Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship?
Abstract
1. Introduction
2. Psoriasis as an Autoinflammatory Disease
3. Mast Cells
4. Mast Cells and Psychological Stress
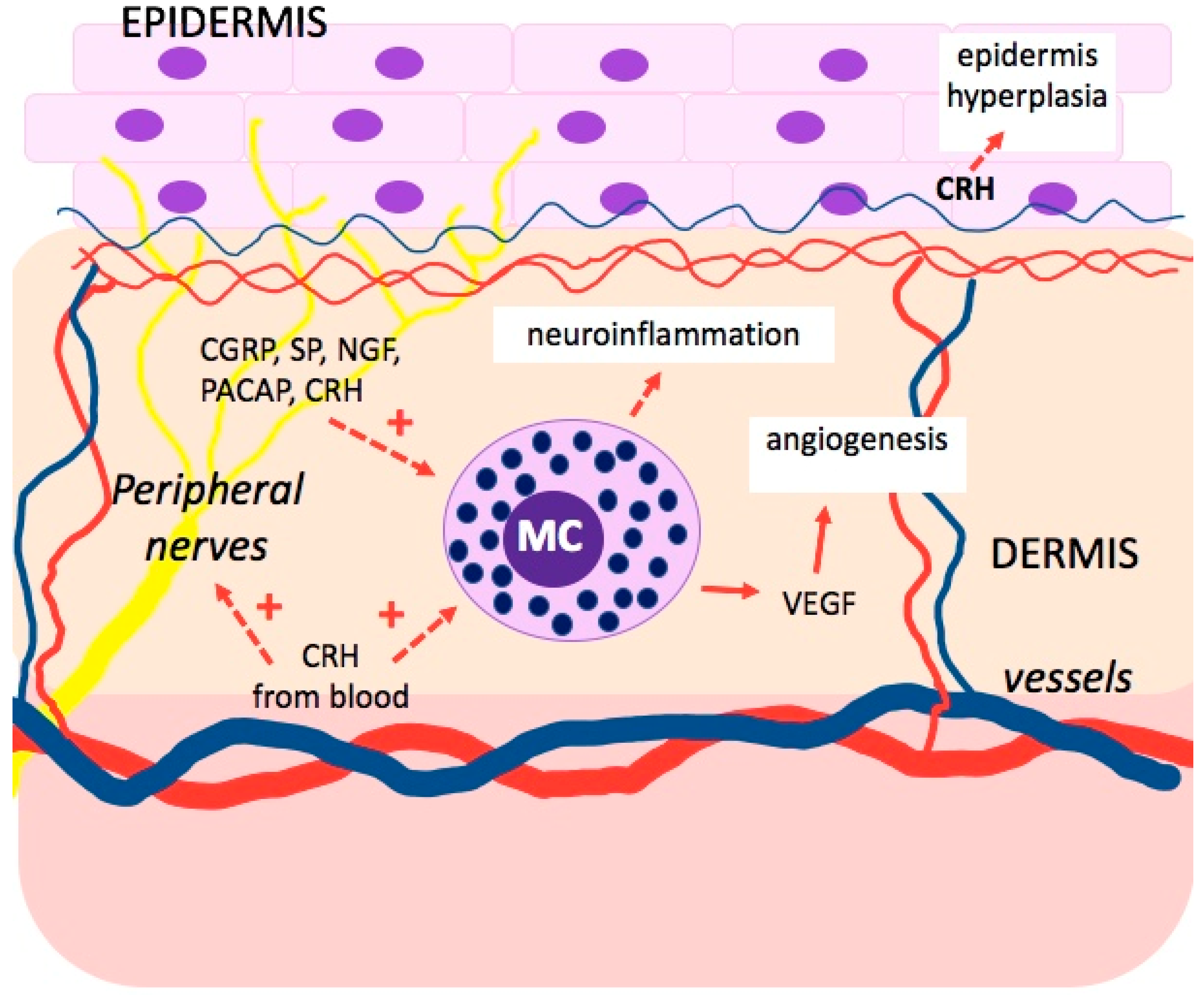
5. Nervous–Endocrine–Immune Networks in the Skin
6. The Role of the Hypothalamic–Pituitary–Adrenal Axis in the Skin
6.1. Corticotropin-Releasing Hormone (CRH)
6.2. Proopiomelanocortin and Its Derivatives
7. Psychological Stress and Psoriasis Skin
8. Psoriasis and Mental Disorders
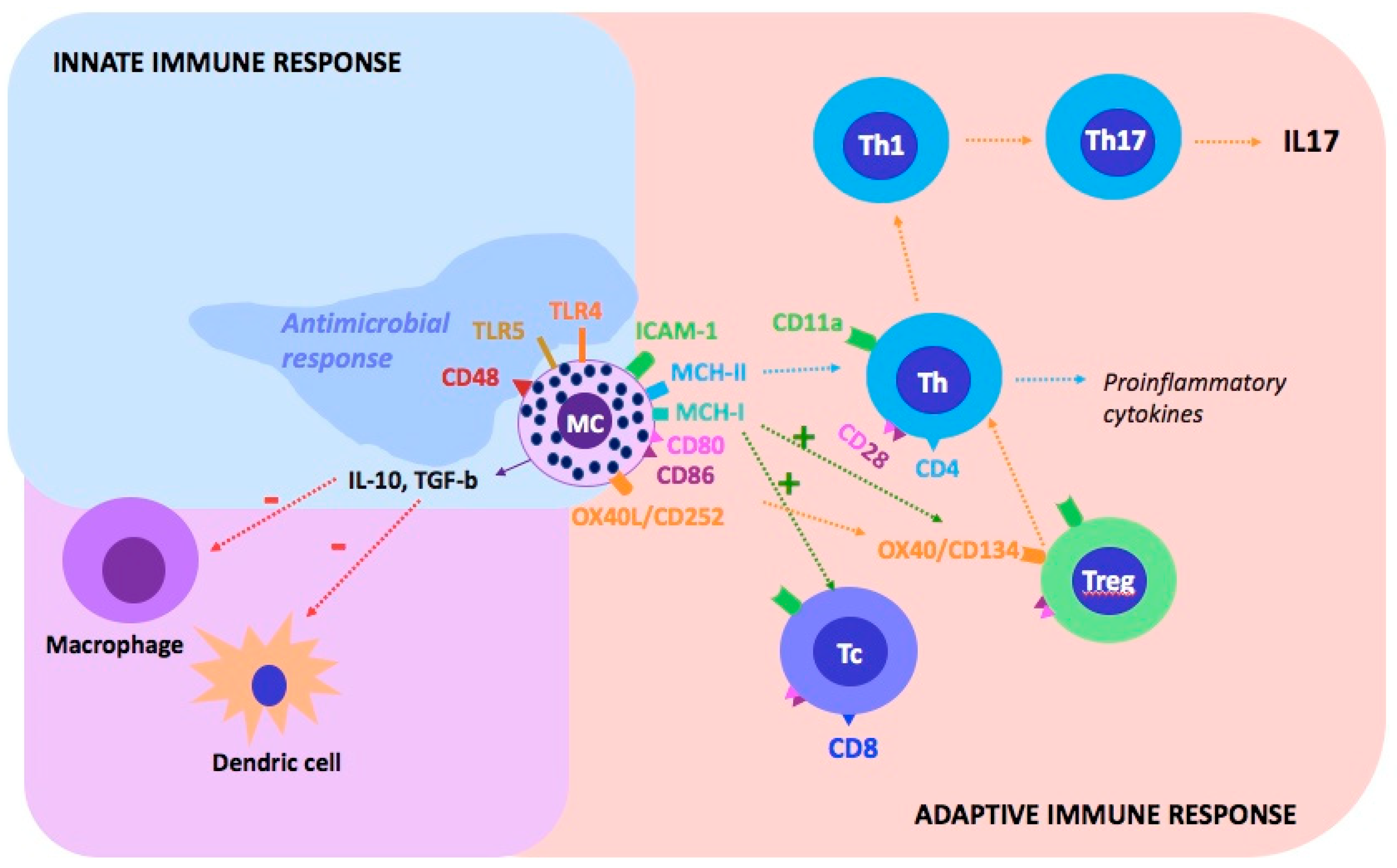
9. Interaction between Mast Cells and Different Immunological Molecules in Psoriasis
10. The Influence of Psychological Therapies and Relaxation Techniques on the Therapy of Various Autoimmune Diseases
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seneviratne, S.L.; Maitland, A.; Afrin, L. Mast Cell Disorders in Ehlers-Danlos Syndrome. Am. J. Med. Genet. 2017, 175, 226–236. [Google Scholar] [CrossRef]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human Mast Cells Are Major IL-22 Producers in Patients with Psoriasis and Atopic Dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359.e1. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast Cells and Inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Mohammadi, A.; Rhatigan, R. A Comparative Analysis of Mast Cell Quantification in Five Common Dermatoses: Lichen Simplex Chronicus, Psoriasis, Lichen Planus, Lupus, and Insect Bite/Allergic Contact Dermatitis/Nummular Dermatitis. ISRN Dermatol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945. [Google Scholar] [CrossRef] [PubMed]
- Tillett, W.; Charlton, R.; Nightingale, A.; Snowball, J.; Green, A.; Smith, C.; Shaddick, G.; McHugh, N. Interval between Onset of Psoriasis and Psoriatic Arthritis Comparing the UK Clinical Practice Research Datalink with a Hospital-Based Cohort. Rheumatology 2017, 56, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on Psoriasis Immunopathogenesis and Targeted Immunotherapy. Semin. Immunopathol. 2016, 38, 11–27. [Google Scholar] [CrossRef]
- Ansarimoghaddam, A.; Adineh, H.A.; Zareban, I.; Iranpour, S.; HosseinZadeh, A.; Kh, F. Prevalence of Metabolic Syndrome in Middle-East Countries: Meta-Analysis of Cross-Sectional Studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 195–201. [Google Scholar] [CrossRef]
- El-Boghdady, N.A.; Ismail, M.F.; Abd-Alhameed, M.F.; Ahmed, A.S.; Ahmed, H.H. Bidirectional Association Between Psoriasis and Obesity: Benefits and Risks. J. Interferon Cytokine Res. 2018, 38, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Deplus, R.; Brenner, C.; Burgers, W.A.; Putmans, P.; Kouzarides, T.; de Launoit, Y.; Fuks, F. Dnmt3L Is a Transcriptional Repressor That Recruits Histone Deacetylase. Nucleic Acids Res. 2002, 30, 3831–3838. [Google Scholar] [CrossRef]
