3. Discussion
Before becoming fertilization competent, testicular mammalian sperm require to undergo epididymal maturation in males followed by capacitation in females. Epididymal maturation involves a series of biochemical and physiological changes that render the sperm able to move and to capacitate
in vivo in the female tract or in vitro in appropriate media (reviewed in [
1]). Epididymal maturation is a slow process that requires several days in which sperm transit through different regions of the epididymis. The complexity of this process has prevented development of in vitro systems that could successfully mimic the compartmentalized epididymal environments. Therefore, the study of sperm maturation relies on the comparison between sperm recovered from different regions of the epididymis (i.e., caput, corpus, cauda). Here, we show that ligation of the mouse epididymis at the distal caput can be used as a tool to investigate some aspects of sperm maturation. This approach has been used in the past in other species such as rabbits and rats [
25,
27,
28,
29]. Our results using a mouse system are consistent with these previous reports and show that a significant fraction of the immature sperm population acquire the capacity to move when retained in the caput epididymis for a period of at least 7 days. It has been proposed that epididymal maturation is triggered by a complex combination of exposure to a compartmentalized epididymal milieu and intrinsic sperm mechanisms depending on the total time spent in the epididymis [
30]. In this regard, mouse sperm take 7–10 days to transit through the epididymis while maturing [
31]. Our results indicate that caput epididymal ligation might be promoting intrinsic time-dependent sperm maturation events in caput sperm, and this technique could be a useful tool to dissect those processes that occur to sperm during maturation that are time- but not region-specific dependent.
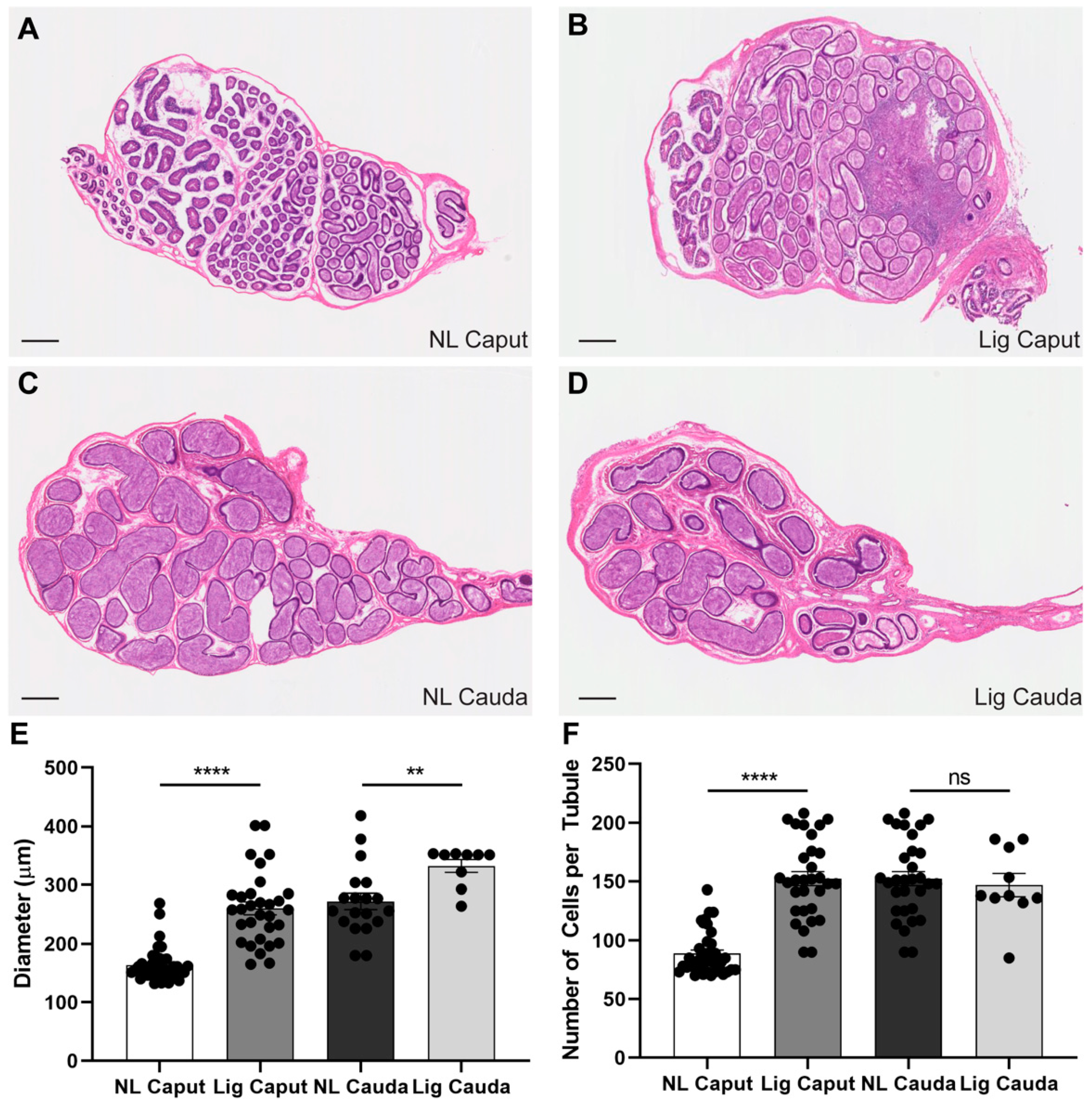
Epididymal ligation did not affect sperm viability up to 7 days post-ligation, nor acrosomal integrity. The ligation procedure induced considerable tissue damage to a region in the caput epididymis as well as the enlargement of the remaining intact caput epididymal tubules. In addition, the epithelial composition of the caput epididymis changed after 7 days of ligation. This was evidenced by the reduction of AQP9 expression, a marker for principal cells, in the initial segment and caput epithelial cells. Principal cells are the main contributors of protein synthesis and secretions in the proximal epididymis [
32,
33,
34]. Therefore, the observed reduction of AQP9 expression in the ligated initial segment and caput epididymis may indicate deficient principal cell function that could result in drastic changes in the luminal content to which sperm are exposed to in the post-ligation period and may contribute to the sperm gain in motility.
