Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease
Abstract
:1. Introduction
2. Pathogenesis and Histology of Osteoarthritis Disease
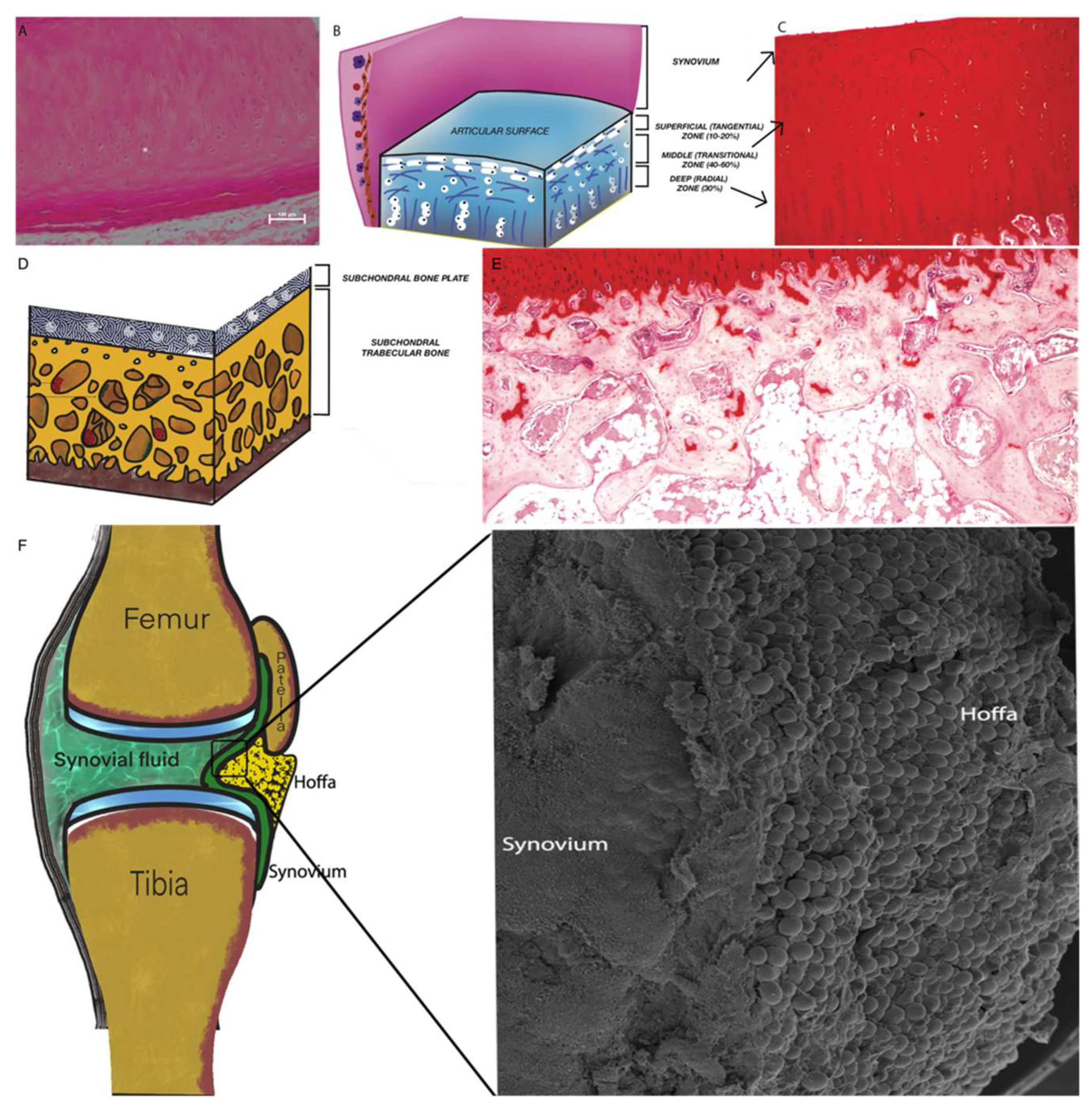
2.1. Articular Cartilage (AC)
2.2. Subchondral Bone (SB)
2.3. Synovium and Synovial Fluid
2.4. Intra-Articular Adipose Tissue Structure
2.5. Osteoarthritis: Cartilage Damage, Repair and Regeneration
3. Regenerative Treatment for Osteoarthritis Disease
3.1. Platelet-Rich Plasma (PRP)
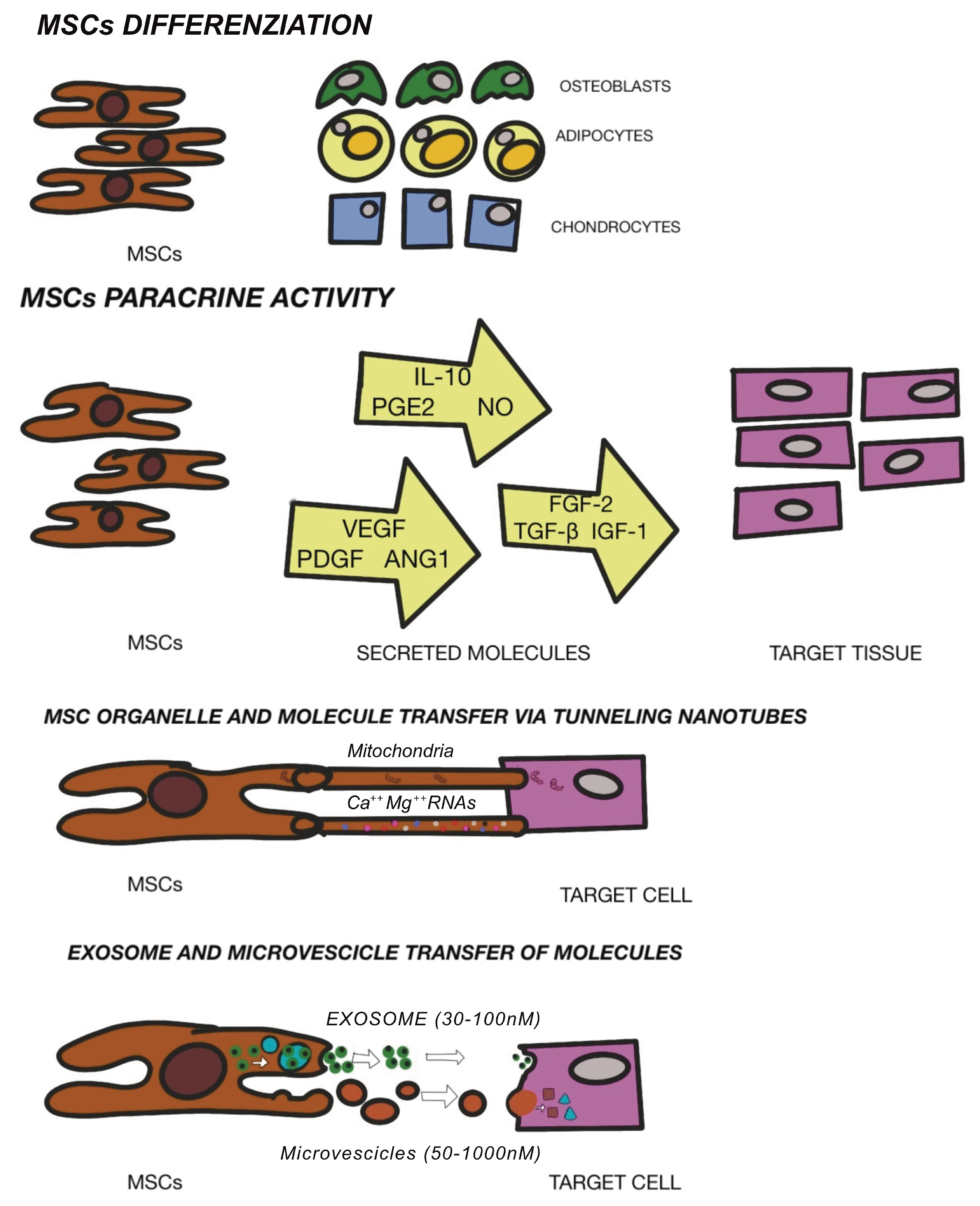
3.2. Mesenchymal Stem Cells Therapy
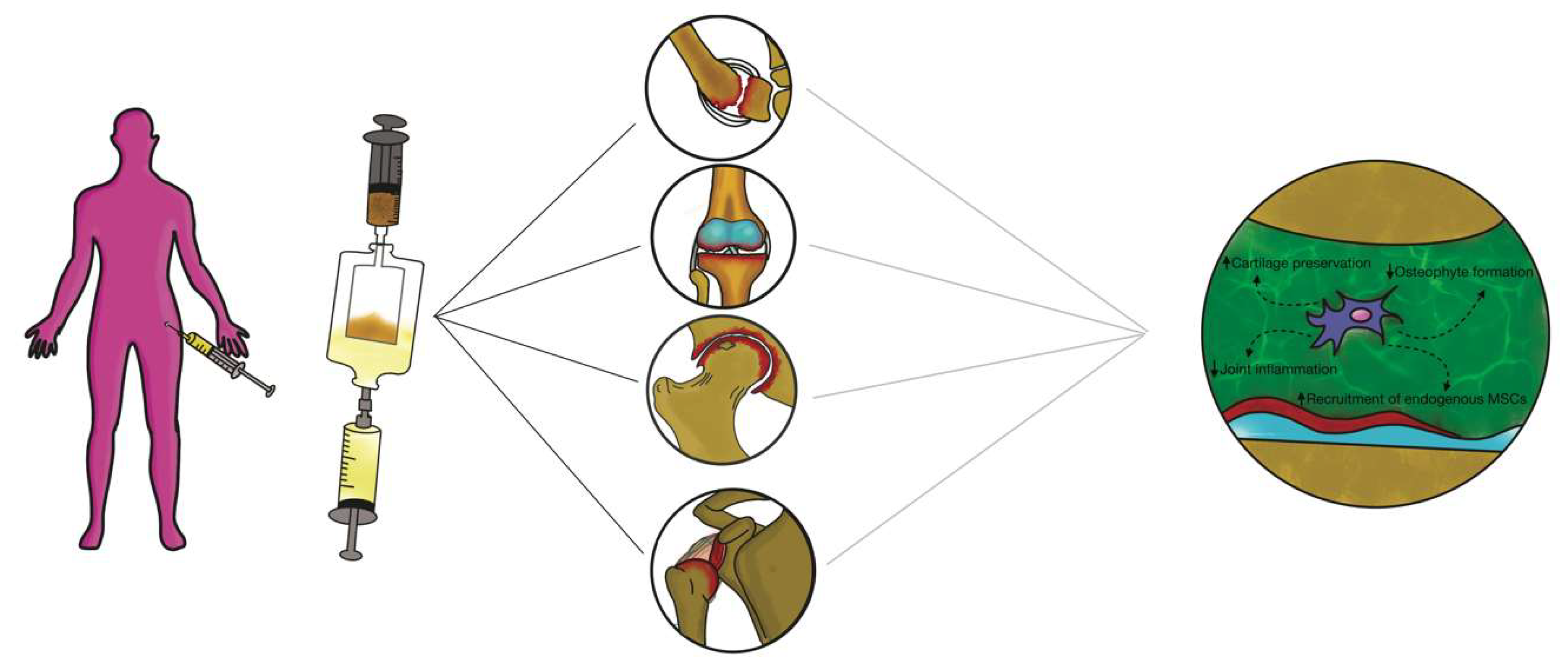
3.3. Intra-articular Application of Autologous Microfragmented Adipose Tissue with Stromal Vascular Fraction
3.4. Exosome and Extracellular Vescicles (EVs)
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McAlindon, T. Osteoarthritis Research Society International (OARSI) Classification and Guidelines. HSS J. 2012, 8, 66–67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell-related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Midgley, J. Osteoarthritis and obesity; conservative management, multi-morbidity, surgery and the implications of restricted access to knee or hip replacement: A literature review. Int. J. Orthop. Trauma Nurs. 2020, 8, 100840. [Google Scholar]
- Tan, S.H.S.; Tan, B.S.W.; Tham, W.Y.W.; Lim, A.K.S.; Huiu, J.H. The incidence and risk factors of osteoarthritis following osteochondritis dissecans of the knees: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2020, 29, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Bernotiene, E.; Bagdonas, E.; Kirdaite, G.; Bernotas, P.; Kalvaityte, U.; Uzieliene, I.; Thudium, C.S.; Hannula, H.; Lorite, G.S.; Dvir-Ginzberg, M.; et al. Emerging technologies and platforms for immunodetection of multiple biochemical markers in Osteoarthritis Research and Therapy. Front. Med. 2020, 7, 572977. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the US bone and joint initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, B.; McMeekin, P.; Bate, A. Systematic review of studies using conjoint analysis techniques to investigate patients’ preferences regarding osteoarthritis treatment. Patient Prefer. Adherence 2021, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyere, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-articular injections in knee osteoarthritis: A review of literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Phys. Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alford, J.W.; Cole, B.J. Cartilage restoration, part I: Basic science, historical perspective, patient evaluation and treatment options. Am. J. Sports Med. 2005, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Li, X.; Chen, Y.; Cai, H.; Cao, W.; Chen, X.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. Exracellular matrix powder from cultured cartilage-like tissue as cell carrier for cartilage repair. J. Mater. Chem. B 2017, 5, 3283–3292. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Carter, S.D.; Martin-Vasallo, P.; Shakibaei, M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol. Int. 2002, 26, 1–18. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as promising target for treatment of osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The role of chondrocyte hypertrophy and science in osteoarthritis initiation and progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef] [Green Version]
