, Switzerland; Boston, MA, USA, 1998; p. 21. 196 p. [Google Scholar]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Mack, M.; Schneider, M.A.; Moll, C.; Cihak, J.; Bruhl, H.; Ellwart, J.W.; Hogarth, M.P.; Stangassinger, M.; Schlondorff, D. Identification of antigen-capturing cells as basophils. J. Immunol. 2005, 174, 735–741. [Google Scholar] [CrossRef]
- Denzel, A.; Maus, U.A.; Rodriguez Gomez, M.; Moll, C.; Niedermeier, M.; Winter, C.; Maus, R.; Hollingshead, S.; Briles, D.E.; Kunz-Schughart, L.A.; et al. Basophils enhance immunological memory responses. Nat. Immunol. 2008, 9, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Voehringer, D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J. Immunol. 2010, 184, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, C.; Voehringer, D. Basophils: Important emerging players in allergic and anti-parasite responses. Bioessays 2011, 33, 423–426. [Google Scholar] [CrossRef]
- Glatman Zaretsky, A.; Engiles, J.B.; Hunter, C.A. Infection-induced changes in hematopoiesis. J. Immunol. 2014, 192, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grainger, J.R.; Grencis, R.K. Neutrophils worm their way into macrophage long-term memory. Nat. Immunol. 2014, 15, 902–904. [Google Scholar] [CrossRef]
- Chen, F.; Wu, W.; Millman, A.; Craft, J.F.; Chen, E.; Patel, N.; Boucher, J.L.; Urban, J.F., Jr.; Kim, C.C.; Gause, W.C. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 2014, 15, 938–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchery, T.; Kyle, R.; Camberis, M.; Shepherd, A.; Filbey, K.; Smith, A.; Harvie, M.; Painter, G.; Johnston, K.; Ferguson, P.; et al. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat. Commun. 2015, 6, 6970. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Bistoni, F.; Verducci, G.; Perito, S.; Vecchiarelli, A.; Puccetti, P.; Marconi, P.; Cassone, A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J. Med. Vet. Mycol. 1988, 26, 285–299. [Google Scholar] [CrossRef]
- Garcia-Valtanen, P.; Guzman-Genuino, R.M.; Williams, D.L.; Hayball, J.D.; Diener, K.R. Evaluation of trained immunity by β-1, 3 (d)-glucan on murine monocytes in vitro and duration of response in vivo. Immunol. Cell Biol. 2017, 95, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Bando, J.K.; Colonna, M. Innate lymphoid cell function in the context of adaptive immunity. Nat. Immunol. 2016, 17, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Crinier, A.; Vely, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V.S.; Robinette, M.L.; Colonna, M. Innate lymphoid cells: New insights into function and development. Curr. Opin. Immunol. 2015, 32, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kansler, E.R.; Li, M.O. Innate lymphocytes-lineage, localization and timing of differentiation. Cell. Mol. Immunol. 2019, 16, 627–633. [Google Scholar] [CrossRef]
- Askenase, M.H.; Han, S.J.; Byrd, A.L.; Morais da Fonseca, D.; Bouladoux, N.; Wilhelm, C.; Konkel, J.E.; Hand, T.W.; Lacerda-Queiroz, N.; Su, X.Z.; et al. Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity 2015, 42, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; von Andrian, U.H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Sun, J.C.; Madera, S.; Bezman, N.A.; Beilke, J.N.; Kaplan, M.H.; Lanier, L.L. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 2012, 209, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [Green Version]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Hao, S.; Zheng, M.; Lin, D.; Jiang, P.; Zhao, J.; Shi, H.; Yang, X.; Li, X.; Yu, Y. Oat-derived β-glucans induced trained immunity through metabolic reprogramming. Inflammation 2020, 43, 1323–1336. [Google Scholar] [CrossRef]
- Keating, S.T.; Groh, L.; van der Heijden, C.; Rodriguez, H.; Dos Santos, J.C.; Fanucchi, S.; Okabe, J.; Kaipananickal, H.; van Puffelen, J.H.; Helder, L.; et al. The Set7 lysine methyltransferase regulates plasticity in oxidative phosphorylation necessary for trained immunity induced by β-glucan. Cell Rep. 2020, 31, 107548. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J. Fungal mediated innate immune memory, what have we learned? Semin. Cell Dev. Biol. 2019, 89, 71–77. [Google Scholar] [CrossRef]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; O’Neill, L.A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 2016, 46, 13–21. [Google Scholar] [CrossRef]
