Evidence for a Novel Antiviral Mechanism of Teleost Fish: Serum-Derived Exosomes Inhibit Virus Replication through Incorporating Mx1 Protein
Abstract
:1. Introduction
2. Results
2.1. Exosomes Were Isolated from Mandarin Fish Serum
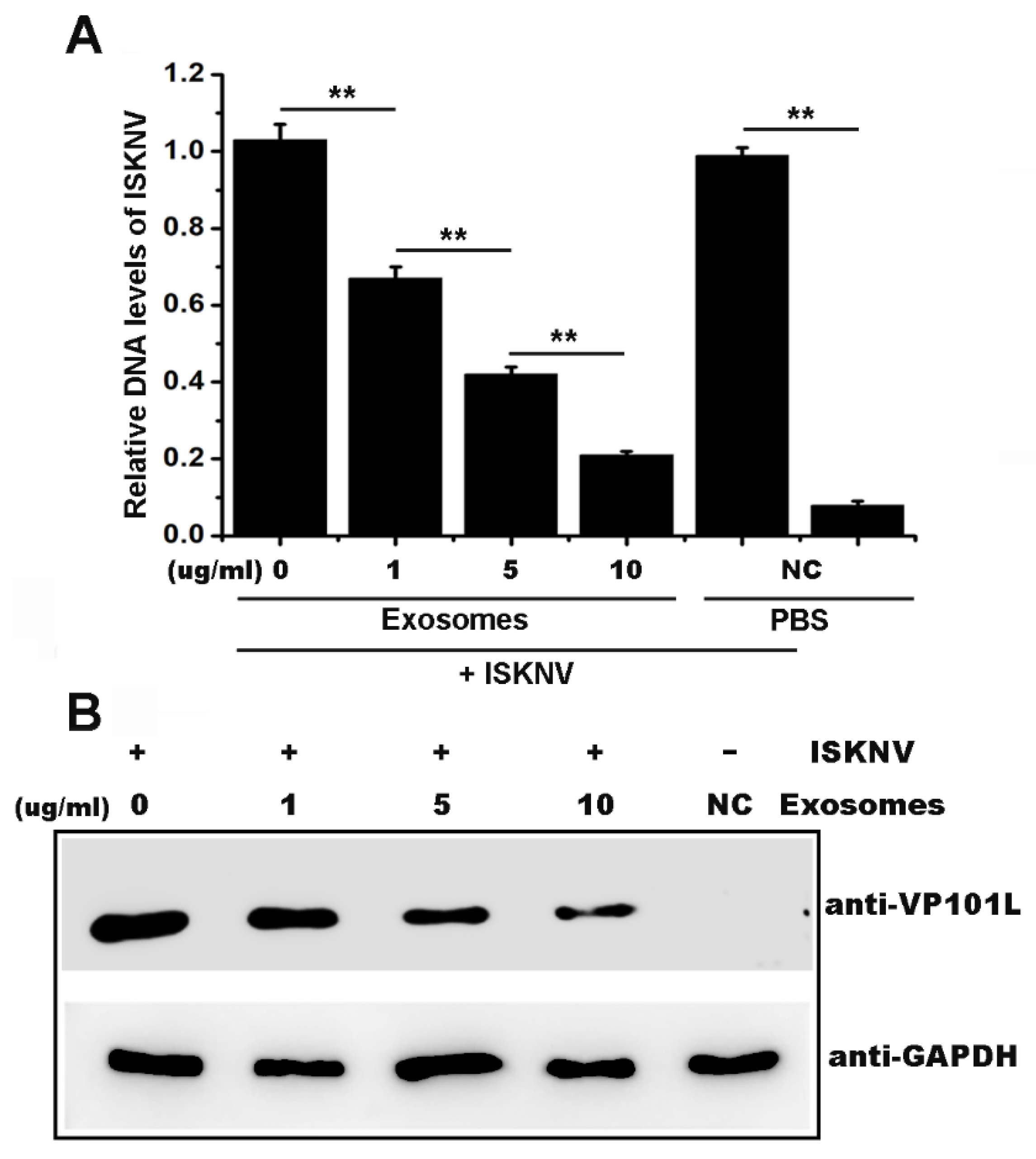
2.2. Serum-Derived Exosomes from Mandarin Fish Inhibited ISKNV Infection
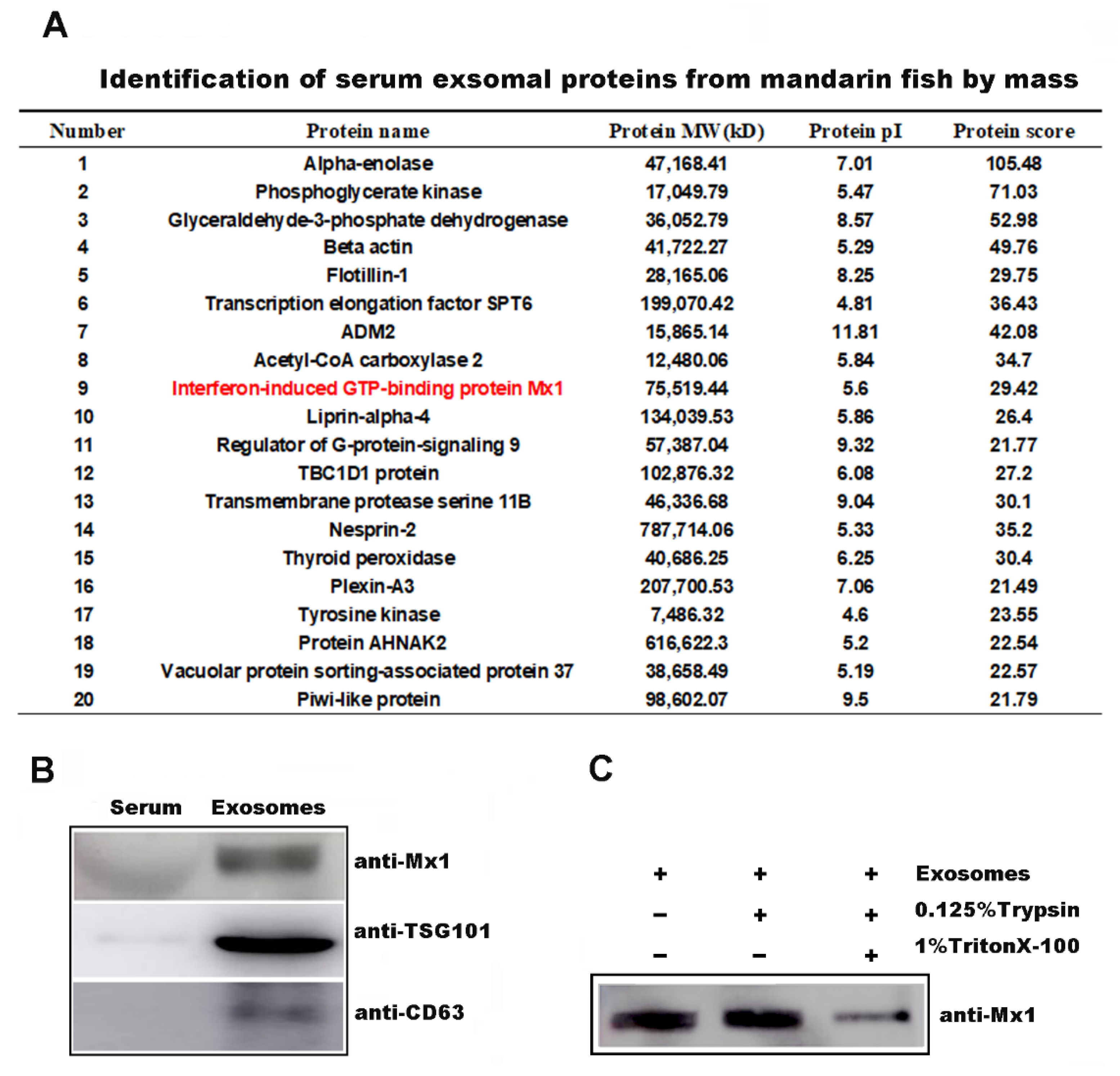
2.3. Mx1 Protein Was Incorporated into the Serum-Derived Exosomes
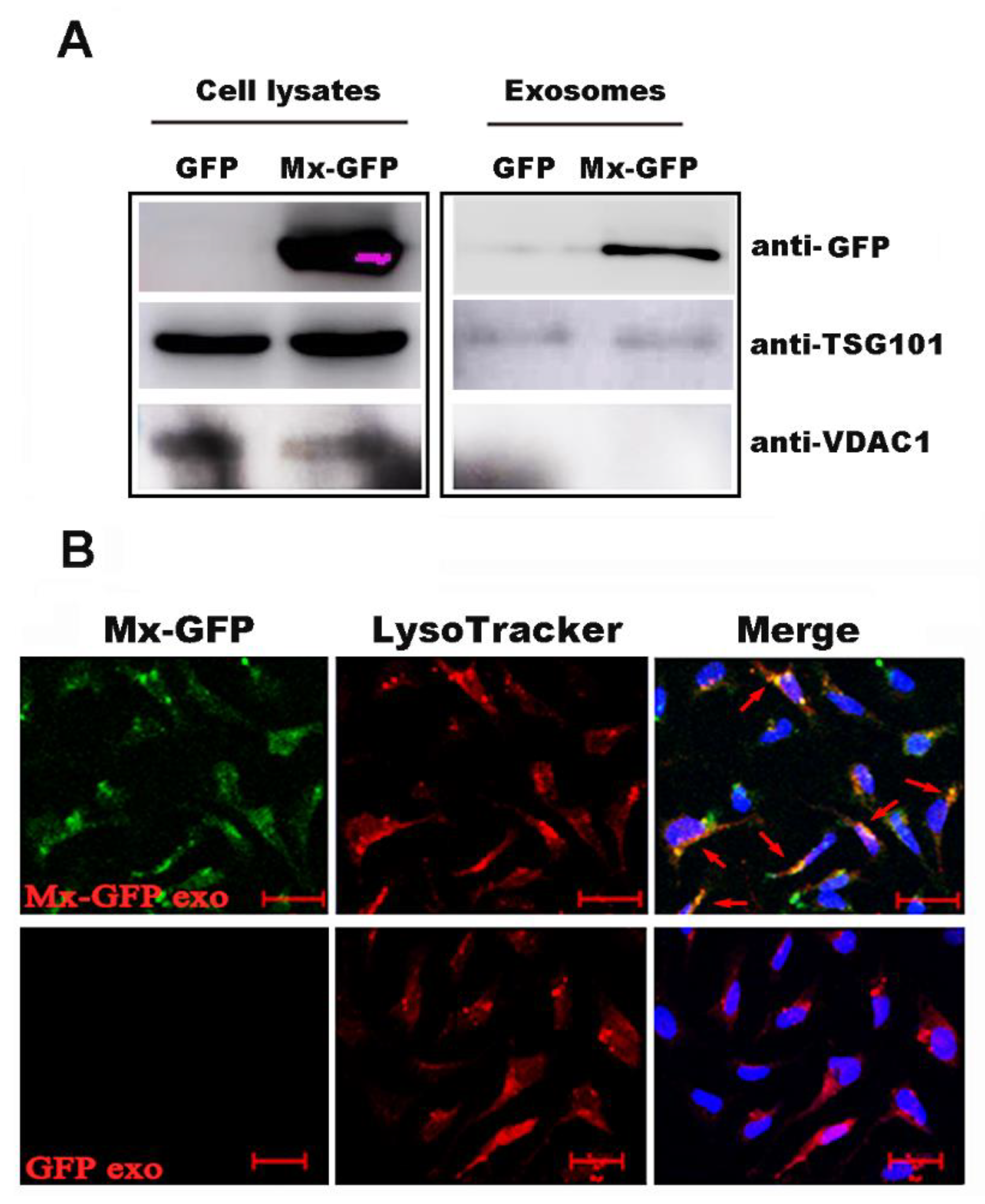
2.4. Mx1 Protein Could Be Transferred to the Recipient Cells through the Exosomes
3. Discussion
4. Materials and Methods
4.1. Cells, Viruses, and Animals
4.2. Plasmid Construction and Transient Transfection
4.3. Exosome Isolation from Fish Serum and Electron Microscopy Analysis
4.4. Exosome Isolation from Transfection Cultured Cells
4.5. Western Blot Analysis
4.6. ISKNV Genome Equivalent (GE) Level Determined by qPCR
4.7. Proteomics and Data Analysis
4.8. Observation of Exosomes in the MFF-1 Cells by Confocal Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, L.; Wang, C.; Xu, J.; Niu, Z. Serum-derived exosomes function as tumor antigens in patients with advanced hepatocellular carcinoma. Mol. Immunol. 2021, 134, 210–217. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic. Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [Green Version]
- Bello-Morales, R.; Ripa, I.; Lopez-Guerrero, J.A. Extracellular Vesicles in Viral Spread and Antiviral Response. Viruses 2020, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.J.; Gu, L.; Sims, B.; Matthews, Q.L. Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol. J. 2018, 12, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Tseng, C.-H.; Chen, Y.-C.; Yu, W.-Y.; Ho, M.-Y.; Ho, C.-Y.; Lai, M.M.C.; Su, W.