Carbenoxolon Is Capable to Regulate the Mitochondrial Permeability Transition Pore Opening in Chronic Alcohol Intoxication
Abstract
1. Introduction
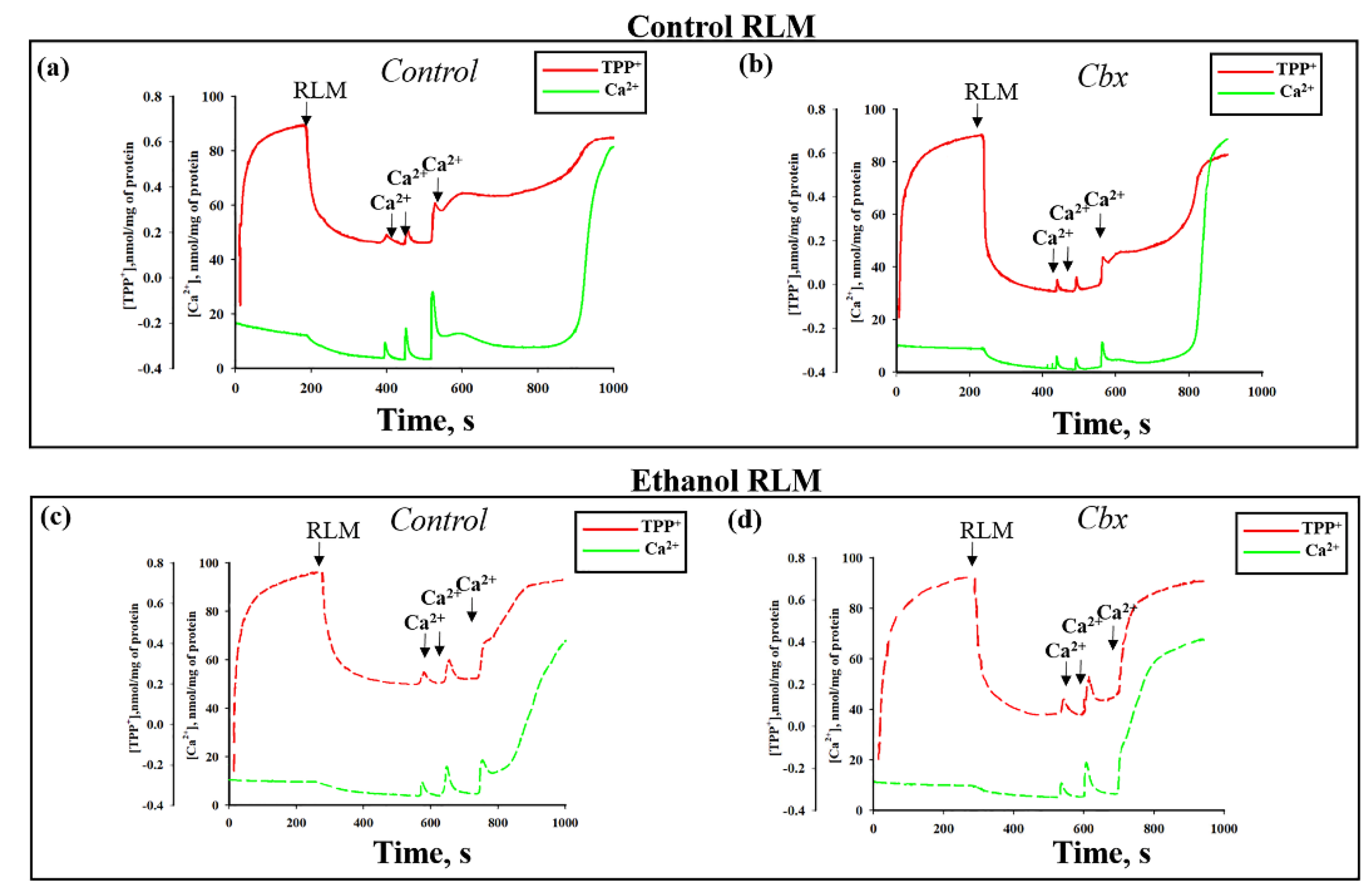
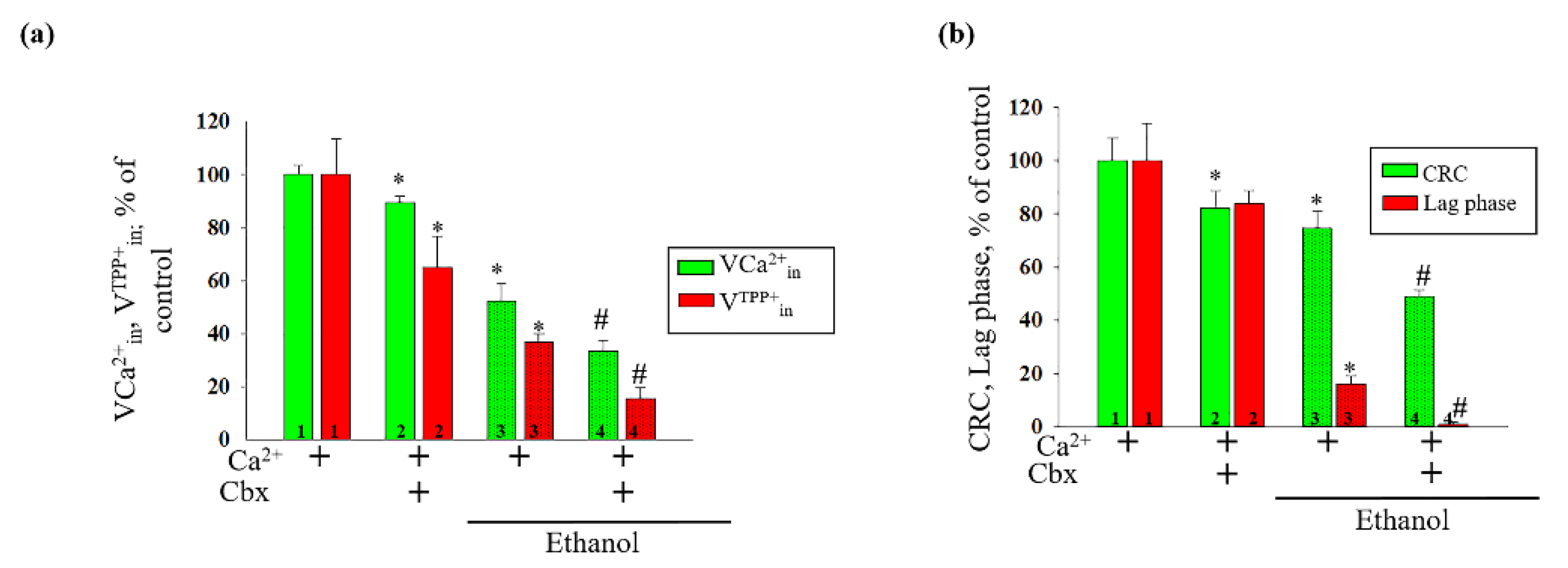
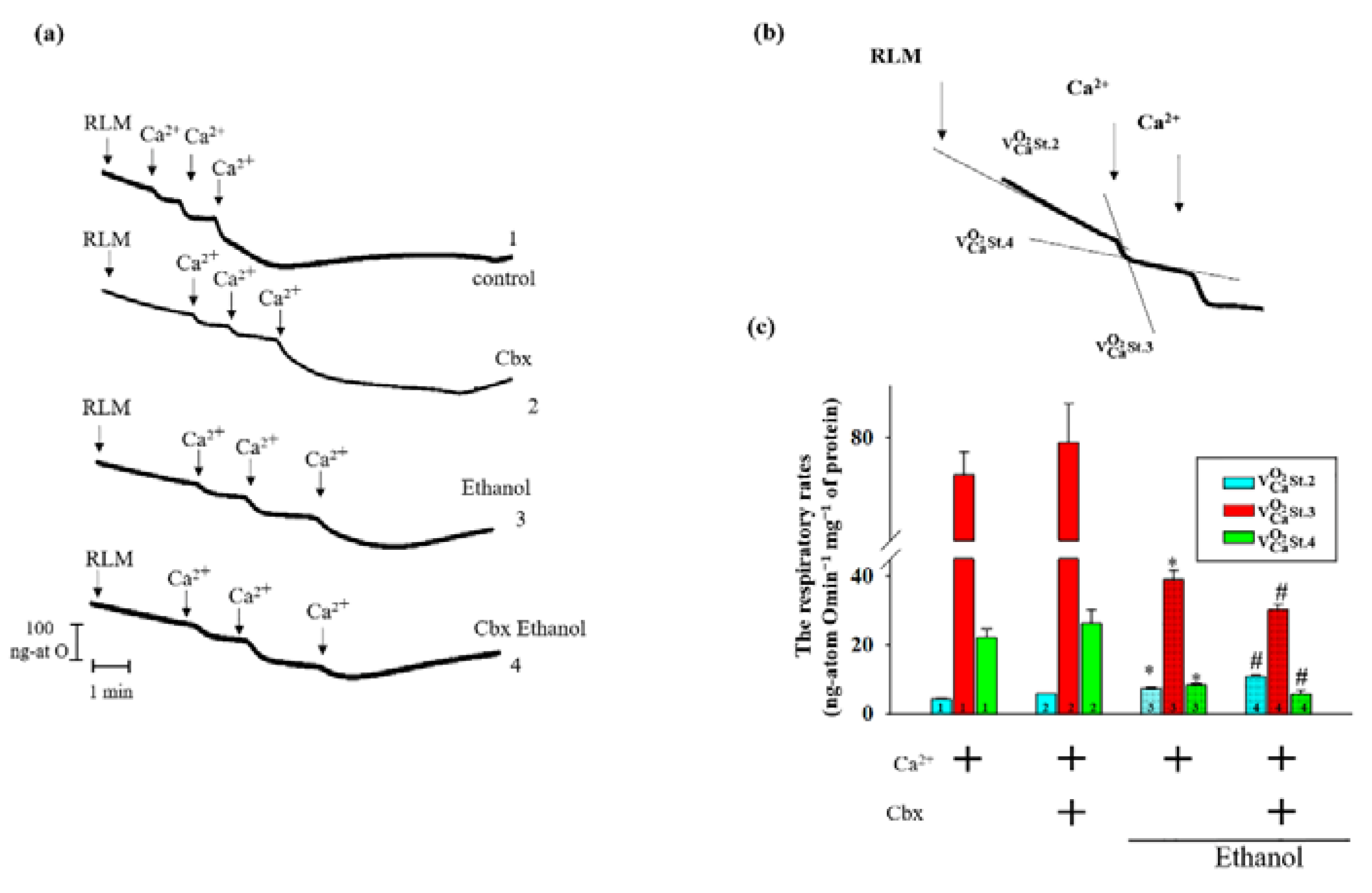
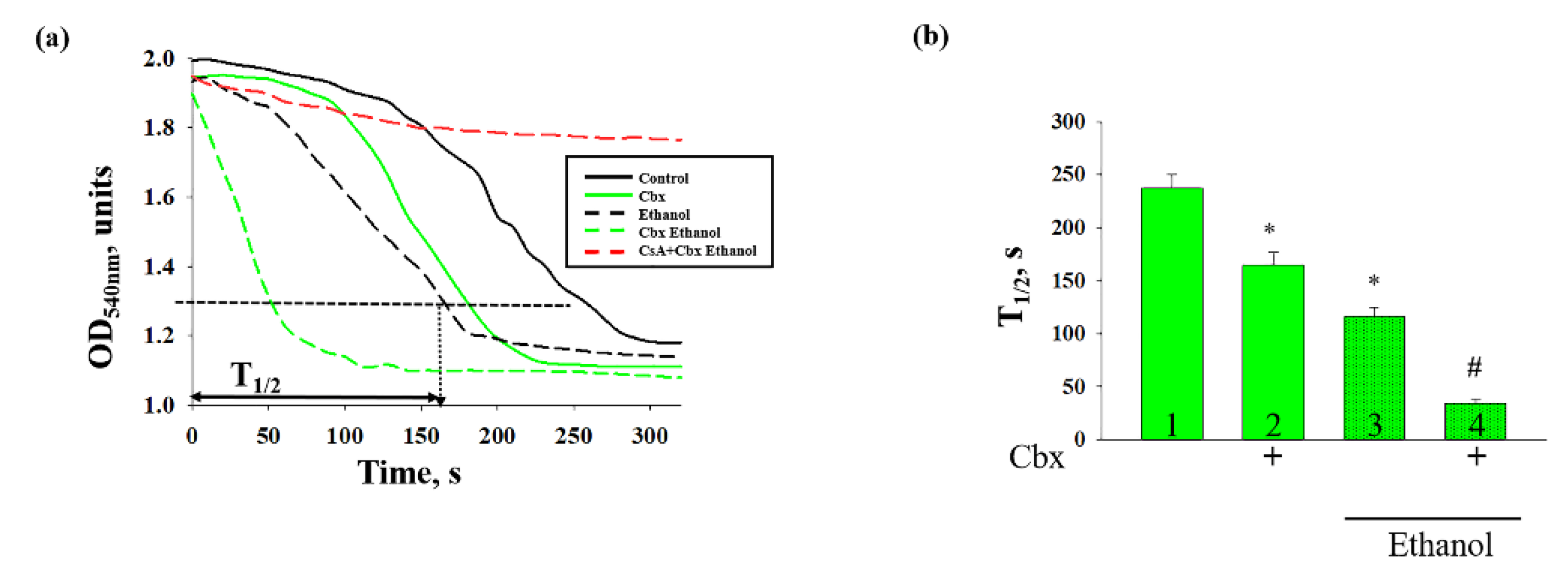
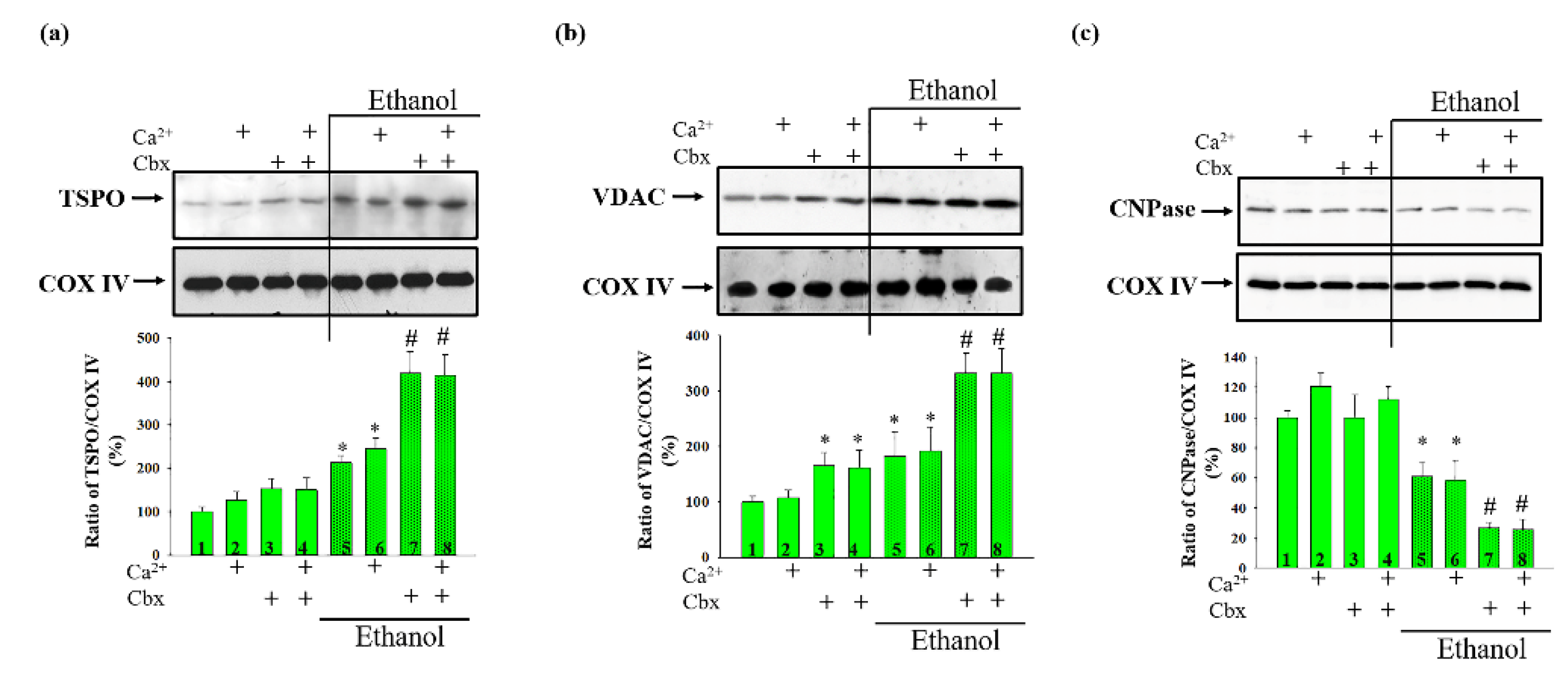
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Isolation of Rat Liver Mitochondria
4.3. Electrophoresis and Immunoblotting of the Mitochondrial Proteins
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, R.E.; Smart, R.G.; Govoni, R. The epidemiology of alcoholic liver disease. Alcohol Res. Health 2003, 27, 209–219. [Google Scholar]
- Miranda-Mendez, A.; Lugo-Baruqui, A.; Armendariz-Borunda, J. Molecular basis and current treatment for alcoholic liver disease. Int. J. Environ. Res. Public Health 2010, 7, 1872–1888. [Google Scholar] [CrossRef]
- Bergheim, I.; McClain, C.J.; Arteel, G.E. Treatment of alcoholic liver disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Nestler, E.J. Molecular mechanisms of drug addiction. J. Neurosci. 1992, 12, 2439–2450. [Google Scholar] [CrossRef]
- Lelevich, S.V. Comparative characteristics of glucose metabolism in the liver of rats under acute alcohol and morphine intoxication. Biomed. Khimiia 2011, 57, 615–623. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.; Swain, T.M.; Mao, Z.; Udoh, U.S.; Oliva, C.R.; Betancourt, A.M.; Griguer, C.E.; Crowe, D.R.; Lesort, M.; Bailey, S.M. Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G265–G277. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Hoek, J.B. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology 2000, 31, 1141–1152. [Google Scholar] [CrossRef]
- Rimessi, A.; Giorgi, C.; Pinton, P.; Rizzuto, R. The versatility of mitochondrial calcium signals: From stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta 2008, 1777, 808–816. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Marcineviciute, A.; Cahill, A.; Hoek, J.B. Potentiation by chronic ethanol treatment of the mitochondrial permeability transition. Biochem. Biophys. Res. Commun. 1999, 265, 405–409. [Google Scholar] [CrossRef]