- Slieker, R.C.; Relton, C.L.; Gaunt, T.R.; Slagboom, P.E.; Heijmans, B.T. Age-Related DNA Methylation Changes Are Tissue-Specific with ELOVL2 Promoter Methylation as Exception. Epigenetics Chromatin 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, L.C.; Stuart, P.E.; Tian, C.; Gudjonsson, J.E.; Das, S.; Zawistowski, M.; Ellinghaus, E.; Barker, J.N.; Chandran, V.; Dand, N.; et al. Large Scale Meta-Analysis Characterizes Genetic Architecture for Common Psoriasis Associated Variants. Nat. Commun. 2017, 8, 15382. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pradhan, D.; Puri, P.; Ramesh, V.; Aggarwal, S.; Nayek, A.; Jain, A.K. Genomic Alterations Driving Psoriasis Pathogenesis. Gene 2019, 683, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Al’Abadie, M.S.; Kent, G.G.; Gawkrodger, D.J. The Relationship between Stress and the Onset and Exacerbation of Psoriasis and Other Skin Conditions. Br. J. Dermatol. 1994, 130, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Richards, H.L. Psychological Influences in Psoriasis: Psychological Influences in Psoriasis. Clin. Exp. Dermatol. 2001, 26, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Romanelli, P.; Volpe, E.; Borsellino, G.; Romanelli, M. Scanning the Immunopathogenesis of Psoriasis. Int. J. Mol. Sci. 2018, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Spain, S.L.; Knight, J.; Ellinghaus, E.; Stuart, P.E.; Capon, F.; Ding, J.; Li, Y.; Tejasvi, T.; Gudjonsson, J.E.; et al. Identification of Fifteen New Psoriasis Susceptibility Loci Highlights the Role of Innate Immunity. Nat. Genet. 2012, 44, 1341–1348. [Google Scholar] [CrossRef]
- Strange, A.; Capon, F.; Spencer, C.C.; Knight, J.; Weale, M.E.; Allen, M.H.; Barton, A.; Band, G.; Bellenguez, C.; Bergboer, J.G.; et al. Genome-Wide Association Study Identifies New Psoriasis Susceptibility Loci and an Interaction between HLA-C and ERAP1. Nat. Genet. 2010, 42, 985–990. [Google Scholar] [CrossRef]
- Deapen, D.; Escalante, A.; Weinrib, L.; Horwitz, D.; Bachman, B.; Roy-Burman, P.; Walker, A.; Mack, T.M. A Revised Estimate of Twin Concordance in Systemic Lupus Erythematosus. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1992, 35, 311–318. [Google Scholar] [CrossRef]
- Ulff-Møller, C.J.; Asmar, F.; Liu, Y.; Svendsen, A.J.; Busato, F.; Grønbaek, K.; Tost, J.; Jacobsen, S. Twin DNA Methylation Profiling Reveals Flare-Dependent Interferon Signature and B Cell Promoter Hypermethylation in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018, 70, 878–890. [Google Scholar] [CrossRef]