- Di Luzio, N.R.; Williams, D.L. Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect. Immun. 1978, 20, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Marakalala, M.J.; Williams, D.L.; Hoving, J.C.; Engstad, R.; Netea, M.G.; Brown, G.D. Dectin-1 plays a redundant role in the immunomodulatory activities of β-glucan-rich ligands in vivo. Microbes Infect. 2013, 15, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Krahenbuhl, J.L.; Sharma, S.D.; Ferraresi, R.W.; Remington, J.S. Effects of muramyl dipeptide treatment on resistance to infection with Toxoplasma gondii in mice. Infect. Immun. 1981, 31, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Munoz, N.; Van Maele, L.; Marques, J.M.; Rial, A.; Sirard, J.C.; Chabalgoity, J.A. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect. Immun. 2010, 78, 4226–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribes, S.; Meister, T.; Ott, M.; Redlich, S.; Janova, H.; Hanisch, U.K.; Nessler, S.; Nau, R. Intraperitoneal prophylaxis with CpG oligodeoxynucleotides protects neutropenic mice against intracerebral Escherichia coli K1 infection. J. Neuroinflammation 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chassaing, B.; Shi, Z.; Uchiyama, R.; Zhang, Z.; Denning, T.L.; Crawford, S.E.; Pruijssers, A.J.; Iskarpatyoti, J.A.; Estes, M.K.; et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 2014, 346, 861–865. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, J.W.; Barza, M.; Wolff, S.M.; Dinarello, C.A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc. Natl. Acad. Sci. USA 1988, 85, 1620–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribouley, J.; Tribouley-Duret, J.; Appriou, M. Effect of Bacillus Callmette Guerin (BCG) on the receptivity of nude mice to Schistosoma mansoni. C. R. Seances Soc. Biol. Fil. 1978, 172, 902–904. [Google Scholar]
- Van’t Wout, J.W.; Poell, R.; van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992, 36, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bistoni, F.; Vecchiarelli, A.; Cenci, E.; Puccetti, P.; Marconi, P.; Cassone, A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 1986, 51, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchiarelli, A.; Cenci, E.; Puliti, M.; Blasi, E.; Puccetti, P.; Cassone, A.; Bistoni, F. Protective immunity induced by low-virulence Candida albicans: Cytokine production in the development of the anti-infectious state. Cell Immunol. 1989, 124, 334–344. [Google Scholar] [CrossRef]
- Barton, E.S.; White, D.W.; Cathelyn, J.S.; Brett-McClellan, K.A.; Engle, M.; Diamond, M.S.; Miller, V.L.; Virgin, H.W.t. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007, 447, 326–329. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [Green Version]
- Walk, J.; de Bree, L.C.J.; Graumans, W.; Stoter, R.; van Gemert, G.J.; van de Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freyne, B.; Donath, S.; Germano, S.; Gardiner, K.; Casalaz, D.; Robins-Browne, R.M.; Amenyogbe, N.; Messina, N.L.; Netea, M.G.; Flanagan, K.L.; et al. Neonatal BCG vaccination influences cytokine responses to Toll-like receptor ligands and heterologous antigens. J. Infect. Dis. 2018, 217, 1798–1808. [Google Scholar] [CrossRef]
- Jensen, K.J.; Larsen, N.; Biering-Sorensen, S.; Andersen, A.; Eriksen, H.B.; Monteiro, I.; Hougaard, D.; Aaby, P.; Netea, M.G.; Flanagan, K.L.; et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: A randomized-controlled trial. J. Infect. Dis. 2015, 211, 956–967. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer—A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.H.t.; Levine, E.A. Role of bacillus Calmette-Guerin in the treatment of advanced melanoma. Expert Rev. Anticancer Ther. 2011, 11, 1671–1676. [Google Scholar] [CrossRef]