The ligation procedure did not have consequences in the overall cell composition of the cauda epididymal epithelium. It is worth mentioning that the epithelium of the corpus epididymis adjacent to the ligation site seemed to be affected, which would undermine analyses of corpus sperm. Additionally, longer periods of epididymal ligation (i.e., 10 and 14 days) were detrimental for sperm viability suggesting that the accumulation of sperm and lack of flow within the caput for extended periods of time induced sperm death. Altogether, these results support the utilization of 7-days epididymal ligation in the distal caput as a tool for the study of sperm maturation.
One of the hallmarks of epididymal maturation is the acquisition of sperm motility (reviewed in [
1]). It is known that the machinery required for motility is assembled but maintained inhibited in caput sperm as demembranated caput sperm start to be motile when exposed to ATP or cAMP [
35,
36,
37,
38]. Here, we show that after 7 days of epidydimal ligation, caput sperm acquire progressive motility. It has been proposed that the acquisition of motility during epididymal maturation depends on inactivation of the sperm-specific alternative spliced isoform of the ser/thr phosphatase PP1γ known as PP1γ2 which becomes inactivated in cauda sperm [
20]. This inactivation has consequences in the ability of cauda sperm to undergo capacitation-associated phosphorylation cascades. Consistently, caput sperm incubated in capacitation-supporting media (containing HCO
3− and BSA) failed to undergo phosphorylation of PKA substrates [
12] and do not display the hallmark capacitation-associated increase in tyrosine phosphorylation [
11,
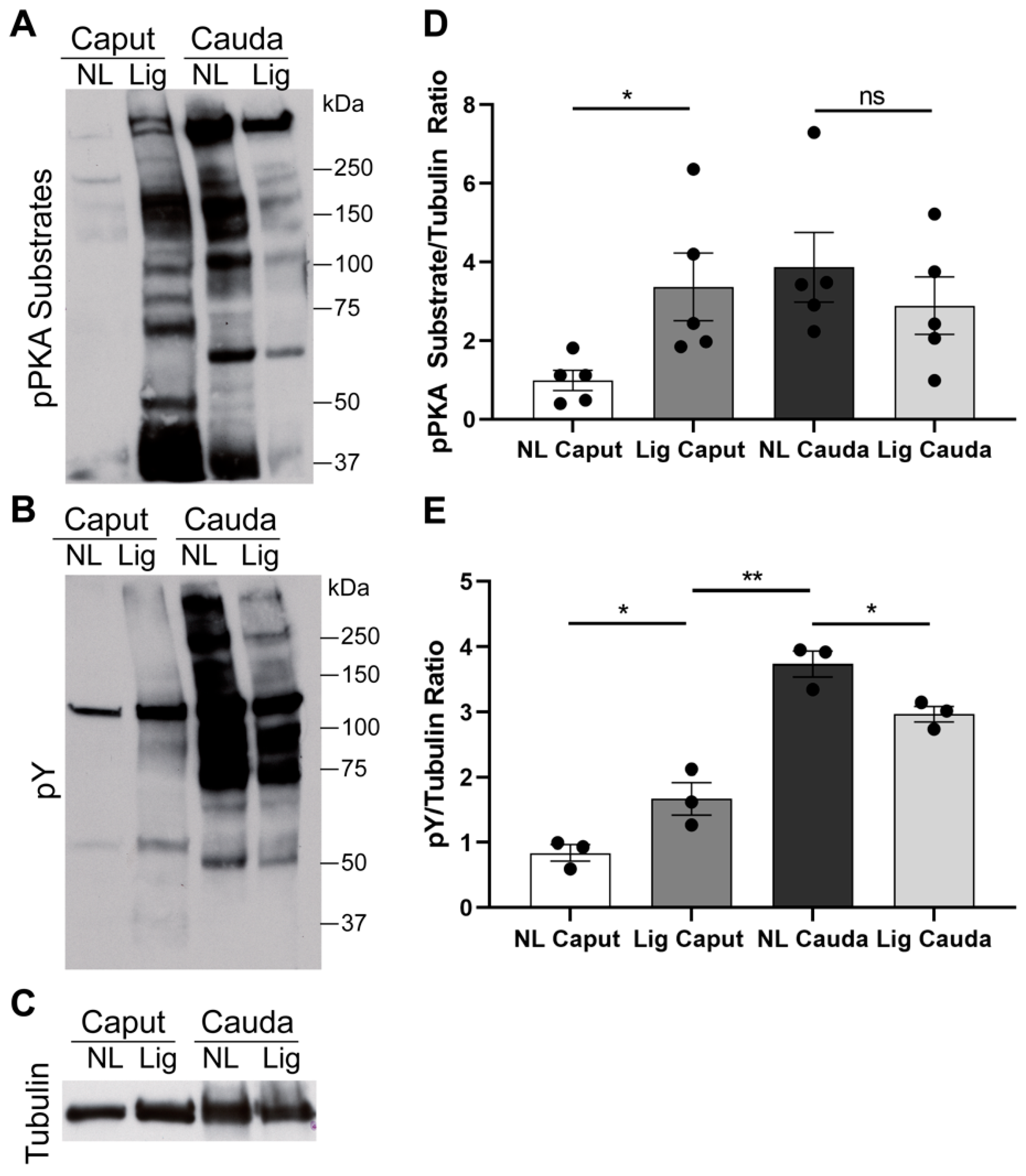
12]. In contrast, sperm from ligated caput undergo phosphorylation of PKA substrates to levels comparable to the ones observed for cauda sperm. These data indicate that PKA-dependent phosphorylation can occur in ligated caput sperm. However, differences in the molecular weight of several phosphorylated proteins observed with the anti-pPKAs antibody indicate that the phosphorylation pattern observed in ligated caput sperm is different to the one obtained for cauda sperm suggesting that ligation does not completely mimic the maturation