Psoriasis and Atherosclerosis—Skin, Joints, and Cardiovascular Story of Two Plaques in Relation to the Treatment with Biologics
Abstract
1. Introduction and Methods
- Epidemiology and pathophysiology of cardiovascular risk factors and complications in patients with PSO/PSA;
- Molecular background of links between PSO and Ath development;
- Impact of traditional and new treatments on cardiovascular risk in PSO;
- Possibilities of early diagnosis, prevention strategies and effective cardiovascular treatment as well as novel therapeutic perspectives in PSO/PSA.
2. Cardiovascular Risk Factors and Complications in PSO/PSA
3. Molecular Background of Links between PSO and Ath Development
4. Impact of Traditional and New Treatment on Cardiovascular Risk in PSO
5. Novel Diagnostic and Therapeutic Perspectives in PSO/PSA
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koppikar, S.; Colaco, K.; Harvey, P.; Akhtari, S.; Chandran, V.; Gladman, D.D.; Cook, R.; Eder, L. Incidence of and Risk Factors for Heart Failure in Patients with Psoriatic Disease—A Cohort Study. Arthritis Care Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Lam, C.S.P.; Lund, L.H.; Maurer, M.S.; Borlaug, B.A. Characterization of the Inflammatory-Metabolic Phenotype of Heart Failure with a Preserved Ejection Fraction: A Hypothesis to Explain Influence of Sex on the Evolution and Potential Treatment of the Disease. Eur. J. Heart Fail. 2020, 22, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Masson, W.; Lobo, M.; Molinero, G. Psoriasis and Cardiovascular Risk: A Comprehensive Review. Adv. Ther. 2020, 37, 2017–2033. [Google Scholar] [CrossRef]
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.J.; Calabresi, P. Occlusive Vascular Disease in Psoriatic Patients. N. Engl. J. Med. 1973, 288, 912. [Google Scholar]
- Samarasekera, E.J.; Neilson, J.M.; Warren, R.B.; Parnham, J.; Smith, C.H. Incidence of Cardiovascular Disease in Individuals with Psoriasis: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2013, 133, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.A.; Li, L.; Jick, S.S. Incidence of Risk Factors for Myocardial Infarction and Other Vascular Diseases in Patients with Psoriasis. Br. J. Dermatol. 2008, 159, 895–902. [Google Scholar] [CrossRef]
- Wakkee, M.; Herings, R.M.C.; Nijsten, T. Psoriasis May Not Be an Independent Risk Factor for Acute Ischemic Heart Disease Hospitalizations: Results of a Large Population-Based Dutch Cohort. J. Investig. Dermatol. 2010, 130, 962–967. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Gislason, G.H.; Charlot, M.; Jørgensen, C.H.; Lindhardsen, J.; Olesen, J.B.; Abildstrøm, S.Z.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R. Psoriasis Is Associated with Clinically Significant Cardiovascular Risk: A Danish Nationwide Cohort Study: Psoriasis and Cardiovascular Risk. J. Intern. Med. 2011, 270, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, Y.-H. Association of Psoriasis with Stroke and Myocardial Infarction: Meta-Analysis of Cohort Studies: Psoriasis, Stroke and Myocardial Infarction. Br. J. Dermatol. 2012, 167, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.J.; Herzog, C.; Rostock, A.; Ochsendorf, F.R.; Zollner, T.M.; Thaci, D.; Kaufmann, R.; Vogl, T.J.; Boehncke, W.-H. Psoriasis: A Possible Risk Factor for Development of Coronary Artery Calcification. Br. J. Dermatol. 2007, 156, 271–276. [Google Scholar] [CrossRef]
- Gisondi, P.; Fantin, F.; Del Giglio, M.; Valbusa, F.; Marino, F.; Zamboni, M.; Girolomoni, G. Chronic Plaque Psoriasis Is Associated with Increased Arterial Stiffness. Dermatology 2009, 218, 110–113. [Google Scholar] [CrossRef]
- Jókai, H.; Szakonyi, J.; Kontár, O.; Marschalkó, M.; Szalai, K.; Kárpáti, S.; Holló, P. Impact of Effective Tumor Necrosis Factor-Alfa Inhibitor Treatment on Arterial Intima-Media Thickness in Psoriasis: Results of a Pilot Study. J. Am. Acad. Dermatol. 2013, 69, 523–529. [Google Scholar] [CrossRef] [PubMed]
- González-Cantero, A.; Gonzalez-Cantero, J.; Sanchez-Moya, A.I.; Perez-Hortet, C.; Arias-Santiago, S.; Martin-Rodriguez, J.L.; Schoendorff-Ortega, C.; Gonzalez-Calvin, J.L. Femoral Artery Ultrasound for Improving the Detection of Atherosclerosis in Psoriasis. J. Am. Acad. Dermatol. 2019, 80, 784–786. [Google Scholar] [CrossRef]
- Fang, N.; Jiang, M.; Fan, Y. Association between Psoriasis and Subclinical Atherosclerosis: A Meta-Analysis. Medicine 2016, 95, e3576. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef]
- Milaniuk, S.; Pietrzak, A.; Mosiewicz, B.; Mosiewicz, J.; Reich, K. Influence of Psoriasis on Circulatory System Function Assessed in Echocardiography. Arch. Derm. Res. 2015, 307, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Atas, H.; Kepez, A.; Bozbay, M.; Gencosmanoglu, D.S.; Cincin, A.; Sunbul, M.; Bozbay, A.Y.; Darvishova, R.; Ergun, T. Assessment of Left Atrial Volume and Function in Patients with Psoriasis by Using Real Time Three-Dimensional Echocardiography. Wien. Klin. Wochenschr. 2015, 127, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Kumar, M.; Sharma, A.; Sharma, R.; Dhattarwal, N.; Sondhi, M. Cardiomyopathy and Echocardiographic Abnormalities in Indian Patients with Psoriasis: Results of a Pilot Study. Int. J. Clin. Pract. 2021, 75, e13756. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Rutter, M.K.; Lunt, M.; Young, H.S.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M.; Identification and Management of Psoriasis Associated ComorbidiTy (IMPACT) project team. Psoriasis and the Risk of Major Cardiovascular Events: Cohort Study Using the Clinical Practice Research Datalink. J. Investig. Dermatol. 2015, 135, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Zinger, H.; Sherer, Y.; Shoenfeld, Y. Atherosclerosis in Autoimmune Rheumatic Diseases-Mechanisms and Clinical Findings. Clin. Rev. Allergy Immunol. 2009, 37, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From Endothelial Dysfunction to Atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Zeiher, A.M. Endothelial Function: Cardiac Events. Circulation 2005, 111, 363–368. [Google Scholar] [CrossRef]
- Mehta, N.N.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Patients with Severe Psoriasis Are at Increased Risk of Cardiovascular Mortality: Cohort Study Using the General Practice Research Database. Eur. Heart J. 2010, 31, 1000–1006. [Google Scholar] [CrossRef]
- Siegel, D.; Devaraj, S.; Mitra, A.; Raychaudhuri, S.P.; Raychaudhuri, S.K.; Jialal, I. Inflammation, Atherosclerosis, and Psoriasis. Clin. Rev. Allergy Immunol. 2013, 44, 194–204. [Google Scholar] [CrossRef]
