Protective and Healing Effects of Ghrelin and Risk of Cancer in the Digestive System
Abstract
1. Ghrelin and Its Synthesis
2. Physiological Action of Ghrelin
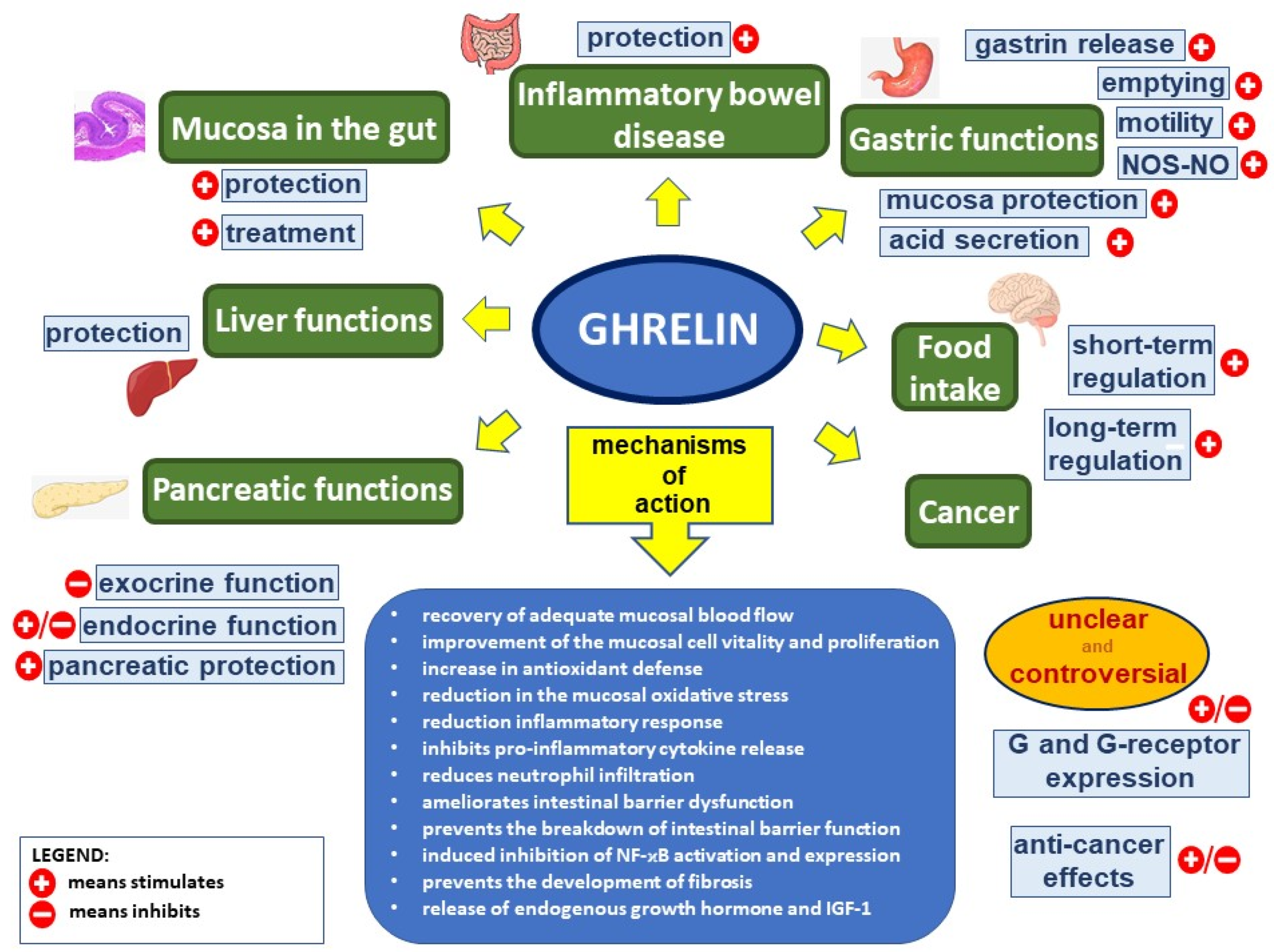
3. Protective, Anti-Inflammatory, and Healing Effects of Ghrelin in the Digestive System
3.1. The Oral Cavity
3.2. The Esophagus
3.3. The Stomach
3.4. The Small Intestine
3.5. The Liver
3.6. The Pancreas
3.7. The Large Bowel
4. Mechanisms of Protective and Therapeutic Effects of Ghrelin in the Digestive System
4.1. Anti-Inflammatory Effects
4.2. Antioxidative Effects
4.3. Antiapoptotic and Proproliferative Effects
4.4. Improvement of Blood Flow
4.5. Direct or Indirect Effects of Ghrelin
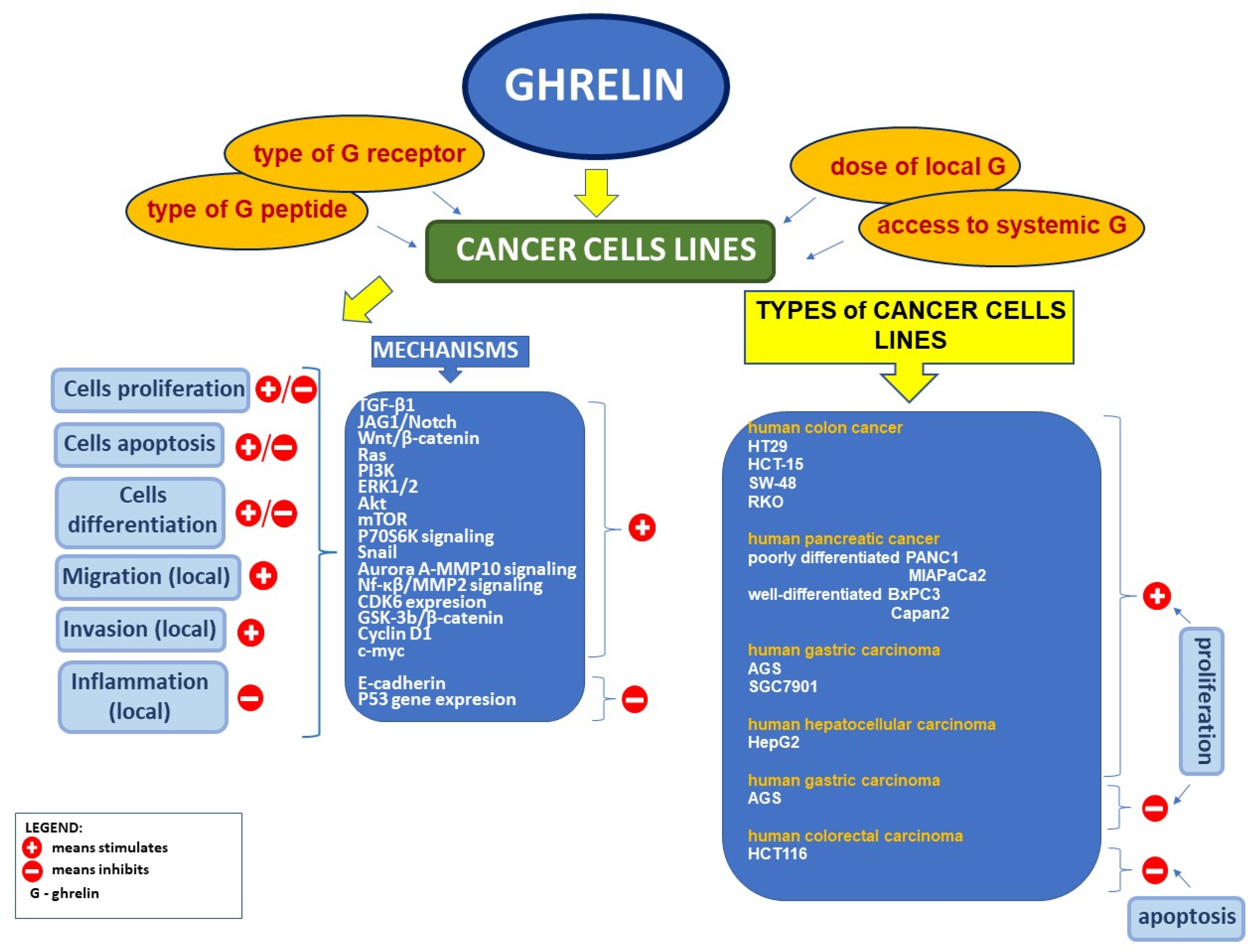
5. Ghrelin and Cancer
5.1. Expression of Ghrelin and Its Receptor in Clinical Neoplasms
5.2. Effect of Ghrelin on Cell Proliferation and Apoptosis in Tumor Cell Lines
5.3. Relationship between the Serum Level of Ghrelin and the Risk of Cancer in the Digestive System
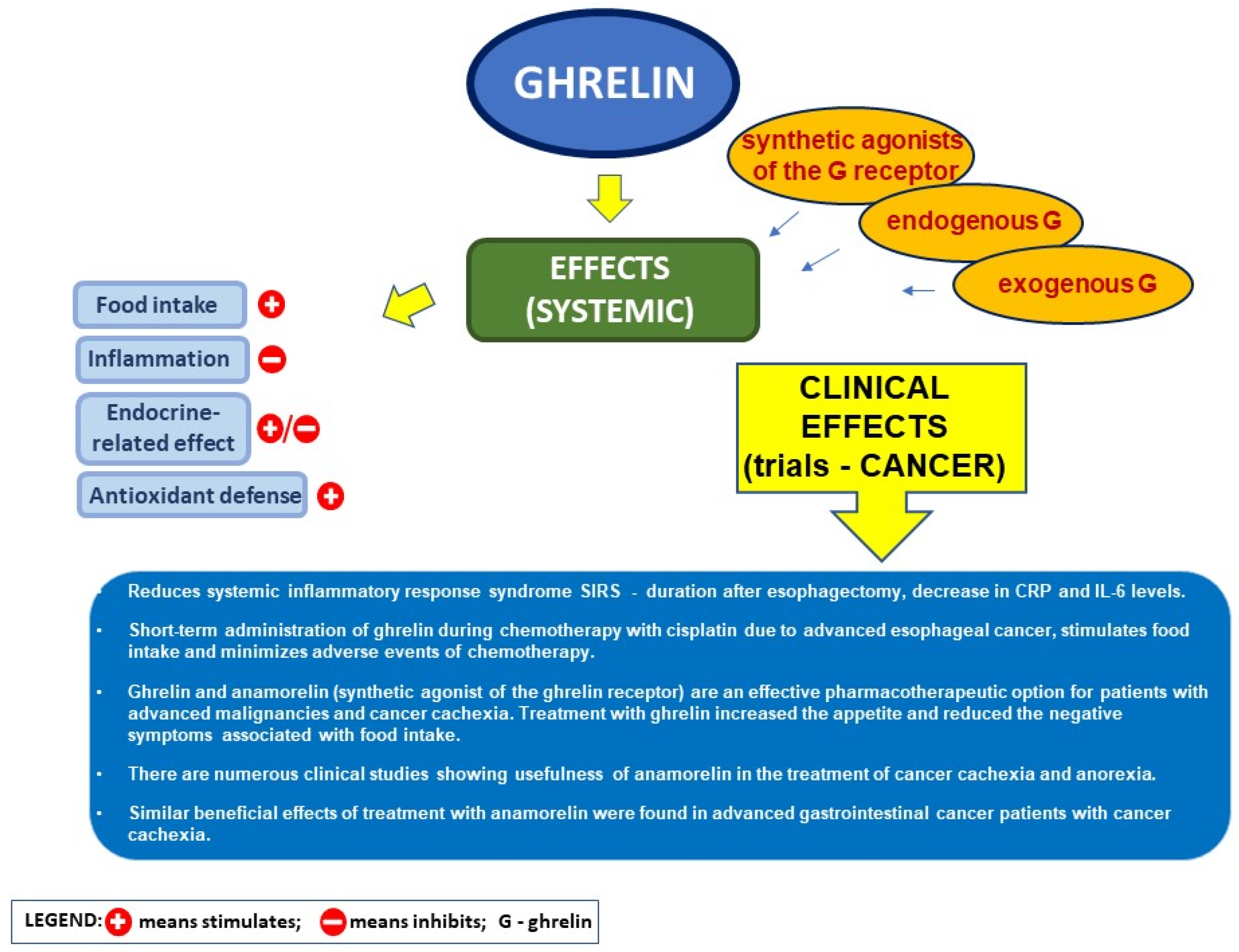
5.4. Ghrelin and the Postoperative Course after Heavy Surgery in Cancer Disease
5.5. Ghrelin and Its Analog in Cancer Cachexia
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A. Protective and Therapeutic Effects of Ghrelin in the Gut. Curr. Med. Chem. 2012, 19, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S.; et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758. [Google Scholar] [CrossRef]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A. Peptidyl hormones of endocrine cells origin in the gut—Their discovery and physiological relevance. J. Physiol. Pharmacol. 2015, 66, 11–27. [Google Scholar]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef]
- Patterson, M.; Murphy, K.G.; Le Roux, C.W.; Ghatei, M.A.; Bloom, S.R. Characterization of ghrelin-like immunoreactivity in human plasma. J. Clin. Endocrinol. Metab. 2005, 90, 2205–2211. [Google Scholar] [CrossRef]
- Yoshimoto, A.; Mori, K.; Sugawara, A.; Mukoyama, M.; Yahata, K.; Suganami, T.; Takaya, K.; Hosoda, H.; Kojima, M.; Kangawa, K.; et al. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J. Am. Soc. Nephrol. 2002, 13, 2748–2752. [Google Scholar] [CrossRef]
- Ariyasu, H.; Takaya, K.; Hosoda, H.; Iwakura, H.; Ebihara, K.; Mori, K.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Kojima, M.; et al. Delayed short-term secretory regulation of ghrelin in obese animals: Evidenced by a specific RIA for the active form of ghrelin. Endocrinology 2002, 143, 3341–3350. [Google Scholar] [CrossRef][Green Version]
- Blatnik, M.; Soderstrom, C.I.; Dysinger, M.; Fraser, S.A. Prandial ghrelin attenuation provides evidence that des-acyl ghrelin may be an artifact of sample handling in human plasma. Bioanalysis 2012, 4, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C. Structure and Physiological Actions of Ghrelin. Scientifica 2013, 2013, 1–25. [Google Scholar] [CrossRef]
- Davenport, A.P.; Bonner, T.I.; Foord, S.M.; Harmar, A.J.; Neubig, R.R.; Pin, J.P.; Spedding, M.; Kojima, M.; Kangawa, K. International union of pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol. Rev. 2005, 57, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Saito, T.; Yagyu, T.; Jiang, B.H.; Kitagawa, K.; Inagaki, C. GH, GH receptor, GH secretagogue receptor, and Ghrelin expression in human T cells, B cells, and neutrophils. J. Clin. Endocrinol. Metab. 2001, 86, 4284–4291. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The Tissue Distribution of the mRNA of Ghrelin and Subtypes of Its Receptor, GHS-R, in Humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988–2991. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.A.; Thomas, G.B.; Howard, A.D.; Feighner, S.D.; van der Ploeg, L.H.; Smith, R.G.; Robinson, I.C. Hypothalamic growth hormone secretagogue-receptor (GHS-R) expression is regulated by growth hormone in the rat. Endocrinology 1997, 138, 4552–4557. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the Acyltransferase that Octanoylates Ghrelin, an Appetite-Stimulating Peptide Hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef]
- Zhao, T.J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef]
- Baldanzi, G.; Filigheddu, N.; Cutrupi, S.; Catapano, F.; Bonissoni, S.; Fubini, A.; Malan, D.; Baj, G.; Granata, R.; Broglio, F.; et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002, 159, 1029–1037. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Reano, S.; Ferrara, M.; Angelino, E.; Gnocchi, V.F.; Prodam, F.; Ronchi, G.; Fagoonee, S.; Fornaro, M.; et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J. Clin. Investig. 2013, 123, 611–622. [Google Scholar] [CrossRef]
- Tannenbaum, G.S.; Lapointe, M.; Beaudet, A.; Howard, A.D. Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology 1998, 139, 4420–4423. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, M.A.; Holst, B. The Complex Signaling Pathways of the Ghrelin Receptor. Endocrinology 2020, 161. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e6. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.D.; Weeraratna, A.T.; Yang, H.; Bertak, D.; Cooper-Jenkins, A.; Riggins, G.J.; Eberhart, C.G.; Taub, D.D. Ghrelin and the growth hormone secretagogue receptor constitute a novel autocrine pathway in astrocytoma motility. J. Biol. Chem. 2006, 281, 16681–16690. [Google Scholar] [CrossRef]
- Duxbury, M.S.; Waseem, T.; Ito, H.; Robinson, M.K.; Zinner, M.J.; Ashley, S.W.; Whang, E.E. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 2003, 309, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Huang, S.M.; Chen, C.C.; Tsai, C.F.; Yeh, W.L.; Chou, S.J.; Hsieh, W.T.; Lu, D.Y. Ghrelin induces cell migration through GHS-R, CaMKII, AMPK, and NF-κB signaling pathway in glioma cells. J. Cell. Biochem. 2011, 112, 2931–2941. [Google Scholar] [CrossRef]
- Hou, Z.; Miao, Y.; Gao, L.; Pan, H.; Zhu, S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul. Pept. 2006, 134, 126–131. [Google Scholar] [CrossRef]
- Raghay, K.; García-Caballero, T.; Nogueiras, R.; Morel, G.; Beiras, A.; Diéguez, C.; Gallego, R. Ghrelin localization in rat and human thyroid and parathyroid glands and tumours. Histochem. Cell Biol. 2006, 125, 239–246. [Google Scholar] [CrossRef]
- Widmayer, P.; Partsch, V.; Pospiech, J.; Kusumakshi, S.; Boehm, U.; Breer, H. Distinct Cell Types With the Bitter Receptor Tas2r126 in Different Compartments of the Stomach. Front. Physiol. 2020, 11, 32. [Google Scholar] [CrossRef]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef]
- Andralojc, K.M.; Mercalli, A.; Nowak, K.W.; Albarello, L.; Calcagno, R.; Luzi, L.; Bonifacio, E.; Doglioni, C.; Piemonti, L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 2009, 52, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Wierup, N.; Sundler, F.; Heller, R.S. The islet ghrelin cell. J. Mol. Endocrinol. 2013, 52, R35–R49. [Google Scholar] [CrossRef] [PubMed]
- Sista, F.; Abruzzese, V.; Clementi, M.; Carandina, S.; Amicucci, G. Effect of Resected Gastric Volume on Ghrelin and GLP-1 Plasma Levels: A Prospective Study. J. Gastrointest. Surg. 2016, 20, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Dogan, U.; Ellidag, H.Y.; Aslaner, A.; Cakir, T.; Oruc, M.T.; Koc, U.; Mayir, B.; Gomceli, I.; Bulbuller, N.; Yilmaz, N. The impact of laparoscopic sleeve gastrectomy on plasma obestatin and ghrelin levels. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2113–2122. [Google Scholar]
- Camacho-Ramírez, A.; Mayo-Ossorio, M.Á.; Pacheco-García, J.M.; Almorza-Gomar, D.; Ribelles-García, A.; Belmonte-Núñez, A.; Prada-Oliveira, J.A.; Pérez-Arana, G.M. Pancreas is a preeminent source of ghrelin after sleeve gastrectomy in wistar rats. Histol. Histopathol. 2020, 35, 801–809. [Google Scholar] [CrossRef]
