Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots
Abstract
:1. Introduction
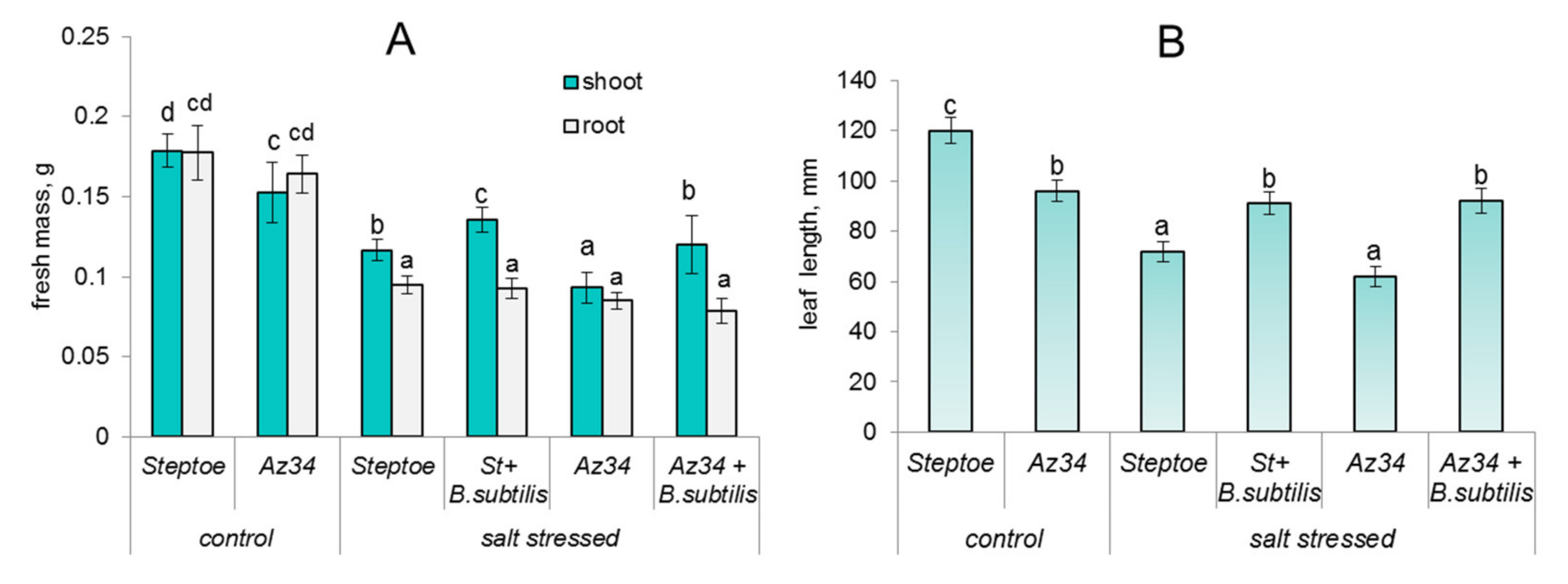
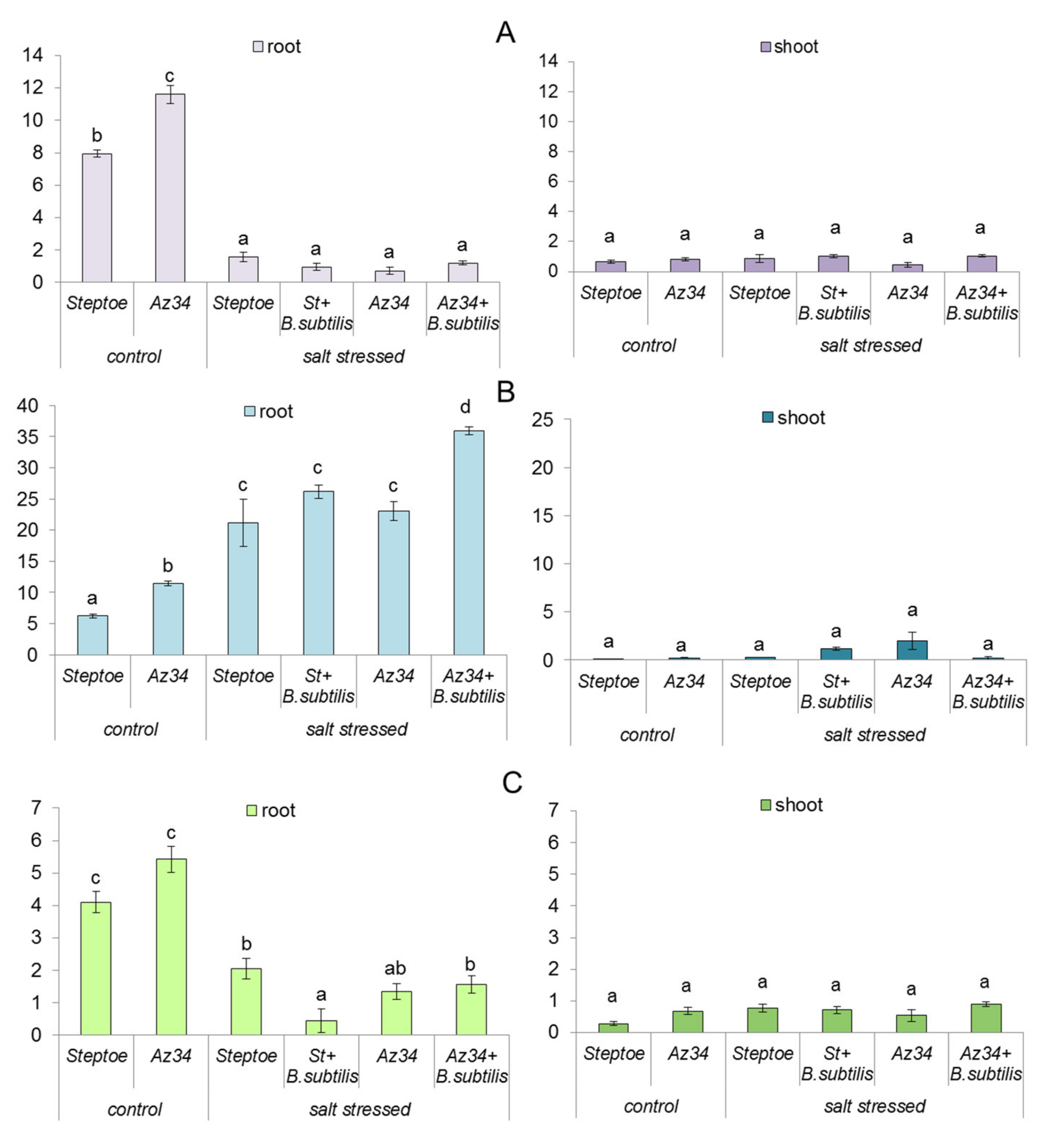
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Bacterial Strain and Culture Media
4.3. ABA Assay
4.4. Total Barley RNA
4.5. Water Relation Measurements
4.5.1. Transpiration
4.5.2. Relative Water Content (RWC)
4.6. Stomatal Conductance
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dodd, I.C.; Perez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [Green Version]
- Mokrani, S.; Nabti, E.; Cruz, C. Current advances in plant growth promoting bacteria alleviating salt stress for sustainable agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- He, A.-L.; Niu, S.-Q.; Zhao, Q.; Li, Y.-S.; Gou, J.-Y.; Gao, H.-J.; Suo, S.-Z.; Zhang, J.-L. Induced Salt Tolerance of Perennial Ryegrass by a Novel Bacterium Strain from the Rhizosphere of a Desert Shrub Haloxylon ammodendron. Int. J. Mol. Sci. 2018, 19, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzzia, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hort. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Shi, T.-Q.; Peng, H.; Zeng, S.-Y.; Ji, R.-Y.; Shi, K.; Huang, H.; Ji, X.-J. Microbial production of plant hormones: Opportunities and challenges. Bioengineered 2017, 8, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Poupin, M.J.; Greve, M.; Carmona, V.; Pinedo, I. A complex molecular interplay of auxin and ethylene signaling pathways is involved in arabidopsis growth promotion by Burkholderia phytofirmans PsJN. Front. Plant Sci. 2016, 7, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Singh, P.C.; Chaudhry, V.; Shirke, P.A.; Chakrabarty, D.; Farooqui, A.; Nautiya, C.S.; Sane, A.P.; Sane, V.A. PGPR-induced OsASR6 improves plant growth and yield by altering root auxin sensitivity and the xylem structure in transgenic Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153010. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arabidopsis Book 2013, 11, e0166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in arabidopsis plants. Plant Growth Regul. 2008, 54, 97–103. [Google Scholar] [CrossRef]