- Abuabara, K.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Cause-Specific Mortality in Patients with Severe Psoriasis: A Population-Based Cohort Study in the U.K: Cause-Specific Mortality in Patients with Severe Psoriasis. Br. J. Dermatol. 2010, 163, 586–592. [Google Scholar] [CrossRef]
- Li, L.; Hagberg, K.W.; Peng, M.; Shah, K.; Paris, M.; Jick, S. Rates of Cardiovascular Disease and Major Adverse Cardiovascular Events in Patients with Psoriatic Arthritis Compared to Patients without Psoriatic Arthritis. J. Clin. Rheumatol. 2015, 21, 405–410. [Google Scholar] [CrossRef]
- Polachek, A.; Touma, Z.; Anderson, M.; Eder, L. Risk of Cardiovascular Morbidity in Patients with Psoriatic Arthritis: A Meta-Analysis of Observational Studies. Arthritis Care Res. 2017, 69, 67–74. [Google Scholar] [CrossRef]
- Shen, J.; Wong, K.-T.; Cheng, I.T.; Shang, Q.; Li, E.K.; Wong, P.; Kun, E.W.; Law, M.Y.; Yip, R.; Yim, I.; et al. Increased Prevalence of Coronary Plaque in Patients with Psoriatic Arthritis without Prior Diagnosis of Coronary Artery Disease. Ann. Rheum. Dis. 2017, 76, 1237–1244. [Google Scholar] [CrossRef]
- Chin, Y.-Y.; Yu, H.-S.; Li, W.-C.; Ko, Y.-C.; Chen, G.-S.; Wu, C.-S.; Lu, Y.-W.; Yang, Y.-H.; Lan, C.-C.E. Arthritis as an Important Determinant for Psoriatic Patients to Develop Severe Vascular Events in Taiwan: A Nation-Wide Study: Psoriasis with or without Joint Involvement. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1262–1268. [Google Scholar] [CrossRef]
- Langan, S.M.; Seminara, N.M.; Shin, D.B.; Troxel, A.B.; Kimmel, S.E.; Mehta, N.N.; Margolis, D.J.; Gelfand, J.M. Prevalence of Metabolic Syndrome in Patients with Psoriasis: A Population-Based Study in the United Kingdom. J. Investig. Dermatol. 2012, 132, 556–562. [Google Scholar] [CrossRef]
- Tom, W.L.; Playford, M.P.; Admani, S.; Natarajan, B.; Joshi, A.A.; Eichenfield, L.F.; Mehta, N.N. Characterization of Lipoprotein Composition and Function in Pediatric Psoriasis Reveals a More Atherogenic Profile. J. Investig. Dermatol. 2016, 136, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, W.-Q.; Han, J.; Sun, Q.; Qureshi, A.A. Hypercholesterolemia and Risk of Incident Psoriasis and Psoriatic Arthritis in US Women: Hypercholesterolemia and Risk of Incident Psoriasis and PsA. Arthritis Rheumatol. 2014, 66, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Wang, S.; Shin, D.B.; Mehta, N.N.; Kimmel, S.E.; Margolis, D.J.; Troxel, A.B.; Gelfand, J.M. Effect of Psoriasis Severity on Hypertension Control: A Population-Based Study in the United Kingdom: A Population-Based Study in the United Kingdom. JAMA Dermatol. 2015, 151, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, G. Psoriasis Alters HDL Composition and Cholesterol Efflux Capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef]
- Ramezani, M.; Zavattaro, E.; Sadeghi, M. Evaluation of Serum Lipid, Lipoprotein, and Apolipoprotein Levels in Psoriatic Patients: A Systematic Review and Meta-Analysis of Case-Control Studies. Postepy Dermatol. Alergol. 2019, 36, 692–702. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Zhu, Z.; Bao, Y.; Yang, B. Epicardial Fat Tissue in Patients with Psoriasis: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2016, 15, 103. [Google Scholar] [CrossRef]
- Ip, W.; Kirchhof, M.G. Glycemic Control in the Treatment of Psoriasis. Dermatology 2017, 233, 23–29. [Google Scholar] [CrossRef]
- Myśliwiec, H.; Baran, A.; Harasim-Symbor, E.; Myśliwiec, P.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Serum Fatty Acid Profile in Psoriasis and Its Comorbidity. Arch. Derm. Res. 2017, 309, 371–380. [Google Scholar] [CrossRef]
- Setty, A.R.; Curhan, G.; Choi, H.K. Obesity, Waist Circumference, Weight Change, and the Risk of Psoriasis in Women: Nurses’ Health Study II: Nurses’ Health Study II. Arch. Intern. Med. 2007, 167, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Gutmark-Little, I.; Shah, K.N. Obesity and the Metabolic Syndrome in Pediatric Psoriasis. Clin. Dermatol. 2015, 33, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Babino, G.; Giunta, A.; Bianchi, L.; Esposito, M. Morbid Obesity and Psoriasis: Disease Remission after Laparoscopic Sleeve Gastrectomy. Obes. Res. Clin. Pract. 2017, 11, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, N.; Nour, T. The Effect of Weight Reduction on Treatment Outcomes in Obese Patients with Psoriasis on Biologic Therapy: A Randomized Controlled Prospective Trial. Expert Opin. Biol. Ther. 2014, 14, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased Expression of Interleukin 17 in Inflammatory Bowel Disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.; Quaranta, M.; Prescott, N.J.; Allen, M.; Smith, R.; Burden, A.D.; Worthington, J.; Griffiths, C.E.M.; Mathew, C.G.; Barker, J.N.; et al. Psoriasis Is Associated with Pleiotropic Susceptibility Loci Identified in Type II Diabetes and Crohn Disease. J. Med. Genet. 2008, 45, 114–116. [Google Scholar] [CrossRef]
- Cargill, M.; Schrodi, S.J.; Chang, M.; Garcia, V.E.; Brandon, R.; Callis, K.P.; Matsunami, N.; Ardlie, K.G.; Civello, D.; Catanese, J.J.; et al. A Large-Scale Genetic Association Study Confirms IL12B and Leads to the Identification of IL23R as Psoriasis-Risk Genes. Am. J. Hum. Genet. 2007, 80, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Skroza, N.; Proietti, I.; Pampena, R.; La Viola, G.; Bernardini, N.; Nicolucci, F.; Tolino, E.; Zuber, S.; Soccodato, V.; Potenza, C. Correlations between Psoriasis and Inflammatory Bowel Diseases. Biomed. Res. Int. 2013, 2013, 983902. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease: Gut Microbiota in PsA. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lee, C.-H.; Chi, C.-C. Association of Psoriasis with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2018, 154, 1417–1423. [Google Scholar] [CrossRef]
- Wu, S.; Han, J.; Li, W.-Q.; Qureshi, A.A. Hypertension, Antihypertensive Medication Use, and Risk of Psoriasis. JAMA Dermatol. 2014, 150, 957–963. [Google Scholar] [CrossRef]
- Egeberg, A.; Gisondi, P.; Carrascosa, J.M.; Warren, R.B.; Mrowietz, U. The Role of the Interleukin-23/Th17 Pathway in Cardiometabolic Comorbidity Associated with Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1695–1706. [Google Scholar] [CrossRef]