- Takaya, K.; Ariyasu, H.; Kanamoto, N.; Iwakura, H.; Yoshimoto, A.; Harada, M.; Mori, K.; Komatsu, Y.; Usui, T.; Shimatsu, A.; et al. Ghrelin strongly stimulates growth hormone (GH) release in humans. J. Clin. Endocrinol. Metab. 2000, 85, 4908–4911. [Google Scholar] [CrossRef]
- Broglio, F.; Benso, A.; Castiglioni, C.; Gottero, C.; Prodam, F.; Destefanis, S.; Gauna, C.; Van Der Lely, A.J.; Deghenghi, R.; Bo, M.; et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J. Clin. Endocrinol. Metab. 2003, 88, 1537–1542. [Google Scholar] [CrossRef]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992–5997. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Dhillo, W.S.; Seal, L.J.; Cohen, M.A.; Batterham, R.L.; Taheri, S.; Stanley, S.A.; Ghatei, M.A.; et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001, 50, 2540–2547. [Google Scholar] [CrossRef]
- Kamegai, J.; Tamura, H.; Shimizu, T.; Ishii, S.; Sugihara, H.; Wakabayashi, I. Chronic Central Infusion of Ghrelin Increases Hypothalamic Neuropeptide Y and Agouti-Related Protein mRNA Levels and Body Weight in Rats. Diabetes 2001, 50, 2438–2443. [Google Scholar] [CrossRef]
- Riediger, T.; Traebert, M.; Schmid, H.A.; Scheel, C.; Lutz, T.A.; Scharrer, E. Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci. Lett. 2003, 341, 151–155. [Google Scholar] [CrossRef]
- Toshinai, K.; Date, Y.; Murakami, N.; Shimada, M.; Mondal, M.S.; Shimbara, T.; Guan, J.L.; Wang, Q.P.; Funahashi, H.; Sakurai, T.; et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003, 144, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.J.; Coiro, C.D.; Duenas, R.; Guss, J.L.; Mirza, A.; Tal, N. Urocortin I inhibits the effects of ghrelin and neuropeptide Y on feeding and energy substrate utilization. Brain Res. 2011, 1385, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Stempniewicz, A.; Ceranowicz, P.; Warzecha, Z. Potential therapeutic effects of gut hormones, ghrelin and obestatin in oral mucositis. Int. J. Mol. Sci. 2019, 20, 1534. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; Overduin, J.; Prudom, C.E.; Liu, J.; Callahan, H.S.; Gaylinn, B.D.; Thorner, M.O.; Cummings, D.E. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 2008, 93, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Tanaka, T.; Inomata, N.; Ohnuma, N.; Tanaka, S.; Itoh, Z.; Hosoda, H.; Kojima, M.; Kangawa, K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun. 2000, 276, 905–908. [Google Scholar] [CrossRef] [PubMed]
- De La Cour, C.D.; Lindström, E.; Norlén, P.; Håkanson, R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul. Pept. 2004, 120, 23–32. [Google Scholar] [CrossRef]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in Ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef]
- Asakawa, A.; Inui, A.; Kaga, T.; Yuzuriha, H.; Nagata, T.; Ueno, N.; Makino, S.; Fujimiya, M.; Niijima, A.; Fujino, M.A.; et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001, 120, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yakabi, K.; Ro, S.; Onouhi, T.; Tanaka, T.; Ohno, S.; Miura, S.; Johno, Y.; Takayama, K. Histamine mediates the stimulatory action of ghrelin on acid secretion in rat stomach. Dig. Dis. Sci. 2006, 51, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Ro, S.; Onouchi, T.; Ohno, S.; Aoyama, T.; Chinen, K.; Takabayashi, H.; Kato, S.; Takayama, K.; Yakabi, K. Comparison of the actions of acylated and desacylated ghrelin on acid secretion in the rat stomach. J. Gastroenterol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef]
- Fukumoto, K.; Nakahara, K.; Katayama, T.; Miyazatao, M.; Kangawa, K.; Murakami, N. Synergistic action of gastrin and ghrelin on gastric acid secretion in rats. Biochem. Biophys. Res. Commun. 2008, 374, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Levin, F.; Edholm, T.; Ehrström, M.; Wallin, B.; Schmidt, P.T.; Kirchgessner, A.M.; Hilsted, L.M.; Hellström, P.M.; Näslund, E. Effect of peripherally administered ghrelin on gastric emptying and acid secretion in the rat. Regul. Pept. 2005, 131, 59–65. [Google Scholar] [CrossRef]
- Sibilia, V.; Pagani, F.; Guidobono, F.; Locatelli, V.; Torsello, A.; Deghenghi, R.; Netti, C. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology 2002, 75, 92–97. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Chen, X.; Segura, B.J.; Mulholland, M.W. Inhibition of pancreatic protein secretion by ghrelin in the rat. J. Physiol. 2001, 537, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kanai, S.; Takano, S.; Kurosawa, M.; Funakoshi, A.; Miyasaka, K. Central Administration of Ghrelin Stimulates Pancreatic Exocrine Secretion via the Vagus in Conscious Rats. Jpn. J. Physiol. 2003, 53, 443–449. [Google Scholar] [CrossRef]
- Lee, H.-M.; Wang, G.; Englander, E.W.; Kojima, M.; Greeley, G.H., Jr. Ghrelin, A New Gastrointestinal Endocrine Peptide that Stimulates Insulin Secretion: Enteric Distribution, Ontogeny, Influence of Endocrine, and Dietary Manipulations. Endocrinology 2002, 143, 185–190. [Google Scholar] [CrossRef]
- Date, Y.; Nakazato, M.; Hashiguchi, S.; Dezaki, K.; Mondal, M.S.; Hosoda, H.; Kojima, M.; Kangawa, K.; Arima, T.; Matsuo, H.; et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes 2002, 51, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.K.; Pacini, G.; Ahrén, B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003, 144, 916–921. [Google Scholar] [CrossRef]
- Broglio, F.; Arvat, E.; Benso, A.; Gottero, C.; Muccioli, G.; Papotti, M.; van der Lely, A.J.; Deghenghi, R.; Ghigo, E. Ghrelin, a Natural GH Secretagogue Produced by the Stomach, Induces Hyperglycemia and Reduces Insulin Secretion in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 5083. [Google Scholar] [CrossRef]
- Dezaki, K. Ghrelin function in insulin release and glucose metabolism. Endocr. Dev. 2013, 25, 135–143. [Google Scholar] [CrossRef]
- Lindqvist, A.; Shcherbina, L.; Prasad, R.B.; Miskelly, M.G.; Abels, M.; Martínez-Lopéz, J.A.; Fred, R.G.; Nergård, B.J.; Hedenbro, J.; Groop, L.; et al. Ghrelin suppresses insulin secretion in human islets and type 2 diabetes patients have diminished islet ghrelin cell number and lower plasma ghrelin levels. Mol. Cell. Endocrinol. 2020, 511, 110835. [Google Scholar] [CrossRef]
- Dezaki, K.; Yada, T. Islet β-cell ghrelin signaling for inhibition of insulin secretion. Methods Enzymol. 2012, 514, 317–331. [Google Scholar] [CrossRef]
- Kurashina, T.; Dezaki, K.; Yoshida, M.; Sukma Rita, R.; Ito, K.; Taguchi, M.; Miura, R.; Tominaga, M.; Ishibashi, S.; Kakei, M.; et al. The β-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Sci. Rep. 2015, 5, 14041. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, E.T.; Møller, N.; Andersen, R.F.; Rittig, S.; Jørgensen, J.O.L. Acute intravenous acyl ghrelin infusion induces thirst but does not affect sodium excretion: Two randomized, double-blind, placebo-controlled crossover studies in hypopituitary patients. Eur. J. Endocrinol. 2019, 181, 23–30. [Google Scholar] [CrossRef]
- Han, Q.Q.; Huang, H.J.; Wang, Y.L.; Yang, L.; Pilot, A.; Zhu, X.C.; Yu, R.; Wang, J.; Chen, X.R.; Liu, Q.; et al. Ghrelin exhibited antidepressant and anxiolytic effect via the p38-MAPK signaling pathway in hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.B.; Pan, Y.M.; Liu, Y.S.; Hu, J.H.; Zhang, X.D.; Zhang, D.W.; Wang, Y.; Feng, Y.K.; Yu, J.B.; Cheng, Y.X. Ghrelin promotes neural differentiation of adipose tissue-derived mesenchymal stem cell via AKT/mTOR and β-catenin signaling pathways. Kaohsiung J. Med. Sci. 2020, 36, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Koutouratsas, T.; Kalli, T.; Karamanolis, G.; Gazouli, M. Contribution of ghrelin to functional gastrointestinal disorders’ pathogenesis. World J. Gastroenterol. 2019, 25, 539–551. [Google Scholar] [CrossRef]