- Emerging Effects of Early Environmental Factors over Genetic Background for Type 1 Diabetes Susceptibility: Evidence from a Nationwide Italian Twin Study-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22569240/ (accessed on 17 September 2021).
- Lønnberg, A.S.; Skov, L.; Skytthe, A.; Kyvik, K.O.; Pedersen, O.B.; Thomsen, S.F. Heritability of Psoriasis in a Large Twin Sample. Br. J. Dermatol. 2013, 169, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Gauderman, W.J.; Cozen, W.; Hamilton, A.S.; Burnett, M.E.; Mack, T.M. Differential Twin Concordance for Multiple Sclerosis by Latitude of Birthplace. Ann. Neurol. 2006, 60, 56–64. [Google Scholar] [CrossRef]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A Mechanism Converting Psychosocial Stress into Mononuclear Cell Activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; Velissaris, D.; Tzanis, G.; Hahalis, G. Kounis Syndrome—Not a Single-Organ Arterial Disorder but a Multisystem and Multidisciplinary Disease. Balk. Med. J. 2019, 36, 212. [Google Scholar] [CrossRef]
- Kerl, K.; Wolf, I.H.; Cerroni, L.; Wolf, P.; French, L.E.; Kerl, H. Hemophagocytosis in Cutaneous Autoimmune Disease. Am. J. Dermatopathol. 2015, 37, 539–543. [Google Scholar] [CrossRef]
- Walker, M.E.; Hatfield, J.K.; Brown, M.A. New Insights into the Role of Mast Cells in Autoimmunity: Evidence for a Common Mechanism of Action? Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 57–65. [Google Scholar] [CrossRef]
- Conti, P.; Gallenga, C.E.; Ronconi, G.; Caraffa, A.; Kritas, S.K. Activation of Mast Cells Mediates Inflammatory Response in Psoriasis: Potential New Therapeutic Approach with IL-37. Dermatol. Ther. 2019, e12943. [Google Scholar] [CrossRef]
- Beghdadi, W. Mast Cells as Cellular Sensors in Inflammation and Immunity. Front. Immun. 2011, 2, 37. [Google Scholar] [CrossRef]
- Suurmond, J.; van Heemst, J.; van Heiningen, J.; Dorjée, A.L.; Schilham, M.W.; van der Beek, F.B.; Huizinga, T.W.J.; Schuerwegh, A.J.M.; Toes, R.E.M. Communication between Human Mast Cells and CD4+ T Cells through Antigen-Dependent Interactions. Eur. J. Immunol. 2013, 43, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Gri, G.; Frossi, B.; D’Inca, F.; Danelli, L.; Betto, E.; Mion, F.; Sibilano, R.; Pucillo, C. Mast Cell: An Emerging Partner in Immune Interaction. Front. Immunol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast Cell Mediators: Their Differential Release and the Secretory Pathways Involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. The Impact of Psychological Stress on Mast Cells. Ann. Allergy Asthma Immunol. 2020, 125, 388–392. [Google Scholar] [CrossRef]