-C. Exosome-delivered and Y RNA-derived small RNA suppresses influenza virus replication. J. Biomed. Sci. 2019, 26, 58. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yu, Y.; Qin, X.-W.; Zeng, R.-Y.; Wang, Y.-Y.; Li, Z.-M.; Mi, S.; Weng, S.-P.; Guo, C.-J. Identification and functional analysis of the Mandarin fish (Siniperca chuatsi) hypoxia-inducible factor-1alpha involved in the immune response. Fish Shellfish. Immunol. 2019, 92, 141–150. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.; Whittington, R.; Williams, T.; et al. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef]
- He, J.G.; Denga, M.; Weng, S.P.; Lia, Z.; Zhou, S.Y.; Long, Q.X.; Wang, X.Z.; Chanb, S.M. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology 2001, 291, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Liu, Y.; Qin, H.; Tong, S.; Sun, Q.; Wang, T.; Zhang, H.; Cui, M.; Guo, S. Osteogenically-induced exosomes stimulate osteogenesis of human adipose-derived stem cells. Cell Tissue Bank 2021, 22, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.F.; Xiong, X.P.; Shuang, F.; Weng, S.P.; Zhang, J.; Zhang, Y.; Luo, Y.W.; He, J.G. Global landscape of structural proteins of Infectious spleen and kidney necrosis virus. J. Virol. 2011, 85, 2869–2877. [Google Scholar] [CrossRef] [Green Version]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends. Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, N.; Jia, L.; Peng, K.; Che, J.; Li, K.; He, X.; Sun, J.; Bao, B. Seminal Plasma Exosomes: Promising Biomarkers for Identification of Male and Pseudo-Males in Cynoglossus semilaevis. Mar. Biotechnol. (N. Y.) 2019, 21, 310–319. [Google Scholar] [CrossRef]