- Saltzman, B.M.; Riboh, J.C. Subchondral bone and the osteochondral unit: Basic science and clin ical implications in sports medicine. Sports Health 2018, 10, 412–418. [Google Scholar] [CrossRef]
- Radin, E.L.; Paul, I.L.; Tolkoff, M.J. Subchondral bone changes in patients with early degenerative joint disease. Arthritis Rheum. 1970, 13, 400–405. [Google Scholar] [CrossRef]
- Lajeunesse, D.; Massicotte, F.; Pelletier, J.P.; Martel-Pelletier, J. Subchondral bone sclerosis in osteoarthritis: Not just an innocent bystander. Mod. Rheumatol. 2003, 13, 7–14. [Google Scholar] [CrossRef]
- Donell, S. Subchondral bone remodelling in osteoarthritis. EFFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Pap, T.; Dankbar, B.; Wehmeyer, C.; Korb-Pap, A.; Sherwood, J. Synovial fibrobasts and articular tissue remodelling: Role and mechanisms. Semin. Cell Dev. Biol. 2020, 101, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Noss, E.H.; Watts, G.F.M.; Zocco, D.; Keller, T.L.; Whitman, M.; Blobel, C.P.; Lee, D.M.; Brenner, M.B. Evidence for cadherin-11 cleavage in the synovium and partial characterization of its mechanism. Arthritis Res. Ther. 2015, 17, 126. [Google Scholar] [CrossRef] [Green Version]
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutic. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar] [PubMed]
- Nigrovic, P.A.; Lee, D.M. Mast cells in inflammatory arthritis. Arthritis Res. Ther. 2004, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labusca, L.; Zugun-Eloae, F. The unexplored role of intra-articular adipose tissue in the homeostasis and pathology of articular joints. Front. Vet. Sci. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; McCormick, C.C. Lumbar facet joint fat pads: Their normal anatomy and their appearance when enlarged. Neuroradiology 1991, 33, 38–42. [Google Scholar] [CrossRef]
- Clavert, P.; Dosch, J.C.; Wolfram-Gabel, R.; Kahn, J.L. New findings on intermetacarpal fat pads: Anatomy and imaging. Surg. Radiol. Anat. 2006, 28, 351–354. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Toussirot, E. Mini-review: The contribution of adipokines to joint inflammation in inflammatory rheumatic diseases. Front. Endocrinol. 2020, 11, 606560. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Lenz, K.L.; Pollitt, E.N.; Ferguson, D.; Hutson, I.; Springer, L.E.; Oetreich, A.K.; Tang, R.; Choi, Y.R.; Meyer, G.A.; et al. Adipose tissue is a critical regulator of osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 5, 118. [Google Scholar]