- Baburina, Y.; Odinokova, I.; Krestinina, O. The effects of pk11195 and protoporphyrin ix can modulate chronic alcohol intoxication in rat liver mitochondria under the opening of the mitochondrial permeability transition pore. Cells 2020, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.K.; Shaw, S.G.; Prendergast, M.A.; Little, H.J. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcohol Clin. Exp. Res. 2010, 34, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Little, H.J.; Richardson, H.N.; Vendruscolo, L.F. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol 2015, 49, 811–816. [Google Scholar] [CrossRef]
- Sanna, P.P.; Kawamura, T.; Chen, J.; Koob, G.F.; Roberts, A.J.; Vendruscolo, L.F.; Repunte-Canonigo, V. 11beta-hydroxysteroid dehydrogenase inhibition as a new potential therapeutic target for alcohol abuse. Transl. Psychiatry 2016, 6, e760. [Google Scholar] [CrossRef]
- Sandeep, T.C.; Yau, J.L.; MacLullich, A.M.; Noble, J.; Deary, I.J.; Walker, B.R.; Seckl, J.R. 11beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc. Natl. Acad. Sci. USA 2004, 101, 6734–6739. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri Asl, M. Anticonvulsant, sedative and muscle relaxant effects of carbenoxolone in mice. BMC Pharmacol. 2003, 3, 3. [Google Scholar] [CrossRef][Green Version]
- Pivato, L.S.; Constantin, R.P.; Ishii-Iwamoto, E.L.; Kelmer-Bracht, A.M.; Yamamoto, N.S.; Constantin, J.; Bracht, A. Metabolic effects of carbenoxolone in rat liver. J. Biochem. Mol. Toxicol. 2006, 20, 230–240. [Google Scholar] [CrossRef]
- Azarashvili, T.; Baburina, Y.; Grachev, D.; Krestinina, O.; Papadopoulos, V.; Lemasters, J.J.; Odinokova, I.; Reiser, G. Carbenoxolone induces permeability transition pore opening in rat mitochondria via the translocator protein tspo and connexin43. Arch. Biochem. Biophys. 2014, 558, 87–94. [Google Scholar] [CrossRef]
- Salvi, M.; Fiore, C.; Battaglia, V.; Palermo, M.; Armanini, D.; Toninello, A. Carbenoxolone induces oxidative stress in liver mitochondria, which is responsible for transition pore opening. Endocrinology 2005, 146, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Zhang, X.; Wilson, C.; McCarron, J.G. Carbenoxolone and 18beta-glycyrrhetinic acid inhibit inositol 1,4,5-trisphosphate-mediated endothelial cell calcium signalling and depolarise mitochondria. Br. J. Pharmacol. 2021, 178, 896–912. [Google Scholar] [CrossRef]