- Faught, E.; Henrickson, L.; Vijayan, M.M. Plasma exosomes are enriched in Hsp70 and modulated by stress and cortisol in rainbow trout. J. Endocrinol. 2017, 232, 237–246. [Google Scholar] [CrossRef]
- Bai, X.; Guo, Y.; Shi, Y.; Lin, J.; Tarique, I.; Wang, X.; Vistro, W.A.; Huang, Y.; Chen, H.; Haseeb, A.; et al. In vivo multivesicular bodies and their exosomes in the absorptive cells of the zebrafish (Danio Rerio) gut. Fish Shellfish. Immunol. 2019, 88, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hao, T.; Tian, J. Identification of exosomes and its signature miRNAs of male and female Cynoglossus semilaevis. Sci. Rep. 2017, 7, 860. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; He, Z.; Yuan, J.; Wen, W.; Huang, X.; Hu, Y.; Lin, C.; Pan, J.; Li, R.; Deng, H.; et al. IFITM3-containing exosome as a novel mediator for anti-viral response in dengue virus infection. Cell Microbiol. 2015, 17, 105–118. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, F.; Xia, B.; Yuan, Y.; Yu, F.; Wang, G.; Chen, Q.; Wang, Q.; Li, Y.; Li, R.; et al. The interferon-stimulated exosomal hACE2 potently inhibits SARS-CoV-2 replication through competitively blocking the virus entry. Signal Transduct. Target. Ther. 2021, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Q.; Zhang, B.; Guo, L.; Liu, Y.; Meng, F.-Z.; Wang, X.; Hu, W.-H.; Khan, A.I.; Ho, W.-Z. Exosomes Transport Anti-Human Immunodeficiency Virus Factors from Human Cervical Epithelial Cells to Macrophages. J. Innate Immun. 2021, 13, 269–279. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.N.; Laghari, Z.A.; Huo, H.J.; Hou, J.; Huang, L.; Li, N.; Nie, P. Myxovirus resistance (Mx) gene and its differential expression regulated by three type I and two type II IFNs in mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2020, 105, 103604. [Google Scholar] [CrossRef]