- Richer, V.; Roubille, C.; Fleming, P.; Starnino, T.; McCourt, C.; McFarlane, A.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.E.; et al. Psoriasis and Smoking: A Systematic Literature Review and Meta-Analysis with Qualitative Analysis of Effect of Smoking on Psoriasis Severity: A Systematic Literature Review and Meta-Analysis with Qualitative Analysis of Effect of Smoking on Psoriasis Severity. J. Cutan. Med. Surg. 2016, 20, 221–227. [Google Scholar] [CrossRef]
- Torii, K.; Saito, C.; Furuhashi, T.; Nishioka, A.; Shintani, Y.; Kawashima, K.; Kato, H.; Morita, A. Tobacco Smoke Is Related to Th17 Generation with Clinical Implications for Psoriasis Patients: Letter to the Editor. Exp. Dermatol. 2011, 20, 371–373. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of Myocardial Infarction in Patients with Psoriasis. JAMA 2006, 296, 1735–1741. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Dommasch, E.D.; Shin, D.B.; Azfar, R.S.; Kurd, S.K.; Wang, X.; Troxel, A.B. The Risk of Stroke in Patients with Psoriasis. J. Investig. Dermatol. 2009, 129, 2411–2418. [Google Scholar] [CrossRef]
- Ogdie, A.; Yu, Y.; Haynes, K.; Love, T.J.; Maliha, S.; Jiang, Y.; Troxel, A.B.; Hennessy, S.; Kimmel, S.E.; Margolis, D.J.; et al. Risk of Major Cardiovascular Events in Patients with Psoriatic Arthritis, Psoriasis and Rheumatoid Arthritis: A Population-Based Cohort Study. Ann. Rheum. Dis. 2015, 74, 326–332. [Google Scholar] [CrossRef]
- Chung, W.-S.; Lin, C.-L. Increased Risks of Venous Thromboembolism in Patients with Psoriasis. A Nationwide Cohort Study: A Nationwide Cohort Study. Thromb. Haemost. 2017, 117, 1637–1643. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Prizment, A.E.; Folsom, A.R. Psoriasis Is Associated with a Greater Risk of Incident Venous Thromboembolism: The Iowa Women’s Health Study: Letters to the Editor. J. Thromb. Haemost. 2012, 10, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Ahlehoff, O.; Gislason, G.H.; Lindhardsen, J.; Charlot, M.G.; Jørgensen, C.H.; Olesen, J.B.; Bretler, D.-M.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R. Psoriasis Carries an Increased Risk of Venous Thromboembolism: A Danish Nationwide Cohort Study. PLoS ONE 2011, 6, e18125. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.R.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF Guidelines of Care for the Management and Treatment of Psoriasis with Awareness and Attention to Comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Hillary, T.; Clijmans, J.; Vermeire, S.; Lambert, J.; Garmyn, M.; Imbrechts, M.; Vanassche, T. Venous Thrombotic Events in Psoriasis Patients: A Systematic Review with Meta-Analysis. Ann. Med. 2021, 53, 1074–1081. [Google Scholar] [CrossRef]
- Khalid, U.; Ahlehoff, O.; Gislason, G.H.; Kristensen, S.L.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R. Psoriasis and Risk of Heart Failure: A Nationwide Cohort Study: Psoriasis and Risk of Heart Failure: A Nationwide Cohort Study. Eur. J. Heart Fail. 2014, 16, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Eliakim-Raz, N.; Shuvy, M.; Lotan, C.; Planer, D. Psoriasis and Dilated Cardiomyopathy: Coincidence or Associated Diseases? Cardiology 2008, 111, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Vaengebjerg, S.; Skov, L.; Egeberg, A.; Loft, N.D. Prevalence, Incidence, and Risk of Cancer in Patients with Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2020, 156, 421–429. [Google Scholar] [CrossRef]
- Geller, S.; Xu, H.; Lebwohl, M.; Nardone, B.; Lacouture, M.E.; Kheterpal, M. Malignancy Risk and Recurrence with Psoriasis and Its Treatments: A Concise Update. Am. J. Clin. Dermatol. 2018, 19, 363–375. [Google Scholar] [CrossRef]
- Egeberg, A.; Thyssen, J.P.; Gislason, G.H.; Skov, L. Skin Cancer in Patients with Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1349–1353. [Google Scholar] [CrossRef]
- Kamstrup, M.R.; Skov, L.; Zachariae, C.; Thyssen, J.P.; Egeberg, A. Psoriasis and Risk of Malignant Lymphoma: A Population-Based Cohort Study. Br. J. Dermatol. 2018, 178, 1435–1436. [Google Scholar] [CrossRef]
- Stern, R.S.; PUVA Follow-Up Study. The Risk of Squamous Cell and Basal Cell Cancer Associated with Psoralen and Ultraviolet A Therapy: A 30-Year Prospective Study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.; Marinos, L.; Moustou, E.; Papadavid, E.; Economidi, A.; Christofidou, E.; Gerochristou, M.; Tasidou, A.; Economaki, E.; Stratigos, A.; et al. Psoriasis in Patients with Mycosis Fungoides: A Clinicopathological Study of 25 Patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Pouplard, C.; Brenaut, E.; Horreau, C.; Barnetche, T.; Misery, L.; Richard, M.-A.; Aractingi, S.; Aubin, F.; Cribier, B.; Joly, P.; et al. Risk of Cancer in Psoriasis: A Systematic Review and Meta-Analysis of Epidemiological Studies. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.L.; Schwartzman-Morris, J.S.; Krathen, M.; Reed, G.; Chang, H.; Saunders, K.C.; Fisher, M.C.; Greenberg, J.D.; Putterman, C.; Mease, P.J.; et al. A Comparison of the Malignancy Incidence among Patients with Psoriatic Arthritis and Patients with Rheumatoid Arthritis in a Large US Cohort: Malignancy in Psoriatic Arthritis and Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.J.; Han, K.D.; Kim, H.-N.; Park, Y.M.; Lee, J.Y.; Park, Y.-G.; Lee, Y.B. Cancer Risk in 892 089 Patients with Psoriasis in Korea: A Nationwide Population-Based Cohort Study. J. Dermatol. 2019, 46, 95–102. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Hsieh, C.-F.; Chiang, Y.-T.; Tsai, Y.-W.; Huang, W.-F.; Li, C.-Y.; Wang, T.-S.; Tsai, T.-F. Concomitant Sleep Disorders Significantly Increase the Risk of Cardiovascular Disease in Patients with Psoriasis. PLoS ONE 2016, 11, e0146462. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Yu, H.-S.; Li, W.-C.; Ko, Y.-C.; Wu, C.-S.; Lu, Y.-W.; Yang, Y.-H.; Chen, G.-S. Anxiety Contributes to the Development of Cerebrovascular Disease in Taiwanese Patients with Psoriasis: A Population-Based Study. Eur. J. Dermatol. 2013, 23, 290–292. [Google Scholar] [CrossRef]
- Egeberg, A.; Khalid, U.; Gislason, G.H.; Mallbris, L.; Skov, L.; Hansen, P.R. Impact of Depression on Risk of Myocardial Infarction, Stroke and Cardiovascular Death in Patients with Psoriasis: A Danish Nationwide Study. Acta Derm. Venereol. 2016, 96, 218–221. [Google Scholar] [CrossRef]
- Augustin, M.; Radtke, M.A. Quality of Life in Psoriasis Patients. Expert Rev. Pharmacoecon. Outcomes Res. 2014, 14, 559–568. [Google Scholar] [CrossRef]
- Dowlatshahi, E.A.; Wakkee, M.; Arends, L.R.; Nijsten, T. The Prevalence and Odds of Depressive Symptoms and Clinical Depression in Psoriasis Patients: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2014, 134, 1542–1551. [Google Scholar] [CrossRef]