process. Moreover, although we were able to observe a slight increase in tyrosine phosphorylation between ligated and non-ligated caput sperm, the level of phosphorylation does not reach to the one observed in cauda sperm. These results suggest that, in addition to the time of residence in the caput, interactions with other epididymal regions are necessary.
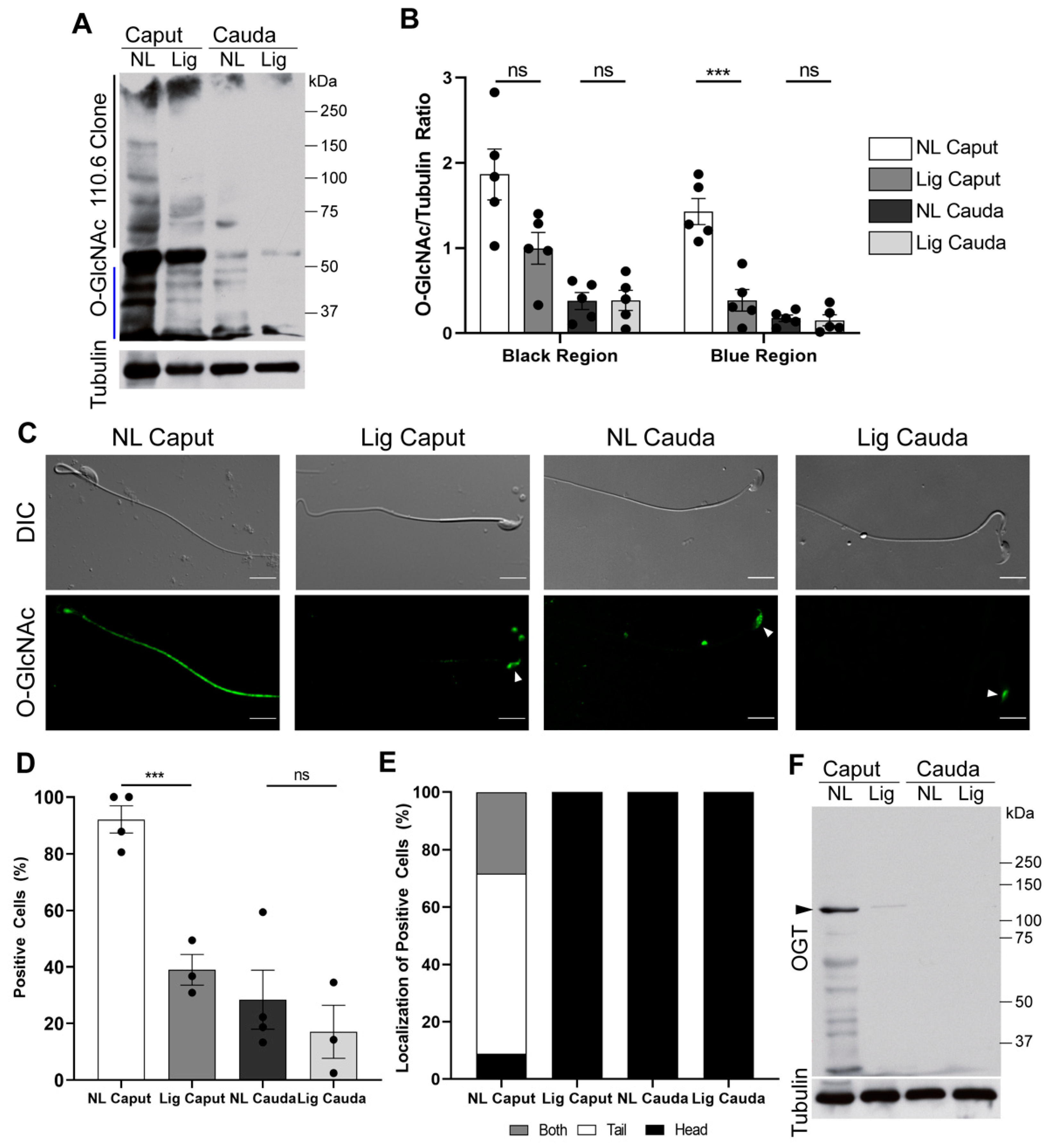
Phosphorylation pathways are thought to be connected with another post-translational modification known as O-GlcNAc, this modification also occurs in ser and thr residues. Current hypotheses state that O-GlcNAc prevents phosphorylation on these residues, and, therefore, prevents activation of phosphorylation pathways. We have previously shown that protein O-GlcNAc decreases in mouse sperm during epididymal maturation [
12]. Here we show that, similar to cauda sperm, sperm recovered from ligated caput also have a significant decrease in O-GlcNAc. As previously observed, the decrease in O-GlcNAc is mostly observed in the sperm tail in a localization reminiscent to the one observed for PKA [
39]. Additionally, as previously reported [
12], levels of OGT, the enzyme responsible for O-GlcNAc, were significantly reduced in ligated caput sperm. These results indicate that OGT and O-GlcNAcylation levels decrease over time during epididymal maturation independently of the epididymal milieu. The mechanisms that drive OGT degradation during epididymal maturation are still unknown.
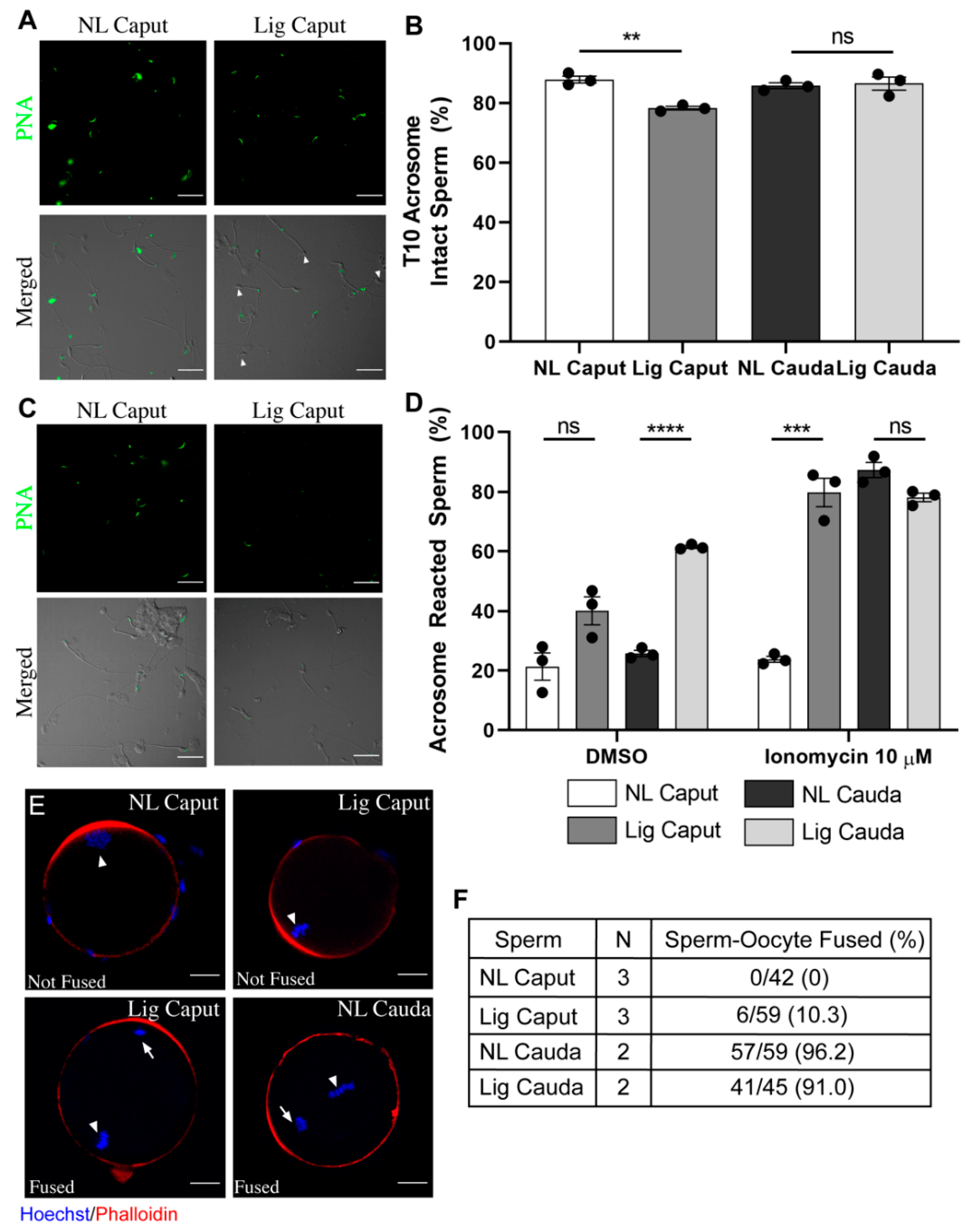
As shown, our results indicate that when sperm movement from the caput to other epididymal regions is restricted, those sperm acquire the ability to move progressively. Those sperm also undergo changes in signaling pathways such as the possibility to activate PKA-dependent phosphorylation and decrease in O-GlcNAc. Although these findings suggest that a fraction of the capacitation-associated pathways are possible without passage through other epididymal regions, to be fully mature, changes in other functional parameters are necessary. Physiological changes that occur to sperm during maturation include rearrangements of the sperm plasma membrane that give them the ability to acrosome react and to fuse with an MII oocyte. Consistent with previous reports in dogs and monkeys, immature non-ligated caput sperm were not able to acrosome react when challenged with the Ca