- Li, B.B.; Chen, Z.B.; Li, B.C.; Lin, Q.; Li, X.X.; Li, S.L.; Ding, C.; Wu, L.L.; Yu, G.Y. Expression of ghrelin in human salivary glands and its levels in saliva and serum in Chinese obese children and adolescents. Arch. Oral Biol. 2011, 56, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ozercan, I.H.; Geckil, H.; Dagli, F.; Aydin, S.; Kumru, S.; Kilic, N.; Sahin, I.; Ozercan, M.R. Ghrelin is present in teeth. J. Biochem. Mol. Biol. 2007, 40, 368–372. [Google Scholar] [CrossRef]
- Ohta, K.; Laborde, N.J.; Kajiya, M.; Shin, J.; Zhu, T.; Thondukolam, A.K.; Min, C.; Kamata, N.; Karimbux, N.Y.; Stashenko, P.; et al. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J. Dent. Res. 2011, 90, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sévigny, J.; Tschöp, M.H.; Maudsley, S.; et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE 2010, 5, e0121327. [Google Scholar] [CrossRef]
- Liu, B.; Han, X.; Feng, W.; Cui, J.; Hasegawa, T.; Amizuka, N.; Xu, X.; Li, M. Altered distribution of Ghrelin protein in mice molar development. Arch. Oral Biol. 2016, 65, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Topf, H.G.; Bohlender, J.; Zenk, J.; Klussmann, S.; Dötsch, J.; Rascher, W.; Rauh, M. Identification of ghrelin in human saliva: Production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 2005, 51, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Halifeoglu, I.; Ozercan, I.H.; Erman, F.; Kilic, N.; Aydin, S.; Ilhan, N.; Ilhan, N.; Ozkan, Y.; Akpolat, N.; et al. A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects. Peptides 2005, 26, 647–652. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Memmert, S.; Damanaki, A.; Nanayakkara, S.; Zhou, X.; Jäger, A.; Deschner, J. Effect of interleukin-1β on ghrelin receptor in periodontal cells. Clin. Oral Investig. 2019, 23, 113–122. [Google Scholar] [CrossRef]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. 2013, 64, 657–668. [Google Scholar]
- Konturek, P.C.; Burnat, G.; Rau, T.; Hahn, E.G.; Konturek, S. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig. Dis. Sci. 2008, 53, 597–605. [Google Scholar] [CrossRef]
- Thomas, S.J.; Almers, L.; Schneider, J.; Graham, J.E.; Havel, P.J.; Corley, D.A. Ghrelin and Leptin Have a Complex Relationship with Risk of Barrett’s Esophagus. Dig. Dis. Sci. 2016, 61, 70–79. [Google Scholar] [CrossRef]
- Takata, A.; Takiguchi, S.; Miyazaki, Y.; Miyata, H.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Mori, M.; Kangawa, K.; et al. Randomized Phase II Study of the Anti-inflammatory Effect of Ghrelin During the Postoperative Period of Esophagectomy. Ann. Surg. 2015, 262, 230–236. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamamoto, K.; Takata, A.; Miyazaki, Y.; Saito, T.; Tanaka, K.; Makino, T.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; et al. Continuous ghrelin infusion attenuates the postoperative inflammatory response in patients with esophageal cancer. Esophagus 2021, 18, 239–247. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takiguchi, S.; Miyata, H.; Miyazaki, Y.; Hiura, Y.; Yamasaki, M.; Nakajima, K.; Fujiwara, Y.; Mori, M.; Kangawa, K.; et al. Reduced plasma ghrelin levels on day 1 after esophagectomy: A new predictor of prolonged systemic inflammatory response syndrome. Surg. Today 2013, 43, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Kamangar, F.; Dawsey, S.M.; Stanczyk, F.Z.; Weinstein, S.J.; Taylor, P.R.; Virtamo, J.; Abnet, C.C.; Albanes, D.; Freedman, N.D. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J. Natl. Cancer Inst. 2011, 103, 1123–1129. [Google Scholar] [CrossRef]
- Sibilia, V.; Rindi, G.; Pagani, F.; Rapetti, D.; Locatelli, V.; Torsello, A.; Campanini, N.; Deghenghi, R.; Netti, C. Ghrelin protects against ethanol-induced gastric ulcers in rats: Studies on the mechanisms of action. Endocrinology 2003, 144, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Pajdo, R.; Nikiforuk, A.; Kwiecien, S.; Harsch, I.; Drozdowicz, D.; Hahn, E.G.; Konturek, S.J. Ghrelin—A new gastroprotective factor in gastric mucosa. J. Physiol. Pharmacol. 2004, 55, 325–336. [Google Scholar] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Kwiecień, S.; Drozdowicz, D.; Bielanski, W.; Pajdo, R.; Ptak, A.; Nikiforuk, A.; Pawlik, W.W.; et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul. Pept. 2004, 120, 39–51. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.C.; Drozdowicz, D.; Konturek, S.J.; Pawlik, M.; Sliwowski, Z.; Pawlik, W.W.; Hahn, E.G. Role of central and peripheral ghrelin in the mechanism of gastric mucosal defence. Inflammopharmacology 2005, 13, 45–62. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Walter, B.; Burnat, G.; Hess, T.; Hahn, E.G.; Konturek, S.J. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. Eur. J. Pharmacol. 2006, 536, 171–181. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.C.; Sliwowski, Z.; Pajdo, R.; Drozdowicz, D.; Kwiecien, S.; Burnat, G.; Konturek, S.J.; Pawlik, W.W. Prostaglandin/cyclooxygenase pathway in ghrelin-induced gastroprotection against ischemia-reperfusion injury. J. Pharmacol. Exp. Ther. 2006, 319, 477–487. [Google Scholar] [CrossRef]
- Adami, M.; Pozzoli, C.; Leurs, R.; Stark, H.; Coruzzi, G. Histamine H(3) receptors are involved in the protective effect of ghrelin against HCl-induced gastric damage in rats. Pharmacology 2010, 86, 259–266. [Google Scholar] [CrossRef]
- Íşeri, S.Ö.; Şener, G.; Yüksel, M.; Contuk, G.; Çetinel, Ş.; Gedik, N.; Yeǧen, B.Ç. Ghrelin against alendronate-induced gastric damage in rats. J. Endocrinol. 2005, 187, 399–406. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Ginter, G.; Ptak-Belowska, A.; Dembinski, A. Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J. Physiol. Pharmacol. 2014, 65, 95–106. [Google Scholar]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar] [PubMed]
- Wu, R.; Dong, W.; Ji, Y.; Zhou, M.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE 2008, 3, e2026. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, M.W.; Obuchowicz, R.; Biernat, J.; Szczepanski, W.; Pajdo, R.; Kwiecień, S.; Brzozowski, T.; Konturek, S.J.; Pawlik, W.W. Effects of peripherally and centrally applied ghrelin in the pathogenesis of ischemia-reperfusion induced injury of the small intestine. J. Physiol. Pharmacol. 2011, 62, 429–439. [Google Scholar] [PubMed]
- Zhang, H.; Cui, Z.; Luo, G.; Zhang, J.; Ma, T.; Hu, N.; Cui, T. Ghrelin attenuates intestinal ischemia/reperfusion injury in mice by activating the mTOR signaling pathway. Int. J. Mol. Med. 2013, 32, 851–859. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.L.; Jacob, A.; Aziz, M.; Wang, P. Human ghrelin mitigates intestinal injury and mortality after whole body irradiation in rats. PLoS ONE 2015, 10, e0118213. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, Y.; Yang, W.; Cai, Y.; Chen, B.; Yang, G.; Shang, H.; Zhao, W. Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice. Int. J. Mol. Sci. 2016, 17, 2032. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Dembiński, M.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med. Sci. Monit. 2012, 18, BR181. [Google Scholar] [CrossRef]
- Onishi, S.; Kaji, T.; Yamada, W.; Nakame, K.; Machigashira, S.; Kawano, M.; Yano, K.; Harumatsu, T.; Yamada, K.; Masuya, R.; et al. Ghrelin stimulates intestinal adaptation following massive small bowel resection in parenterally fed rats. Peptides 2018, 106, 59–67. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Ponciano-Rodriguez, G.; Bermejo-Martinez, L.; Villa, A.R.; Chavez-Tapia, N.C.; Zamora-Valdes, D.; Pichardo-Bahena, R.; Barredo-Prieto, B.; Uribe-Ramos, M.H.; Ramos, M.H.; et al. Low serum levels of ghrelin are associated with gallstone disease. World J. Gastroenterol. 2006, 12, 3096–3100. [Google Scholar] [CrossRef]
- Gutierrez-Grobe, Y.; Villalobos-Blasquez, I.; Sánchez-Lara, K.; Villa, A.R.; Ponciano-Rodríguez, G.; Ramos, M.H.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. High ghrelin and obestatin levels and low risk of developing fatty liver. Ann. Hepatol. 2010, 9, 52–57. [Google Scholar] [CrossRef]