- Yan, M.; Zhang, J.; Yang, H.; Sun, Y. The role of leptin in osteoarthritis. Medicine 2018, 97, e0257. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, Z.C.; Shen, L.Y.; Shang, P.; Xu, H.Z.; Liu, H.X. Association of osteoarthritis and circulating adiponectin levels: A systematic review and meta-analysis. Lipids Health Dis. 2018, 17, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front. Genet. 2016, 7, 213. [Google Scholar] [CrossRef] [Green Version]
- De l’Escalopier, N.; Anract, P.; Biau, D. Surgical treatments for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef]
- Mobasheri, A. Future Cell and Gene Therapy for Osteoarthritis (OA): Potential for Using Mammalian Protein Production Platforms, Irradiated and Transfected Protein Packaging Cell Lines for Over-Production of Therapeutic Proteins and Growth Factors. In Cell Biology and Translational Medicine; Turksen, K., Ed.; Advances in Experimental Medicine and Biology, Volume 1247; Springer: Berlin/Heidelberg, Germany, 2019; Volume 8, pp. 17–31. [Google Scholar]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Baciut, M.; Baciut, G.; Bran, S.; Onisor, F.; Piciu, A.; Pasca, R.D.; et al. Systemic drugs with impact on osteoarthritis. Drug Metab. Rev. 2019, 51, 498–523. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Gheban, D.; Benea, H.R.C. Tibolone, alendronate, and simvastatin enhance implant osseointegration in a preclinical in vivo model. Clin. Oral Implant. Res. 2020, 31, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. IJMS 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Russell, R.P.; Apostolakos, J.; Hirose, T.; Cote, M.P.; Mazzocca, A.D. Variability of platelet-rich plasma preparations. Sports Med. Arthrosc. Rev. 2013, 21, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yin, W.; Zhang, Y. Comparative evaluation of leukocyte and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci. Rep. 2017, 7, 43301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wu, Y.; Meng, J.; Wu, F.; Li, S.; Lin, M.; Gao, X.; Hong, J.; Chen, W.; Yan, S.; et al. Comparison of leukocyte-rich platelet -rich plasma and leukocyte-poor platelet-rich plasma on Achilles Tendinopathy at an early stage in a rabbit model. Am. J. Sports Med. 2020, 48, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Saita, Y.; Nishio, H.; Ikeda, H.; Takazawa, Y.; Nagao, M.; Takaku, T.; Komatsu, N.; Kaneko, K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J. Orthop. Sci. 2016, 21, 683–689. [Google Scholar] [CrossRef]
- Kenmochi, M. Clinical outcomes following injections of leukocyte-rich platelet-rich plasma in osteoarthritis patients. J. Orthop. 2020, 18, 143–149. [Google Scholar] [CrossRef]
- Marmotti, A.; Rossi, R.; Castoldi, F.; Roveda, E.; Michielon, G.; Peretti, G.M. PRP and articular cartilage: A clinical update. Biomed. Res. Int. 2015, 2015, 542502. [Google Scholar] [CrossRef]
