Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium (IV) Oxide Particles
2.2. Simulated Body Fluids, Blood Plasma, and Cultivation Media
2.3. Preparation of TiO2 Dispersions
2.4. Characterization
2.4.1. Dynamic Light Scattering
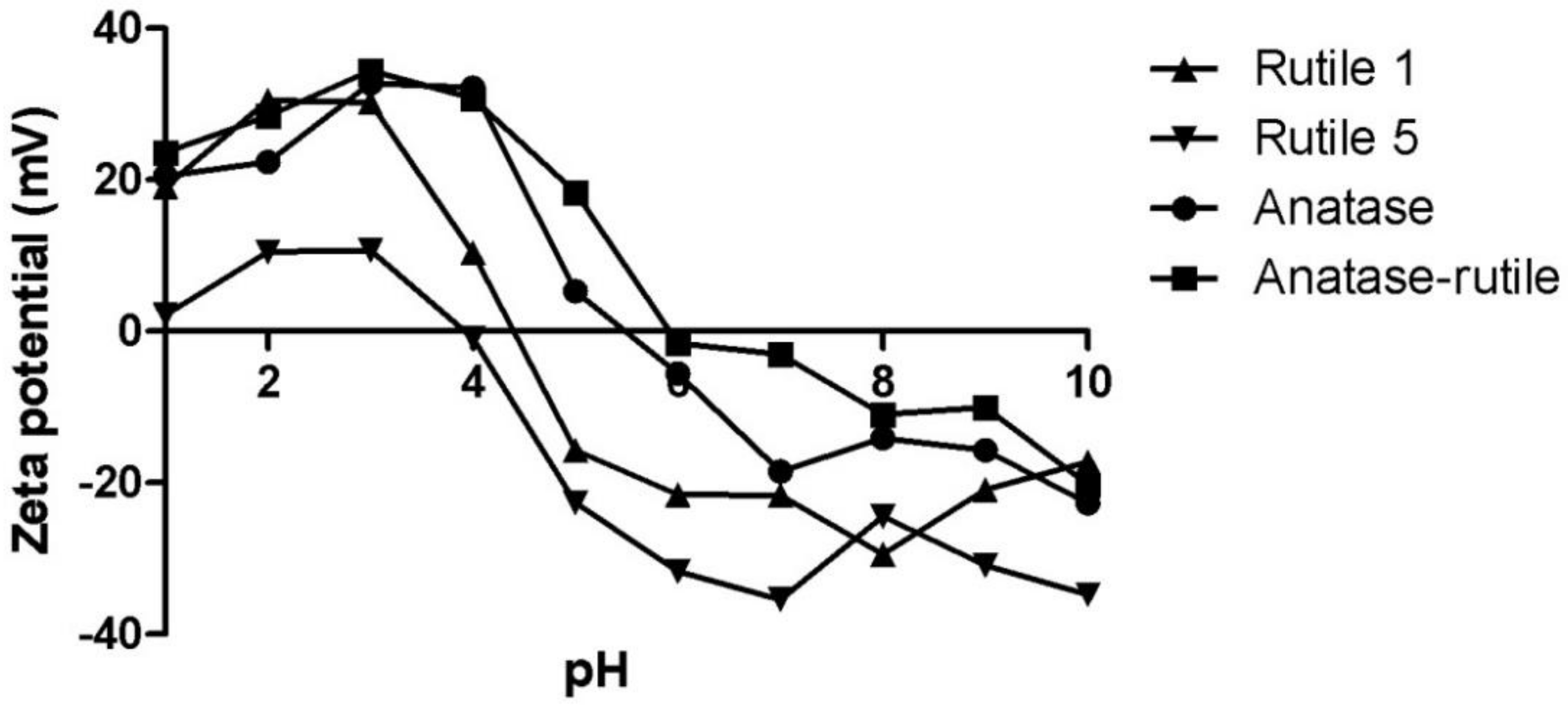
2.4.2. Zeta Potential
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Cytotoxicity
3. Results and Discussion
3.1. Morphology of TiO2 Particles
3.2. Behaviour of TiO2 Particles in Water
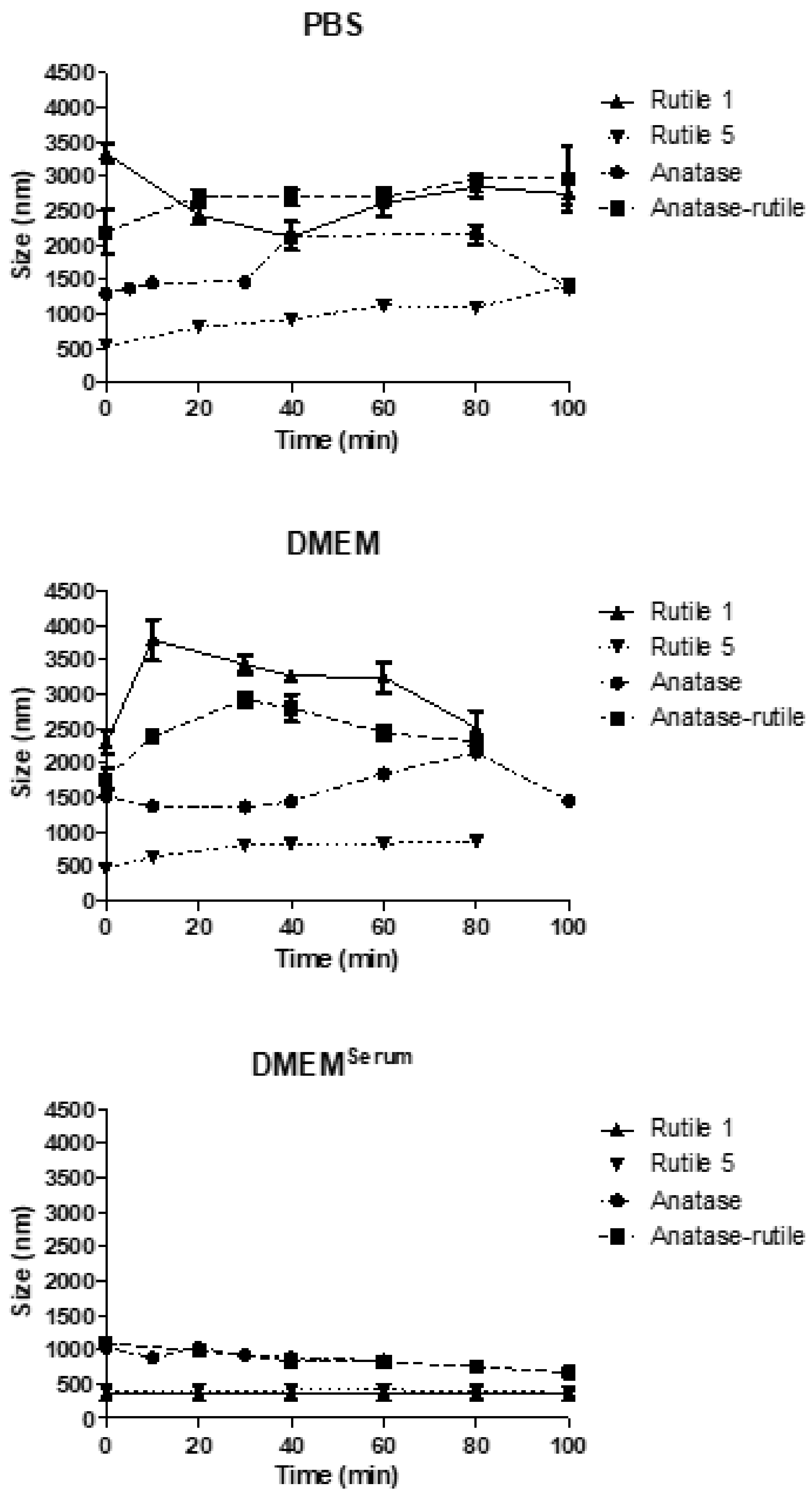
3.3. TiO2 Particles in Cultivation Media
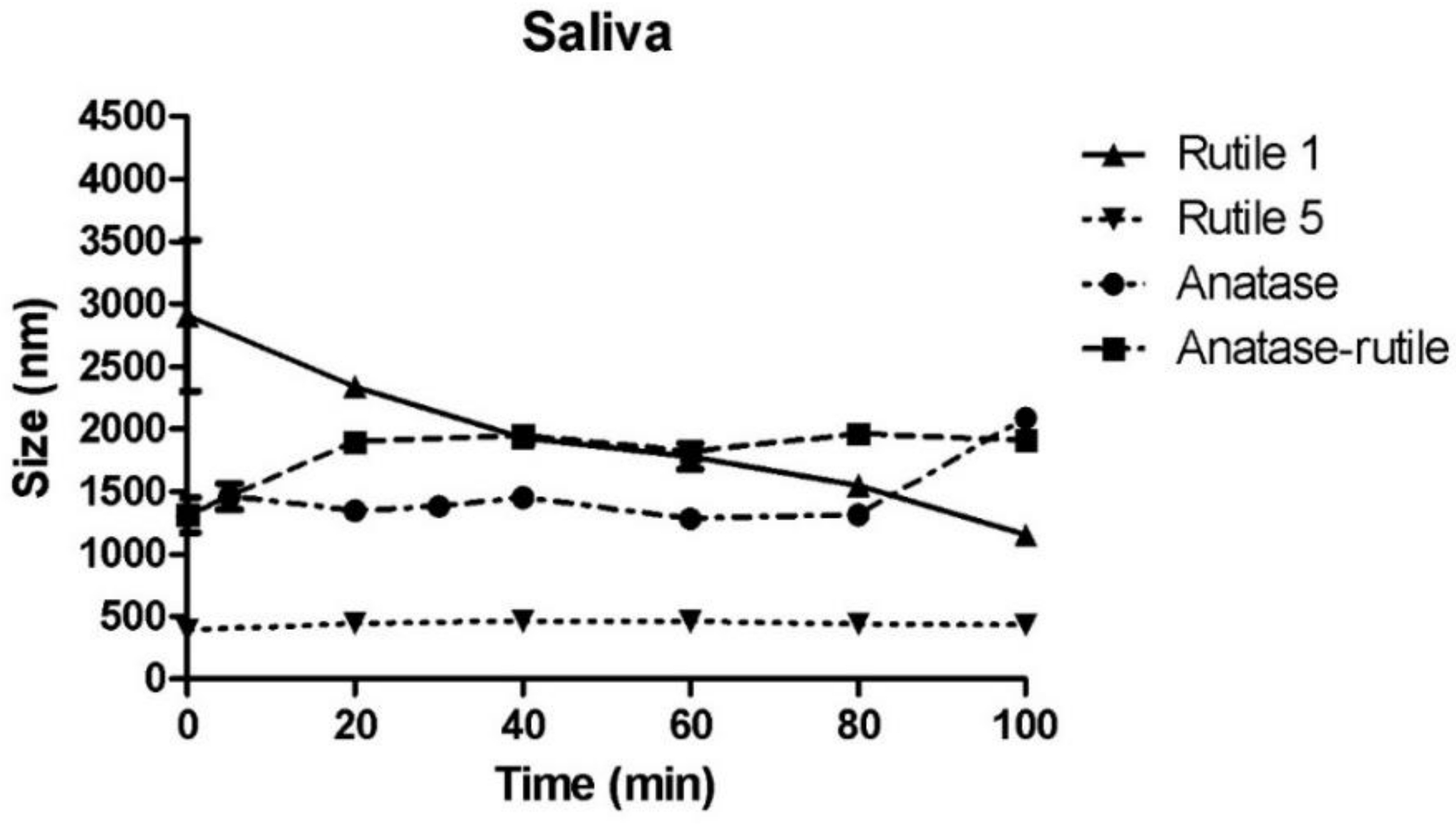
3.3.1. Simulated Saliva
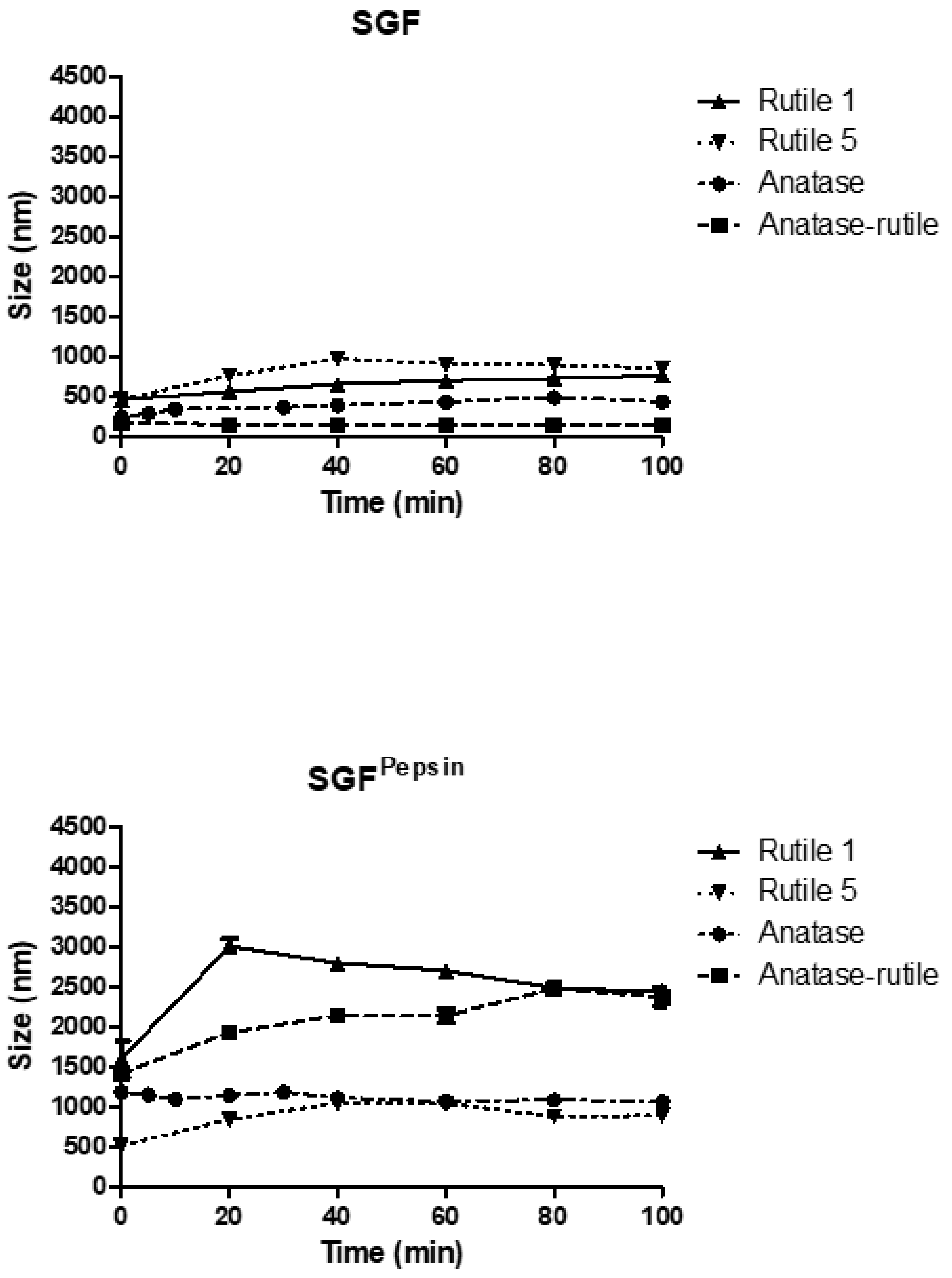
3.3.2. Simulated Gastric Fluid
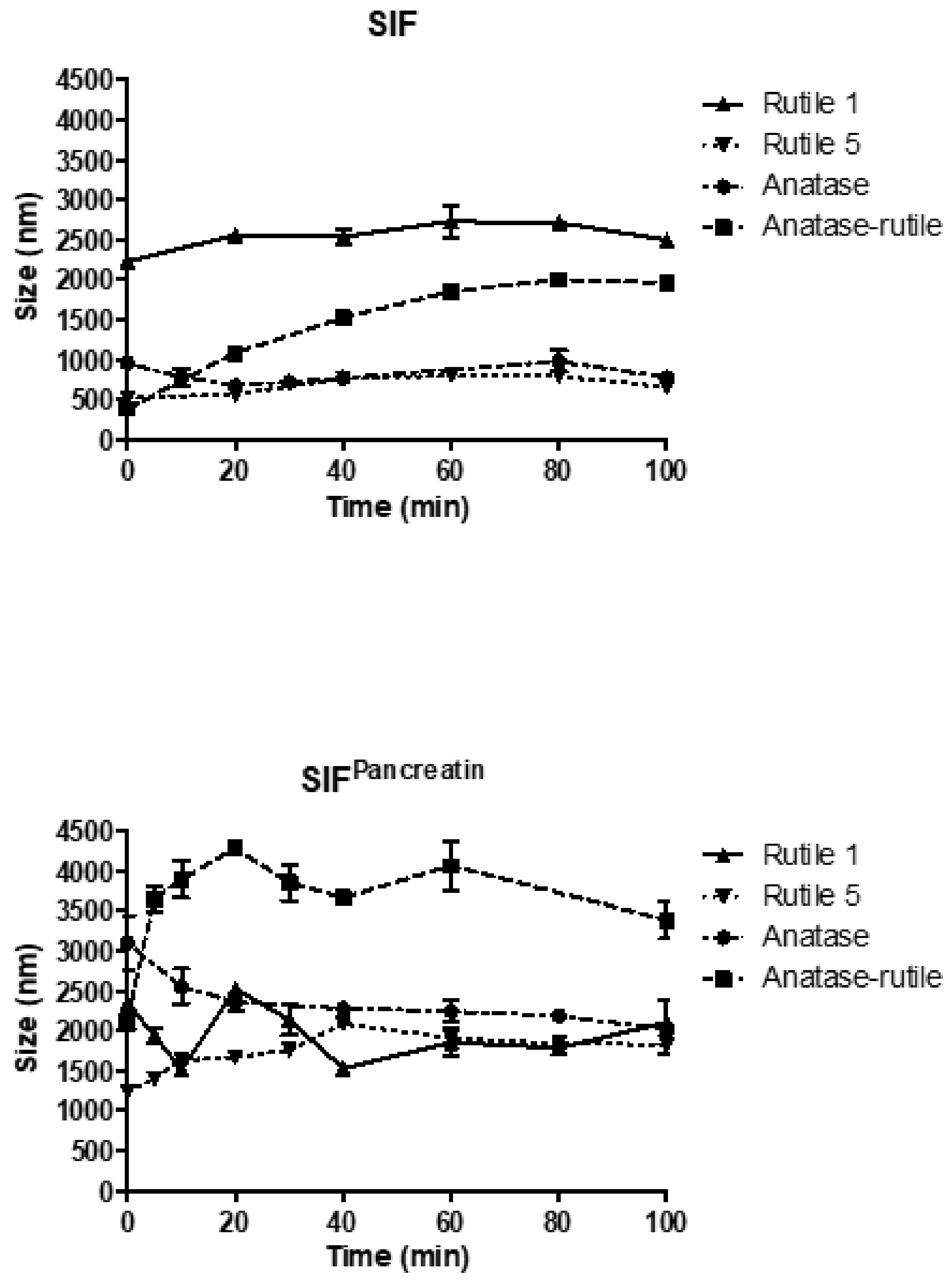
3.3.3. Simulated Intestinal Fluid
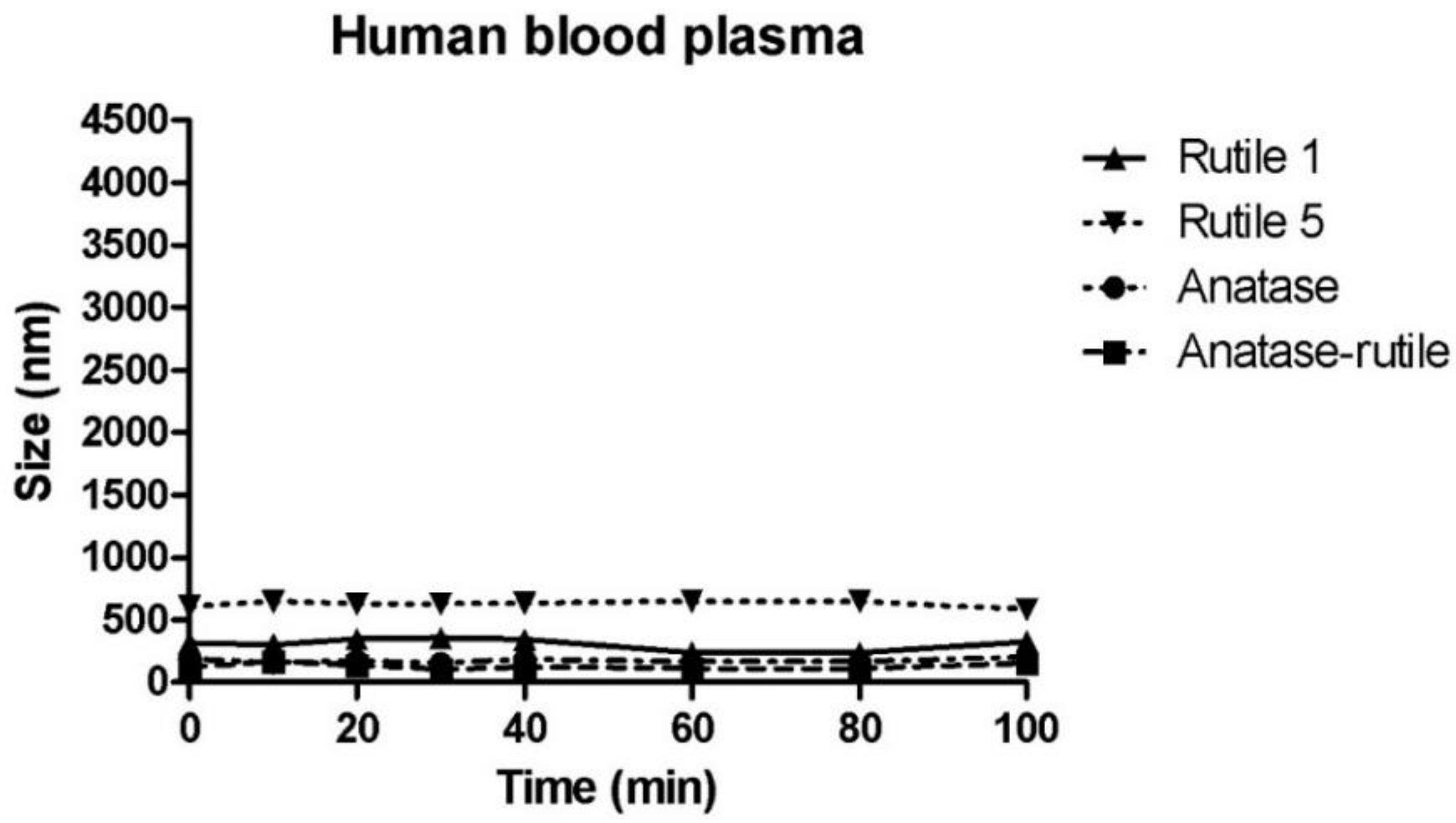
3.4. Behaviour of TiO2 Particles in Human Blood Plasma
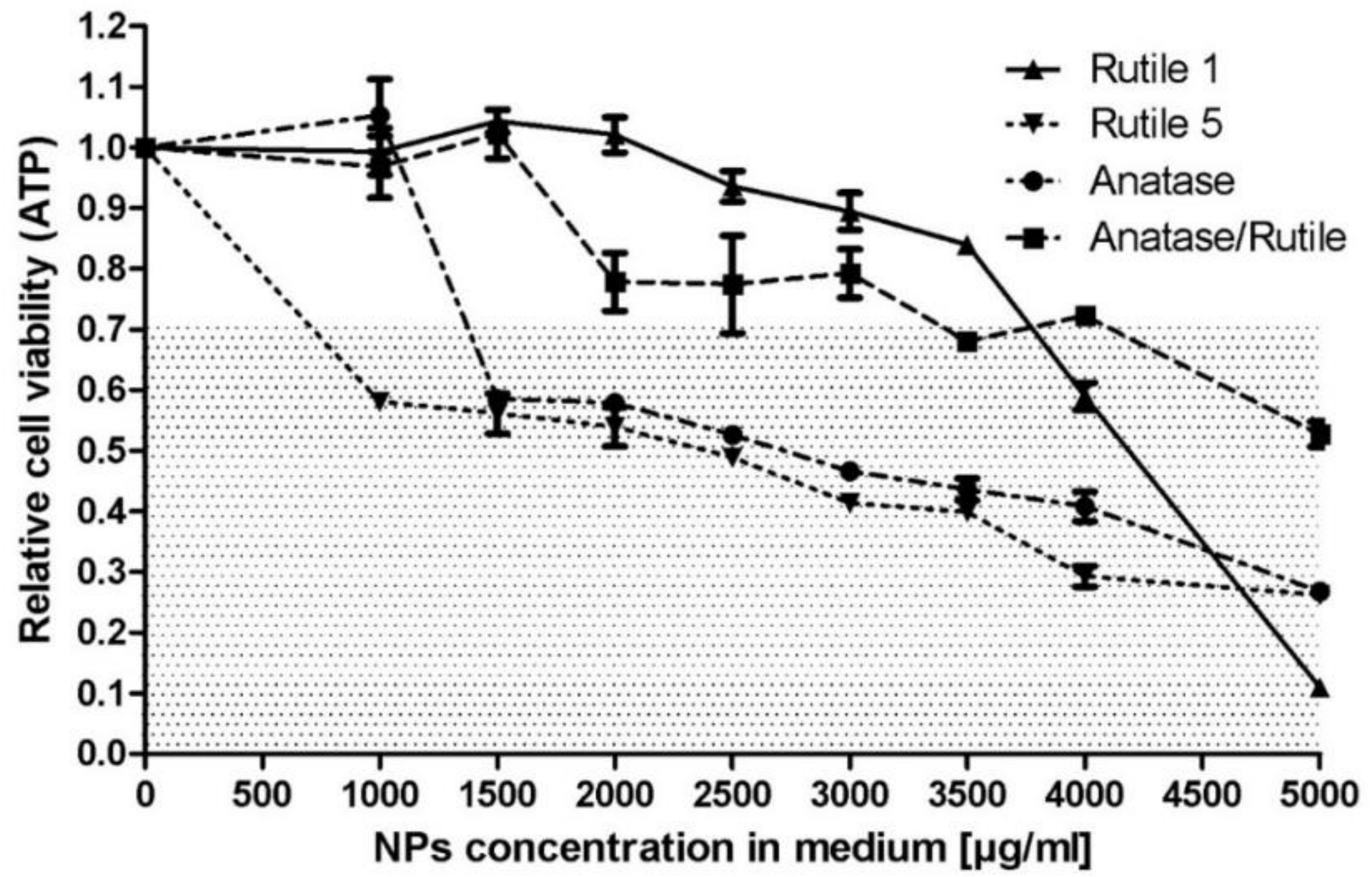
3.5. Biological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, X.; Chen, X. Titanium Dioxide Nanomaterials. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–38. [Google Scholar] [CrossRef]
- Cho, W.-S.; Kang, B.-C.; Lee, J.K.; Jeong, J.; Che, J.-H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Haghi, M.; Hekmatafshar, M.; Janipour, M.B.; Seyyed, S. Antibacterial Effect of TiO2 Nanoparticles on Pathogenic Strain of E. coli. IJABR 2012, 3, 621–624. [Google Scholar]
- Ahmad, R. Antibacterial Agents Against E. coli. IJIRSET 2013, 2, 3569–3574. [Google Scholar]
- Abdulazeem, L.; L-Amiedi, B.H.A.; Alrubaei, H.A.; L-Mawlah, Y.H.A. Titanium dioxide nanoparticles as antibacterial agents against some pathogenic bacteria. Drug Invent. Today 2019, 12, 5. [Google Scholar]