- Amin, M.; Lee, E.B.; Bhutani, T.; Wu, J.J. Do Psoriasis Patients Engage in Vigorous Physical Activity? Cutis 2018, 101, 198–200. [Google Scholar] [PubMed]
- Frankel, H.C.; Han, J.; Li, T.; Qureshi, A.A. The Association between Physical Activity and the Risk of Incident Psoriasis. Arch. Dermatol. 2012, 148, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, A.; Balato, A.; Megna, M.; Balato, N. Limitations of Current Monoclonal Antibodies for Plaque-Type Psoriasis and an Outlook for the Future. Expert Opin. Biol. Ther. 2018, 18, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Boehncke, S.; Tobin, A.-M.; Kirby, B. The “Psoriatic March”: A Concept of How Severe Psoriasis May Drive Cardiovascular Comorbidity: The ‘Psoriatic March‘. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. A Tale of Two Plaques: Convergent Mechanisms of T-Cell-Mediated Inflammation in Psoriasis and Atherosclerosis: T-Cell Immunology in Psoriasis and Atherosclerosis. Exp. Dermatol. 2011, 20, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Ruschitzka, F. Psoriasis and Atherosclerosis: Two Plaques, One Syndrome? Eur. Heart J. 2012, 33, 1989–1991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davidovici, B.B.; Sattar, N.; Prinz, J.C.; Puig, L.; Emery, P.; Barker, J.N.; van de Kerkhof, P.; Ståhle, M.; Nestle, F.O.; Girolomoni, G.; et al. Psoriasis and Systemic Inflammatory Diseases: Potential Mechanistic Links between Skin Disease and Co-Morbid Conditions. J. Investig. Dermatol. 2010, 130, 1785–1796. [Google Scholar] [CrossRef]
- Ryan, C.; Kirby, B. Psoriasis Is a Systemic Disease with Multiple Cardiovascular and Metabolic Comorbidities. Dermatol. Clin. 2015, 33, 41–55. [Google Scholar] [CrossRef]
- Ghazizadeh, R.; Shimizu, H.; Tosa, M.; Ghazizadeh, M. Pathogenic Mechanisms Shared between Psoriasis and Cardiovascular Disease. Int. J. Med. Sci. 2010, 7, 284–289. [Google Scholar] [CrossRef]
- Chen, S.; Crother, T.R.; Arditi, M. Emerging Role of IL-17 in Atherosclerosis. J. Innate Immun. 2010, 2, 325–333. [Google Scholar] [CrossRef]
- Mehta, N.N.; Li, K.; Szapary, P.; Krueger, J.; Brodmerkel, C. Modulation of Cardiometabolic Pathways in Skin and Serum from Patients with Psoriasis. J. Transl. Med. 2013, 11, 194. [Google Scholar] [CrossRef]
- Hashmi, S.; Zeng, Q.T. Role of Interleukin-17 and Interleukin-17-Induced Cytokines Interleukin-6 and Interleukin-8 in Unstable Coronary Artery Disease. Coron. Artery Dis. 2006, 17, 699–706. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Placek, W. Interleukin-17 as a factor linking the pathogenesis of psoriasiswith metabolic disorders. Int. J. Dermatol. 2017, 56, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kolliker Frers, R.A.; Cosentino, V.; Tau, J.; Kerzberg, E.M.; Urdapilleta, A.; Chiocconi, M.; Kogan, N.; Otero-Losada, M.; Capani, F. Immune-Mediated Inflammation Promotes Subclinical Atherosclerosis in Recent-Onset Psoriatic Arthritis Patients without Conventional Cardiovascular Risk Factors. Front. Immunol. 2018, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Hjuler, K.F.; Gormsen, L.C.; Vendelbo, M.H.; Egeberg, A.; Nielsen, J.; Iversen, L. Increased Global Arterial and Subcutaneous Adipose Tissue Inflammation in Patients with Moderate-to-Severe Psoriasis. Br. J. Dermatol. 2017, 176, 732–740. [Google Scholar] [CrossRef]
- Youn, S.W.; Kang, S.Y.; Kim, S.A.; Park, G.Y.; Lee, W.W. Subclinical Systemic and Vascular Inflammation Detected by (18) F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Mild Psoriasis. J. Dermatol. 2015, 42, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, H.; Loyd, C.M.; Fu, W.; Diaconu, D.; Liu, S.; Cooper, K.D.; McCormick, T.S.; Simon, D.I.; Ward, N.L. Chronic Skin-Specific Inflammation Promotes Vascular Inflammation and Thrombosis. J. Investig. Dermatol. 2012, 132, 2067–2075. [Google Scholar] [CrossRef]
- Hu, J.; Yang, R.; Wen, C.; Li, H.; Zhao, H. Expression of NLRP3 inflammasome in BALB/c mice with imiquimod-induced psoriasis-like inflammation and therapeutic effect of mustard seed (Sinapis Alba Linn). Nan Fang Yi Ke Da Xue Xue Bao 2013, 33, 1394–1398. [Google Scholar] [PubMed]
- Jiang, W.; Zhu, F.-G.; Bhagat, L.; Yu, D.; Tang, J.X.; Kandimalla, E.R.; La Monica, N.; Agrawal, S. A Toll-like Receptor 7, 8, and 9 Antagonist Inhibits Th1 and Th17 Responses and Inflammasome Activation in a Model of IL-23-Induced Psoriasis. J. Investig. Dermatol. 2013, 133, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. The Role of NLRP1, NLRP3, and AIM2 Inflammasomes in Psoriasis: Review. Int. J. Mol. Sci. 2021, 22, 5898. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, H.; Nikamo, P.; Qi Low, H.; Helms, C.; Seielstad, M.; Liu, J.; Bowcock, A.M.; Stahle, M.; Liao, W. Association of Cardiovascular and Metabolic Disease Genes with Psoriasis. J. Investig. Dermatol. 2013, 133, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Campalani, E.; Allen, M.H.; Fairhurst, D.; Young, H.S.; Mendonca, C.O.; Burden, A.D.; Griffiths, C.E.M.; Crook, M.A.; Barker, J.N.W.N.; Smith, C.H. Apolipoprotein E Gene Polymorphisms Are Associated with Psoriasis but Do Not Determine Disease Response to Acitretin: ApoE Gene Polymorphisms, Psoriasis and Acitretin. Br. J. Dermatol. 2006, 154, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Eirís, N.; González-Lara, L.; Santos-Juanes, J.; Queiro, R.; Coto, E.; Coto-Segura, P. Genetic Variation at IL12B, IL23R and IL23A Is Associated with Psoriasis Severity, Psoriatic Arthritis and Type 2 Diabetes Mellitus. J. Dermatol. Sci. 2014, 75, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Kadono, T. “Inflammatory Skin March” in Atopic Dermatitis and Psoriasis. Inflamm. Res. 2017, 66, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K.; Leducq Transatlantic Network on Atherothrombosis. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Hu, S.; Lan, C.-C.E. Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment. Int. J. Mol. Sci. 2017, 18, 2211. [Google Scholar] [CrossRef]
- Mahmoudi, M.J.; Saboor-Yaraghi, A.-A.; Zabetian-Targhi, F.; Siassi, F.; Zarnani, A.H.; Eshraghian, M.R.; Shokri, F.; Rezaei, N.; Kalikias, Y.; Mahmoudi, M. Vitamin A Decreases Cytotoxicity of Oxidized Low-Density Lipoprotein in Patients with Atherosclerosis. Immunol. Investig. 2016, 45, 52–62. [Google Scholar] [CrossRef]