- Krueger, K.E.; Papadopoulos, V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J. Biol. Chem. 1990, 265, 15015–15022. [Google Scholar] [CrossRef]
- Lim, S.; Smith, K.R.; Lim, S.T.; Tian, R.; Lu, J.; Tan, M. Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation. Cell Biosci. 2016, 6, 25. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (gsk3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Rasola, A.; Bernardi, P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 2007, 12, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 2020, 126, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The mitochondrial permeability transition pore: Channel formation by f-atp synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef]
- Chinopoulos, C.; Adam-Vizi, V. Mitochondria as atp consumers in cellular pathology. Biochim. Biophys. Acta 2010, 1802, 221–227. [Google Scholar] [CrossRef]
- Sileikyte, J.; Blachly-Dyson, E.; Sewell, R.; Carpi, A.; Menabo, R.; Di Lisa, F.; Ricchelli, F.; Bernardi, P.; Forte, M. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (translocator protein of 18 kda (tspo)). J. Biol. Chem. 2014, 289, 13769–13781. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, P.; Liu, H.M.; Zhang, H.T.; Liu, L. Ethanol induced mitochondria injury and permeability transition pore opening: Role of mitochondria in alcoholic liver disease. World J. Gastroenterol. 2007, 13, 2352–2356. [Google Scholar] [CrossRef]
- Shalbueva, N.; Mareninova, O.A.; Gerloff, A.; Yuan, J.; Waldron, R.T.; Pandol, S.J.; Gukovskaya, A.S. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 2013, 144, 437–446. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Baburina, Y.; Odinokova, I.; Grachev, D.; Papadopoulos, V.; Akatov, V.; Lemasters, J.J.; Reiser, G. Combined effect of g3139 and tspo ligands on Ca2+-induced permeability transition in rat brain mitochondria. Arch. Biochem. Biophys. 2015, 587, 70–77. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Yurkov, I.; Evtodienko, Y.; Reiser, G. High-affinity peripheral benzodiazepine receptor ligand, pk11195, regulates protein phosphorylation in rat brain mitochondria under control of Ca2+. J. Neurochem. 