- Powles, R.L.; Russell, J.; Lister, T.A.; Oliver, T.; Whitehouse, J.M.; Malpas, J.; Chapuis, B.; Crowther, D.; Alexander, P. Immunotherapy for acute myelogenous leukaemia: A controlled clinical study 2 1/2 years after entry of the last patient. Br. J. Cancer 1977, 35, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Villumsen, M.; Sorup, S.; Jess, T.; Ravn, H.; Relander, T.; Baker, J.L.; Benn, C.S.; Sorensen, T.I.; Aaby, P.; Roth, A. Risk of lymphoma and leukaemia after bacille Calmette-Guerin and smallpox vaccination: A Danish case-cohort study. Vaccine 2009, 27, 6950–6958. [Google Scholar] [CrossRef]
- Walk, J.; Keramati, F.; de Bree, L.C.J.; Arts, R.J.W.; Blok, B.; Netea, M.G.; Stunnenberg, H.G.; Sauerwein, R.W. Controlled human malaria infection induces long-term functional changes in monocytes. Front. Mol. Biosci. 2020, 7, 604553. [Google Scholar] [CrossRef]
- Petit, J.; Embregts, C.W.E.; Forlenza, M.; Wiegertjes, G.F. Evidence of trained immunity in a fish: Conserved features in carp macrophages. J. Immunol. 2019, 203, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.; Weber, K.S.; Johnson, S.M. Exposome and Immunity Training: How Pathogen Exposure Order Influences Innate Immune Cell Lineage Commitment and Function. Int. J. Mol. Sci. 2020, 21, 8462. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Miura, N.N.; Ohno, N.; Aketagawa, J.; Tamura, H.; Tanaka, S.; Yadomae, T. Blood clearance of (1-->3)-β-D-glucan in MRL lpr/lpr mice. FEMS Immunol. Med. Microbiol. 1996, 13, 51–57. [Google Scholar] [CrossRef]
- Schwartz, B.; Vetvicka, V. Review: β-glucans as effective antibiotic alternatives in poultry. Molecules 2021, 26, 3560. [Google Scholar] [CrossRef]
- Sima, P.; Richter, J.; Vetvicka, V. Glucans as new anticancer agents. Anticancer Res. 2019, 39, 3373–3378. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, J.; Grosse, S.; Marx, C.; Siwczak, F.; Stengel, S.; Bruns, T.; Bauer, R.; Kiehntopf, M.; Williams, D.L.; Wang, Z.Q.; et al. Candida albicans β-glucan differentiates human monocytes into a specific subset of macrophages. Front. Immunol. 2018, 9, 2818. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Effects of yeast-derived β-glucans on blood cholesterol and macrophage functionality. J. Immunotoxicol. 2009, 6, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sima, P.; Vetvicka, V.; Vannucci, L. Ambiguous role of immunity in malignant neoplasms. J. Tumor 2020, 8, 560–564. [Google Scholar]
- Namakula, R.; de Bree, L.C.J.; TH, A.T.; Netea, M.G.; Cose, S.; Hanevik, K. Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and β-glucan. PLoS One 2020, 15, e0229287. [Google Scholar] [CrossRef] [Green Version]
- Moorlag, S.; Khan, N.; Novakovic, B.; Kaufmann, E.; Jansen, T.; van Crevel, R.; Divangahi, M.; Netea, M.G. β-Glucan induces protective trained immunity against Mycobacterium tuberculosis infection: A key role for IL-1. Cell Rep. 2020, 31, 107634. [Google Scholar] [CrossRef]
- Walachowski, S.; Tabouret, G.; Fabre, M.; Foucras, G. Molecular analysis of a short-term model of β-glucans-trained immunity highlights the accessory contribution of GM-CSF in priming mouse macrophages response. Front. Immunol. 2017, 8, 1089. [Google Scholar] [CrossRef] [Green Version]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef] [PubMed]
- Isoda, N.; Eguchi, Y.; Nukaya, H.; Hosho, K.; Suga, Y.; Suga, T.; Nakazawa, S.; Sugano, K. Clinical efficacy of superfine dispersed lentinan (beta-1,3-glucan) in patients with hepatocellular carcinoma. Hepatogastroenterology 2009, 56, 437–441. [Google Scholar] [PubMed]
- Wu, L.; Zhao, J.; Zhang, X.; Liu, S.; Zhao, C. Antitumor effect of soluble β-glucan as an immune stimulant. Int. J. Biol. Macromol. 2021, 179, 116–124. [Google Scholar] [CrossRef]