2+ ionophore A
23187 [
40,
41]. Here, we show that sperm recovered from non-ligated caput epididymis did not undergo acrosomal exocytosis when exposed to ionomycin, another Ca
2+ ionophore able to induce high levels of acrosome reaction even in non-capacitated cauda sperm [
42]. On the other hand, most sperm from ligated caput undergo the acrosome reaction when challenged with ionomycin. It was recently shown that AQPs expression in the different regions of the epididymal epithelium changes due to cryptorchidism in the dog [
43]. The study of AQPs expression in the mouse caput epithelium after epididymal ligation and its relation to the acquisition of ability of sperm to acrosome react needs to be explored. Finally, our results evidence that caput epididymal ligation induces tissue damage of the caput epididymis. Then, we cannot discard that sperm in the ligated caput are exposed to higher levels of radical oxygen species (ROS). However, recent work from our laboratory indicates that, in the mouse, ROS generation does not influence cAMP-dependent pathways during sperm capacitation [
44]. These results suggest that the increase in phosphorylation of PKA substrates observed in caput sperm upon epididymal ligation is not a consequence of increased ROS production. More work will be needed to further evaluate the role of ROS during this process.
Although the ability to acrosome react is an essential component of epididymal maturation, to be considered complete, it is necessary that sperm gain fertilizing potential. To explore this possibility, we performed in vitro fertilization with ligated and non-ligated caput sperm using eggs in three different protocols: cumulus-enclosed, cumulus-free and zona-pellucida-free. Although ligated caput sperm can move progressively, they failed to undergo hyperactivation; therefore, it was not surprising to find that these sperm were not able to fertilize zona-intact eggs. When the assay was conducted with zona-free eggs, contrary to non-ligated caput sperm, a small fraction of the ligated caput sperm population was able to fuse. Fusibility of these sperm might be related to their ability to acrosome react. Interestingly, those eggs with fused ligated caput sperm were not able to cleave. These data are consistent with recent work indicating that caput sperm are not able to maintain early embryo development [