2005, 94, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.; Musman, J.; Pons, S.; Berdeaux, A.; Ghaleh, B. Mitochondrial translocator protein (tspo): From physiology to cardioprotection. Biochem. Pharmacol. 2016, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tian, M.; Yin, S.; Lai, S.; Zhou, Y.; Chen, J.; He, M.; Liao, Z. Downregulation of tspo expression inhibits oxidative stress and maintains mitochondrial homeostasis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed. Pharmacother. 2020, 121, 109588. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, N.; Nagler, R.; Gabay, M.; Weizman, A.; Gavish, M. Effects of cigarette smoke on tspo-related mitochondrial processes. Cells 2019, 8, 694. [Google Scholar] [CrossRef]
- Miyazawa, N.; Hamel, E.; Diksic, M. Assessment of the peripheral benzodiazepine receptors in human gliomas by two methods. J. Neurooncol. 1998, 38, 19–26. [Google Scholar] [CrossRef]
- Miettinen, H.; Kononen, J.; Haapasalo, H.; Helen, P.; Sallinen, P.; Harjuntausta, T.; Helin, H.; Alho, H. Expression of peripheral-type benzodiazepine receptor and diazepam binding inhibitor in human astrocytomas: Relationship to cell proliferation. Cancer Res. 1995, 55, 2691–2695. [Google Scholar]
- Galiegue, S.; Casellas, P.; Kramar, A.; Tinel, N.; Simony-Lafontaine, J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin. Cancer Res. 2004, 10, 2058–2064. [Google Scholar] [CrossRef]
- Beinlich, A.; Strohmeier, R.; Kaufmann, M.; Kuhl, H. Relation of cell proliferation to expression of peripheral benzodiazepine receptors in human breast cancer cell lines. Biochem. Pharmacol. 2000, 60, 397–402. [Google Scholar] [CrossRef]
- Fafalios, A.; Akhavan, A.; Parwani, A.V.; Bies, R.R.; McHugh, K.J.; Pflug, B.R. Translocator protein blockade reduces prostate tumor growth. Clin. Cancer Res. 2009, 15, 6177–6184. [Google Scholar] [CrossRef] [PubMed]
- Konigsrainer, I.; Vogel, U.F.; Beckert, S.; Sotlar, K.; Coerper, S.; Braun, A.; Lembert, N.; Steurer, W.; Konigsrainer, A.; Kupka, S. Increased translocator protein (tspo) mrna levels in colon but not in rectum carcinoma. Eur. Surg. Res. 2007, 39, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Maaser, K.; Grabowski, P.; Sutter, A.P.; Hopfner, M.; Foss, H.D.; Stein, H.; Berger, G.; Gavish, M.; Zeitz, M.; Scherubl, H. Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage iii colorectal cancer. Clin. Cancer Res. 2002, 8, 3205–3209. [Google Scholar] [PubMed]