- Abbasi, A. TiO2-Based Nanocarriers for Drug Delivery. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–248. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Uboldi, C.; Urbán, P.; Gilliland, D.; Bajak, E.; Valsami-Jones, E.; Ponti, J.; Rossi, F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol. Vitr. 2016, 31, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Allouni, Z.E.; Cimpan, M.R.; Høl, P.J.; Skodvin, T.; Gjerdet, N.R. Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Colloids Surf. B Biointerfaces 2009, 68, 83–87. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Tiancheng, W.; Yu, H.; Wang, T.; Ma, Y.; Jiangxue, W.; Gao, Y.; Li, Y.-F.; Sun, J. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef]
- Xie, G.; Lu, W.; Lu, D. Penetration of titanium dioxide nanoparticles through slightly damaged skin in vitro and in vivo. JABFM 2015, 13, 356–361. [Google Scholar] [CrossRef]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef]
- Pelclova, D.; Navratil, T.; Kacerova, T.; Zamostna, B.; Fenclova, Z.; Vlckova, S.; Kacer, P. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials 2019, 9, 888. [Google Scholar] [CrossRef]
- Jones, K.; Morton, J.; Smith, I.; Jurkschat, K.; Harding, A.-H.; Evans, G. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2015, 233, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Marucco, A.; Prono, M.; Beal, D.; Alasonati, E.; Fisicaro, P.; Bergamaschi, E.; Carriere, M.; Fenoglio, I. Biotransformation of Food-Grade and Nanometric TiO2 in the Oral–Gastro–Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials 2020, 10, 2132. [Google Scholar] [CrossRef] [PubMed]