- Netherton, C.L.; Simpson, J.; Haller, O.; Wileman, T.E.; Takamatsu, H.H.; Monaghan, P.; Taylor, G. Inhibition of a large double-stranded DNA virus by MxA protein. J. Virol. 2009, 83, 2310–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, O.; Staeheli, P.; Kochs, G. Interferon-induced Mx proteins in antiviral host defense. Biochimie 2007, 89, 812–818. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhou, Y.; Jiang, N.; Fan, Y.; Zeng, L. Characterization, Expression Pattern and Antiviral Activities of Mx Gene in Chinese Giant Salamander, Andrias davidianus. Int. J. Mol. Sci. 2020, 21, 2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Shi, N.; He, J. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of Infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; He, J.; Zeng, R.-Y.; Li, Z.-M.; Luo, Z.-Y.; Pan, W.-Q.; Weng, S.-P.; Guo, C.-J. Deletion of the Infectious spleen and kidney necrosis virus ORF069L reduces virulence to mandarin fish Siniperca chuatsi. Fish. Shellfish Immunol. 2019, 95, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method OF estimating fifty per cent ENDPOINTS12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Zeng, R.; Pan, W.; Lin, Y.; He, J.; Luo, Z.; Li, Z.; Weng, S.; He, J.; Guo, C. Development of a gene-deleted live attenuated candidate vaccine against fish virus (ISKNV) with low pathogenicity and high protection. iScience 2021, 24, 102750. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lotvall, J.; Lasser, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesiclesv. 2014, 3, 23111. [Google Scholar] [CrossRef]
- He, J.-G.; Zheng, Y.-W.; Lin, Y.-F.; Mi, S.; Qin, X.-W.; Weng, S.-P.; Guo, C.-J. Caveolae Restrict Tiger Frog Virus Release in HepG2 cells and Caveolae-Associated Proteins Incorporated into Virus Particles. Sci. Rep. 2016, 6, 21663. [Google Scholar] [CrossRef] [Green Version]