- Theoharides, T.C. Neuroendocrinology of Mast Cells: Challenges and Controversies. Exp. Dermatol. 2017, 26, 751–759. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.L.; Lipper, G.; Lerner, E.A. The Neuro-Immuno-Cutaneous-Endocrine Network: Relationship of Mind and Skin. Arch. Dermatol. 1998, 134, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Vidal Yucha, S.E.; Tamamoto, K.A.; Kaplan, D.L. The Importance of the Neuro-Immuno-Cutaneous System on Human Skin Equivalent Design. Cell Prolif. 2019, 52, e12677. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin1. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Pisarchik, A.; Slominski, R.M.; Zmijewski, M.A.; Wortsman, J. CRH Functions as a Growth Factor/Cytokine in the Skin. J. Cell. Physiol. 2006, 206, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin Neuroendocrine System. Adv. Anat. Embryol. Cell Biol. 2012, 212, v-115. [Google Scholar]
- Traks, T.; Keermann, M.; Karelson, M.; Rätsep, R.; Reimann, E.; Silm, H.; Vasar, E.; Kõks, S.; Kingo, K. Polymorphisms in Corticotrophin-Releasing Hormone-Proopiomelanocortin (CRH-POMC) System Genes Are Associated with Plaque Psoriasis. Acta Derm. Venerol. 2019, 99, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Nagata, H.; Umemura, S.; Kawana, S.; Osamura, R.Y. In Situ Expression of Corticotropin-releasing Hormone (CRH) and Proopiomelanocortin (POMC) Genes in Human Skin. FASEB J. 2001, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Stewart, J.M.; Taracanova, A.; Conti, P.; Zouboulis, C.C. Neuroendocrinology of the Skin. Rev. Endocr. Metab. Disord. 2016, 17, 287–294. [Google Scholar] [CrossRef]
- Crompton, R.; Clifton, V.L.; Bisits, A.T.; Read, M.A.; Smith, R.; Wright, I.M.R. Corticotropin-Releasing Hormone Causes Vasodilation in Human Skin via Mast Cell-Dependent Pathways. J. Clin. Endocrinol. Metab. 2003, 88, 5427–5432. [Google Scholar] [CrossRef][Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key Role of CRF in the Skin Stress Response System. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef]
- McEwen, B.S. Brain on Stress: How the Social Environment Gets under the Skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17180–17185. [Google Scholar] [CrossRef]
- Polenghi, M.M.; Molinari, E.; Gala, C.; Guzzi, R.; Garutti, C.; Finzi, A.F. Experience with Psoriasis in a Psychosomatic Dermatology Clinic. Acta Derm. Venereol. Suppl. 1994, 186, 65–66. [Google Scholar] [CrossRef]