- Dudefoi, W.; Rabesona, H.; Rivard, C.; Mercier-Bonin, M.; Humbert, B.; Terrisse, H.; Ropers, M.-H. In vitro digestion of food grade TiO2 (E171) and TiO2 nanoparticles: Physicochemical characterization and impact on the activity of digestive enzymes. Food Funct. 2021, 12, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2019, 193, 118–129. [Google Scholar] [CrossRef]
- Warheit, D.B.; Donner, E.M. Risk assessment strategies for nanoscale and fine-sized titanium dioxide particles: Recognizing hazard and exposure issues. Food Chem. Toxicol. 2015, 85, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Iosi, F.; Maranghi, F.; Tassinari, R.; Cubadda, F.; Aureli, F.; Raggi, A.; Superti, F.; Mantovani, A.; De Berardis, B. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells. Food Chem. Toxicol. 2017, 102, 63–75. [Google Scholar] [CrossRef]
- Barrett, A.J.; Woessner, J.F.; Rawlings, N.D. Handbook of Proteolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Showing Compound Pancreatin (FDB001084)—FooDB. Available online: https://foodb.ca/compounds/FDB001084 (accessed on 16 September 2021).
- Pinďáková, L.; Kašpárková, V.; Kejlová, K.; Dvorakova, M.; Krsek, D.; Jírová, D.; Kašparová, L. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int. J. Pharm. 2017, 527, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C.; De Mattos, C.N.; Rêgo, F.M.P.D.; Cardim, L.N.; Altran, S.C.; Paggiaro, A.O.; Tutihashi, R.M.C.; Mathor, M.B.; Ferreira, M.C. Replacement of fetal calf serum by human serum as supplementation for human fibroblast culture. Rev. Bras. Cir. Plást. 2011, 26, 379–384. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, J.; Zhai, W. Effect of Crystallographic Phases of TiO2 on Hepatocyte Attachment, Proliferation and Morphology. J. Biomater. Appl. 2005, 19, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Hezam, M.; Qaid, S.M.H.; Bedja, I.M.; Alharbi, F.; Nazeeruddin, M.K.; Aldwayyan, A. Synthesis of Pure Brookite Nanorods in a Nonaqueous Growth Environment. Crystals 2019, 9, 562. [Google Scholar] [CrossRef]
- Sean, N.A.; Leaw, W.L.; Nur, H. Effect of calcination temperature on the photocatalytic activity of carbon-doped titanium dioxide revealed by photoluminescence study. J. Chin. Chem. Soc. 2019, 66, 1277–1283. [Google Scholar] [CrossRef]
- Li, C.-C.; Chang, S.-J.; Tai, M.-Y. Surface Chemistry and Dispersion Property of TiO2 Nanoparticles. J. Am. Ceram. Soc. 2010, 93, 4008–4010. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar] [CrossRef]
- Kosmulski, M. The significance of the difference in the point of zero charge between rutile and anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef]
- Teubl, B.J.; Schimpel, C.; Leitinger, G.; Bauer, B.; Fröhlich, E.; Zimmer, A.; Roblegg, E. Interactions between nano-TiO2 and the oral cavity: Impact of nanomaterial surface hydrophilicity/hydrophobicity. J. Hazard. Mater. 2015, 286, 298–305. [Google Scholar] [CrossRef]
- Sager, T.M.; Porter, D.W.; Robinson, V.A.; Lindsley, W.G.; Schwegler-Berry, D.E.; Castranova, V. Improved method to disperse nanoparticles forin vitroandin vivoinvestigation of toxicity. Nanotoxicology 2007, 1, 118–129. [Google Scholar] [CrossRef]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.; Godwin, H.; et al. Dispersion and Stability Optimization of TiO2 Nanoparticles in Cell Culture Media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef]