19]. In this regard, it has been shown that during passage through the epididymis sperm acquire small non-coding RNAs that can affect fertilization and early embryo development [
14,
18].
Overall, our results indicate that ligated caput sperm can achieve some extent of maturation at the physiological level. Associated to the changes in motility, levels of O-GlcNAcylation were found to decrease in the ligated caput sperm as well as the levels of the enzyme OGT. Moreover, changes in phosphorylation of PKA substrates indicated that ligated caput sperm were able to activate pathways related to sperm capacitation. Despite these changes, sperm from ligated caput did not acquire the ability to fertilize in vitro. However, a small fraction of ligated caput sperm was capable of fusing with zona-free eggs. Altogether, these results show that the acquisition of motility and the capacity to undergo capacitation-associated changes in phosphorylation can occur in the absence of corpus or cauda epididymal secretions.
Application of distal caput epididymal ligation has the potential to be useful to study certain aspects of sperm maturation beyond the comparison of sperm recovered from different regions of the epididymis. By applying this technique, we showed that molecular changes that induce sperm progressive motility can develop without transit and without secretions from corpus or cauda epididymis. Our results also indicate that ligated caput sperm cannot undergo full capacitation, and therefore cannot be considered completely mature without passage through all the different regions of the epididymis. These data highlight the complexity of epididymal maturation and the importance of epididymal transit to achieve full fertilizing potential. In this regard, it is well established that epithelial cells in different epididymal regions are transcriptionally and functionally different [
45,
46,
47,
48]. Further studies to evaluate functional changes in caput epithelial cells following ligation, and their role in sperm maturation are warranted.