- Ezquerro, S.; Mocha, F.; Frühbeck, G.; Guzmán-Ruiz, R.; Valentí, V.; Mugueta, C.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; et al. Ghrelin Reduces TNF-α-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J. Clin. Endocrinol. Metab. 2019, 104, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Nagoya, T.; Kamimura, K.; Inoue, R.; Ko, M.; Owaki, T.; Niwa, Y.; Sakai, N.; Setsu, T.; Sakamaki, A.; Yokoo, T.; et al. Ghrelin-insulin-like growth factor-1 axis is activated via autonomic neural circuits in the non-alcoholic fatty liver disease. Neurogastroenterol. Motil. 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hai, J.; Li, L.; Chen, X.; Peng, H.; Cao, M.; Zhang, Q. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 2013, 43, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Golestan Jahromi, M.; Nabavizadeh, F.; Vahedian, J.; Nahrevanian, H.; Dehpour, A.R.; Zare-Mehrjardi, A. Protective effect of ghrelin on acetaminophen-induced liver injury in rat. Peptides 2010, 31, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Chaves, J.F.; Sancho-Bru, P.; Ramalho, F.; Ramalho, L.N.; Mansego, M.L.; Ivorra, C.; Dominguez, M.; Conde, L.; Millán, C.; et al. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology 2010, 51, 974–985. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Z.; Wang, Z.; Li, Y.; Zhao, J.; Mulholland, M.; Zhang, W. Ghrelin contributes to protection of hepatocellular injury induced by ischaemia/reperfusion. Liver Int. 2014, 34, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.; Kanbur, M.; Cetin, N.; Eraslan, G.; Atasever, A. Hepatoprotective effect of ghrelin on carbon tetrachloride-induced acute liver injury in rats. Regul. Pept. 2011, 171, 1–5. [Google Scholar] [CrossRef]
- Arıcı, O.F.; Cetin, N. Protective role of ghrelin against carbon tetrachloride (CCl₄)-induced coagulation disturbances in rats. Regul. Pept. 2011, 166, 139–142. [Google Scholar] [CrossRef]
- Granata, R.; Settanni, F.; Trovato, L.; Destefanis, S.; Gallo, D.; Martinetti, M.; Ghigo, E.; Muccioli, G. Unacylated as well as acylated ghrelin promotes cell survival and inhibit apoptosis in HIT-T15 pancreatic beta-cells. J. Endocrinol. Investig. 2006, 29. [Google Scholar] [CrossRef]
- Granata, R.; Settanni, F.; Biancone, L.; Trovato, L.; Nano, R.; Bertuzzi, F.; Destefanis, S.; Annunziata, M.; Martinetti, M.; Catapano, F.; et al. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: Involvement of 3’,5’-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology 2007, 148, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Granata, R.; Volante, M.; Settanni, F.; Gauna, C.; Ghé, C.; Annunziata, M.; Deidda, B.; Gesmundo, I.; Abribat, T.; van der Lely, A.J.; et al. Unacylated ghrelin and obestatin increase islet cell mass and prevent diabetes in streptozotocin-treated newborn rats. J. Mol. Endocrinol. 2010, 45, 9–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Zhang, D.; Zhao, H.; Chen, Y.; Liu, Y.; Cao, C.; Han, L.; Liu, G. Ghrelin inhibits cell apoptosis induced by lipotoxicity in pancreatic beta-cell line. Regul. Pept. 2010, 161, 43–50. [Google Scholar] [CrossRef]
- Diaz-Ganete, A.; Baena-Nieto, G.; Lomas-Romero, I.M.; Lopez-Acosta, J.F.; Cozar-Castellano, I.; Medina, F.; Segundo, C.; Lechuga-Sancho, A.M. Ghrelin’s Effects on Proinflammatory Cytokine Mediated Apoptosis and Their Impact on β-Cell Functionality. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef]
- Baena-Nieto, G.; Lomas-Romero, I.M.; Mateos, R.M.; Leal-Cosme, N.; Perez-Arana, G.; Aguilar-Diosdado, M.; Segundo, C.; Lechuga-Sancho, A.M. Ghrelin mitigates β-cell mass loss during insulitis in an animal model of autoimmune diabetes mellitus, the BioBreeding/Worcester rat. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Tomaszewska, R.; Stachura, J.; Konturek, S.J.; Konturek, P.C. Ghrelin attenuates the development of acute pancreatitis in rats. J. Physiol. Pharmacol. 2003, 54, 561–573. [Google Scholar]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Cieszkowski, J.; Pawlik, W.W.; Tomaszewska, R.; Kuśnierz-Cabala, B.; Naskalski, J.W.; Kuwahara, A.; Kato, I. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm. IGF Res. 2006, 16, 348–356. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, C. Ghrelin attenuates acute pancreatitis-induced lung injury and inhibits substance P expression. Am. J. Med. Sci. 2010, 339, 49–54. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, C. Ghrelin inhibits the development of acute pancreatitis and nuclear factor kappaB activation in pancreas and liver. Pancreas 2009, 38, 752–757. [Google Scholar] [CrossRef]
- Bonior, J.; Ceranowicz, P.; Gajdosz, R.; Kuśnierz-Cabala, B.; Pierzchalski, P.; Warzecha, Z.; Dembiński, A.; Pędziwiatr, M.; Kot, M.; Szpak, A.L.; et al. Molecular ghrelin system in the pancreatic acinar cells: The role of the polypeptide, caerulein and sensory nerves. Int. J. Mol. Sci. 2017, 18, 929. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.J.; Wang, H.L.; Qin, M.B.; Liang, Z.H.; He, J.P.; Wei, Y.L.; Fu, H.Z.; Tang, G.D. Ghrelin inhibits IKKβ/NF-κB activation and reduces pro-inflammatory cytokine production in pancreatic acinar AR42J cells treated with cerulein. Hepatobiliary Pancreat. Dis. Int. 2020, 20, 366–375. [Google Scholar] [CrossRef]
- Bonior, J.; Warzecha, Z.; Ceranowicz, P.; Gajdosz, R.; Pierzchalski, P.; Kot, M.; Leja-Szpak, A.; Nawrot-Porąbka, K.; Link-Lenczowski, P.; Pędziwiatr, M.; et al. Capsaicin-Sensitive Sensory Nerves Are Necessary for the Protective Effect of Ghrelin in Cerulein-Induced Acute Pancreatitis in Rats. Int. J. Mol. Sci. 2017, 18, 1402. [Google Scholar] [CrossRef]
- Tang, X.; Tang, G.; Liang, Z.; Qin, M.; Fang, C.; Zhang, L. Effects of Ghrelin miRNA on Inflammation and Calcium Pathway in Pancreatic Acinar Cells of Acute Pancreatitis. Pancreas 2017, 46, 1305–1313. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, A.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Therapeutic effect of ghrelin in the course of cerulein induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2010, 61, 419–427. [Google Scholar]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qin, M.; Liang, Z.; Chang, R.; Fu, H.; Wei, Y.; Tang, G. Serum ghrelin, but not obestatin, is a potential predictor of acute pancreatitis severity. Medicine 2017, 96, e7963. [Google Scholar] [CrossRef]
- Panek, J.; Bonior, J.; Pieton, J.; Jaworek, J. Serum leptin and ghrelin levels in patients in the early stages of acute biliary pancreatitis and different degrees of severity. Pol. Przegl. Chir. 2014, 86, 211–217. [Google Scholar] [CrossRef]
- Daniel, P.; Leśniowski, B.; Jasińska, A.; Pietruczuk, M.; Małecka-Panas, E. Usefulness of assessing circulating levels of resistin, ghrelin, and IL-18 in alcoholic acute pancreatitis. Dig. Dis. Sci. 2010, 55, 2982–2987. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Böyük, A.; Tanrıverdi, M.H.; Gündüz, E.; Dusak, A.; Kaplan, İ.; Gümüş, M. The potential role of BMI, plasma leptin, nesfatin-1 and ghrelin levels in the early detection of pancreatic necrosis and severe acute pancreatitis: A prospective cohort study. Int. J. Surg. 2014, 12, 1310–1313. [Google Scholar] [CrossRef]
- Sasaki, K.; Asaoka, T.; Eguchi, H.; Fukuda, Y.; Iwagami, Y.; Yamada, D.; Miyazaki, Y.; Noda, T.; Takahashi, T.; Gotoh, K.; et al. Plasma ghrelin suppression as an early predictor for postoperative complications after pancreatoduodenectomy. Pancreatology 2018, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kerem, M.; Salman, B.; Ozsoy, S.; Pasaoglu, H.; Bedirli, A.; Haziroglu, R.; Yilmaz, T.U. Exogenous ghrelin enhances endocrine and exocrine regeneration in pancreatectomized rats. J. Gastrointest. Surg. 2009, 13, 775–783. [Google Scholar] [CrossRef]