- Pareek, V.; Bhargava, A.; Bhanot, V.; Gupta, R.; Jain, N.; Panwar, J. Formation and Characterization of Protein Corona Around Nanoparticles: A Review. J. Nanosci. Nanotechnol. 2018, 18, 6653–6670. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef]
- Nierenberg, D.; Khaled, A.R.; Flores, O. Formation of a protein corona influences the biological identity of nanomaterials. Rep. Pract. Oncol. Radiother. 2018, 23, 300–308. [Google Scholar] [CrossRef]
- Chen, E.Y.; Liu, W.F.; Megido, L.; Díez, P.; Fuentes, M.; Fager, C.; Olsson, E.; Gessner, I.; Mathur, S. Understanding and utilizing the biomolecule/nanosystems interface. In Nanotechnologies in Preventive and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 207–297. [Google Scholar] [CrossRef]
- Capjak, I.; Goreta, S.; Jurašin, D.D.; Vrček, I.V. How protein coronas determine the fate of engineered nanoparticles in biological environment. Arh. Hig. Rada Toksikol. 2017, 68, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sohal, I.S.; Cho, Y.K.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Dissolution Behavior and Biodurability of Ingested Engineered Nanomaterials in the Gastrointestinal Environment. ACS Nano 2018, 12, 8115–8128. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Oral uptake of nanoparticles: Human relevance and the role of in vitro systems. Arch. Toxicol. 2016, 90, 2297–2314. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhen, T.; Li, Y.; Wang, Y.; Wang, M.; Li, X.; Sun, Q. Interaction of food-grade titanium dioxide nanoparticles with pepsin in simulated gastric fluid. LWT 2020, 134, 110208. [Google Scholar] [CrossRef]