- Venturini, I.; Alho, H.; Podkletnova, I.; Corsi, L.; Rybnikova, E.; Pellicci, R.; Baraldi, M.; Pelto-Huikko, M.; Helen, P.; Zeneroli, M.L. Increased expression of peripheral benzodiazepine receptors and diazepam binding inhibitor in human tumors sited in the liver. Life Sci. 1999, 65, 2223–2231. [Google Scholar] [CrossRef]
- Bhoola, N.H.; Mbita, Z.; Hull, R.; Dlamini, Z. Translocator protein (tspo) as a potential biomarker in human cancers. Int. J. Mol. Sci. 2018, 19, 2176. [Google Scholar] [CrossRef]
- Austin, C.J.; Kahlert, J.; Kassiou, M.; Rendina, L.M. The translocator protein (tspo): A novel target for cancer chemotherapy. Int. J. Biochem. Cell Biol. 2013, 45, 1212–1216. [Google Scholar] [CrossRef]
- Gatliff, J.; East, D.; Crosby, J.; Abeti, R.; Harvey, R.; Craigen, W.; Parker, P.; Campanella, M. Tspo interacts with vdac1 and triggers a ros-mediated inhibition of mitochondrial quality control. Autophagy 2014, 10, 2279–2296. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Pittala, S.; Mizrachi, D. Vdac1 and the tspo: Expression, interactions, and associated functions in health and disease states. Int. J. Mol. Sci. 2019, 20, 3348. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Sheiko, T.; Craigen, W.J.; Molkentin, J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007, 9, 550–555. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, S.; Jin, Q.; Shi, C.; Zhang, Y.; Zhu, P.; Ma, Q.; Tian, F.; Chen, Y. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mros-mediated cardiolipin oxidation and hk2/vdac1 disassociation-involved mptp opening. J. Am. Heart Assoc. 2017, 6, e005328. [Google Scholar] [CrossRef]
- Camara, A.K.S.; Zhou, Y.; Wen, P.C.; Tajkhorshid, E.; Kwok, W.M. Mitochondrial vdac1: A key gatekeeper as potential therapeutic target. Front. Physiol. 2017, 8, 460. [Google Scholar] [CrossRef]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. Mptp opening caused by cdk5 loss is due to increased mitochondrial Ca2+ uptake. Oncogene 2020, 39, 2797–2806. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Choi, D.C.; Kabaria, S.; Tran, A.; Junn, E. Microrna-7 regulates the function of mitochondrial permeability transition pore by targeting vdac1 expression. J. Biol. Chem. 2016, 291, 6483–6493. [Google Scholar] [CrossRef]