- Ates, Y.; Degertekin, B.; Erdil, A.; Yaman, H.; Dagalp, K. Serum ghrelin levels in inflammatory bowel disease with relation to disease activity and nutritional status. Dig. Dis. Sci. 2008, 53, 2215–2221. [Google Scholar] [CrossRef]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef]
- Peracchi, M.; Bardella, M.T.; Caprioli, F.; Massironi, S.; Conte, D.; Valenti, L.; Ronchi, C.; Beck-Peccoz, P.; Arosio, M.; Piodi, L. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut 2006, 55, 432–433. [Google Scholar] [CrossRef]
- Hosomi, S.; Oshitani, N.; Kamata, N.; Sogawa, M.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Watanabe, T.; Fujiwara, Y.; Maeda, K.; et al. Phenotypical and functional study of ghrelin and its receptor in the pathogenesis of Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2009, 60, 41–47. [Google Scholar]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic Action of Ghrelin in a Mouse Model of Colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, J.; Shen, J.; Wang, S.; Guo, C.; Fan, X. Ghrelin Inhibits Intestinal Epithelial Cell Apoptosis Through the Unfolded Protein Response Pathway in Ulcerative Colitis. Front. Pharmacol. 2021, 12, 405. [Google Scholar] [CrossRef]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Kúsnierz-Cabala, B.; Bonior, J.; Jaworek, J.; Ambroży, T.; Gil, K.; Olszanecki, R.; et al. Essential role of growth hormone and IGF-1 in therapeutic effect of ghrelin in the course of acetic acid-induced colitis. Int. J. Mol. Sci. 2017, 18, 1118. [Google Scholar] [CrossRef]
- Ozturk, C.C.; Oktay, S.; Yuksel, M.; Akakin, D.; Yarat, A.; Kasimay Cakir, O. Anti-inflammatory effects of nesfatin-1 in rats with acetic acid-induced colitis and underlying mechanisms. J. Physiol. Pharmacol. 2015, 66, 741–750. [Google Scholar] [PubMed]
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The Influence of Ghrelin on the Development of Dextran Sodium Sulfate-Induced Colitis in Rats. BioMed Res. Int. 2015, 2015, 718314. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, L.; Dai, W.; Mao, Y.; Li, S.; Wang, J.; Li, H.; Guo, C.; Fan, X. Ghrelin ameliorates intestinal barrier dysfunction in experimental colitis by inhibiting the activation of nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 2015, 458, 140–147. [Google Scholar] [CrossRef] [PubMed]
- De Smet, B.; Thijs, T.; Moechars, D.; Colsoul, B.; Polders, L.; Ver Donck, L.; Coulie, B.; Peeters, T.L.; Depoortere, I. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol. Motil. 2009, 21, 59–70. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wang, W.G.; Li, Q.; Tang, M.; Li, J.; Wu, W.T.; Wan, Y.H.; Wang, Z.G.; Bao, S.S.; Fei, J. Growth hormone secretagogue receptor is important in the development of experimental colitis. Cell Biosci. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Tian, P.; Lu, X.; Jin, N.; Shi, J. Knockdown of ghrelin-O-acyltransferase attenuates colitis through the modulation of inflammatory factors and tight junction proteins in the intestinal epithelium. Cell Biol. Int. 2020, 44, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Mehdi, S.F.; Anipindi, M.; Aziz, M.; Khan, S.A.; Kondakindi, H.; Lowell, B.; Wang, P.; Roth, J. Ghrelin as an Anti-Sepsis Peptide: Review. Front. Immunol. 2021, 11, 3620. [Google Scholar] [CrossRef] [PubMed]
- Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Ceranowicz, D.; Kusnierz-Cabala, B.; Pedziwiatr, M.; Dembinski, M.; Ambrozy, T.; Kaczmarzyk, T.; Pihut, M.; et al. Therapeutic effect of exogenous ghrelin in the healing of gingival ulcers is mediated by the release of endogenous growth hormone and insulin-like growth factor-1. J. Physiol. Pharmacol. 2017, 68, 609–617. [Google Scholar]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 and interleukin-1 antagonism. Blood 1991, 77, 1627–1652. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef]
- Jaworek, J.; Konturek, S.J. Hormonal protection in acute pancreatitis by ghrelin, leptin and melatonin. World J. Gastroenterol. 2014, 20, 16902–16912. [Google Scholar] [CrossRef]
- Jacob, A.; Rajan, D.; Pathickal, B.; Balouch, I.; Hartman, A.; Wu, R.; Zhou, M.; Wang, P. The inhibitory effect of ghrelin on sepsis-induced inflammation is mediated by the MAPK phosphatase-1. Int. J. Mol. Med. 2010, 25, 159–164. [Google Scholar] [CrossRef]
- Norman, J.G.; Fink, G.W.; Denham, W.; Yang, J.; Carter, G.; Sexton, C.; Falkner, J.; Gower, W.R.; Franz, M.G. Tissue-specific cytokine production during experimental acute pancreatitis: A probable mechanism for distant organ dysfunction. Dig. Dis. Sci. 1997, 42, 1783–1788. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106, 1229–1234. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsuzaki, J.; Hibi, T. Ghrelin and oxidative stress in gastrointestinal tract. J. Clin. Biochem. Nutr. 2011, 48, 122–125. [Google Scholar] [CrossRef]
- Parks, D.A. Oxygen radicals: Mediators of gastrointestinal pathophysiology. Gut 1989, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Tudek, B.; Kowalczyk, P.; Kot, M.; Szklarczyk, J.; Leja-Szpak, A.; Pierzchalski, P.; Bonior, J.; Dembiński, A.; Ceranowicz, P.; et al. Effect of Endotoxemia in Suckling Rats on Pancreatic Integrity and Exocrine Function in Adults: A Review Report. Gastroenterol. Res. Pract. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kownacki, P.; Konturek, P.C. Role of sensory nerves in gastroprotective effect of anandamide in rats. J. Physiol. Pharmacol. 2011, 62, 207–217. [Google Scholar]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed cell death in animal development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczynska, E.; Rak, A. Effect of ghrelin on the apoptosis of various cells. A critical review. J. Physiol. Pharmacol. 2019, 70, 1–11. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: Ten minutes to dead. Cell 2005, 121, 671–674. [Google Scholar] [CrossRef]
- Galluzzi, L.; López-Soto, A.; Kumar, S.; Kroemer, G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity 2016, 44, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kakimoto, T.; Kuroki, T.; Shiraishi, R.; Fujise, T.; Iwakiri, R.; Fujimoto, K. Suppression of intestinal mucosal apoptosis by ghrelin in fasting rats. Exp. Biol. Med. 2008, 233, 48–56. [Google Scholar] [CrossRef] [PubMed]
- de Segura, I.A.; Vallejo-Cremades, M.T.; Lomas, J.; Sánchez, M.F.; Caballero, M.I.; Largo, C.; De Miguel, E. Exogenous ghrelin regulates proliferation and apoptosis in the hypotrophic gut mucosa of the rat. Exp. Biol. Med. 2010, 235, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ercan, S.; Basaranlar, G.; Gungor, N.E.; Kencebay, C.; Sahin, P.; Celik-Ozenci, C.; Derin, N. Ghrelin inhibits sodium metabisulfite induced oxidative stress and apoptosis in rat gastric mucosa. Food Chem. Toxicol. 2013, 56, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Slomiany, A. Ghrelin protection against lipopolysaccharide-induced gastric mucosal cell apoptosis involves constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef][Green Version]
- Slomiany, B.L.; Slomiany, A. Constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation in ghrelin protection against Porphyromonas gingivalis-induced salivary gland acinar cell apoptosis. Inflammopharmacology 2010, 18, 119–125. [Google Scholar] [CrossRef]
- He, X.T.; Fan, X.M.; Zha, X.L. Ghrelin inhibits 5-fluorouracil-induced apoptosis in colonic cancer cells. J. Gastroenterol. Hepatol. 2011, 26, 1169–1173. [Google Scholar] [CrossRef]
- Bonfili, L.; Cuccioloni, M.; Cecarini, V.; Mozzicafreddo, M.; Palermo, F.A.; Cocci, P.; Angeletti, M.; Eleuteri, A.M. Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis 2013, 18, 1188–1200. [Google Scholar] [CrossRef]