4. Materials and Methods
Animals. Mouse sperm samples were collected from male CD1 retired breeders (Charles River Laboratories, Wilmington, MA, USA). Mouse oocytes were collected from 8–10-week-old super ovulated CD1 females (Charles River Laboratories, Wilmington, MA, USA). For superovulation, approximately 10 females per round of IVF were injected with 5 IU pregnant mare serum gonadotropin (PMSG) (Lee BioSolutions, cat # 493-10, Maryland Heights, MO, USA) and 5 IU human chorionic gonadotrophin (hCG) (Sigma, cat # CG5, St. Louis, MO, USA) 48 h later. Cumulus-Oocytes-Complexes (COCs) were collected 13 h post hCG injection. Animal care and use of experimental animals were conducted in accordance with specific guidelines and standards dictated by the Office of Laboratory Animal Welfare (OLAW) and approved by the Institutional Animal Use and Care Committee (IACUC), University of Massachusetts-Amherst (Protocol #2019-0008).
Media. Medium used for sperm capacitation was HEPES-based, modified Toyoda-Yokoyama-Hosi (m-TYH) medium consisting of 119.37 NaCl, 4.78 KCl, 1.19 KH2PO4, 1.19 MgSO4, 5.56 glucose, 1.71 CaCl2, 20 HEPES, 0.51 Na-pyruvate, 10 µg/mL gentamicin, 0.0006 % phenol red supplemented with 25 mM NaHCO3− and 5 mg/mL of bovine serum albumin (BSA) (Sigma cat # A0281, St. Louis, MO, USA) at pH 7.2–7.4. Medium used for sperm fertilization assay and fusion assay was Toyoda-Yokoyama-Hosi (IVF-TYH) medium, consisting of 119.37 NaCl, 4.78 KCl, 1.19 KH2PO4, 1.19 MgSO4. 7 H2O, 5.56 glucose, 1.71 CaCl2.2 H2O, 25.1 mM NaHCO3−, 0.51 Na-pyruvate, 4 mg/mL BSA, 10 µg/mL gentamicin, 0.0006 % phenol red at pH 7.4 equilibrated with 5% CO2. This medium does not contain HEPES. Medium used for oocyte collection was Tyrodes’s lactate-HEPES (TL-HEPES), consisting of 114 mM NaCl, 3.22 mM KCl, 2.04 mM CaCl2.2 H2O, 0.35 mM NaH2PO4.2H2O, 0.49 mM MgCl2.6H2O, 2.02 mM NaHCO3−, 10 mM Lactic acid (sodium salt), and 10.1 mM HEPES at pH 7.4).
Epididymal Ligation Surgery. Male CD1 retired breeders were given analgesic meloxicam at a dose of 10 mg/kg via intraperitoneal (IP) injection five minutes prior to being anesthetized with 2–3% isoflurane via inhalation. Once the animal was non-responsive per IACUC protocol, a small 5 mm incision was made lateral to the penis on either ventral-left or ventral-right of the animal. Experiments were conducted ligating either the ventral-left or the ventral-right to account for asymmetrical differences in the epididymis. After dissecting through the skin and muscle layer, the caput region of the epididymis was identified, and two sutures were placed between the distal caput and proximal corpus. Animals received one suture to close the muscle layer and an additional two sutures to close the skin layer before being removed from isoflurane. Post-operative care was monitored for 2–14 days in relationship to collection time point.
Sperm sample collection. Time points of collection were conducted 2-, 5-, 7-, 10-, and 14-days post ligation surgery dependent on the experimental endpoint. Mice were euthanized by exposure to carbon dioxide (CO
2). Then, the epididymides were dissected and the sperm samples were collected from the following regions: non-ligated caput (NL Caput), non-ligated cauda (NL Cauda), ligated caput (Lig Caput) and ligated cauda (Lig Cauda) (
Figure S1). Each region was isolated in 500 µL of capacitating media. Sperm recovered from the NL and Lig Caput epididymides were obtained by squeezing the tissue in capacitating m-TYH or IVF-TYH media approximately 10–15 times. Sperm recovered from the NL and Lig Cauda epididymides were obtained by the “swim-out” method using the same capacitating media in a 10 min period. All samples were counted using a hemocytometer and adjusted to have a final concentration of 500,000–2,000,000 sperm per mL concentration for protein analysis. Samples used for fertilization assays and fusion assays were adjusted to have a final concentration of 100,000 sperm per fertilizing droplet (90 μL). Samples were capacitated for 40 min unless otherwise stated.
Motility Assay and CASA Analysis. Samples were analyzed at two time points of incubation: 10 and 40 min of capacitation. Sperm suspensions (35 μL) were loaded into a pre-warmed chamber slide (depth 100 μm) (Leja slide, Spectrum Technologies, Aurora, IL, USA) and placed on a microscope stage at 37 °C. Sperm motility was examined using the CEROS computer-assisted sperm analysis (CASA) system (Hamilton Thorne Research, Beverly, MA, USA). The default settings include the following: frames acquired: 90; frame rate 60 Hz; minimum cell size: 4 pixels; static head size: 0.13–2.43; static head intensity: 0.10–1.52; static head elongation: 5–100. At least 5 microscopy fields corresponding to the minimum of 500 sperm were analyzed for each treatment in each experiment.