- Geller, A.; Shrestha, R.; Yan, J. Yeast-derived β-glucan in cancer: Novel uses of a traditional therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourits, V.P.; Arts, R.J.W.; Novakovic, B.; Matzaraki, V.; de Bree, L.C.J.; Koeken, V.; Moorlag, S.; van Puffelen, J.H.; Groh, L.; van der Heijden, C.; et al. The role of Toll-like receptor 10 in modulation of trained immunity. Immunology 2020, 159, 289–297. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Z.; Zhang, W.; Xie, X.; Song, N.; Lv, T.; Wu, D.; Cao, Y. The preventable efficacy of β-glucan against leptospirosis. PLoS Negl. Trop. Dis. 2019, 13, e0007789. [Google Scholar] [CrossRef]
- Vetvicka, V.; Fernandez-Botran, R. β-Glucan and parasites. Helminthologia 2018, 55, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, J.C.; Barroso de Figueiredo, A.M.; Teodoro Silva, M.V.; Cirovic, B.; de Bree, L.C.J.; Damen, M.; Moorlag, S.; Gomes, R.S.; Helsen, M.M.; Oosting, M.; et al. β-glucan-induced trained immunity protects against Leishmania braziliensis infection: A crucial role for IL-32. Cell Rep. 2019, 28, 2659–2672.e6. [Google Scholar] [CrossRef] [Green Version]
- Paris, S.; Chapat, L.; Martin-Cagnon, N.; Durand, P.Y.; Piney, L.; Cariou, C.; Bergamo, P.; Bonnet, J.M.; Poulet, H.; Freyburger, L.; et al. β-glucan as trained immunity-based adjuvants for rabies vaccines in dogs. Front. Immunol. 2020, 11, 564497. [Google Scholar] [CrossRef]
- Verwoolde, M.B.; van den Biggelaar, R.; van Baal, J.; Jansen, C.A.; Lammers, A. Training of primary chicken monocytes results in enhanced pro-inflammatory responses. Vet. Sci. 2020, 7, 115. [Google Scholar] [CrossRef]
- Verwoolde, M.B.; van den Biggelaar, R.; de Vries Reilingh, G.; Arts, J.A.J.; van Baal, J.; Lammers, A.; Jansen, C.A. Innate immune training and metabolic reprogramming in primary monocytes of broiler and laying hens. Dev. Comp. Immunol. 2021, 114, 103811. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Angulo, C. Oral administration of Debaryomyces hansenii CBS8339-β-glucan induces trained immunity in newborn goats. Dev. Comp. Immunol. 2020, 105, 103597. [Google Scholar] [CrossRef]
- Libran-Perez, M.; Costa, M.M.; Figueras, A.; Novoa, B. β-glucan administration induces metabolic changes and differential survival rates after bacterial or viral infection in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2018, 82, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Chi, H.; Dalmo, R.A. Trained innate immunity of fish is a viable approach in larval aquaculture. Front. Immunol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.X.; Zhang, J. Alternative pre-mRNA splicing in mammals and teleost fish: A effective strategy for the regulation of immune responses against pathogen infection. Int. J. Mol. Sci. 2017, 18, 1530. [Google Scholar] [CrossRef]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Dominguez-Andres, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained immunity: A tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. Could the induction of trained immunity by β-glucan serve as a defense against COVID-19? Front. Immunol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Bono, C.; Martinez, A.; Megias, J.; Gozalbo, D.; Yanez, A.; Gil, M.L. Dectin-1 Stimulation of Hematopoietic Stem and Progenitor Cells Occurs In Vivo and Promotes Differentiation Toward Trained Macrophages via an Indirect Cell-Autonomous Mechanism. mBio 2020, 11, e00781-20. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetvicka, V.; Sima, P.; Vannucci, L. Trained Immunity as an Adaptive Branch of Innate Immunity. Int. J. Mol. Sci. 2021, 22, 10684. https://doi.org/10.3390/ijms221910684
Vetvicka V, Sima P, Vannucci L. Trained Immunity as an Adaptive Branch of Innate Immunity. International Journal of Molecular Sciences. 2021; 22(19):10684. https://doi.org/10.3390/ijms221910684
Chicago/Turabian StyleVetvicka, Vaclav, Petr Sima, and Luca Vannucci. 2021. "Trained Immunity as an Adaptive Branch of Innate Immunity" International Journal of Molecular Sciences 22, no. 19: 10684. https://doi.org/10.3390/ijms221910684
APA StyleVetvicka, V., Sima, P., & Vannucci, L. (2021). Trained Immunity as an Adaptive Branch of Innate Immunity. International Journal of Molecular Sciences, 22(19), 10684. https://doi.org/10.3390/ijms221910684