- Macdonald, W.C.; Trier, J.S.; Everett, N.B. Cell proliferation and migration in the stomach, duodenum, and rectum of man: Radioautographic studies. Gastroenterology 1964, 46, 405–417. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Bielański, W.; Cieszkowski, J.; Dembiński, M.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Variable effect of ghrelin administration on pancreatic development in young rats. role of insulin-like growth factor-1. J. Physiol. Pharmacol. 2005, 56, 555–570. [Google Scholar]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Bielański, W.; Pawlik, W.W.; Kuwahara, A.; Kato, I. Dual age-dependent effect of ghrelin administration on serum level of insulin-like growth factor-1 and gastric growth in young rats. Eur. J. Pharmacol. 2006, 529, 145–150. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Konturek, S.J.; Polus, A.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; et al. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J. Physiol. Pharmacol. 2006, 57, 425–437. [Google Scholar] [PubMed]
- Saito, E.S.; Kaiya, H.; Takagi, T.; Yamasaki, I.; Denbow, D.M.; Kangawa, K.; Furuse, M. Chicken ghrelin and growth hormone-releasing peptide-2 inhibit food intake of neonatal chicks. Eur. J. Pharmacol. 2002, 453, 75–79. [Google Scholar] [CrossRef]
- Bellone, S.; Castellino, N.; Broglio, F.; Rapa, A.; Vivenza, D.; Radetti, G.; Bellone, J.; Gottero, C.; Ghigo, E.; Bona, G. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J. Clin. Endocrinol. Metab. 2004, 89, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Dörr, W.; Kummermehr, J. Proliferation kinetics of mouse tongue epithelium under normal conditions and following single dose irradiation. Virchows Arch. B 1991, 60, 287–294. [Google Scholar] [CrossRef]
- Konarska, K.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Chmura, A.; Kuśnierz-Cabala, B.; Gałązka, K.; Kowalczyk, P.; Miskiewicz, A.; Konturek, T.J.; et al. Treatment with Obestatin-A Ghrelin Gene-Encoded Peptide-Reduces the Severity of Experimental Colitis Evoked by Trinitrobenzene Sulfonic Acid. Int. J. Mol. Sci. 2018, 19, 1643. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Tomaszewska, R.; Dembiński, M.; Pabiańczyk, M.; Stachura, J.; Konturek, S.J. Ischemic preconditioning reduces the severity of ischemia/reperfusion-induced pancreatitis. Eur. J. Pharmacol. 2003, 473, 207–216. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Konturek, P.C.; Stachura, J.; Tomaszewska, R.; Konturek, S.J. Calcitonin gene-related peptide can attenuate or augment pancreatic damage in caerulein-induced pancreatitis in rats. J. Physiol. Pharmacol. 1999, 50, 49–62. [Google Scholar]
- Leung, F.W.; Su, K.C.; Pique, J.M.; Thiefin, G.; Passaro, E.; Guth, P.H. Superior mesenteric artery is more important than inferior mesenteric artery in maintaining colonic mucosal perfusion and integrity in rats. Dig. Dis. Sci. 1992, 37, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Svanes, K. The role of blood flow in gastric mucosal defence, damage and healing. Dig. Dis. 1994, 12, 305–317. [Google Scholar] [CrossRef] [PubMed]
- West, S.D.; Helmer, K.S.; Chang, L.K.; Cui, Y.; Greeley, G.H.; Mercer, D.W. Cholecystokinin secretagogue-induced gastroprotection: Role of nitric oxide and blood flow. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G399–G410. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Gałązka, K.; Bonior, J.; Jaworek, J.; Konturek, P.C.; Gil, K.; Dembiński, A. Pretreatment with obestatin inhibits the development of acetic acid-induced colitis in rats. Arch. Med. Sci. 2018, 14, 920–929. [Google Scholar] [CrossRef]
- Orlando, R.C. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembiński, A.; Brzozowski, T.; Ceranowicz, P.; Dembiński, M.; Stachura, J.; Konturek, S.J. Histamine in stress ulcer prophylaxis in rats. J. Physiol. Pharmacol. 2001, 52, 407–421. [Google Scholar] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Brzozowski, T.; Dembiński, M.; Konturek, S.J.; Pawlik, W.W. Role of capsaicin-sensitive nerves and histamine H1, H2, and H3 receptors in the gastroprotective effect of histamine against stress ulcers in rats. Eur. J. Pharmacol. 2005, 508, 211–221. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Brzozowski, T.; Ceranowicz, P.; Pajdo, R.; Niemiec, J.; Drozdowicz, D.; Mitis-Musioł, M.; Konturek, S.J. Gastroprotective effect of histamine and acid secretion on ammonia-induced gastric lesions in rats. Scand. J. Gastroenterol. 2000, 35, 916–924. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Gosiewski, T.; Bulanda, M.; Kuśnierz-Cabala, B.; Gałązka, K.; Konturek, P.C. Synergic Interaction of Rifaximin and Mutaflor (Escherichia coli Nissle 1917) in the Treatment of Acetic Acid-Induced Colitis in Rats. Gastroenterol. Res. Pract. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Kuśnierz-Cabala, B.; Konturek, P.; Ambroży, T.; Dembiński, A. Obestatin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Lonardo, A. Ischaemic necrotizing pancreatitis after cardiac surgery. A case report and review of the literature. Ital. J. Gastroenterol. Hepatol. 1999, 31, 872–875. [Google Scholar]
- Sakorafas, G.H.; Tsiotos, G.G.; Bower, T.C.; Sarr, M.G. Ischemic necrotizing pancreatitis. Two case reports and review of the literature. Int. J. Pancreatol. 1998, 24, 117–121. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J.; Sendur, R.; Dembiński, M.; Pawlik, W.W. Pancreatic damage and regeneration in the course of ischemia-reperfusion induced pancreatitis in rats. J. Physiol. Pharmacol. 2001, 52, 221–235. [Google Scholar]
- Waldner, H. Vascular mechanisms to induce acute pancreatitis. Eur. Surg. Res. 1992, 24, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Chmura, A.; Galazka, K.; Kusnierz-Cabala, B.; Warzecha, Z. Administration of warfarin accelerates the recovery in ischemia/reperfusion-induced acute pancreatitis. J. Physiol. Pharmacol. 2020, 71, 417–427. [Google Scholar] [CrossRef]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Gałązka, K.; Kuśnierz-Cabala, B.; Warzecha, Z. Pretreatment with Warfarin Attenuates the Development of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. Molecules 2020, 25, 2493. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Jaworek, J.; Ceranowicz, P.; Szlachcic, A.; Walocha, J.; Konturek, S.J. Role of sensory nerves in pancreatic secretion and caerulein-induced pancreatitis. J. Physiol. Pharmacol. 1997, 48, 43–58. [Google Scholar]
- Kageyama, H.; Funahashi, H.; Hirayama, M.; Takenoya, F.; Kita, T.; Kato, S.; Sakurai, J.; Lee, E.Y.; Inoue, S.; Date, Y.; et al. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul. Pept. 2005, 126, 67–71. [Google Scholar] [CrossRef]
- Volante, M.; Allìa, E.; Gugliotta, P.; Funaro, A.; Broglio, F.; Deghenghi, R.; Muccioli, G.; Ghigo, E.; Papotti, M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J. Clin. Endocrinol. Metab. 2002, 87, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.D.; Yang, H.; Sun, Y.; Weeraratna, A.T.; Youm, Y.H.; Smith, R.G.; Taub, D.D. Ghrelin promotes thymopoiesis during aging. J. Clin. Investig. 2007, 117, 2778–2790. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Pang, W.; Pan, H.; Zheng, Y.; Kang, J.S.; Zhu, S.G. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul. Pept. 2004, 122, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, K.; Hosoda, H.; Kakei, M.; Hashiguchi, S.; Watanabe, M.; Kangawa, K.; Yada, T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: Implication in the glycemic control in rodents. Diabetes 2004, 53, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Lobie, P.E.; Breipohl, W.; Waters, M.J.; Lobie, P.E. Growth hormone receptor expression in the rat gastrointestinal tract. Endocrinology 1990, 126, 299–306. [Google Scholar] [CrossRef]