- Baburina, Y.; Azarashvili, T.; Grachev, D.; Krestinina, O.; Galvita, A.; Stricker, R.; Reiser, G. Mitochondrial 2′,3′-cyclic nucleotide 3′-phosphodiesterase (cnp) interacts with mptp modulators and functional complexes (i-v) coupled with release of apoptotic factors. Neurochem. Int. 2015, 90, 46–55. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Evtodienko, Y.; Reiser, G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am. J. Physiol. Cell Physiol. 2009, 296, C1428–C1439. [Google Scholar] [CrossRef]
- Meissner, G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium 2004, 35, 621–628. [Google Scholar] [CrossRef]
- Kerner, J.; Lee, K.; Tandler, B.; Hoppel, C.L. Vdac proteomics: Post-translation modifications. Biochim. Biophys. Acta 2012, 1818, 1520–1525. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Reiser, G. Identification of phosphorylated form of 2′,3′-cyclic nucleotide 3′-phosphodiesterase (cnpase) as 46 kda phosphoprotein in brain non-synaptic mitochondria overloaded by calcium. J. Bioenerg. Biomembr. 2014, 46, 135–145. [Google Scholar] [CrossRef]
- Azarashvili, T.; Grachev, D.; Krestinina, O.; Evtodienko, Y.; Yurkov, I.; Papadopoulos, V.; Reiser, G. The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium 2007, 42, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Odinokova, I.; Krestinina, O.; Baburina, Y.; Teplova, V.; Jahangir, A.; Holmuhamedov, E. Ethanol exposure increases the activity of mitochondria-associated glycogen synthase kinase-3 beta (gsk-3β): Role in phosphorylation of mitochondrial proteins. Innov. J. Med. Health Sci. 2013, 3, 163–170. [Google Scholar]
- Das, S.; Wong, R.; Rajapakse, N.; Murphy, E.; Steenbergen, C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ. Res. 2008, 103, 983–991. [Google Scholar] [CrossRef]
- Xi, J.; Wang, H.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur. J. Pharmacol. 2009, 604, 111–116. [Google Scholar] [CrossRef]