- Mariani, E.; Canella, V.; Cattini, L.; Kon, E.; Marcacci, M.; Di Matteo, B.; Pulsatelli, L.; Filardo, G. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS ONE 2016, 11, e015613753V. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Paterson, K.L.; Hunter, D.J.; Metcalf, B.R.; Eyles, J.; Duong, V.; Kazsa, J.; Wang, Y.; Buchbinder, R.; Cicuttini, F.; Forbes, A.; et al. Efficacy of intra-articular injections of platelet-rich plasma as a symptom- and disease-modifying treatment for knee osteoarthritis–the RESTORE trial protocol. BMC Musculoskelet. Disord. 2018, 19, 272. [Google Scholar] [CrossRef] [Green Version]
- Delgado, D.; Garate, A.; Vincent, H.; Bilbao, A.M.; Patel, R.; Fiz, N.; Sampson, S.; Sanchez, M. Current concepts in intraosseous platelet-rich plasma injections for knee osteoarthritis. J. Clin. Orthop. Trauma. 2019, 10, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Delgado, D.; Pompei, O.; Perez, J.C.; Sanchez, P.; Garate, A.; Bilbao, A.M.; Fiz, N.; Padilla, S. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: An observational study. Cartilage 2019, 10, 245–253. [Google Scholar] [CrossRef]
- Vyas, C.; Mishbak, H.; Cooper, G.; Peach, C.; Pereira, R.F.; Bartolo, P. Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanuf. Rev. 2020, 5, 2. [Google Scholar] [CrossRef]
- Bianco, P. “Mesenchymal” Stem Cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The international Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic properties of mesenchymal stromal/Stem Cells: The need of cell priming for cell-free therapies in regenerative medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Matula, Z.; Nemeth, A.; Lorincz, P.; Szepesi, A.; Brozik, A.; Buzas, E.I.; Low, P.; Nemet, K.; Uher, F.; Urban, V.S. The role of extracellular vesicle and tunneling nanotubes-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cell. Stem Cell Dev. 2016, 25, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells and their potential for microengineering the chondrocyte niche. EBioMedicine 2015, 2, 1560–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.L.; Wagers, A.J. No place like home: Anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008, 9, 11–21. [Google Scholar] [CrossRef]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human adipose stem cells: From bench to bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef] [Green Version]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef]
- Harrel, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Tirino, V.; Desiderio, V.; Ferraro, G.; D’Andrea, F.; Giuliano, M.; Libondi, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS ONE 2009, 4, e6537. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): An overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived m esenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.A.; De Francesco, F.; Nicoletti, G.; Paino, F.; Desiderio, V.; Tirino, V.; D’Andrea, F. Human adipose CD34+CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissue. J. Cell Biochem. 