- Boehncke, S.; Fichtlscherer, S.; Salgo, R.; Garbaraviciene, J.; Beschmann, H.; Diehl, S.; Hardt, K.; Thaçi, D.; Boehncke, W.-H. Systemic Therapy of Plaque-Type Psoriasis Ameliorates Endothelial Cell Function: Results of a Prospective Longitudinal Pilot Trial. Arch. Derm. Res. 2011, 303, 381–388. [Google Scholar] [CrossRef]
- Micha, R.; Imamura, F.; Wyler von Ballmoos, M.; Solomon, D.H.; Hernán, M.A.; Ridker, P.M.; Mozaffarian, D. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef]
- Caiazzo, G.; Fabbrocini, G.; Di Caprio, R.; Raimondo, A.; Scala, E.; Balato, N.; Balato, A. Psoriasis, Cardiovascular Events, and Biologics: Lights and Shadows. Front. Immunol. 2018, 9, 1668. [Google Scholar] [CrossRef]
- Rich, S.J.; Bello-Quintero, C.E. Advancements in the Treatment of Psoriasis: Role of Biologic Agents. J. Manag. Care Pharm. 2004, 10, 318–325. [Google Scholar] [CrossRef]
- Pina, T.; Armesto, S.; Lopez-Mejias, R.; Genre, F.; Ubilla, B.; Gonzalez-Lopez, M.A.; Gonzalez-Vela, M.C.; Corrales, A.; Blanco, R.; Garcia-Unzueta, M.T.; et al. Anti-TNF-α Therapy Improves Insulin Sensitivity in Non-Diabetic Patients with Psoriasis: A 6-Month Prospective Study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1325–1330. [Google Scholar] [CrossRef]
- Kwiek, B.; Narbutt, J.; Sysa-Jędrzejowska, A.; Langner, A.; Lesiak, A. Long-Term Treatment of Chronic Plaque Psoriasis with Biological Drugs Can Control Platelet Activation: Targeting the Bridge between Inflammation and Atherothrombosis. Postepy Dermatol. Alergol. 2017, 34, 131–137. [Google Scholar] [CrossRef]
- Pina, T.; Genre, F.; Lopez-Mejias, R.; Armesto, S.; Ubilla, B.; Mijares, V.; Dierssen-Sotos, T.; Corrales, A.; Gonzalez-Lopez, M.A.; Gonzalez-Vela, M.C.; et al. Anti-TNF-α Therapy Reduces Retinol-Binding Protein 4 Serum Levels in Non-Diabetic Patients with Psoriasis: A 6-Month Prospective Study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 92–95. [Google Scholar] [CrossRef]
- Pina, T.; Corrales, A.; Lopez-Mejias, R.; Armesto, S.; Gonzalez-Lopez, M.A.; Gómez-Acebo, I.; Ubilla, B.; Remuzgo-Martínez, S.; Gonzalez-Vela, M.C.; Blanco, R.; et al. Anti-Tumor Necrosis Factor-Alpha Therapy Improves Endothelial Function and Arterial Stiffness in Patients with Moderate to Severe Psoriasis: A 6-Month Prospective Study. J. Dermatol. 2016, 43, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Papadavid, E.; Makavos, G.; Andreadou, I.; Varoudi, M.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Moutsatsou, P.; et al. Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared with Tumor Necrosis Factor-a Antagonism or Cyclosporine in Psoriasis. Circ. Cardiovasc. Imaging 2017, 10, e006283. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Langley, R.G.; Gottlieb, A.B.; Papp, K.A.; Krueger, G.G.; Strober, B.E.; Williams, D.A.; Gu, Y.; Valdes, J.M. A Phase III, Randomized, Controlled Trial of the Fully Human IL-12/23 MAb Briakinumab in Moderate-to-Severe Psoriasis. J. Investig. Dermatol. 2012, 132, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Krueger, G.G.; Langley, R.G.; Leonardi, C.; Yeilding, N.; Guzzo, C.; Wang, Y.; Dooley, L.T.; Lebwohl, M.; CNTO 1275 Psoriasis Study Group. A Human Interleukin-12/23 Monoclonal Antibody for the Treatment of Psoriasis. N. Engl. J. Med. 2007, 356, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in Plaque Psoriasis--Results of Two Phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef]
- Bai, F.; Li, G.G.; Liu, Q.; Niu, X.; Li, R.; Ma, H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Immunol. Res. 2019, 2019, 2546161. [Google Scholar] [CrossRef]
- Makavos, G.; Ikonomidis, I.; Andreadou, I.; Varoudi, M.; Kapniari, I.; Loukeri, E.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Thymis, J.; et al. Effects of Interleukin 17A Inhibition on Myocardial Deformation and Vascular Function in Psoriasis. Can. J. Cardiol. 2020, 36, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Tardif, J.-C.; Harel, F.; Pressacco, J.; Bolduc, C.; Guertin, M.-C. Effects of the Tumor Necrosis Factor-α Antagonist Adalimumab on Arterial Inflammation Assessed by Positron Emission Tomography in Patients with Psoriasis: Results of a Randomized Controlled Trial: Results of a Randomized Controlled Trial. Circ. Cardiovasc. Imaging 2013, 6, 83–90. [Google Scholar] [CrossRef]
- Hjuler, K.F.; Bøttcher, M.; Vestergaard, C.; Bøtker, H.E.; Iversen, L.; Kragballe, K. Association between Changes in Coronary Artery Disease Progression and Treatment with Biologic Agents for Severe Psoriasis. JAMA Dermatol. 2016, 152, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahlehoff, O.; Hansen, P.R.; Gislason, G.H.; Frydland, M.; Bryld, L.E.; Elming, H.; Jemec, G.B.E. Myocardial Function and Effects of Biologic Therapy in Patients with Severe Psoriasis: A Prospective Echocardiographic Study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 819–823. [Google Scholar] [CrossRef]
- Herédi, E.; Végh, J.; Pogácsás, L.; Gáspár, K.; Varga, J.; Kincse, G.; Zeher, M.; Szegedi, A.; Gaál, J. Subclinical Cardiovascular Disease and It’s Improvement after Long-Term TNF-α Inhibitor Therapy in Severe Psoriatic Patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1531–1536. [Google Scholar] [CrossRef]
- Mann, D.L.; McMurray, J.J.V.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted Anticytokine Therapy in Patients with Chronic Heart Failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL): Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T.; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-Alpha, in Patients with Moderate-to-Severe Heart Failure: Results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) Trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.R.; Blumenschein, W.; Murphy, E.; Diveu, C.; Wiekowski, M.; Abbondanzo, S.; Lucian, L.; Geissler, R.; Brodie, S.; Kimball, A.B.; et al. IL-23 Stimulates Epidermal Hyperplasia via TNF and IL-20R2-Dependent Mechanisms with Implications for Psoriasis Pathogenesis. J. Exp. Med. 2006, 203, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Gregersen, I.; Holm, S.; Daissormont, I.; Bjerkeli, V.; Krohg-Sørensen, K.; Skagen, K.R.; Dahl, T.B.; Russell, D.; Almås, T.; et al. Interleukin 23 Levels Are Increased in Carotid Atherosclerosis: Possible Role for the Interleukin 23/Interleukin 17 Axis. Stroke 2015, 46, 793–799. [Google Scholar] [CrossRef]