- Gunawan, C.; Lim, M.; Marquis, C.; Amal, R. Nanoparticle–protein corona complexes govern the biological fates and functions of nanoparticles. J. Mater. Chem. B 2014, 2, 2060–2083. [Google Scholar] [CrossRef]
- Zhu, R.-R.; Wang, W.-R.; Sun, X.-Y.; Liu, H.; Wang, S.-L. Enzyme activity inhibition and secondary structure disruption of nano-TiO2 on pepsin. Toxicol. Vitr. 2010, 24, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- McCracken, C.; Zane, A.; Knight, D.A.; Dutta, P.K.; Waldman, W.J. Minimal Intestinal Epithelial Cell Toxicity in Response to Short- and Long-Term Food-Relevant Inorganic Nanoparticle Exposure. Chem. Res. Toxicol. 2013, 26, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Mortimer, G.; Schiller, T.; Musumeci, A.; Martin, D.; Minchin, R.F. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology 2009, 20, 455101. [Google Scholar] [CrossRef] [PubMed]
- Ruh, H.; Kühl, B.; Brenner-Weiss, G.; Hopf, C.; Diabaté, S.; Weiss, C. Identification of serum proteins bound to industrial nanomaterials. Toxicol. Lett. 2012, 208, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Marucco, A.; Fenoglio, I.; Turci, F.; Fubini, B. Interaction of fibrinogen and albumin with titanium dioxide nanoparticles of different crystalline phases. J. Phys. Conf. Ser. 2013, 429. [Google Scholar] [CrossRef]
- Jin, C.-Y.; Zhu, B.-S.; Wang, X.-F.; Lu, Q.-H. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion. Nanomaterials 2020, 10, 1516. [Google Scholar] [CrossRef]