2013, 114, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.; De Francesco, F.; Ferraro, G.A.; Desiderio, V.; Tirino, V.; De Rosa, A.; Papaccio, G. Large-scale production of human adipose tissue from stem cells: A new tool for regenerative medicine and tissue banking. Tissue Eng. Part C Methods 2008, 14, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, G.F.; De Francesco, F.; D’Andrea, F.; Ferraro, G.A. Methods and procedures in adipose stem cells: State of the art and perspective for translation medicine. J. Cell Physiol. 2015, 230, 489–495. [Google Scholar] [CrossRef]
- Pagani, S.; Veronesi, F.; Giavaresi, G.; Filardo, G.; Papio, T.; Romandini, I.; Fini, M. Autologous protein soluction effect on chondrogenic differentiation of mesenchymal stem cells from adipose tissue and bone marrow in an osteoarthritic environment. Cartilage 2021, 15, 1947603521993217. [Google Scholar]
- Gaut, C.; Sugaya, K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen. Med. 2015, 10, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Trumbull, A.; Subramanian, G.; Yildirim-Ayan, E. Mechanoresponsive musculoskeletal tissue differentiation of adipose-derived stem cells. Biomed. Eng. Online 2016, 15, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.A.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal Stem/Stromal Cells: A new “cells as drugs” paradigm. Effic. Crit. Asp. Cell Ther. Curr. Pharm. Design 2013, 19, 13. [Google Scholar]
- De Francesco, F.; Mannucci, S.; Conti, G.; Dai Prè, E.; Sbarbati, A.; Riccio, M. A Non-enzymatic method to obtain a fat tissue derivatite highly enriched in adipose stem cells (ASCs) from human lipoaspirates: Preliminary results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, K.; Speidel, A.T.; Yamato, M. Four Food and Drug Administration draft guidance documents and the REGROW Act: A litmus test for future changes in human cell- and tissue-based products regulatory policy in the United States? J. Tissue Eng. Regen. Med. 2018, 12, 1579–1593. [Google Scholar] [CrossRef] [Green Version]
- Raposio, E.; Ciliberti, R.G. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64. [Google Scholar] [CrossRef]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): Enzymatic digestion versus mechanical centrifugation. Int. J. Mol. Sci. 2019, 20, 5471. [Google Scholar] [CrossRef] [Green Version]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef] [Green Version]
- Tremolada, C.; Colombo, C.; Ventura, C. Adipose tissue and mesenchymal stem cells: State of the art and lipogems technology development. Curr. Stem. Cell. Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef] [Green Version]
- Condè-Green, A.; Kotamarti, V.S.; Sherman, L.S.; Keith, J.D.; Lee, E.S.; Granick, M.S.; Rameshwar, P. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: Review of upcoming techniques. Plast. Reconstr. Surg. Global Open 2016, 4, e1017. [Google Scholar] [CrossRef]