- de Brouwer, S.J.M.; van Middendorp, H.; Stormink, C.; Kraaimaat, F.W.; Sweep, F.C.G.J.; de Jong, E.M.G.J.; Schalkwijk, J.; Eijsbouts, A.; Donders, A.R.T.; van de Kerkhof, P.C.M.; et al. The Psychophysiological Stress Response in Psoriasis and Rheumatoid Arthritis. Br. J. Dermatol. 2014, 170, 824–831. [Google Scholar] [CrossRef]
- Richards, H.L.; Ray, D.W.; Kirby, B.; Mason, D.; Plant, D.; Main, C.J.; Fortune, D.G.; Griffiths, C.E.M. Response of the Hypothalamic-Pituitary-Adrenal Axis to Psychological Stress in Patients with Psoriasis. Br. J. Dermatol. 2005, 153, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zheng, J. Influence of Stress on the Development of Psoriasis. Clin. Exp. Dermatol. 2020, 45, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.F.; desAnges, C.; Podawiltz, A.; Mummert, D.I.; Jones, H.; Mummert, M.E. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol. Res. Pract. 2012, 2012, e403908. [Google Scholar] [CrossRef] [PubMed]
- Căruntu, C.; Boda, D.; Musat, S.; Căruntu, A.; Mandache, E. Stress-Induced Mast Cell Activation in Glabrous and Hairy Skin. Mediat. Inflamm. 2014, 2014, 105950. [Google Scholar] [CrossRef] [PubMed]
- Harvima, I.T.; Nilsson, G.; Naukkarinen, A. Role of Mast Cells and Sensory Nerves in Skin Inflammation. Giornale Ital. Dermatol. Venereol. 2010, 145, 195–204. [Google Scholar]
- Peters, E.M.J.; Kuhlmei, A.; Tobin, D.J.; Müller-Röver, S.; Klapp, B.F.; Arck, P.C. Stress Exposure Modulates Peptidergic Innervation and Degranulates Mast Cells in Murine Skin. Brain Behav. Immun. 2005, 19, 252–262. [Google Scholar] [CrossRef]
- Kulka, M.; Alexopoulou, L.; Flavell, R.A.; Metcalfe, D.D. Activation of Mast Cells by Double-Stranded RNA: Evidence for Activation through Toll-like Receptor 3. J. Allergy Clin. Immunol. 2004, 114, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Skin Mast Cells: Are We Missing the Forest for the Trees? Exp. Dermatol. 2016, 25, 422–423. [Google Scholar] [CrossRef]
- Caraffa, A.; Spinas, E.; Kritas, S.K.; Lessiani, G.; Ronconi, G.; Saggini, A.; Antinolfi, P.; Pizzicannella, J.; Toniato, E.; Theoharides, T.C.; et al. Endocrinology of the Skin: Intradermal Neuroimmune Network, a New Frontier. J. Biol. Regul. Homeost. Agents 2016, 30, 339–343. [Google Scholar] [PubMed]
- Kastelan, M.; Prpić-Massari, L.; Brajac, I. Apoptosis in Psoriasis. Acta Derm. Croat. 2009, 17, 182–186. [Google Scholar]