- Yuzikhin, O.S.; Gogoleva, N.E.; Shaposhnikov, A.I.; Konnova, T.A.; Osipova, E.V.; Syrova, D.S.; Ermakova, E.A.; Shevchenko, V.P.; Nagaev, I.Y.; Shevchenko, K.V.; et al. Rhizosphere bacterium rhodococcus sp. P1Y metabolizes abscisic acid to form dehydrovomifoliol. Biomolecules 2021, 11, 345. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Khana, A.L.; Waqasa, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Porcel, R.; Zamarreno, A.M.; Garcia-Mina, J.M.; Aroca, R. Involvement of plant endogenous ABA in Bacillus megaterium PGPR activity in tomato plants. BMC Plant Biol. 2014, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [Green Version]
- Chono, M.; Honda, I.; Shinoda, S.; Kushiro, T.; Kamiya, Y.; Nambara, E.; Kawakami, N.; Kaneko, S.; Watanabe, Y. Field studies on the regulation of abscisic acid content and germinability during grain development of barley: Molecular and chemical analysis of pre-harvest sprouting. J. Exp. Bot. 2006, 57, 2421–2434. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Arkhipova, T.N.; Shendel, G.V.; Kuzmina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Effect of rhizobacteria on phytohormone status of potato microclones under osmotic stress in vitro. Biomolecules 2020, 10, 1231. [Google Scholar] [CrossRef]
- Wilkinson, S.; Kudoyarova, G.R.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant hormone interactions: Innovative targets for crop breeding and management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef]
- Sharipova, G.; Veselov, D.; Fricke, W.; Dodd, I.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.Y.; Dodd, I.C.; Ivanov, I.; Kudoyarova, G.R. Rapid changes in root HvPIP2;2 aquaporins abundance and ABA concentration are required to enhance root hydraulic conductivity and maintain leaf water potential in response to increased evaporative demand. Funct. Plant Biol. 2018, 45, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Walker-Simmons, M.; Kudrna, D.A.; Warner, R.L. Reduced accumulation of aba during water stress in a molybdenum cofactor mutant of barley. Plant Physiol. 1989, 90, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.; McCarty, D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–12240. [Google Scholar] [CrossRef] [Green Version]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Growth Regul. 2005, 24, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Vandeleur, R.K.; Sullivan, W.; Athman, A.; Jordans, C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ. 