- Bijur, G.N.; Jope, R.S. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport 2003, 14, 2415–2419. [Google Scholar] [CrossRef]
- Nishihara, M.; Miura, T.; Miki, T.; Tanno, M.; Yano, T.; Naitoh, K.; Ohori, K.; Hotta, H.; Terashima, Y.; Shimamoto, K. Modulation of the mitochondrial permeability transition pore complex in gsk-3beta-mediated myocardial protection. J. Mol. Cell. Cardiol. 2007, 43, 564–570. [Google Scholar] [CrossRef]
- Juhaszova, M.; Zorov, D.B.; Kim, S.H.; Pepe, S.; Fu, Q.; Fishbein, K.W.; Ziman, B.D.; Wang, S.; Ytrehus, K.; Antos, C.L.; et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004, 113, 1535–1549. [Google Scholar] [CrossRef]
- Zhou, L.J.; Mo, Y.B.; Bu, X.; Wang, J.J.; Bai, J.; Zhang, J.W.; Cheng, A.B.; Ma, J.H.; Wang, Y.W.; Xie, Y.X. Erinacine facilitates the opening of the mitochondrial permeability transition pore through the inhibition of the pi3k/akt/gsk-3beta signaling pathway in human hepatocellular carcinoma. Cell. Physiol. Biochem. 2018, 50, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.R.; Hsu, A.K.; Gross, G.J. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes 2007, 56, 127–136. [Google Scholar] [CrossRef]
- Cai, W.J.; Chen, Y.; Shi, L.X.; Cheng, H.R.; Banda, I.; Ji, Y.H.; Wang, Y.T.; Li, X.M.; Mao, Y.X.; Zhang, D.F.; et al. Akt-gsk3beta signaling pathway regulates mitochondrial dysfunction-associated opa1 cleavage contributing to osteoblast apoptosis: Preventative effects of hydroxytyrosol. Oxid. Med. Cell. Longev. 2019, 2019, 4101738. [Google Scholar] [CrossRef]
- Doble, B.W.; Woodgett, J.R. Gsk-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Jensen, J.; Brennesvik, E.O.; Lai, Y.C.; Shepherd, P.R. Gsk-3beta regulation in skeletal muscles by adrenaline and insulin: Evidence that pka and pkb regulate different pools of gsk-3. Cell. Signal. 2007, 19, 204–210. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, H.; Zhang, H.; Xu, F.; Zou, Z.; Liu, M.; Wang, Q.; Miao, M.; Shi, X. Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating akt-gsk-3beta signaling and inhibiting mitochondrial permeability transition. PLoS ONE 2013, 8, e74422. [Google Scholar]
- Lieber, C.S.; DeCarli, L.M. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989, 24, 197–211. [Google Scholar]