- Delehaye-Zervas, M.C.; Mertani, H.; Martini, J.F.; Nihoul-Feketé, C.; Morel, G.; Postel-Vinay, M.C. Expression of the growth hormone receptor gene in human digestive tissue. J. Clin. Endocrinol. Metab. 1994, 78, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Barnard, R.T.; Lobie, P.E.; Lim, L.; Hamlin, G.; Spencer, S.A.; Hammonds, R.G.; Leung, D.W.; Wood, W.I. Growth hormone receptors--their structure, location and role. Acta Paediatr. Scand. Suppl. 1990, 366, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Welniak, L.A.; Sun, R.; Murphy, W.J. The role of growth hormone in T-cell development and reconstitution. J. Leukoc. Biol. 2002, 71, 381–387. [Google Scholar] [CrossRef]
- Van Schravendijk, C.F.H.; Foriers, A.; Van Den Brande, J.L.; Pipeleers, D.G. Evidence for the presence of type i insulin-like growth factor receptors on rat pancreatic a and b cells. Endocrinology 1987, 121, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Bailey, A.; Humbel, R.; Goldfine, I.D. Insulinlike growth factors bind to specific receptors in isolated pancreatic acini. Am. J. Physiol.-Gastrointest. Liver Physiol. 1984, 9, G96–G99. [Google Scholar] [CrossRef]
- Xu, X.; Mardell, C.; Xian, C.J.; Zola, H.; Read, L.C. Expression of functional insulin-like growth factor-1 receptor on lymphoid cell subsets of rats. Immunology 1995, 85, 394–399. [Google Scholar]
- Skrtic, S.; Wallenius, K.; Gressner, A.M.; Jansson, J.O. Insulin-like growth factor signaling pathways in rat hepatic stellate cells: Importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology 1999, 140, 5729–5735. [Google Scholar] [CrossRef] [PubMed]
- Zapf, J.; Froesch, E.R. Insulin-like growth factors/somatomedins: Structure, secretion, biological actions and physiological role. Horm. Res. 1986, 24, 121–130. [Google Scholar] [CrossRef]
- Ho, K.K.; O’Sullivan, A.J.; Hoffman, D.M. Metabolic actions of growth hormone in man. Endocr. J. 1996, 43, S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Leja-Szpak, A.; Dembiński, A.; Tomaszewska, R.; Szklarczyk, J.; Kot, M.; Nawrot-Porabka, K.; Bonior, J.; Warzecha, Z.; Pawlik, W.W. Involvement of sensory nerves in the protective effect of growth hormone on acute pancreatitis. Growth Horm. IGF Res. 2009, 19, 517–522. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Wu, J.; Wang, G. Beneficial effects of growth hormone on bacterial translocation during the course of acute necrotizing pancreatitis in rats. Pancreas 2001, 23, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Konturek, S.J.; Tomaszewska, R.; Stachura, J.; Konturek, P.C. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J. Physiol. Pharmacol. 2003, 54, 575–590. [Google Scholar]
- Palestino-Dominguez, M.; Pelaez-Luna, M.; Lazzarini-Lechuga, R.; Rodriguez-Ochoa, I.; Souza, V.; Miranda, R.U.; Perez-Aguilar, B.; Bucio, L.; Marquardt, J.U.; Gomez-Quiroz, L.E.; et al. Recombinant human hepatocyte growth factor provides protective effects in cerulein-induced acute pancreatitis in mice. J. Cell. Physiol. 2018, 233, 9354–9364. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembiński, A.; Konturek, P.C.; Ceranowicz, P.; Konturek, S.J.; Tomaszewska, R.; Schuppan, D.; Stachura, J.; Nakamura, T. Hepatocyte growth factor attenuates pancreatic damage in caerulein-induced pancreatitis in rats. Eur. J. Pharmacol. 2001, 430, 113–121. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Konturek, S.; Tomaszewska, R.; Stachura, J.; Nakamura, T.; Konturek, P.C. Inhibition of cyclooxygenase-2 reduces the protective effect of hepatocyte growth factor in experimental pancreatitis. Eur. J. Pharmacol. 2004, 486, 107–119. [Google Scholar] [CrossRef]
- Tahara, Y.; Ido, A.; Yamamoto, S.; Miyata, Y.; Uto, H.; Hori, T.; Hayashi, K.; Tsubouchi, H. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J. Pharmacol. Exp. Ther. 2003, 307, 146–151. [Google Scholar] [CrossRef]
- Ha, X.; Peng, J.; Zhao, H.; Deng, Z.; Dong, J.; Fan, H.; Zhao, Y.; Li, B.; Feng, Q.; Yang, Z. Enhancement of Gastric Ulcer Healing and Angiogenesis by Hepatocyte Growth Factor Gene Mediated by Attenuated Salmonella in Rats. J. Korean Med. Sci. 2017, 32, 186–194. [Google Scholar] [CrossRef]
- Christensen, H.; Flyvbjerg, A.; Orskov, H.; Laurberg, S. Effect of growth hormone on the inflammatory activity of experimental colitis in rats. Scand. J. Gastroenterol. 1993, 28, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Pajdo, R.; Schuppan, D.; Drozdowicz, D.; Ptak, A.; Pawlik, M.; Nakamura, T.; Hahn, E.G. Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. J. Physiol. Pharmacol. 2000, 51, 751–773. [Google Scholar]
- Beckert, S.; Class, N.; Farrahi, F.; Coerper, S. Growth hormone enhances gastric ulcer healing in rats. Med. Sci. Monit. 2004, 10, BR255–BR258. [Google Scholar]
- Ceranowicz, D.; Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Role of hormonal axis, growth hormone—IGF-1, in therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis. J. Physiol. Pharmacol. 2010, 61, 599–606. [Google Scholar]
- Lin, T.C.; Hsiao, M. Ghrelin and cancer progression. Biochim. Biophys. Acta-Rev. Cancer 2017, 1868, 51–57. [Google Scholar] [CrossRef]
- Chopin, L.K.; Seim, I.; Walpole, C.M.; Herington, A.C. The Ghrelin axis-does it have an appetite for cancer progression? Endocr. Rev. 2012, 33, 849–891. [Google Scholar] [CrossRef]
- Lin, T.C.; Liu, Y.P.; Chan, Y.C.; Su, C.Y.; Lin, Y.F.; Hsu, S.L.; Yang, C.S.; Hsiao, M. Ghrelin promotes renal cell carcinoma metastasis via Snail activation and is associated with poor prognosis. J. Pathol. 2015, 237, 50–61. [Google Scholar] [CrossRef]
- Chopin, L.; Walpole, C.; Seim, I.; Cunningham, P.; Murray, R.; Whiteside, E.; Josh, P.; Herington, A. Ghrelin and cancer. Mol. Cell. Endocrinol. 2011, 340, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sanborn, J.Z.; Benz, S.; Szeto, C.; Hsu, F.; Kuhn, R.M.; Karolchik, D.; Archie, J.; Lenburg, M.E.; Esserman, L.J.; et al. The UCSC Cancer Genomics Browser. Nat. Methods 2009, 6, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.S.; Lin, C.H.; Yang, Y.L.; Wu, M.S.; Chen, B.C. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur. J. Pharmacol. 2016, 776, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Waseem, T.; Ahmad, F.; Azam, M.; Qureshi, M.A. Role of ghrelin axis in colorectal cancer: A novel association. Peptides 2008, 29, 1369–1376. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, L.; Hu, D.; Ji, J. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-κB signaling pathway. Mol. Cell. Biochem. 2013, 382, 163–172. [Google Scholar] [CrossRef]
- Jeffery, P.L.; Murray, R.E.; Yeh, A.H.; McNamara, J.F.; Duncan, R.P.; Francis, G.D.; Herington, A.C.; Chopin, L.K. Expression and function of the ghrelin axis, including a novel preproghrelin isoform, in human breast cancer tissues and cell lines. Endocr. Relat. Cancer 2005, 12, 839–850. [Google Scholar] [CrossRef]
- Fung, J.N.; Seim, I.; Wang, D.; Obermair, A.; Chopin, L.K.; Chen, C. Expression and in vitro functions of the ghrelin axis in endometrial cancer. Horm. Cancer 2010, 1, 245–255. [Google Scholar] [CrossRef]
- Yeh, A.H.; Jeffery, P.L.; Duncan, R.P.; Herington, A.C.; Chopin, L.K. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogen-activated protein kinase in prostate cancer. Clin. Cancer Res. 2005, 11, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Okimura, Y.; Iida, K.; Matsumoto, M.; Sowa, H.; Kaji, H.; Kojima, M.; Kangawa, K.; Chihara, K. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J. Biol. Chem. 2002, 277, 5667–5674. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhu, Y.J.; Hu, C.H.; You, L.; Wu, J.; He, X.Y.; Huang, W.J.; Wu, Z.H. Ghrelin Affects Gastric Cancer Progression by Activating AMPK Signaling Pathway. Biochem. Genet. 2021, 59, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Allia, E.; Fulcheri, E.; Cassoni, P.; Ghigo, E.; Muccioli, G.; Papotti, M. Ghrelin in fetal thyroid and follicular tumors and cell lines: Expression and effects on tumor growth. Am. J. Pathol. 2003, 162, 645–654. [Google Scholar] [CrossRef]