- Shimoura, N.; Nagai, H.; Fujiwara, S.; Jimbo, H.; Yoshimoto, T.; Nishigori, C. Interleukin (IL)-18, Cooperatively with IL-23, Induces Prominent Inflammation and Enhances Psoriasis-like Epidermal Hyperplasia. Arch. Dermatol. Res. 2017, 309, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Liang, Z.; Suzuki, H.; Kawana, S. Inhibitory Effects of Antipsychotic and Anxiolytic Agents on Stress-Induced Degranulation of Mouse Dermal Mast Cells: The Effects of Antipsychotic and Anxiolytic Agents on Mouse Dermal Mast Cells. Clin. Exp. Dermatol. 2009, 35, 531–536. [Google Scholar] [CrossRef]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental Cell Programs Are Co-Opted in Inflammatory Skin Disease. Science 2021, 371, eaba6500. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Schilit, A.; Lee, F.S. Stress Effects on BDNF Expression: Effects of Age, Sex, and Form of Stress. Neuroscience 2013, 239, 149–156. [Google Scholar] [CrossRef]
- JiaWen, W.; Hong, S.; ShengXiang, X.; Jing, L. Depression- and Anxiety-like Behaviour Is Related to BDNF/TrkB Signalling in a Mouse Model of Psoriasis. Clin. Exp. Dermatol. 2018, 43, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Marconi, A.; Atzei, P.; Panza, M.C.; Lotti, R.; Dallaglio, K.; Tiberio, R.; Palazzo, E.; Vaschieri, C.; Pincelli, C. P75 Neurotrophin Receptor Mediates Apoptosis in Transit-Amplifying Cells and Its Overexpression Restores Cell Death in Psoriatic Keratinocytes. Cell Death Differ. 2011, 18, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Roque Ferreira, B.; Pio-Abreu, J.L.; Reis, J.P.; Figueiredo, A. Analysis of the Prevalence of Mental Disorders in Psoriasis: The Relevance of Psychiatric Assessment in Dermatology. Psychiat. Danub. 2017, 29, 401–406. [Google Scholar] [CrossRef]
- Olivier, C.; Robert, P.D.; Daihung, D.; Urbà, G.; Catalin, M.P.; Hywel, W.; Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The Risk of Depression, Anxiety, and Suicidality in Patients With Psoriasis: A Population-Based Cohort Study. Arch. Dermatol. 2010, 146. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast Cells in Neuroinflammation and Brain Disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef]
- Mitchell, R.H.B.; Goldstein, B.I. Inflammation in Children and Adolescents With Neuropsychiatric Disorders: A Systematic Review. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Ronconi, G.; Conti, C.M.; Kritas, S.K.; Mastrangelo, F.; Tettamanti, L.; Theoharides, T.C. Impact of Mast Cells in Depression Disorder: Inhibitory Effect of IL-37 (New Frontiers). Immunol. Res. 2018, 66, 323–331. [Google Scholar] [CrossRef]