- Gandamalla, D.; Lingabathula, H.; Yellu, N.R. Cytotoxicity Evaluation of Titanium and Zinc Oxide Nanoparticles on Human Cell Lines. Int. J. Pharm. Pharm. Sci. 2017, 9, 240–246. [Google Scholar] [CrossRef][Green Version]
- Hamzeh, M.; Sunahara, G.I. In vitro cytotoxicity and genotoxicity studies of titanium dioxide (TiO2) nanoparticles in Chinese hamster lung fibroblast cells. Toxicol. Vitr. 2013, 27, 864–873. [Google Scholar] [CrossRef]
- Hanot-Roy, M.; Tubeuf, E.; Guilbert, A.; Bado-Nilles, A.; Vigneron, P.; Trouiller, B.; Braun, A.; Lacroix, G. Oxidative stress pathways involved in cytotoxicity and genotoxicity of titanium dioxide (TiO2) nanoparticles on cells constitutive of alveolo-capillary barrier in vitro. Toxicol. Vitr. 2016, 33, 125–135. [Google Scholar] [CrossRef]
- He, P.; Tao, J.; Xue, J.; Chen, Y. Cytotoxicity Property of Nano-TiO2 Sol and Nano-TiO2 Powder. J. Nanomater. 2011, 2011, 261605. [Google Scholar] [CrossRef]
- Kongseng, S.; Yoovathaworn, K.; Wongprasert, K.; Chunhabundit, R.; Sukwong, P.; Pissuwan, D. Cytotoxic and inflammatory responses of TiO2 nanoparticles on human peripheral blood mononuclear cells: High concentrations of TiO2–NPs could induce cytotoxicity in PBMCs. J. Appl. Toxicol. 2016, 36, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Suker, D.K.; Albadran, R.M. Cytotoxic Effects of Titanium Dioxide Nanoparticles on Rat Embryo Fibroblast REF-3 Cell Line In Vitro. Eur. J. Exp. Biol. 2013, 3. Available online: https://www.imedpub.com/abstract/cytotoxic-effects-of-titanium-dioxide-nanoparticles-on-rat-embryo-fibroblast-ref3-cell-line-in-vitro-11714.html (accessed on 17 April 2021).