- Cassoni, P.; Papotti, M.; Ghè, C.; Catapano, F.; Sapino, A.; Graziani, A.; Deghenghi, R.; Reissmann, T.; Ghigo, E.; Muccioli, G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 2001, 86, 1738–1745. [Google Scholar] [CrossRef]
- Xu, Y.; Pang, X.; Dong, M.; Wen, F.; Zhang, Y. Ghrelin inhibits ovarian epithelial carcinoma cell proliferation in vitro. Oncol. Rep. 2013, 30, 2063–2070. [Google Scholar] [CrossRef]
- Bai, R.X.; Wang, W.P.; Zhao, P.W.; Li, C.B. Ghrelin attenuates the growth of HO-8910 ovarian cancer cells through the ERK pathway. Braz. J. Med Biol. Res. 2016, 49. [Google Scholar] [CrossRef]
- Díaz-Lezama, N.; Hernández-Elvira, M.; Sandoval, A.; Monroy, A.; Felix, R.; Monjaraz, E. Ghrelin inhibits proliferation and increases T-type Ca2+ channel expression in PC-3 human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2010, 403, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Gilrain, J.; Anderson, S.; Gaylinn, B.; Dalkin, A.; Day, R.; Peruggia, M.; Thorner, M.O. High plasma growth hormone (GH) levels inhibit expression of GH secretagogue receptor messenger ribonucleic acid levels in the rat pituitary. Endocrinology 2000, 141, 2084–2089. [Google Scholar] [CrossRef]
- Kamegai, J.; Tamura, H.; Shimizu, T.; Ishii, S.; Sugihara, H.; Oikawa, S. Insulin-like growth factor-I down-regulates ghrelin receptor (growth hormone secretagogue receptor) expression in the rat pituitary. Regul. Pept. 2005, 127, 203–206. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Y.; Zhang, W. The growth hormone secretagogue receptor: Its intracellular signaling and regulation. Int. J. Mol. Sci. 2014, 15, 4837–4855. [Google Scholar] [CrossRef]
- Luque, R.M.; Kineman, R.D.; Park, S.; Peng, X.D.; Gracia-Navarro, F.; Castaño, J.P.; Malagon, M.M. Homologous and heterologous regulation of pituitary receptors for ghrelin and growth hormone-releasing hormone. Endocrinology 2004, 145, 3182–3189. [Google Scholar] [CrossRef] [PubMed]
- Tolle, V.; Zizzari, P.; Tomasetto, C.; Rio, M.C.; Epelbaum, J.; Bluet-Pajot, M.T. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology 2001, 73, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Camiña, J.P.; Carreira, M.C.; El Messari, S.; Llorens-Cortes, C.; Smith, R.G.; Casanueva, F.F. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology 2004, 145, 930–940. [Google Scholar] [CrossRef]
- Majchrzak, K.; Pawłowski, K.M.; Orzechowska, E.J.; Dolka, I.; Mucha, J.; Motyl, T.; Król, M. A role of ghrelin in canine mammary carcinoma cells proliferation, apoptosis and migration. BMC Vet. Res. 2012, 8, 170. [Google Scholar] [CrossRef]
- Lin, T.C.; Yeh, Y.M.; Fan, W.L.; Chang, Y.C.; Lin, W.M.; Yang, T.Y.; Hsiao, M. Ghrelin Upregulates Oncogenic Aurora A to Promote Renal Cell Carcinoma Invasion. Cancers 2019, 11, 303. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, T. Ghrelin inhibits cisplatin-induced MDA-MB-231 breast cancer cell apoptosis via PI3K/Akt/mTOR signaling. Exp. Ther. Med. 2020, 19, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.N.; Jeffery, P.L.; Lee, J.D.; Seim, I.; Roche, D.; Obermair, A.; Chopin, L.K.; Chen, C. Silencing of ghrelin receptor expression inhibits endometrial cancer cell growth in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E305–E313. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kanemaru, A.; Fukushima, T.; Yamamoto, K.; Tanaka, H.; Haruyama, Y.; Itoh, H.; Matsumoto, N.; Kangawa, K.; Nakazato, M.; et al. Ghrelin administration suppresses inflammation-associated colorectal carcinogenesis in mice. Cancer Sci. 2015, 106, 1130–1136. [Google Scholar] [CrossRef]
- Murphy, G.; Kamangar, F.; Albanes, D.; Stanczyk, F.Z.; Weinstein, S.J.; Taylor, P.R.; Virtamo, J.; Abnet, C.C.; Dawsey, S.M.; Freedman, N.D. Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut 2012, 61, 1533–1537. [Google Scholar] [CrossRef]
- Murphy, G.; Cross, A.J.; Dawsey, S.M.; Stanczyk, F.Z.; Kamangar, F.; Weinstein, S.J.; Taylor, P.R.; Männistö, S.; Albanes, D.; Abnet, C.C.; et al. Serum ghrelin is associated with risk of colorectal adenocarcinomas in the ATBC study. Gut 2018, 67, 1646–1651. [Google Scholar] [CrossRef]
- Pritchett, N.R.; Maziarz, M.; Shu, X.O.; Kamangar, F.; Dawsey, S.M.; Fan, J.H.; Ji, B.T.; Gao, Y.T.; Xiang, Y.B.; Qiao, Y.L. Serum ghrelin and esophageal and gastric cancer in two cohorts in China. Int. J. Cancer 2020, 146, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- D’Onghia, V.; Leoncini, R.; Carli, R.; Santoro, A.; Giglioni, S.; Sorbellini, F.; Marzocca, G.; Bernini, A.; Campagna, S.; Marinello, E.; et al. Circulating gastrin and ghrelin levels in patients with colorectal cancer: Correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed. Pharmacother. 2007, 61, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hiura, Y.; Takiguchi, S.; Yamamoto, K.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Miyata, H.; Fujiwara, Y.; Mori, M.; et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: A prospective, randomized, placebo-controlled phase 2 study. Cancer 2012, 118, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia--pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; de Wolf-Linder, S.; Oberholzer, R.; Brändle, M.; Hundsberger, T.; Strasser, F. Natural ghrelin in advanced cancer patients with cachexia, a case series. J. Cachexia Sarcopenia Muscle 2021, 12, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Boccia, R.V.; Graham, C.D.; Yan, Y.; Duus, E.M.; Allen, S.; Friend, J. Anamorelin for patients with cancer cachexia: An integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet. Oncol. 2015, 16, 108–116. [Google Scholar] [CrossRef]
- Temel, J.S.; Abernethy, A.P.; Currow, D.C.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet. Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef]
- Takayama, K.; Katakami, N.; Yokoyama, T.; Atagi, S.; Yoshimori, K.; Kagamu, H.; Saito, H.; Takiguchi, Y.; Aoe, K.; Koyama, A.; et al. Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: Results of a randomized phase 2 trial. Support. Care Cancer 2016, 24, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.; Temel, J.S.; Abernethy, A.; Milanowski, J.; Friend, J.; Fearon, K.C. ROMANA 3: A phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Uchino, J.; Yokoyama, T.; Naito, T.; Kondo, M.; Yamada, K.; Kitajima, H.; Yoshimori, K.; Sato, K.; Saito, H.; et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018, 124, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Hamauchi, S.; Furuse, J.; Takano, T.; Munemoto, Y.; Furuya, K.; Baba, H.; Takeuchi, M.; Choda, Y.; Higashiguchi, T.; Naito, T.; et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019, 125, 4294–4302. [Google Scholar] [CrossRef]
- Malik, J.S.; Yennurajalingam, S. Prokinetics and ghrelin for the management of cancer cachexia syndrome. Ann. Palliat. Med. 2019, 8, 80–85. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginter, G.; Ceranowicz, P.; Warzecha, Z. Protective and Healing Effects of Ghrelin and Risk of Cancer in the Digestive System. Int. J. Mol. Sci. 2021, 22, 10571. https://doi.org/10.3390/ijms221910571
Ginter G, Ceranowicz P, Warzecha Z. Protective and Healing Effects of Ghrelin and Risk of Cancer in the Digestive System. International Journal of Molecular Sciences. 2021; 22(19):10571. https://doi.org/10.3390/ijms221910571
Chicago/Turabian StyleGinter, Grzegorz, Piotr Ceranowicz, and Zygmunt Warzecha. 2021. "Protective and Healing Effects of Ghrelin and Risk of Cancer in the Digestive System" International Journal of Molecular Sciences 22, no. 19: 10571. https://doi.org/10.3390/ijms221910571
APA StyleGinter, G., Ceranowicz, P., & Warzecha, Z. (2021). Protective and Healing Effects of Ghrelin and Risk of Cancer in the Digestive System. International Journal of Molecular Sciences, 22(19), 10571. https://doi.org/10.3390/ijms221910571