- Ferguson, R.E.H.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L.Q. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast. Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cohen, S.R.; Hicok, K.C.; Shanahan, R.K.; Strem, B.M.; Yu, J.C.; Arm, D.M.; Fraser, J.K. Comparison of three different fat graft preparation methods: Gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast. Reconstr. Surg. 2013, 131, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Patel, P.; Li, H.; Huang, L.T.; Wan, H.; Collins, S.; Connell, T.L.; Xu, H. Physical, biochemical, and biologic properties of fat graft processed via different methods. Plast. Reconstr. Surg. Global Open 2020, 8, e3010. [Google Scholar]
- De Fazio, D.; Cingozoglu, C.A.C. Combined mastopexy and augmentation with autologous fat grafting: First results with lipopexy. Plast. Reconstr. Surg. Global Open 2020, 8, e1957. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raddaini, M.; Tremolada, C.; et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Peault, B. Higher Perycite content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Randelli, P.; Menon, A.; Ragone, V.; Creo, P.; Bergante, S.; Randelli, F.; De Girolamo, L.; Montrasio, U.A.; Banfi, G.; Cabitza, P.; et al. Lipogems product treatment increases the proliferation rate of human tendon stem cells without affecting their stemness and differentiation capability. Stem Cells Int. 2016, 2016, 4373410. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.A.; Wilson, M.; Togashi, R.; Han, B.; Mircheff, A.K.; Thomas Vangsness, C., Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: A study protocol. BMC Musculoskelet. Disord. 2018, 19, 383. [Google Scholar] [CrossRef]
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New medical device rigeneracons allows to obtain viable micrografts from mechanical disaggregation of human tissues. J. Cell Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef]
- Dai Prè, E.; Busato, A.; Mannucci, S.; Vurro, F.; De Francesco, F.; Riccio, V.; Solito, S.; Biswas, R.; Bernardi, P.; Riccio, M.; et al. In Vitro characterization of adipose stem cells non-enzymatically extracted from the thigh and abdomen. Int. J. Mol. Sci. 2020, 21, 3081. [Google Scholar] [CrossRef]
- Raposio, E.; Caruana, G.; Petrella, M.; Bonomini, M.P.; Grieco, A. A standardized method of isolating adipose-derived stem cells for clinical application. Ann. Plast. Surg. 2016, 76, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Simonacci, F.; Perrotta, R.E. Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann. Med. Surg. 2017, 20, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A.; et al. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: Preliminary results. Front. Cell Dev. Biol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, A.; Riccio, V.; Riccio, M.; Sbarbati, A. Simple and Rapid Non-enzymatic procedure allows the isolation of structurally preserved connective tissue micro-fragments enriched with SVF. Cells 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Isola, A.; Chen, S. Exosomes: The messengers of health and disease. Curr. Neuropharmacol. 