- Jia, K.-T.; Wu, Y.-Y.; Liu, Z.-Y.; Mi, S.; Zheng, Y.-W.; He, J.; Weng, S.-P.; Li, S.C.; Guo, C.-J. Mandarin fish caveolin 1 interaction with major capsid protein of Infectious spleen and kidney necrosis virus and its role in early stages of infection. J. Virol. 2013, 87, 3027–3038. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Chen, N.-N.; Li, Z.-M.; Wang, Y.-Y.; Weng, S.-P.; Guo, C.-J.; He, J.-G. Evidence for a Novel Antiviral Mechanism of Teleost Fish: Serum-Derived Exosomes Inhibit Virus Replication through Incorporating Mx1 Protein. Int. J. Mol. Sci. 2021, 22, 10346. https://doi.org/10.3390/ijms221910346
He J, Chen N-N, Li Z-M, Wang Y-Y, Weng S-P, Guo C-J, He J-G. Evidence for a Novel Antiviral Mechanism of Teleost Fish: Serum-Derived Exosomes Inhibit Virus Replication through Incorporating Mx1 Protein. International Journal of Molecular Sciences. 2021; 22(19):10346. https://doi.org/10.3390/ijms221910346
Chicago/Turabian StyleHe, Jian, Nan-Nan Chen, Zhi-Min Li, Yuan-Yuan Wang, Shao-Ping Weng, Chang-Jun Guo, and Jian-Guo He. 2021. "Evidence for a Novel Antiviral Mechanism of Teleost Fish: Serum-Derived Exosomes Inhibit Virus Replication through Incorporating Mx1 Protein" International Journal of Molecular Sciences 22, no. 19: 10346. https://doi.org/10.3390/ijms221910346
APA StyleHe, J., Chen, N.-N., Li, Z.-M., Wang, Y.-Y., Weng, S.-P., Guo, C.-J., & He, J.-G. (2021). Evidence for a Novel Antiviral Mechanism of Teleost Fish: Serum-Derived Exosomes Inhibit Virus Replication through Incorporating Mx1 Protein. International Journal of Molecular Sciences, 22(19), 10346. https://doi.org/10.3390/ijms221910346