- Mitra, A.; Fallen, R.S.; Lima, H.C. Cytokine-based therapy in psoriasis. Clin. Rev. Allergy Immunol. 2013, 44, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Langley, R.G.; Lebwohl, M.; Szapary, P.; Guzzo, C.; Yeilding, N.; Li, S.; Hsu, M.-C.; Griffiths, C.E.M. Cardiovascular Safety of Ustekinumab in Patients with Moderate to Severe Psoriasis: Results of Integrated Analyses of Data from Phase II and III Clinical Studies: Cardiovascular Safety of Ustekinumab in Clinical Trials of Psoriasis. Br. J. Dermatol. 2011, 164, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Torres, G.; McCormick, T.; Marano, C.; Cooper, K.; Yeilding, N.; Wang, Y.; Pendley, C.; Prabhakar, U.; Wong, J.; et al. Positive Treatment Effects of Ustekinumab in Psoriasis: Analysis of Lesional and Systemic Parameters: Ustekinumab Has Minimal Systemic Effects. J. Dermatol. 2010, 37, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kagami, S.; Rizzo, H.L.; Lee, J.J.; Koguchi, Y.; Blauvelt, A. Circulating Th17, Th22, and Th1 Cells Are Increased in Psoriasis. J. Investig. Dermatol. 2010, 130, 1373–1383. [Google Scholar] [CrossRef]
- Wu, D.; Hou, S.-Y.; Zhao, S.; Hou, L.-X.; Jiao, T.; Xu, N.-N.; Zhang, N. Efficacy and Safety of Interleukin-17 Antagonists in Patients with Plaque Psoriasis: A Meta-Analysis from Phase 3 Randomized Controlled Trials. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- van de Kerkhof, P.C.M.; Griffiths, C.E.M.; Reich, K.; Leonardi, C.L.; Blauvelt, A.; Tsai, T.-F.; Gong, Y.; Huang, J.; Papavassilis, C.; Fox, T. Secukinumab Long-Term Safety Experience: A Pooled Analysis of 10 Phase II and III Clinical Studies in Patients with Moderate to Severe Plaque Psoriasis. J. Am. Acad. Dermatol. 2016, 75, 83–98.e4. [Google Scholar] [CrossRef]
- Strober, B.; Leonardi, C.; Papp, K.A.; Mrowietz, U.; Ohtsuki, M.; Bissonnette, R.; Ferris, L.K.; Paul, C.; Lebwohl, M.; Braun, D.K.; et al. Short- and Long-Term Safety Outcomes with Ixekizumab from 7 Clinical Trials in Psoriasis: Etanercept Comparisons and Integrated Data. J. Am. Acad. Dermatol. 2017, 76, 432–440.e7. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Amoruso, G.F.; Nisticò, S.P.; Iannone, L.; Russo, E.; Rago, G.; Patruno, C.; Bennardo, L. Ixekizumab May Improve Renal Function in Psoriasis. Healthcare 2021, 9, 543. [Google Scholar] [CrossRef]
- Papp, K.A.; Reich, K.; Paul, C.; Blauvelt, A.; Baran, W.; Bolduc, C.; Toth, D.; Langley, R.G.; Cather, J.; Gottlieb, A.B.; et al. A Prospective Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Brodalumab in Patients with Moderate-to-Severe Plaque Psoriasis. Br. J. Dermatol. 2016, 175, 273–286. [Google Scholar] [CrossRef]
- Lebwohl, M.; Strober, B.; Menter, A.; Gordon, K.; Weglowska, J.; Puig, L.; Papp, K.; Spelman, L.; Toth, D.; Kerdel, F.; et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N. Engl. J. Med. 2015, 373, 1318–1328. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristics—Bimzelx. Available online: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf (accessed on 19 September 2021).
- Blauvelt, A.; Papp, K.A.; Merola, J.F.; Gottlieb, A.B.; Cross, N.; Madden, C.; Wang, M.; Cioffi, C.; Griffiths, C.E.M. Bimekizumab for Patients with Moderate to Severe Plaque Psoriasis: 60-Week Results from BE ABLE 2, a Randomized, Double-Blinded, Placebo-Controlled, Phase 2b Extension Study. J. Am. Acad. Dermatol. 2020, 83, 1367–1374. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Kavanaugh, A.; Merola, J.F.; Schett, G.; Scher, J.U.; Warren, R.B.; Gottlieb, A.B.; Assudani, D.; Bedford-Rice, K.; Coarse, J.; et al. Bimekizumab in patients with active psoriatic arthritis: Results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 2020, 395, 427–440. [Google Scholar] [CrossRef]
- Rungapiromnan, W.; Yiu, Z.Z.N.; Warren, R.B.; Griffiths, C.E.M.; Ashcroft, D.M. Impact of Biologic Therapies on Risk of Major Adverse Cardiovascular Events in Patients with Psoriasis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Br. J. Dermatol. 2017, 176, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Patel, R.; Pradhan, D.; Deval, R.; Singh, H.; Thomas, G.; Jain, A.K. Psoriasis and Cardiovascular Disorders: Association or Epiphenomenon? Meta-Analysis of Observational Studies. 3 Biotech 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Eder, L.; Chandran, V.; Gladman, D.D. The Framingham Risk Score Underestimates the Extent of Subclinical Atherosclerosis in Patients with Psoriatic Disease. Ann. Rheum. Dis. 2014, 73, 1990–1996. [Google Scholar] [CrossRef]
- Fernández-Torres, R.; Pita-Fernández, S.; Fonseca, E. Psoriasis and Cardiovascular Risk. Assessment by Different Cardiovascular Risk Scores: Psoriasis and Cardiovascular Risk Scores. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR Recommendations for Cardiovascular Disease Risk Management in Patients with Rheumatoid Arthritis and Other Forms of Inflammatory Joint Disorders: 2015/2016 Update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Koo, J. The Mechanism of Lithium and Beta-Blocking Agents in Inducing and Exacerbating Psoriasis. J. Drugs Dermatol. 2006, 5, 426–432. [Google Scholar]
- Baccino, D.; Merlo, G.; Cozzani, E.; Rosa, G.M.; Tini, G.; Burlando, M.; Parodi, A. Cutaneous Effects of Antihypertensive Drugs. G. Ital. Dermatol. Venereol. 2020, 155, 202–211. [Google Scholar] [CrossRef]
- Singh, S.; Bhansali, A. Randomized Placebo Control Study of Insulin Sensitizers (Metformin and Pioglitazone) in Psoriasis Patients with Metabolic Syndrome (Topical Treatment Cohort). BMC Dermatol. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Buysschaert, M.; Baeck, M.; Preumont, V.; Marot, L.; Hendrickx, E.; Van Belle, A.; Dumoutier, L. Improvement of Psoriasis during Glucagon-like Peptide-1 Analogue Therapy in Type 2 Diabetes Is Associated with Decreasing Dermal Γδ T-Cell Number: A Prospective Case-Series Study. Br. J. Dermatol. 2014, 171, 155–161. [Google Scholar] [CrossRef]
- Kim, S.C.; Schneeweiss, S.; Glynn, R.J.; Doherty, M.; Goldfine, A.B.; Solomon, D.H. Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes May Reduce the Risk of Autoimmune Diseases: A Population-Based Cohort Study. Ann. Rheum. Dis. 2015, 74, 1968–1975. [Google Scholar] [CrossRef]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and Safety of Risankizumab in Moderate-to-Severe Plaque Psoriasis (UltIMMa-1 and UltIMMa-2): Results from Two Double-Blind, Randomised, Placebo-Controlled and Ustekinumab-Controlled Phase 3 Trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef]
- Lilly’s Mirikizumab Superior to Cosentyx (Secukinumab) in a Phase 3 Study for Patients with Moderate to Severe Plaque Psoriasis. Available online: https://investor.lilly.com/node/43481/pdf (accessed on 19 September 2021).