2016, 15, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017, 9, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagn, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, M.; Cosenza, S.; Maumus, M.; Jorgensen, C.; Noel, D. Therapeutic application of mesenchymal stem cells in osteoarthritis. Expert Opin. Biol. Ther. 2016, 16, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tofino-Vian, M.; Guillen, M.I.; Perez Del Caz, M.D.; Castejon, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid. Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [Green Version]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef] [Green Version]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Lugano, G.; Viganò, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; De Girolamo, L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 2019, 10, 109. [Google Scholar] [CrossRef]
- Daher, S.R.; Johnstone, B.H.; Phinney, D.G.; March, K.L. Adipose stromal/stem cells: Basic and translational advances. The IFATS collection. Stem Cells 2008, 26, 2664–2665. [Google Scholar] [CrossRef]
- Tang, T.T.; Wang, B.; Lv, L.L.; Liu, B.C. Extracellular vesicles-based nanotherapeutics: Emerging frontiers in anti-inflammatory therapy. Theranostics 2020, 10, 8111. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, L.; Pekała, P.; Zabrzynski, J.; Kruczy, J. Genetics in Cartilage Lesions: Basic Science and Therapy Approaches. IJMS 2020, 21, 5430. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Zhang, W.; Riches, P.; Li, B.; Shu, W. 3D biofabrication for soft tissue and cartilage engineering. Med. Eng. Phys. 2020, 82, 13–39. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; O’Connor, A.J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: A path towords the development of a clinical treatment. Biofabrication 2018, 10, 045006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.P.; Moses, J.C.; Bhardwaj, N.; Mandal, B.B. Overcoming the dependence on animal models for osteoarthtitis therapeutics- the promises and prospects of in vitro model. Adv. Healthc. Mater. 2021, 24, e2100961. [Google Scholar] [CrossRef]
- Brun, P.; Cortivo, R.; Zavan, B.; Vecchiato, N.; Abatangelo, G. In vitro reconstructed tissues on hyaluronan-based temporary scaffolding. J. Mater. Sci. Mater. Med. 1999, 10, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Figallo, E.; Flaibani, M.; Zavan, B.; Abatangelo, G.; Elvassore, N. Micropatterned Biopolymer 3D Scaffold for Static and Dynamic Culture of Human Fibroblasts. Biotechnol. Prog. 2007, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Bressan, E.; Ferroni, L.; Nalesso, E.; Vindigni, V.; Stellini, E.; Pinton, P.; Sivolella, S.; Zavan, B. In Vitro Concurrent Endothelial and Osteogenic Commitment of Adipose-Derived Stem Cells and Their Genomical Analyses Through Comparative Genomic Hybridization Array: Novel Strategies to Increase the Successful Engraftment of Tissue-Engineered Bone Grafts. Stem Cells Dev. 2012, 21, 767–777. [Google Scholar] [CrossRef]