- John, A.E.; Zhu, Y.M.; Brightling, C.E.; Pang, L.; Knox, A.J. Human Airway Smooth Muscle Cells from Asthmatic Individuals Have CXCL8 Hypersecretion Due to Increased NF-ΚB P65, C/EBPβ, and RNA Polymerase II Binding to the CXCL8 Promoter. J. Immunol. 2009, 183, 4682–4692. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Kee, W.J.; Strijbos, P.J.L.M. Potentiation by Histamine of Synaptically Mediated Excitotoxicity in Cultured Hippocampal Neurones: A Possible Role for Mast Cells. J. Neurochem. 2001, 76, 47–55. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Romanello, S.; Leon, A. Mast Cell Activation Causes Delayed Neurodegeneration in Mixed Hippocampal Cultures via the Nitric Oxide Pathway. J. Neurochem. 2002, 66, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Camargo, C.M.; Brotas, A.M.; Ramos-e-Silva, M.; Carneiro, S. Isomorphic Phenomenon of Koebner: Facts and Controversies. Clin. Dermatol. 2013, 31, 741–749. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast Cell and Macrophage Chemokines CXCL1/CXCL2 Control the Early Stage of Neutrophil Recruitment during Tissue Inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Chattedee, A.D.; Raychaudhuri, S.P.; Raychaudhuri, S.K.; Farber, E.M. Mast Cell Density and IL-8 Expression in Nonlesional and Lesional Psoriatic Skin. Int. J. Dermatol. 2001, 40, 699–703. [Google Scholar] [CrossRef]
- Kandere-Grzybowska, K.; Kempuraj, D.; Cao, J.; Cetrulo, C.L.; Theoharides, T.C. Regulation of IL-1-Induced Selective IL-6 Release from Human Mast Cells and Inhibition by Quercetin. Br. J. Pharmacol. 2006, 148, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Bertino, L.; Di Salvo, E.; Papaianni, V.; Ventura-Spagnolo, E.; Gangemi, S. Possible Roles of IL-33 in the Innate-Adaptive Immune Crosstalk of Psoriasis Pathogenesis. Mediators Inflamm. 2019, 2019, 7158014. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Patel, D.D.; Lee, D.M.; Kolbinger, F.; Antoni, C. Effect of IL-17A Blockade with Secukinumab in Autoimmune Diseases. Ann. Rheum. Dis. 2013, 72, iii116–iii123. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef]
- Brembilla, N.C.; Stalder, R.; Senra, L.; Boehncke, W.-H. IL -17A Localizes in the Exocytic Compartment of Mast Cells in Psoriatic Skin. Br. J. Dermatol. 2017, 177, 1458–1460. [Google Scholar] [CrossRef]
- Dyring-Andersen, B.; Honoré, T.V.; Madelung, A.; Bzorek, M.; Simonsen, S.; Clemmensen, S.N.; Clark, R.A.; Borregaard, N.; Skov, L. IL-17A and IL-22 Producing Neutrophils in Psoriatic Skin. Br. J. Dermatol. 2017, 177, e321–e322. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Aroni, K.; Tsagroni, E.; Kavantzas, N.; Patsouris, E.; Ioannidis, E. A Study of the Pathogenesis of Rosacea: How Angiogenesis and Mast Cells May Participate in a Complex Multifactorial Process. Arch. Dermatol. Res. 2008, 300, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.-J.; Hao, D.; Wen, X.; Du, D.; He, G.; Jiang, X. The Theranostics Role of Mast Cells in the Pathophysiology of Rosacea. Front. Med. 2019, 6, 324. [Google Scholar] [CrossRef]