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2014, 22, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, W.; Guo, J.; Zhang, J.; Sun, Z.; Li, L.; Ding, F.; Gao, M. Cytotoxicity of different sized TiO2 nanoparticles in mouse macrophages. Toxicol. Ind. Health 2012, 29, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Pittol, M.; Tomacheski, D.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Evaluation of the Toxicity of Silver/Silica and Titanium Dioxide Particles in Mammalian Cells. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Rosłon, M.; Jastrzębska, A.; Sitarz, K.; Książek, I.; Koronkiewicz, M.; Anuszewska, E.; Jaworska, M.; Dudkiewicz-Wilczyńska, J.; Ziemkowska, W.; Basiak, D.; et al. The toxicity in vitro of titanium dioxide nanoparticles modified with noble metals on mammalian cells. Int. J. Appl. Ceram. Technol. 2018, 16, 481–493. [Google Scholar] [CrossRef]
- Wagner, S.; Münzer, S.; Behrens, P.; Scheper, T.; Bahnemann, D.B.D.; Kasper, C. Cytotoxicity of titanium and silicon dioxide nanoparticles. J. Phys. Conf. Ser. 2009, 170, 012022. [Google Scholar] [CrossRef]
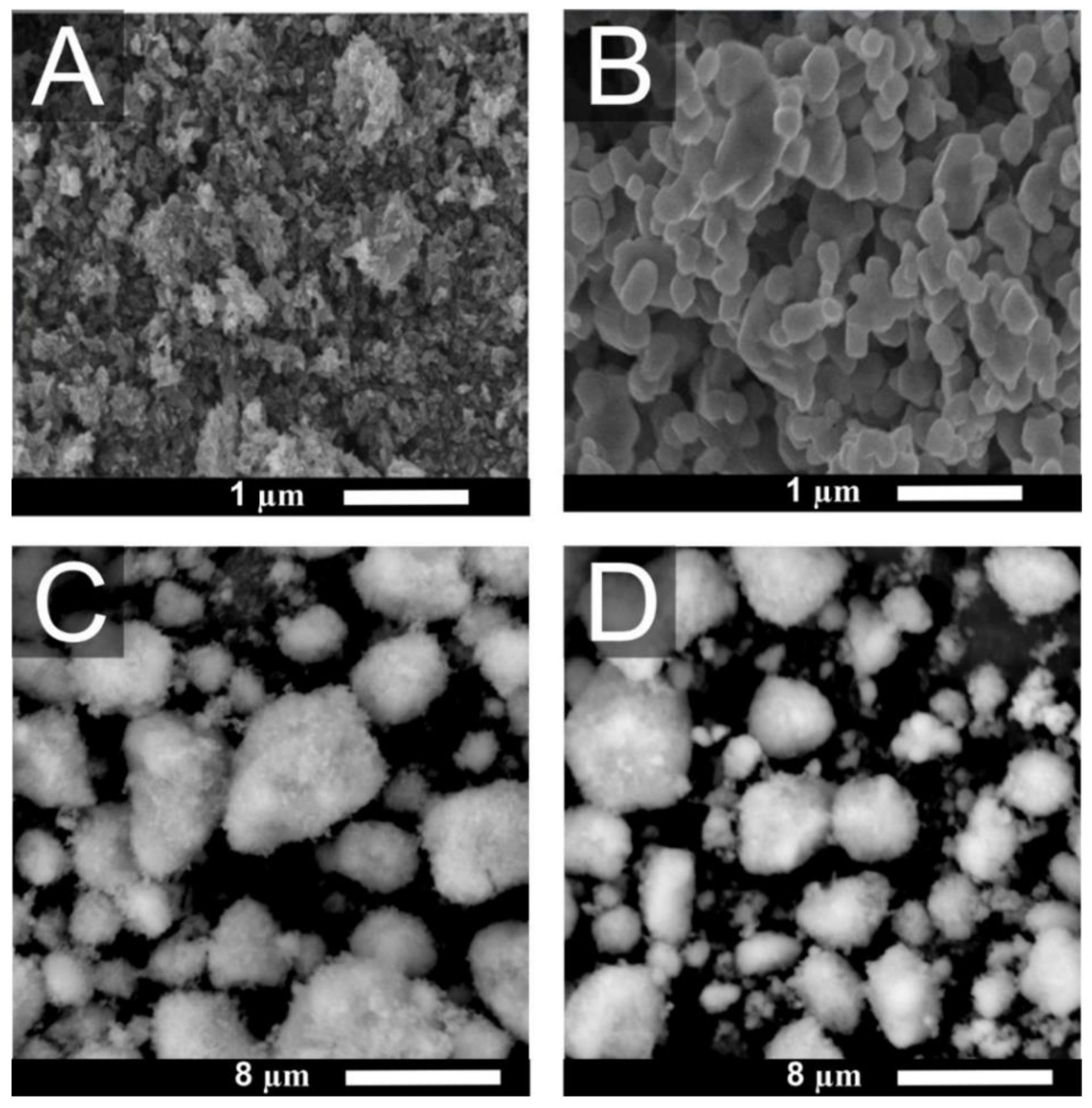
| Sample | Specific Surface Area [m2/g] | Crystalline Form | Crystal Size [nm] |
|---|---|---|---|
| Rutile 1 | 76.02 | rutile | 21 |
| Rutile 5 | 7.11 | rutile | 150 |
| Anatase | 45–55 | anatase | <25 |
| Anatase/Rutile | n. p. | anatase/rutile | <100 a, <50 b |
| Sample | Z-Average [nm] | PDI | Zeta Potential [mV] | IEP |
|---|---|---|---|---|
| Rutile 1 | 215 ± 1 | 0.23 ± 0.01 | −21.6 ± 1.0 | 4.4 |
| Rutile 5 | 287 ± 8 | 0.18 ± 0.01 | −35.5 ± 0.9 | 4.0 |
| Anatase | 553 ± 7 | 0.33 ± 0.02 | −18.5 ± 0.4 | 5.5 |
| Anatase/Rutile | 142 ± 2 | 0.35 ± 0.01 | −3.0 ± 0.6 | 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korábková, E.; Kašpárková, V.; Jasenská, D.; Moricová, D.; Daďová, E.; Truong, T.H.; Capáková, Z.; Vícha, J.; Pelková, J.; Humpolíček, P. Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma. Int. J. Mol. Sci. 2021, 22, 10614. https://doi.org/10.3390/ijms221910614
Korábková E, Kašpárková V, Jasenská D, Moricová D, Daďová E, Truong TH, Capáková Z, Vícha J, Pelková J, Humpolíček P. Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma. International Journal of Molecular Sciences. 2021; 22(19):10614. https://doi.org/10.3390/ijms221910614
Chicago/Turabian StyleKorábková, Eva, Věra Kašpárková, Daniela Jasenská, Dita Moricová, Eliška Daďová, Thanh Huong Truong, Zdenka Capáková, Jan Vícha, Jana Pelková, and Petr Humpolíček. 2021. "Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma" International Journal of Molecular Sciences 22, no. 19: 10614. https://doi.org/10.3390/ijms221910614
APA StyleKorábková, E., Kašpárková, V., Jasenská, D., Moricová, D., Daďová, E., Truong, T. H., Capáková, Z., Vícha, J., Pelková, J., & Humpolíček, P. (2021). Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma. International Journal of Molecular Sciences, 22(19), 10614. https://doi.org/10.3390/ijms221910614