Immunocytochemistry (H&E) and immunofluorescence of the epididymis. Epididymides were removed and fixed by immersion in 4% paraformaldehyde-lysine periodate (PLP) solution for 24 h at 4 °C, then washed in PBS, and stored at 4 °C in PBS containing 0.02% sodium azide. PLP-fixed epididymal slices were cryoprotected in 30% Sucrose/PBS for at least 48 h at room temperature. They were embedded in Tissue-Tek OCT compound 4583 (Sakura Finetek, Torrance, CA) and frozen at −20 °C. Sections were cut at 5 µm using a Leica CM3050S cryostat (Leica, Wetzlar, Germany), and picked up onto Fisherbrand Superfrost Plus microscope slides (Fisher Scientific, Pittsburg, PA, USA). H&E staining (Millipore, Sigma, Burlington, MA, USA) was performed for overall morphology and the sections were scanned by using a digital slide scanner, NanoZoomer 2.0RS (Hamamatsu, Japan). For AQP9 and V-ATPase labeling: epididymal sections were hydrated in PBS for 15 min, and treated with sodium dodecyl sulfate (SDS) for 4 min. Slides were blocked in 1% BSA, and incubated with the primary antibody at 4 °C overnight, and with the secondary antibody for 1 h at room temperature (RT). The primary antibodies used were chicken polyclonal anti-A subunit V-ATPase (0.3 μg/mL) and rabbit polyclonal anti-aquaporin 9 (AQP9, 0.5 μg/mL) made and purified in Breton’s laboratory. The corresponding secondary antibodies (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA) were donkey anti-chicken IgG conjugated to Alexa488 (30 μg/mL, cat. no.: 703-545-155) and donkey anti-rabbit IgG conjugated to indocarbocyanine (Cy3) (7.5 μg/mL cat. no.: 711-165-152). All antibodies were diluted in DAKO medium (Dako, Carpinteria, CA, USA). Slides were mounted with SlowFade Diamond Antifade Mount medium (Thermo Fisher Scientific, Waltham, MA, USA) containing the DNA marker DAPI (Vector Laboratories, Burlingame, CA, USA), and were examined using the 90i Nikon microscope.
Viability Assay. After collection, sperm samples were capacitated for 30 min in capacitating m-TYH. Then the addition of Hoescht 33258 (Molecular Probes, H-1398) at a concentration of 1 μg/μL was added to the incubation for an additional 10 min. Once finished, samples were centrifuged at 800× g for 5 min, washed with filtered PBS and air-dried on Poly-L-Lysine-coated glass coverslips, followed by mounting using VectaShield (H-1000, Vector Laboratories, Burlingame, CA, USA) on slides. Images were taken using a 60X objective (Nikon, PlanApo, NA 1.49) on a fluorescence microscope (Nikon Eclipse T300). Conditions for analysis were the following: uptake of fluorescence probe indicative of dead sperm. Differential interference contract (DIC) images were taken in parallel and served as a control for live sperm. A minimum of 200 sperm were analyzed per condition per experiment.
SDS/PAGE and Western Blotting. After collection, sperm samples were capacitated for 40 min in full capacitation m-TYH media and then extracted for Western blot analysis as previously described [
12]. Western blotting was performed using the following antibodies: anti-pPKA substrates monoclonal antibody (clone 100G7) diluted 1:10,000 (Cell Signaling, # 9624, Danvers, MA, USA); monoclonal anti-pY antibody (clone 4G10) diluted 1:10,000 (Millipore, cat # 05-321, Burlington, MA); O-GlcNAc monoclonal antibody (clone 110.6) diluted 1:2000 (Cell Signaling, cat # 9875, Danvers, MA, USA); OGT polyclonal antibody 1:1000 (Cell Signaling, cat # 5368, Danvers, MA, USA). Optical densitometry analysis was performed in all blots using the Fiji software. Each lane was normalized to tubulin. For pPKA-substrates analyses, the entirety of the lane was compared between conditions. For pY, the region below the hexokinase (110 kDa) was compared between conditions. For O-GlcNAc, two regions were compared as indicated in the figure, the upper region (>56 kDa, indicated with a black line) and lower region (<50 kDA, indicated with a blue line) for comparison between conditions. For OGT, the specific band at 110 kDa was used to compare between conditions.
Sperm Immunolocalization. After collection of sperm samples as described above, samples were fixed in 4% paraformaldehyde (EMS, Hatfield, MA) in PBS for 10 min at room temperature. Sperm samples were centrifuged at 800×
g for 5 min, washed with PBS, and air-dried on Poly-L-Lysine-coated glass slides. For staining with O-GlcNAc or OGT, samples were processed as previously described [
12]. Negative controls were run in parallel by incubation with secondary antibody alone. Images were taken using a 60X objective (Nikon, PlanApo, NA 1.49) on a fluorescence microscope (Nikon Eclipse T300). Differential interference contrast (DIC) images were taken in parallel and served as control for sperm morphology. A minimum of 100 sperm were analyzed per condition per experiment.