- Dattola, A.; Silvestri, M.; Tamburi, F.; Amoruso, G.F.; Bennardo, L.; Nisticò, S.P. Emerging Role of Anti-IL23 in the Treatment of Psoriasis: When Humanized Is Very Promising. Dermatol. Ther. 2020, 33, e14504. [Google Scholar] [CrossRef]
- Kvist-Hansen, A.; Hansen, P.R.; Skov, L. Systemic Treatment of Psoriasis with JAK Inhibitors: A Review. Dermatol. Ther. 2020, 10, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Gordon, K.; Thaçi, D.; Morita, A.; Gooderham, M.; Foley, P.; Girgis, I.G.; Kundu, S.; Banerjee, S. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N. Engl. J. Med. 2018, 379, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Menter, M.A.; Abe, M.; Elewski, B.; Feldman, S.R.; Gottlieb, A.B.; Langley, R.; Luger, T.; Thaci, D.; Buonanno, M.; et al. Tofacitinib, an Oral Janus Kinase Inhibitor, for the Treatment of Chronic Plaque Psoriasis: Results from Two Randomized, Placebo-Controlled, Phase III Trials. Br. J. Dermatol. 2015, 173, 949–961. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Summary of Product Characteristics—Xeljanz. Available online: https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf (accessed on 19 September 2021).
- Wolk, R.; Armstrong, E.J.; Hansen, P.R.; Thiers, B.; Lan, S.; Tallman, A.M.; Kaur, M.; Tatulych, S. Effect of Tofacitinib on Lipid Levels and Lipid-Related Parameters in Patients with Moderate to Severe Psoriasis. J. Clin. Lipidol. 2017, 11, 1243–1256. [Google Scholar] [CrossRef]
- Desai, R.J.; Pawar, A.; Weinblatt, M.E.; Kim, S.C. Comparative Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Receiving Tofacitinib versus Those Receiving Tumor Necrosis Factor Inhibitors: An Observational Cohort Study. Arthritis Rheumatol. 2019, 71, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Piros, É.A.; Szilveszter, B.; Vattay, B.; Maurovich-Horvat, P.; Szalai, K.; Dósa, E.; Merkely, B.; Holló, P. Novel Anti-Inflammatory Therapies to Reduce Cardiovascular Burden of Psoriasis. Dermatol. Ther. 2021, 34, e14721. [Google Scholar] [CrossRef] [PubMed]
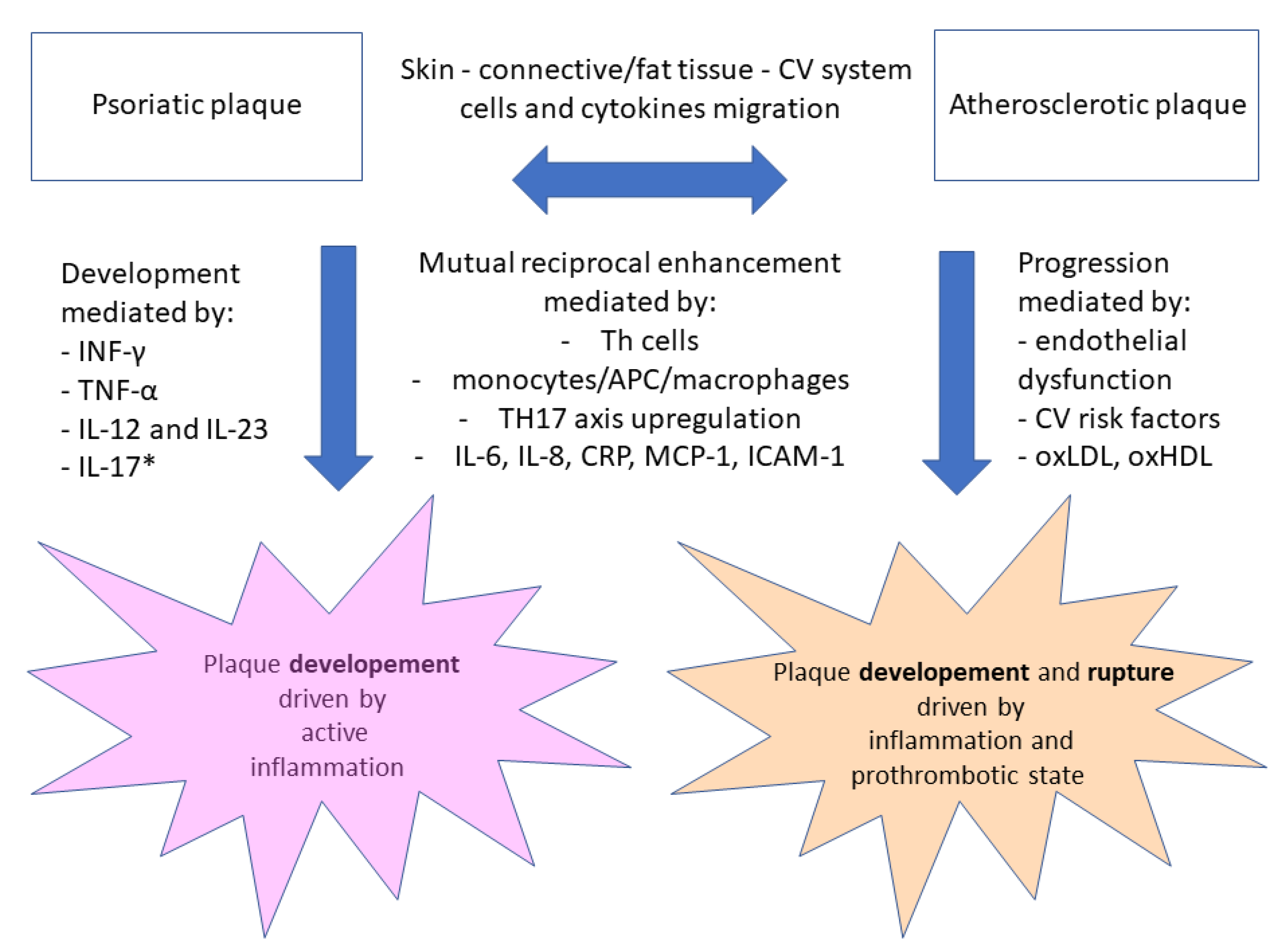
| Disease/State Related to Psoriasis | Pathophysiologic Link and Chosen References |
|---|---|
| Psoriatic arthritis (PSA) | This form of psoriasis (PSO) involving joints is related to higher prevalence of cardiovascular and metabolic risk factors, atherosclerosis progression, as well as cardiovascular complications including myocardial infarction and strokes as compared to psoriasis limited only to the skin [30,31,32,33]. |
| Metabolic syndrome | Increased prevalence of traditional risk factors: arterial hypertension (HTN), dyslipidemia (abnormalities found even in pediatrics populations as well as data suggesting increased risk of PSO development in long-lasting dyslipidemia), obesity, diabetes mellitus and their clustering in psoriatic patients [34,35,36], evidence of more difficult control of risk factors in psoriatic population [37], impaired cholesterol efflux capacity from macrophages observed in psoriasis because of higher level of oxidized high-density lipoprotein (oxHDL) [38], higher lipoprotein (a), apolipoprotein B and oxidized low-density lipoprotein (oxLDL) levels [39], as well as increased epicardial fat amount (expressed as thickness or area) [40]. Well-controlled glycaemia in diabetics was related to better control of skin psoriasis [41]. |
| Nonalcoholic fatty liver disease | High triglycerides and abnormal serum fatty acid profile were also described in PSO [42]. In obese psoriatic patients treated with anti-TNF-alpha medications, dose–response relationship and particular tendency to central obesity were described. Beneficial effect of weight loss on improvement of PSO, including the increase in the efficacy of the treatment, was also observed [43,44,45,46]. |
| Inflammatory bowel disease (IBD): Crohn’s disease and ulcerative colitis | Increased levels of IL-17 in both IBD (both in serum and bowel mucosa) and PSO [47]. Common pleiotropic susceptibility loci identified in PSO, type II diabetes and Crohn’s disease (genes related to locus 6p21 coding the major histocompatibility complex—MHC, genes related to IL23R and IL12B, the latter encoding p40 subunit essential in pathogenesis of IBD and PSO), shared clinical course and immunologic features [48,49,50]. Postulated similar abnormalities of gut microbiota and role of gut–skin axis in IBD and PSO/PSA, characterized generally by decreased bacterial diversity of gut microbiota beyond skin dysbiosis typical for PSO [51,52,53]. Increased risk of Crohn’s disease and ulcerative colitis in PSO as well as in PSA [54]. |
| Cardiovascular disorders | Reciprocal relationship between the increased prevalence of HTN in psoriasis as well as more frequent development of PSO in hypertensives observed, e.g., in a Nurses’ Health Study—the use of beta-blockers for HTN treatment was postulated as a potential mechanism [55]. The amount of adipose tissue which in PSO is increased also around vessel walls is the source of angiotensinogen, converted then to angiotensin II, which further stimulates Th17 cells and enhanced hypertension by vascular dysfunction [56]. Increased cigarette smoking and alcohol use reported in PSO as well as an association between smoking and PSO severity. The increased amount of Th17 cells in blood in psoriatic patients who smoke was observed [57,58]. Evidence for increased number of myocardial infarctions and strokes as well as CV mortality in PSO, PSA, as well as rheumatoid arthritis patients [59,60,61]. Some studies indicated the increased number of venous thrombosis (VTE) in PSO patients. This trend was confirmed by meta-analysis although without reaching statistical significance [62,63,64,65,66]. Augmented risk of new-onset heart failure showing severity-dependence in psoriatic patients was observed in a Danish nationwide cohort study [67]. Increased prevalence of dilated cardiomyopathy was observed in PSO [68]. |