- Reiser, G.; Kunzelmann, U.; Steinhilber, G.; Binmoller, F.J. Generation of a monoclonal antibody against the myelin protein cnp (2′,3′-cyclic nucleotide 3′-phosphodiesterase) suitable for biochemical and for immunohistochemical investigations of cnp. Neurochem. Res. 1994, 19, 1479–1485. [Google Scholar] [CrossRef]

| №, Pair | Control | №, Pair | Ethanol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body Weight, g | Liver Weight Aft. Exp, g | Ratio of Body/Liver weight Aft. Exp, % | Body Weight, g | Liver Weight aft. Exp, g | Ratio of Body/Liver Weight Aft. Exp, % | ||||
| Bef. Exp. | Aft. Exp. | Bef. Exp. | Aft. Exp. | ||||||
| 1 (C1) | 155 | 337 | 10.4 | 3.08 | 1 (E1) | 157 | 312 | 11.5 | 3.08 |
| 2 (C2) | 161 | 342 | 10.2 | 2.99 | 2 (E2) | 160 | 326 | 10.9 | 2.99 |
| 3 (C3) | 164 | 348 | 10.5 | 3.02 | 3 (E3) | 167 | 315 | 12.1 | 3.02 |
| 4 (C4) | 158 | 337 | 10.7 | 3.17 | 4 (E4) | 155 | 314 | 11.7 | 3.17 |
| mean | 159.5 ± 3.9 | 341 ± 5.2 | 10.45 ± 0.2 | 3.06 ± 0.08 | mean | 159.7 ± 5.3 | 316.7 ± 6.3 | 11.5 ± 0.5 | 3.64 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baburina, Y.; Odinokova, I.; Krestinina, O. Carbenoxolon Is Capable to Regulate the Mitochondrial Permeability Transition Pore Opening in Chronic Alcohol Intoxication. Int. J. Mol. Sci. 2021, 22, 10249. https://doi.org/10.3390/ijms221910249
Baburina Y, Odinokova I, Krestinina O. Carbenoxolon Is Capable to Regulate the Mitochondrial Permeability Transition Pore Opening in Chronic Alcohol Intoxication. International Journal of Molecular Sciences. 2021; 22(19):10249. https://doi.org/10.3390/ijms221910249
Chicago/Turabian StyleBaburina, Yulia, Irina Odinokova, and Olga Krestinina. 2021. "Carbenoxolon Is Capable to Regulate the Mitochondrial Permeability Transition Pore Opening in Chronic Alcohol Intoxication" International Journal of Molecular Sciences 22, no. 19: 10249. https://doi.org/10.3390/ijms221910249
APA StyleBaburina, Y., Odinokova, I., & Krestinina, O. (2021). Carbenoxolon Is Capable to Regulate the Mitochondrial Permeability Transition Pore Opening in Chronic Alcohol Intoxication. International Journal of Molecular Sciences, 22(19), 10249. https://doi.org/10.3390/ijms221910249