Acrosome Reaction Assay. After collection, sperm samples were incubated in capacitating m-TYH media. Immediately, 100 μL was taken from each condition (T10) and centrifuged at 800× g for 5 min at room temperature, washed with PBS and fixed with 4% paraformaldehyde (EMS, Hatfield, MA) for 10 min at room temperature. The suspension was then centrifuged at 800× g for 5 min and washed twice in PBS. Samples were stored at 4 °C during the remaining capacitation time of other samples. After 40 min of capacitation, each sperm sample from each region was divided and subjected to either DMSO or Ionomycin (20 μM) (Enzo, cat #ALX-450-007, Farmingdale, NY, USA). After an additional incubation of 30 min, the samples were processed in the same manner as the T10 samples. All samples were left to air dry on Poly-L-Lysine-coated coverslips, followed by permeabilization with 100% methanol for 30 s and washed with filtered PBS three times. Sperm were incubated with Alexa 488-conjugated peanut agglutinin (PNA) 10 μg/mL (Molecular Probes, cat #L-21409, Eugene, OR, USA) for 1 h at room temperature in a humidified chamber and then washed three times with PBS and mounted as described above. Images were taken using a 60X objective (Nikon, PlanApo, NA 1.49) on a fluorescence microscope (Nikon Eclipse T300). Differential interference contrast (DIC) images were taken in parallel and served as control for sperm morphology.
Fusion Assay and analysis. For Zona-Free IVF: cumulus-oocyte-complexes (COCs) were collected 13-h post hCG. Removal of the cumulus cells, COCs were placed in a 30 μL droplet of hyaluronidase for 2–5 min, then proceeded by washing in IVF-TYH media. Followed by removal of the zona pellucida, oocytes were placed in a 10 μL droplet of acid tyrode’s solution for 30–60 s then washed in IVF-TYH media. Oocytes were placed in a 90 μL droplet dish prior to fertilization and allowed to rest for 30 min in the incubator at 37 °C, 5% CO2. After collection of sperm as described above, the insemination droplet was inseminated with approximately 10,000 sperm per 90 μL droplet for 1 h. Oocytes were then washed thoroughly in IVF-TYH to remove extra sperm and placed into 4% PFA for 20 min at room temperature, followed by three washes with PBS. Samples were then permeabilized with 0.5% Triton X-100 for 20 min at RT, followed by three washes with 0.1% Triton X-100 in PBS. Oocytes were then incubated for 1 h at RT with 10% normal goat serum and incubated overnight in the presence of 6.6 μM phalloidin (Invitrogen, cat #A22287, Carlsbad, CA, USA) and Hoechst 33342 (Fisher Scientific, cat #H3570, Waltham, MA, USA). Images were taken using a 40X (Plan Fluor, NA 1.3) objective in a Nikon confocal microscope. Fused embryos were considered when Hoechst-stained sperm heads were visualized within the plasma membrane of the oocyte during 3D-analysis.
In vitro fertilization (IVF) assay. For Cumulus-intact Standard IVF: cumulus-oocyte-complexes (COCs) were collected 13 h post hCG and washed with IVF-TYH prior to being placed in the insemination droplet. After collection of sperm as described above, the insemination droplet was inseminated with approximately 100,000 sperm per 90 μL droplet. After 4 h, oocytes were washed in IVF-TYH and allowed to culture for 20 h. The following day, fertilization was investigated by visualization of 2-cell cleavage. For Cumulus-Free IVF: cumulus-oocyte-complexes (COCs) were collected 13 h post hCG. For removal of the cumulus cells, COCs were placed in a 30 μL droplet of hyaluronidase for 2–5 min, then proceeded by washing in IVF-TYH media. Oocytes were placed in a 90 μL droplet dish prior to fertilization and allowed to rest for 30 min in the incubator at 37 °C, 5% CO2. After collection of sperm as described above, the insemination droplet was inseminated with approximately 100,000 sperm per 90 μL droplet. After 4 h, oocytes were washed in IVF-TYH and allowed to culture for 20 h. The following day, fertilization was assessed by visualization of 2-cell cleavage. For Zona-Free IVF: cumulus-oocyte-complexes (COCs) were collected 13-h post hCG. Removal of the cumulus cells, COCs were placed in a 30 μL droplet of hyaluronidase for 2–5 min, then proceeded by washing in IVF-TYH media. For removal of the zona pellucida, oocytes were placed in a 10 μL droplet of acid tyrode’s solution for 30–60 s then washed in IVF-TYH media. Oocytes were placed in a 90 μL droplet dish prior to fertilization and allowed to rest for 30 min in the incubator at 37 °C, 5% CO2. After collection of sperm as described above, the insemination droplet was inseminated with approximately 10,000 sperm per 90 μL droplet. After 1 h, oocytes were washed in IVF-TYH and cultured for 20 h. The following day, cleavage was assessed by visualization of 2-cell cleavage.
Statistical Analysis. Data from all studies were analyzed using PRISM 8 Graph Pad software (
https://www.graphpad.com/scientific-software/prism/). Data expressed as the mean ± S.E.M with individual experimental values represented by dots. For Caput Data: non-paired
t-tests, or when applicable Sidak–Bonferroni
t-tests were performed between NL Caput and Lig Caput. For Cauda Data: non-paired
t-tests, or when applicable Sidak–Bonferroni
t-tests were performed between NL Cauda and Lig Cauda. Statistical significances are indicated in the figure legends.