2014, 37, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [Green Version]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, L.; Belimov, A.A.; Shaposhnikov, A.I.; Gong, F.; Meng, X.; Hartung, W.; Jeschke, D.W.; Davies, W.J.; Dodd, I.C. Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum. J. Exp. Bot. 2012, 63, 6421–6430. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Veselova, S.; Hartung, W.; Farhutdinov, R.; Veselov, D.; Sharipova, G. Involvement of root ABA and hydraulic conductance in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 2011, 233, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; California Agriculture Experiment Station Circular 347-2; University of California College of Agriculture: Berkeley, CA, USA, 1950. [Google Scholar]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.U.; Kudoyarova, G.R. The effects of NaCl treatment on water relations, growth and ABA content in barley cultivars differing in drought tolerance. J. Plant Growth Regul. 2008, 27, 380–386. [Google Scholar] [CrossRef]


| Genes | Strand | 5′ to 3′ Primer Sequences | GenBank Accession Number |
|---|---|---|---|
| HvNCED1 | Forward | CCCCTATGGCTTCCACGGCACATT | AB239297 |
| Reverse | GTGATGAGTAACCGCCGCTAACTG | ||
| HvNCED2 | Forward | CGGGTACATTCTCACCTTCGTGCA | AB239298 |
| Reverse | CTGGCAACTTCCTCTTTCCATGTCC | ||
| HvCYP707A1 | Forward | CCACCAAGTACAGATGGTCTAC | AB239299 |
| Reverse | TCCGAAGGAGGAAGACATAGA | ||
| HvGADPH | Forward | GCCACTATTCTTCAGGGACTT | EF409626 |
| Reverse | CTTCTTGGCACCACCCTAATA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. Int. J. Mol. Sci. 2021, 22, 10680. https://doi.org/10.3390/ijms221910680
Akhtyamova Z, Arkhipova T, Martynenko E, Nuzhnaya T, Kuzmina L, Kudoyarova G, Veselov D. Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. International Journal of Molecular Sciences. 2021; 22(19):10680. https://doi.org/10.3390/ijms221910680
Chicago/Turabian StyleAkhtyamova, Zarina, Tatiana Arkhipova, Elena Martynenko, Tatyana Nuzhnaya, Ludmila Kuzmina, Guzel Kudoyarova, and Dmitry Veselov. 2021. "Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots" International Journal of Molecular Sciences 22, no. 19: 10680. https://doi.org/10.3390/ijms221910680
APA StyleAkhtyamova, Z., Arkhipova, T., Martynenko, E., Nuzhnaya, T., Kuzmina, L., Kudoyarova, G., & Veselov, D. (2021). Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. International Journal of Molecular Sciences, 22(19), 10680. https://doi.org/10.3390/ijms221910680