| Lymphoma and other malignancies | PSO seem to be related to an increased risk of cancer, especially keratinocyte cancers and lymphomas, including cutaneous T-cell lymphomas (CTCL) [69,70,71,72]. PSO treated with psoralen-UV-A (PUVA) therapy shows highly increased risk of squamous cell carcinomas [73]. The increased risk of CTCL in PSO may be explained by persistent immune activation leading to the development of a dominant clone [74]. Certain known risk factors for cancerogenesis (such as alcohol and smoking) are more prevalent in psoriatic patients and some studies postulated the increased risk of lung, bladder, breast (observed in PSA patients in 3 studies) and colorectal cancer in psoriatic patients [75,76] as well as an increased risk of overall cancers after adjustment for confounders may be connected to this phenomenon [77]. The impact of conventional vs biologic treatment as well as potential differences in patients with PSA needs further analysis. |
| Depression | Chronic inflammation and endothelial dysfunction as well as higher incidence of traditional risk factors in PSO; additionally, sleep and anxiety disorders as well as depression led to further increases in CV complications [78,79,80]. Impaired quality of life, typical for chronic, systemic inflammatory disease, as well as for significant skin and joint involvement [81,82]. Observed reciprocal association between decreased probability of performing more intense physical activity when suffering from PSO, as well as reduced risk of PSO incidence in those performing vigorous physical activity [83,84]. |
| Group of Medication | Name of Drug | Specific Targets and Actions in Immune System/Role in Pathogenesis and Treatment |
|---|---|---|
| TNF-α inhibitors | Infliximab Etanercept Adalimumab Certolizumab-pegol Golimumab | Target elevated levels of TNF-α; cause CRP, VEGF and resistin as well as chemotactics factors’ reduction: VCAM-1, E-selectin, IL-8, MCP-1 [113]. Additionally, decreases Th17 cell count in blood by stopping CD4+ T cells differentiation into Th1, Th17 and Th22. Improvement of insulin sensitivity [114] and showing antiplatelet activity, especially in patients with increased platelet activation prior to treatment, as evidenced by decreases in P-selectin levels [115] (data for TNF inhibitors and ustekinumab). Reducing level of circulating retinol-binding protein 4: RBP4 (showed to be linked to subclinical atherosclerosis with positive correlation with intima-media thickness) [116]. Adalimumab therapy improved pulse wave velocity (being strong predictor of CV events) after 6 months of therapy [117]. |
| Inhibitors of the p40 subunit to IL-12 and IL-23 | Ustekinumab Briakinumab | Human monoclonal antibodies from this group showed improvement of myocardial and vascular function as compared to treatment with cyclosporine or TNF alfa inhibitors [118]. During the initial stage, the temporary increase in pro-atherogenic mediators was observed. Small, randomized trials showed the increase in CV events with briakinumab (but not with ustekinumab), which was then withdrawn from application for psoriasis treatment [119,120]. |
| IL-17A inhibitors | Secukinumab Ixekizumab | Confirmed high, dose-dependent efficacy in improving PSO during short-term 12–16-week treatment [121,122]. Moreover, secukinumab improved myocardial and vascular function as assessed by global longitudinal strain and pulse wave velocity measurements, respectively, which achieved significance in comparison to MTX and cyclosporine therapy after 12 months of follow-up [123]. |
| IL-17 receptor antagonist | Brodalumab | |
| Novel inhibitors of IL-23 (blocking p19 unit of IL23) | Guselkumab Risankizumab Tildrakizumab Mirikizumab | Data are still being gathered; risankizumab being a new type of IL-23 inhibitor, seems to present better short-term efficacy [122]. In comparison with IL-17 antagonists, the novel group seems to show a better safety profile [122]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska-Drabik, K.; Lesiak, A.; Skibińska, M.; Niedźwiedź, M.; Kasprzak, J.D.; Narbutt, J. Psoriasis and Atherosclerosis—Skin, Joints, and Cardiovascular Story of Two Plaques in Relation to the Treatment with Biologics. Int. J. Mol. Sci. 2021, 22, 10402. https://doi.org/10.3390/ijms221910402
Wierzbowska-Drabik K, Lesiak A, Skibińska M, Niedźwiedź M, Kasprzak JD, Narbutt J. Psoriasis and Atherosclerosis—Skin, Joints, and Cardiovascular Story of Two Plaques in Relation to the Treatment with Biologics. International Journal of Molecular Sciences. 2021; 22(19):10402. https://doi.org/10.3390/ijms221910402
Chicago/Turabian StyleWierzbowska-Drabik, Karina, Aleksandra Lesiak, Małgorzata Skibińska, Michał Niedźwiedź, Jarosław D. Kasprzak, and Joanna Narbutt. 2021. "Psoriasis and Atherosclerosis—Skin, Joints, and Cardiovascular Story of Two Plaques in Relation to the Treatment with Biologics" International Journal of Molecular Sciences 22, no. 19: 10402. https://doi.org/10.3390/ijms221910402
APA StyleWierzbowska-Drabik, K., Lesiak, A., Skibińska, M., Niedźwiedź, M., Kasprzak, J. D., & Narbutt, J. (2021). Psoriasis and Atherosclerosis—Skin, Joints, and Cardiovascular Story of Two Plaques in Relation to the Treatment with Biologics. International Journal of Molecular Sciences, 22(19), 10402. https://doi.org/10.3390/ijms221910402