- Azzena, B.; Mazzoleni, F.; Abatangelo, G.; Zavan, B.; Vindigni, V. Autologous Platelet-Rich Plasma as an Adipocyte In Vivo Delivery System: Case Report. Aesthet. Plast. Surg. 2008, 32, 155–158. [Google Scholar] [CrossRef]
- Ettorre, V.; De Marco, P.; Zara, S.; Perrotti, V.; Scarano, A.; Di Crescenzo, A.; Petrini, M.; Hadad, C.; Bosco, D.; Zavan, B.; et al. In vitro and in vivo characterization of graphene oxide coated porcine bone granules. Carbon 2016, 103, 291–298. [Google Scholar] [CrossRef]


| Grade of Osteoarthritis | OARSI Score | Radiographic Score (Ahlback) | Radiographic Score (Kellgren–Lawrence) |
|---|---|---|---|
| Grade 0 | Normal | No radiographic findings of OA | No radiographic findings of OA |
| Grade 1 | Small fibrillations without loss of cartilage | Joint space narrowing <3 mm | Doubtful joint space narrowing and possible osteophytic lipping |
| Grade 2 | Vertical clefts down to the layer immediately below the superficial layer and some loss of surface lamina | Joint space obliterated or almost obliterated | Definite osteophytes and possible joint space narrowing |
| Grade 3 | Vertical clefts/erosion to the calcified cartilage extending to <25% of the articular surface | Minor bone attrition (<5 mm) | Multiple osteophytes, definite joint space narrowing, sclerosis, possible bony deformity |
| Grade 4 | Vertical clefts/erosion to the calcified cartilage extending to 25–50% of the articular surface | Moderate bone attrition (5–15 mm) | Large osteophytes, marked narrowing of joint space, severe sclerosis, and definite deformity of bone ends |
| Grade 5 | Vertical clefts/erosion to the calcified cartilage extending to 50–75% of the articular surface | Severe bone attrition (>15 mm) | |
| Grade 6 | Vertical clefts/erosion to the calcified cartilage extending to >75% of the articular surface |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M.; et al. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. Int. J. Mol. Sci. 2021, 22, 10197. https://doi.org/10.3390/ijms221910197
De Francesco F, Gravina P, Busato A, Farinelli L, Soranzo C, Vidal L, Zingaretti N, Zavan B, Sbarbati A, Riccio M, et al. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. International Journal of Molecular Sciences. 2021; 22(19):10197. https://doi.org/10.3390/ijms221910197
Chicago/Turabian StyleDe Francesco, Francesco, Pasquale Gravina, Alice Busato, Luca Farinelli, Carlo Soranzo, Luis Vidal, Nicola Zingaretti, Barbara Zavan, Andrea Sbarbati, Michele Riccio, and et al. 2021. "Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease" International Journal of Molecular Sciences 22, no. 19: 10197. https://doi.org/10.3390/ijms221910197
APA StyleDe Francesco, F., Gravina, P., Busato, A., Farinelli, L., Soranzo, C., Vidal, L., Zingaretti, N., Zavan, B., Sbarbati, A., Riccio, M., & Gigante, A. (2021). Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. International Journal of Molecular Sciences, 22(19), 10197. https://doi.org/10.3390/ijms221910197