- Nakamura, M.; Toyoda, M.; Morohashi, M. Pruritogenic Mediators in Psoriasis Vulgaris: Comparative Evaluation of Itch-Associated Cutaneous Factors. Br. J. Dermatol. 2003, 149, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Hagforsen, E.; Lampinen, M.; Paivandy, A.; Weström, S.; Velin, H.; Öberg, S.; Pejler, G.; Rollman, O. Siramesine Causes Preferential Apoptosis of Mast Cells in Skin Biopsies from Psoriatic Lesions. Br. J. Dermatol. 2017, 177, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Tamburi, F.; Amoruso, G.F.; Bennardo, L.; Nisticò, S.P. Emerging Role of anti-IL23 in the Treatment of Psoriasis: When Humanized Is Very Promising. Dermatol. Ther. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Tolahunase, M.; Kumar, U.; Dada, R. Impact of Yoga Based Mind-Body Intervention on Systemic Inflammatory Markers and Co-Morbid Depression in Active Rheumatoid Arthritis Patients: A Randomized Controlled Trial. RNN 2019, 37, 41–59. [Google Scholar] [CrossRef] [PubMed]
| System Name | System Components |
|---|---|
| Nervous System | Sensory Neurons (β, Aδ, and C Nerve Fibers) That Secrete Neuropeptides, Neurotrophins, Neurohormones; Autonomic Nerves (Sympathetic and Parasympathetic) |
| Endocrine system | Secretion hormones by skin cells, example: CRH, ACTH, cortisol, α-MSH, and β-endorphin |
| Immune system | Immune cells (Macrophages, monocytes, eosinophils, basophils, neutrophils, T cells, dendritic cells, innate lymphoid cells) |
| CRH-R1 | CRH-R2 |
|---|---|
| Normal and malignant melanocytes Keratinocytes Dermal fibroblasts Squamous cells carcinoma cells | Hair follicle keratinocytes Papilla fibroblasts Sebaceous glands Eccrine glands Dermal blood vessels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, E.; Owczarczyk-Saczonek, A.; Placek, W. Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship? Int. J. Mol. Sci. 2021, 22, 13252. https://doi.org/10.3390/ijms222413252
Woźniak E, Owczarczyk-Saczonek A, Placek W. Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship? International Journal of Molecular Sciences. 2021; 22(24):13252. https://doi.org/10.3390/ijms222413252
Chicago/Turabian StyleWoźniak, Ewelina, Agnieszka Owczarczyk-Saczonek, and Waldemar Placek. 2021. "Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship?" International Journal of Molecular Sciences 22, no. 24: 13252. https://doi.org/10.3390/ijms222413252
APA StyleWoźniak, E., Owczarczyk-Saczonek, A., & Placek, W. (2021). Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship? International Journal of Molecular Sciences, 22(24), 13252. https://doi.org/10.3390/ijms222413252






